Synthesis and Characterization of p-n Junction Ternary Mixed Oxides for Photocatalytic Coprocessing of CO2 and H2O
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis, Composition, and Size of Ternary Oxides
2.2. XPS Analysis
Valence Band Maximum Evaluation by XPS
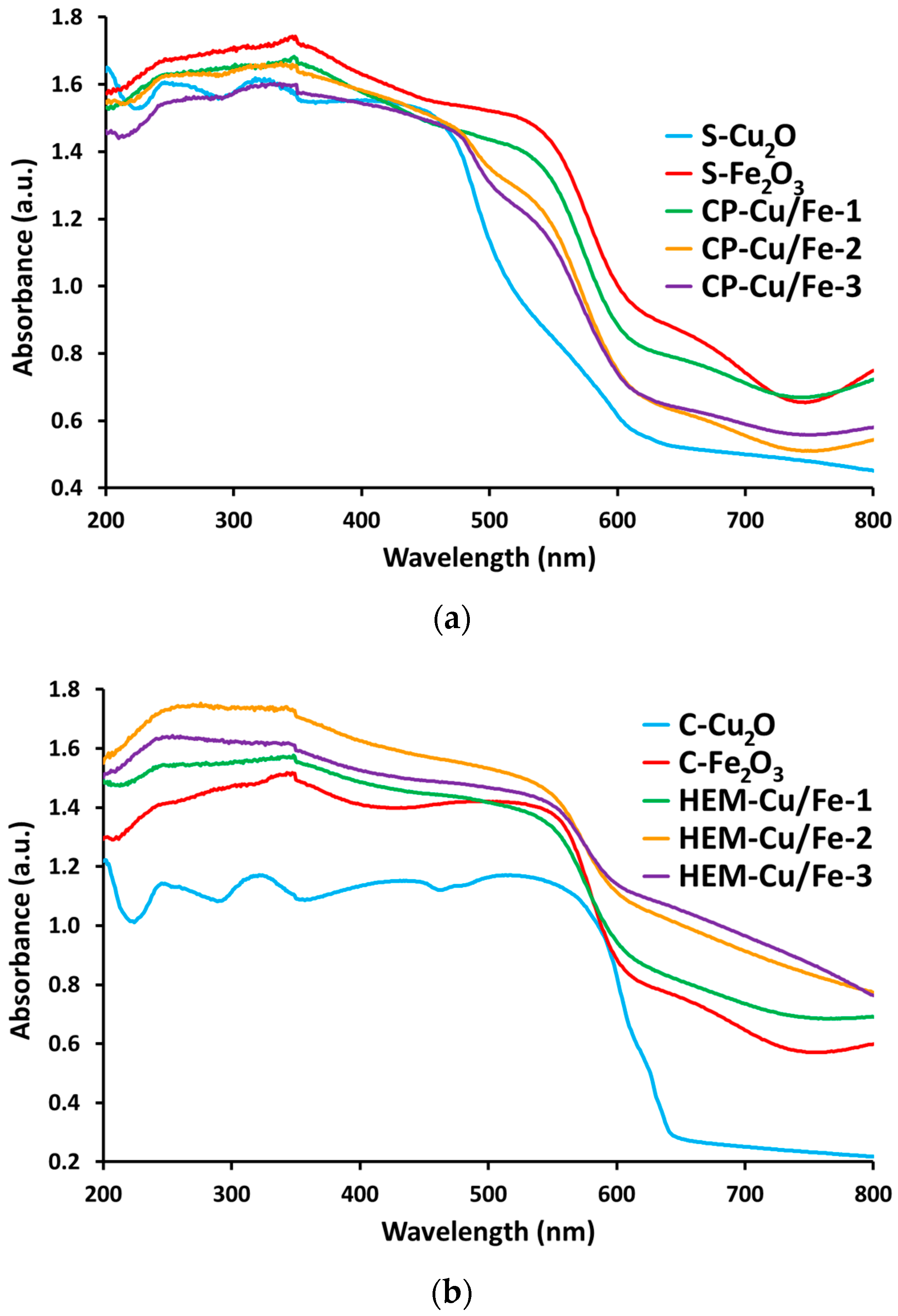
2.3. UV-Visible Spectroscopy Characterization
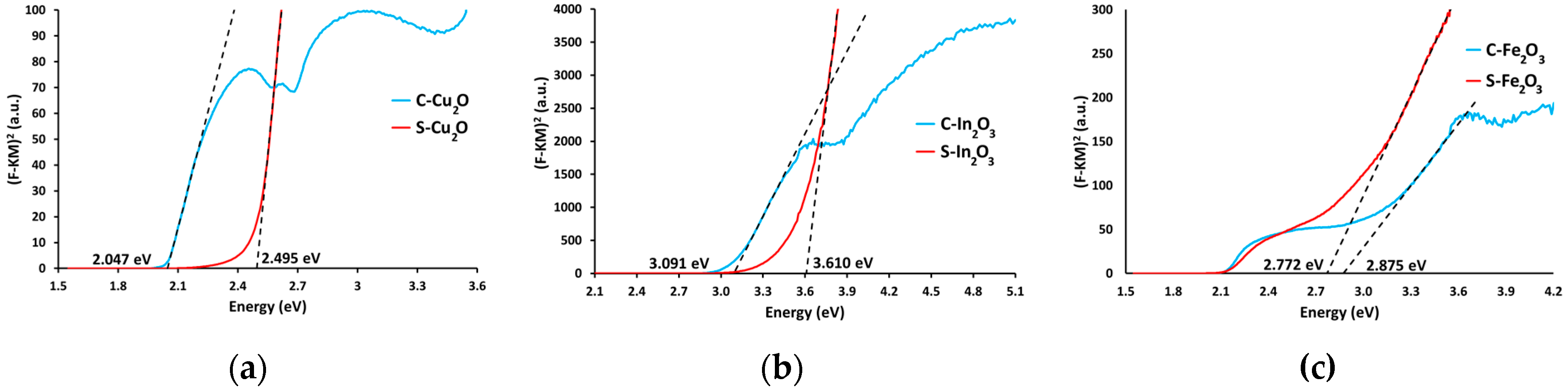
Band-Gap Evaluation by UV-Visible Spectroscopy
2.4. Band Structure Evaluation and Discussion
2.5. Photocatalytic Activity
3. Materials and Methods
3.1. Preparation of Oxides
3.1.1. Cu2O
3.1.2. In2O3
3.1.3. Fe2O3
3.1.4. Preparation of Mixed Oxides by Coprecipitation Using Synthesized Binary Oxides
- (a)
- 3.5929, 1.5698, and 0.8121 g of S-In2O3 nano-powder were dispersed in CuSO4 aqueous solution for the ratios Cu/In-1, 2, and 3, respectively, and treated as reported above.
- (b)
- 2.1781, 0.8799, and 0.7287 g of S-Fe2O3 powder were dispersed in CuSO4 aqueous solution for ratios Cu/Fe-1, 2, and 3, respectively and reacted as reported in the general procedure.
3.1.5. Preparation of Mixed Oxides by High Energy Milling-HEM Using Commercial Samples
- (a)
- for Cu/In-1, 0.5469 g of C-Cu2O and 1.9863 g of C-In2O3 were mixed.
- (b)
- for Cu/In-2, 0.8579 g of C-Cu2O and 1.6354 g of C-In2O3 were mixed.
- (c)
- for Cu/In-3, 1.2705 g of C-Cu2O and 1.2304 g of C-In2O3 were mixed.
- (d)
- For Cu/Fe-1, 0.7740 g of C-Cu2O and 1.7332 g of C-Fe2O3 were mixed.
- (e)
- For Cu/Fe-2, 1.4252 g of C-Cu2O and 1.5915 g of C-Fe2O3 were mixed.
- (f)
- For Cu/Fe-3, 1.6086 g of C-Cu2O and 0.8969 g of C-Fe2O3 were mixed.
3.2. Characterization
3.3. Photocatalytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Global Monitoring Laboratory-Global Greenhouse Gas Reference Network. Available online: https://www.esrl.noaa.gov/gmd/ccgg/trends/global.html (accessed on 8 April 2020).
- Aresta, M.; DiBenedetto, A.; Quaranta, E. State of the art and perspectives in catalytic processes for CO2 conversion into chemicals and fuels: The distinctive contribution of chemical catalysis and biotechnology. J. Catal. 2016, 343, 2–45. [Google Scholar] [CrossRef]
- Aresta, M.; DiBenedetto, A.; Angelini, A. The changing paradigm in CO2 utilization. J. CO2 Util. 2013, 3, 65–73. [Google Scholar] [CrossRef]
- Pesnell, D.; Addison, K. SDO | Solar Dynamics Observatory. Available online: https://sdo.gsfc.nasa.gov/ (accessed on 13 April 2020).
- Ali, S.; Flores, M.C.; Razzaq, A.; Sorcar, S.; Hiragond, C.B.; Kim, H.R.; Park, Y.H.; Hwang, Y.; Kim, H.S.; Kim, H.; et al. Gas phase photocatalytic CO2 reduction, “A brief overview for benchmarking”. Catalysts 2019, 9, 727. [Google Scholar] [CrossRef]
- Clarizia, L.; Russo, D.; Di Somma, I.; Andreozzi, R.; Marotta, R. Hydrogen generation through solar photocatalytic processes: A review of the configuration and the properties of effective metal-based semiconductor nanomaterials. Energies 2017, 10, 1624. [Google Scholar] [CrossRef]
- Babu, V.J.; Vempati, S.; Uyar, T.; Ramakrishna, S. Review of one-dimensional and two-dimensional nanostructured materials for hydrogen generation. Phys. Chem. Chem. Phys. 2015, 17, 2960–2986. [Google Scholar] [CrossRef]
- Inoue, T.; Fujishima, A.; Konishi, S.; Honda, K. Photoelectrocatalytic reduction of carbon dioxide in aqueous suspensions of semiconductor powders. Nature 1979, 277, 637–638. [Google Scholar] [CrossRef]
- Lehn, J.-M.; Ziessel, R. Photochemical generation of carbon monoxide and hydrogen by reduction of carbon dioxide and water under visible light irradiation. Proc. Natl. Acad. Sci. USA 1982, 79, 701–704. [Google Scholar] [CrossRef]
- Stolarczyk, J.K.; Bhattacharyya, S.; Polavarapu, L.; Feldmann, J. Challenges and prospects in solar water splitting and CO2 reduction with inorganic and hybrid nanostructures. ACS Catal. 2018, 8, 3602–3635. [Google Scholar] [CrossRef]
- Li, K.; Peng, B.; Peng, T. Recent advances in heterogeneous photocatalytic CO2 conversion to solar fuels. ACS Catal. 2016, 6, 7485–7527. [Google Scholar] [CrossRef]
- Nikokavoura, A.; Trapalis, C. Alternative photocatalysts to TiO2 for the photocatalytic reduction of CO2. Appl. Surf. Sci. 2017, 391, 149–174. [Google Scholar] [CrossRef]
- Baran, T.; Wojtyła, S.; DiBenedetto, A.; Aresta, M.; Macyk, W. Zinc sulfide functionalized with ruthenium nanoparticles for photocatalytic reduction of CO2. Appl. Catal. B Environ. 2015, 178, 170–176. [Google Scholar] [CrossRef]
- Baran, T.; Wojtyła, S.; DiBenedetto, A.; Aresta, M.; Macyk, W. Photocatalytic carbon dioxide reduction at p-Type Copper(I) Iodide. ChemSusChem 2016, 9, 2933–2938. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yu, J.; Jaroniec, M.; Chen, X.-B. Cocatalysts for selective photoreduction of CO2into solar fuels. Chem. Rev. 2019, 119, 3962–4179. [Google Scholar] [CrossRef]
- Védrine, J.C. Heterogeneous catalysis on metal oxides. Catalysts 2017, 7, 341. [Google Scholar] [CrossRef]
- Roy, D.; Samu, G.F.; Hossain, M.K.; Janáky, C.; Rajeshwar, K. On the measured optical bandgap values of inorganic oxide semiconductors for solar fuels generation. Catal. Today 2018, 300, 136–144. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Y.; Huang, B.; Dai, Y.; Lou, Z.; Wang, G.; Zhang, X.; Qin, X. Progress on extending the light absorption spectra of photocatalysts. Phys. Chem. Chem. Phys. 2014, 16, 2758. [Google Scholar] [CrossRef] [PubMed]
- Christoforidis, K.C.; Fornasiero, P. Photocatalysis for hydrogen production and Co2 reduction: The case of copper-catalysts. ChemCatChem 2018, 11, 368–382. [Google Scholar] [CrossRef]
- Luévano-Hipólito, E.; Torres-Martínez, L.; Martínez, D.S.; Cruz, M.A. Cu2O precipitation-assisted with ultrasound and microwave radiation for photocatalytic hydrogen production. Int. J. Hydrog. Energy 2017, 42, 12997–13010. [Google Scholar] [CrossRef]
- Aguirre, M.E.; Zhou, R.; Eugene, A.J.; Guzman, M.I.; Grela, M.A. Cu2O/TiO2 heterostructures for CO2 reduction through a direct Z-scheme: Protecting Cu2O from photocorrosion. Appl. Catal. B Environ. 2017, 217, 485–493. [Google Scholar] [CrossRef]
- An, X.; Li, K.; Tang, J. Cu2O/reduced graphene oxide composites for the photocatalytic conversion of CO2. ChemSusChem 2014, 7, 1086–1093. [Google Scholar] [CrossRef]
- Xiong, Z.; Lei, Z.; Kuang, C.-C.; Chen, X.; Gong, B.; Zhao, Y.; Zhang, J.; Zheng, C.; Wu, J.C. Selective photocatalytic reduction of CO2 into CH4 over Pt-Cu2O TiO2 nanocrystals: The interaction between Pt and Cu2O cocatalysts. Appl. Catal. B Environ. 2017, 202, 695–703. [Google Scholar] [CrossRef]
- Handoko, A.D.; Tang, J. Controllable proton and CO2 photoreduction over Cu2O with various morphologies. Int. J. Hydrog. Energy 2013, 38, 13017–13022. [Google Scholar] [CrossRef]
- Bierwagen, O. Indium oxide—A transparent, wide-band gap semiconductor for (opto)electronic applications. Semicond. Sci. Technol. 2015, 30, 024001. [Google Scholar] [CrossRef]
- Mishra, M.; Chun, D.-M. α-Fe2O3 as a photocatalytic material: A review. Appl. Catal. A Gen. 2015, 498, 126–141. [Google Scholar] [CrossRef]
- Cao, S.-W.; Liu, X.-F.; Yuan, Y.-P.; Zhang, Z.; Liao, Y.; Fang, J.; Loo, S.C.J.; Sum, T.C.; Xue, C. Solar-to-fuels conversion over In2O3/g-C3N4 hybrid photocatalysts. Appl. Catal. B Environ. 2014, 147, 940–946. [Google Scholar] [CrossRef]
- Tahir, M.; Tahir, B.; Amin, N.A.S.; Muhammad, A. Photocatalytic CO2 methanation over NiO/In2O3 promoted TiO2 nanocatalysts using H2O and/or H2 reductants. Energy Convers Manag. 2016, 119, 368–378. [Google Scholar] [CrossRef]
- Hoch, L.B.; He, L.; Qiao, Q.; Liao, K.; Reyes, L.M.; Zhu, Y.; Ozin, G.A. Effect of precursor selection on the photocatalytic performance of indium oxide nanomaterials for gas-phase CO2 reduction. Chem. Mater. 2016, 28, 4160–4168. [Google Scholar] [CrossRef]
- Hu, B.; Guo, Q.; Wang, K.; Wang, X.T. Enhanced photocatalytic activity of porous In2O3 for reduction of CO2 with H2O. J. Mater. Sci. Mater. Electron. 2019, 30, 7950–7962. [Google Scholar] [CrossRef]
- Ba-Abbad, M.M.; Takriff, M.S.; Benamor, A.; Mohammad, A.W. Size and shape controlled of α-Fe2O3 nanoparticles prepared via sol–gel technique and their photocatalytic activity. J. Sol-Gel Sci. Technol. 2016, 81, 880–893. [Google Scholar] [CrossRef]
- Boumaza, S.; Kabir, H.; Gharbi, I.; Belhadi, A.; Trari, M. Preparation and photocatalytic H2 -production on α-Fe2 O3 prepared by sol-gel. Int. J. Hydrog. Energy 2018, 43, 3424–3430. [Google Scholar] [CrossRef]
- Liu, J.; Ke, J.; Li, D.; Sun, H.; Liang, P.; Duan, X.; Tian, W.; Tade, M.; Liu, S.; Wang, S. Oxygen vacancies in shape controlled Cu2O/reduced graphene oxide/In2O3 hybrid for promoted photocatalytic water oxidation and degradation of environmental pollutants. ACS Appl. Mater. Interfaces 2017, 9, 11678–11688. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Y.; Zhang, J.-N.; Ye, J.-H.; Ma, X.-N.; Ke, J. Construction of Cu₂O/In₂O₃ hybrids with p-n heterojunctions for enhanced photocatalytic performance. J. Nanosci. Nanotechnol. 2019, 19, 7689–7695. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Dong, B. Construction of novel Z-scheme Cu2O/graphene/α-Fe2O3 nanotube arrays composite for enhanced photocatalytic activity. Ceram. Int. 2017, 43, 16007–16012. [Google Scholar] [CrossRef]
- Lakhera, S.K.; Watts, A.; Hafeez, H.Y.; Neppolian, B. Interparticle double charge transfer mechanism of heterojunction α-Fe2O3/Cu2O mixed oxide catalysts and its visible light photocatalytic activity. Catal. Today 2018, 300, 58–70. [Google Scholar] [CrossRef]
- Shen, H.; Liu, G.; Yan, X.; Jiang, J.; Hong, Y.; Yan, M.; Mao, B.; Li, D.; Fan, W.; Shi, W. All-solid-state Z-scheme system of RGO-Cu2O/Fe2O3 for simultaneous hydrogen production and tetracycline degradation. Mater. Today Energy 2017, 5, 312–319. [Google Scholar] [CrossRef]
- Li, P.; Jing, H.; Xu, J.; Wu, C.; Peng, H.; Lu, J.; Lu, F. High-efficiency synergistic conversion of CO2 to methanol using Fe2O3 nanotubes modified with double-layer Cu2O spheres. Nanoscale 2014, 6, 11380–11386. [Google Scholar] [CrossRef]
- Wang, J.-C.; Zhang, L.; Fang, W.-X.; Ren, J.; Li, Y.-Y.; Yao, H.-C.; Wang, J.-S.; Li, Z.-J. Enhanced photoreduction CO2 activity over direct Z-scheme α-Fe2O3/Cu2O heterostructures under visible light irradiation. ACS Appl. Mater. Interfaces 2015, 7, 8631–8639. [Google Scholar] [CrossRef]
- Heinemann, M.; Eifert, B.; Heiliger, C. Band structure and phase stability of the copper oxides Cu2O, CuO, and Cu4O3. Phys. Rev. B 2013, 87, 87. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.; Gerson, A.R.; Smart, R.S. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Biesinger, M.C. Advanced analysis of copper X-ray photoelectron spectra. Surf. Interface Anal. 2017, 49, 1325–1334. [Google Scholar] [CrossRef]
- X-ray Photoelectron Spectroscopy (XPS) Reference Pages: Indium. Available online: http://www.xpsfitting.com/search/label/Indium (accessed on 9 May 2020).
- Barr, T.L.; Ying, L.L. An x-ray photoelectron spectroscopy study of the valence band structure of indium oxides. J. Phys. Chem. Solids 1989, 50, 657–664. [Google Scholar] [CrossRef]
- Grosvenor, A.; Kobe, B.A.; Biesinger, M.C.; McIntyre, N.S. Investigation of multiplet splitting of Fe 2p XPS spectra and bonding in iron compounds. Surf. Interface Anal. 2004, 36, 1564–1574. [Google Scholar] [CrossRef]
- Radu, T.; Iacovita, C.; Benea, D.; Turcu, R. X-ray photoelectron spectroscopic characterization of iron oxide nanoparticles. Appl. Surf. Sci. 2017, 405, 337–343. [Google Scholar] [CrossRef]
- Kraut, E.A.; Grant, R.W.; Waldrop, J.R.; Kowalczyk, S.P. Precise determination of the valence-band edge in x ray photoemission spectra. Phys. Rev. Lett. 1980, 44, 1620–1623. [Google Scholar] [CrossRef]
- Kraut, E.A.; Grant, R.W.; Waldrop, J.R.; Kowalczyk, S.P. Semiconductor core-level to valence-band maximum binding-energy differences: Precise determination by x-ray photoelectron spectroscopy. Phys. Rev. B 1983, 28, 1965–1977. [Google Scholar] [CrossRef]
- Chambers, S.A.; Droubay, T.C.; Kaspar, T.; Gutowski, M. Experimental determination of valence band maxima for SrTiO3, TiO2 and SrO and the associated valence band offsets with Si(001). J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. 2004, 22, 2205. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, D.; Wu, Q.; Diao, P. Cu2O/CuO bilayered composite as a high-efficiency photocathode for photoelectrochemical hydrogen evolution reaction. Sci. Rep. 2016, 6, 35158. [Google Scholar] [CrossRef]
- Wang, Y.; Lany, S.; Ghanbaja, J.; Fagot-Revurat, Y.; Chen, Y.P.; Soldera, F.; Horwat, D.; Mücklich, F.; Pierson, J.-F. Electronic structures of Cu2O,Cu4O3, and CuO: A joint experimental and theoretical study. Phys. Rev. B 2016, 94, 245418. [Google Scholar] [CrossRef]
- Xu, Y.; Schoonen, M.A. The absolute energy positions of conduction and valence bands of selected semiconducting minerals. Am. Miner. 2000, 85, 543–556. [Google Scholar] [CrossRef]
- Erhart, P.; Klein, A.; Egdell, R.G.; Albe, K. Band structure of indium oxide: Indirect versus direct band gap. Phys. Rev. B 2007, 75, 75. [Google Scholar] [CrossRef]
- Dixon, S.C.; Scanlon, D.O.; Carmalt, C.J.; Parkin, I.P. n-Type doped transparent conducting binary oxides: An overview. J. Mater. Chem. C 2016, 4, 6946–6961. [Google Scholar] [CrossRef]
- Piccinin, S. The band structure and optical absorption of hematite (α-Fe2O3): A first-principles GW-BSE study. Phys. Chem. Chem. Phys. 2019, 21, 2957–2967. [Google Scholar] [CrossRef] [PubMed]
- Temesghen, W.; Sherwood, P. Analytical utility of valence band X-ray photoelectron spectroscopy of iron and its oxides, with spectral interpretation by cluster and band structure calculations. Anal. Bioanal. Chem. 2002, 373, 601–608. [Google Scholar] [CrossRef]
- Luan, P.; Xie, M.; Liu, D.; Fu, X.; Jing, L. Effective charge separation in the rutile TiO2 nanorod-coupled α-Fe2O3 with exceptionally high visible activities. Sci. Rep. 2014, 4, 6180. [Google Scholar] [CrossRef]
- Dolgonos, A.; Mason, T.O.; Poeppelmeier, K.R. Direct optical band gap measurement in polycrystalline semiconductors: A critical look at the Tauc method. J. Solid State Chem. 2016, 240, 43–48. [Google Scholar] [CrossRef]
- Zanatta, A.R. Revisiting the optical bandgap of semiconductors and the proposal of a unified methodology to its determination. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
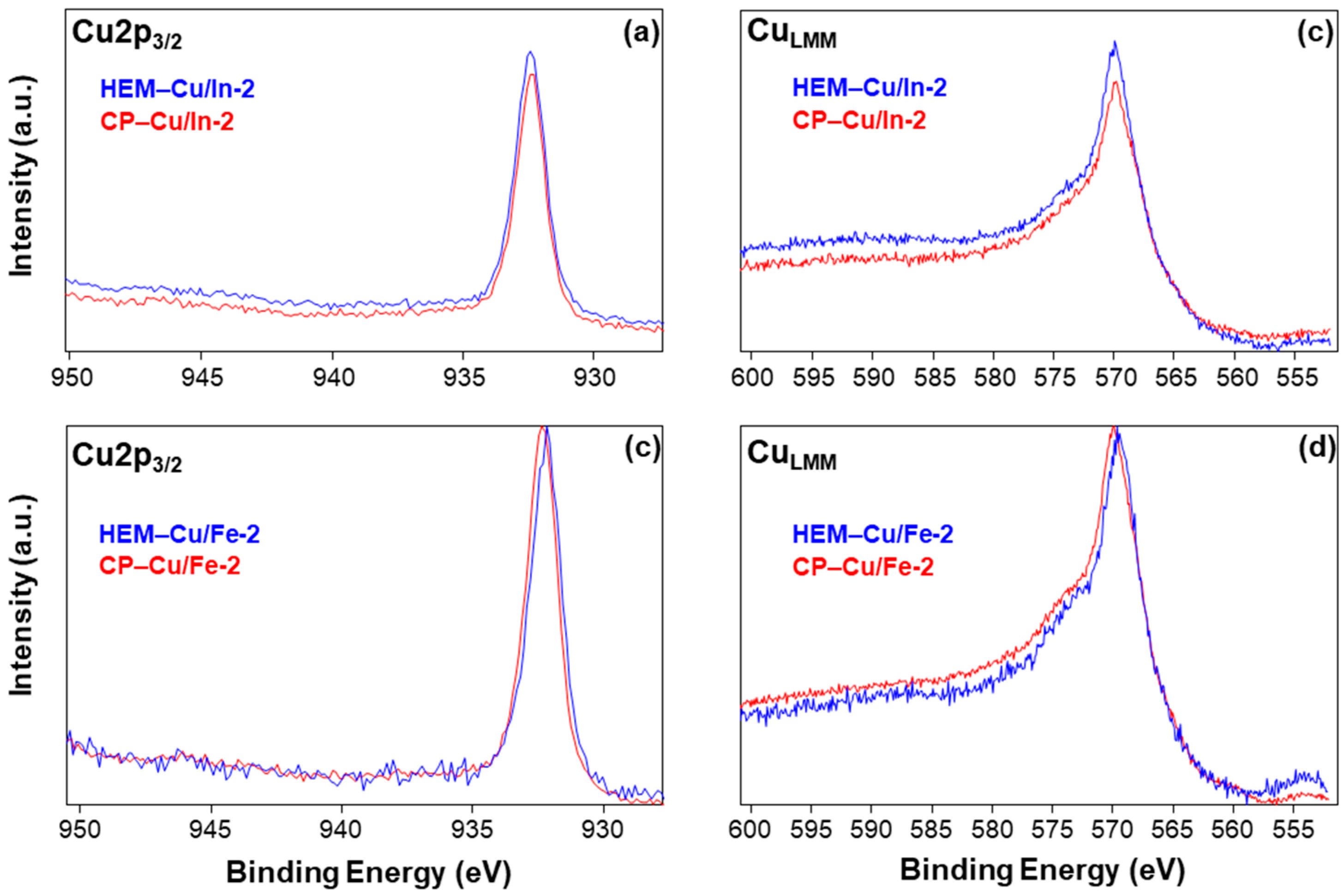
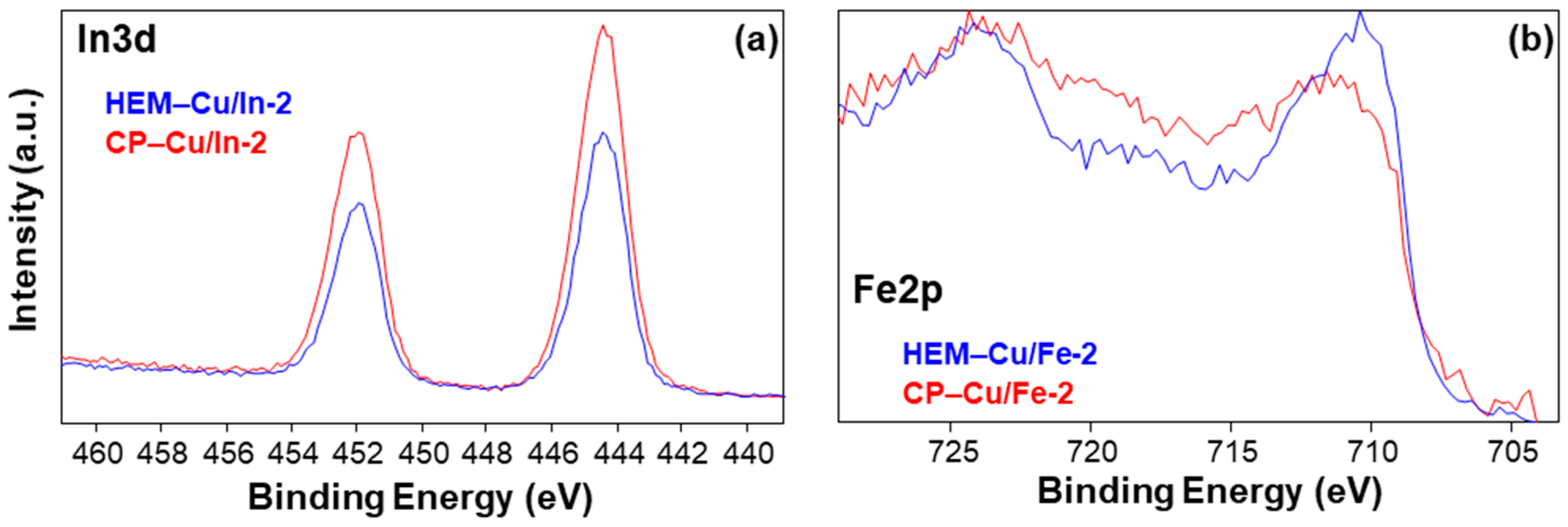
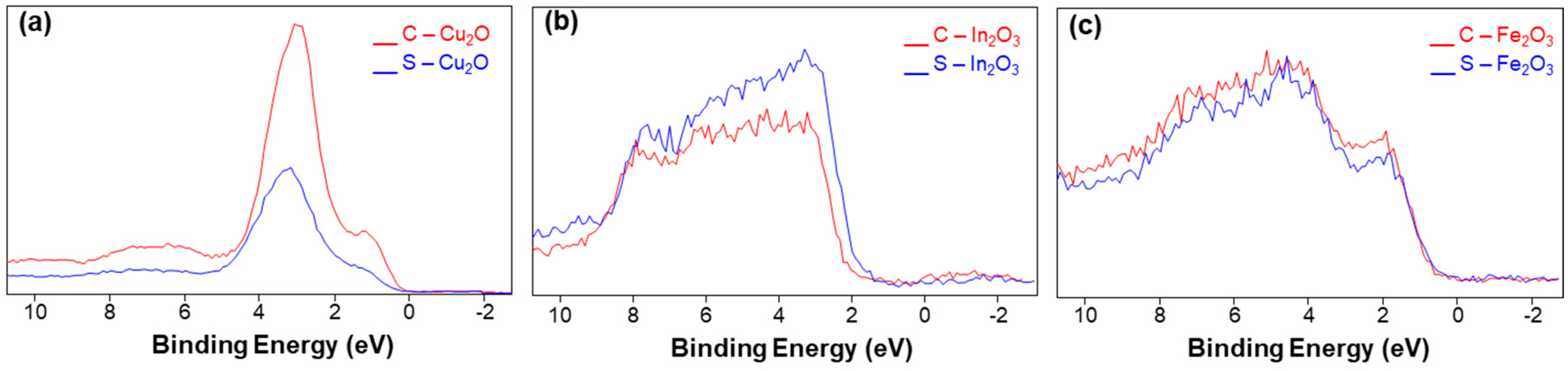
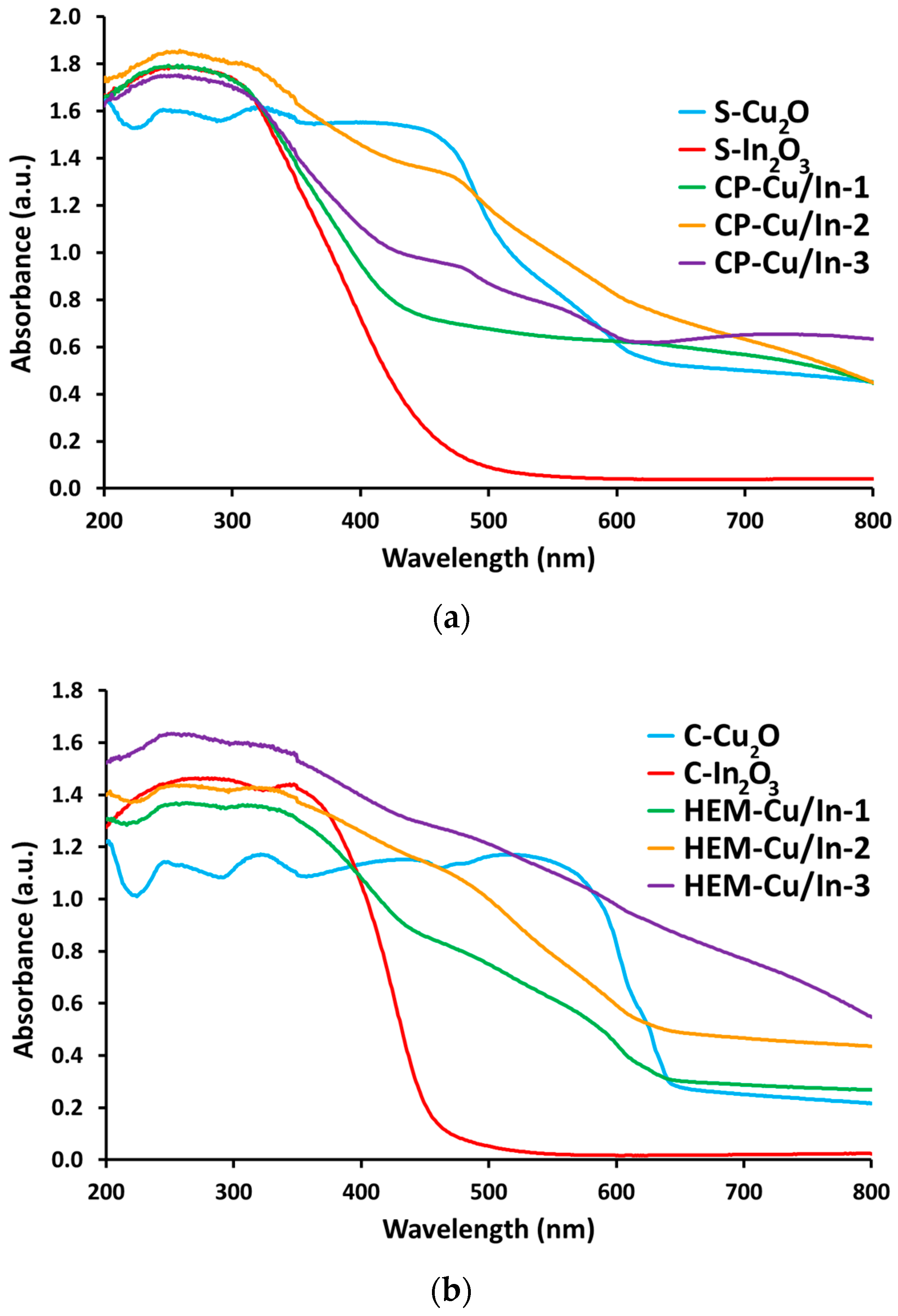

| Sample Name | Cu/In Ratio | Sample Name | Cu/Fe Ratio |
|---|---|---|---|
| HEM-Cu/In-1 | 0.60 | HEM-Cu/Fe-1 | 0.59 |
| HEM-Cu/In-2 | 1.08 | HEM-Cu/Fe-2 | 0.99 |
| HEM-Cu/In-3 | 2.20 | HEM-Cu/Fe-3 | 1.92 |
| CP-Cu/In-1 | 0.25 | CP-Cu/Fe-1 | 0.23 |
| CP-Cu/In-2 | 1.58 | CP-Cu/Fe-2 | 0.66 |
| CP-Cu/In-3 | 2.96 | CP-Cu/Fe-3 | 1.21 |
| Sample | Surface Content (at. %) | Metal Content Ratio | |||||
|---|---|---|---|---|---|---|---|
| C | O | Cu | In | Fe | Cu/In | Cu/Fe | |
| HEM-Cu/In-2 | 42 ± 7 | 39.1 ± 0.7 | 13 ± 5 | 7 ± 2 | - | 1.95 ± 0.20 | - |
| CP-Cu/In-2 | 26 ± 3 | 42.8 ± 1.0 | 17.4 ± 0.9 | 13.5 ± 1.2 | - | 1.29 ± 0.06 | - |
| HEM-Cu/Fe-2 | 40.0 ± 0.5 | 36.4 ± 0.5 | 13.9 ± 0.2 | - | 9.7 ± 0.2 | - | 1.47 ± 0.12 |
| CP-Cu/Fe-2 | 31.3 ± 1.0 | 45.8 ± 1.5 | 9.2 ± 0.2 | - | 14 ± 2 | - | 0.68 ± 0.13 |
| Sample | VBM (eV) | Sample | VBM (eV) |
|---|---|---|---|
| C-Cu2O | 1.65 ± 0.20 | S-Cu2O | 0.86 ± 0.14 |
| C-In2O3 | 2.20 ± 0.11 | S-In2O3 | 1.80 ± 0.15 |
| C-Fe2O3 | 1.53 ± 0.22 | S-Fe2O3 | 1.67 ± 0.23 |
| Sample | Optical Eg (eV) | Absorption Wavelength 1 (nm) |
|---|---|---|
| C-Cu2O | 2.047 | 605.7 |
| S-Cu2O | 2.495 | 497.0 |
| C-In2O3 | 3.091 | 401.1 |
| S-In2O3 | 3.610 | 337.9 |
| C-Fe2O3 | 2.875 | 431.3 |
| S-Fe2O3 | 2.772 | 447.3 |
| Sample | Eg (eV) | VBM (eV) | CBM (eV) | |
|---|---|---|---|---|
| Fundamental | Optical | |||
| C-Cu2O | 2.05 | 2.05 | 1.80 | −0.24 |
| S-Cu2O | 2.49 | 2.49 | 0.83 | −1.67 |
| C-In2O3 | 2.28 | 3.09 | 2.22 | −0.05 |
| S-In2O3 | 2.80 | 3.61 | 1.71 | −1.09 |
| C-Fe2O3 | 2.01 | 2.87 | 1.87 | −0.14 |
| S-Fe2O3 | 1.97 | 2.77 | 1.92 | −0.05 |
| Reaction System | Gaseous Reaction Products | ||
|---|---|---|---|
| H2 | CO | CH4 | |
| (a) | − | − | − |
| (b) | − | − | − |
| (c) | +++ | + | + |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcolongo, D.M.S.; Nocito, F.; Ditaranto, N.; Aresta, M.; Dibenedetto, A. Synthesis and Characterization of p-n Junction Ternary Mixed Oxides for Photocatalytic Coprocessing of CO2 and H2O. Catalysts 2020, 10, 980. https://doi.org/10.3390/catal10090980
Marcolongo DMS, Nocito F, Ditaranto N, Aresta M, Dibenedetto A. Synthesis and Characterization of p-n Junction Ternary Mixed Oxides for Photocatalytic Coprocessing of CO2 and H2O. Catalysts. 2020; 10(9):980. https://doi.org/10.3390/catal10090980
Chicago/Turabian StyleMarcolongo, Davide M. S., Francesco Nocito, Nicoletta Ditaranto, Michele Aresta, and Angela Dibenedetto. 2020. "Synthesis and Characterization of p-n Junction Ternary Mixed Oxides for Photocatalytic Coprocessing of CO2 and H2O" Catalysts 10, no. 9: 980. https://doi.org/10.3390/catal10090980
APA StyleMarcolongo, D. M. S., Nocito, F., Ditaranto, N., Aresta, M., & Dibenedetto, A. (2020). Synthesis and Characterization of p-n Junction Ternary Mixed Oxides for Photocatalytic Coprocessing of CO2 and H2O. Catalysts, 10(9), 980. https://doi.org/10.3390/catal10090980









