Abstract
Low-cost iron-based CO2 hydrogenation catalysts have shown promise as a viable route to the production of value-added hydrocarbon building blocks. It is envisioned that these hydrocarbons will be used to augment industrial chemical processes and produce drop-in replacement operational fuel. To this end, the U.S. Naval Research Laboratory (NRL) has been designing, testing, modeling, and evaluating CO2 hydrogenation catalysts in a laboratory-scale fixed-bed environment. To transition from the laboratory to a commercial process, the catalyst viability and performance must be evaluated at scale. The performance of a Macrolite®-supported iron-based catalyst in a commercial-scale fixed-bed modular reactor prototype was evaluated under different reactor feed rates and product recycling conditions. CO2 conversion increased from 26% to as high as 69% by recycling a portion of the product stream and CO selectivity was greatly reduced from 45% to 9% in favor of hydrocarbon production. In addition, the catalyst was successfully regenerated for optimum performance. Catalyst characterization by X-ray diffraction (XRD) and X-ray photoelectron spectroscopy (XPS), along with modeling and kinetic analysis, highlighted the potential challenges and benefits associated with scaling-up catalyst materials and processes for industrial implementation.
1. Introduction
Operating in a littoral and marine environment provides the U.S. Navy with unique access to a vast environmental resource of carbon. The world’s oceans are the largest carbon reservoirs containing approximately 38,000 gigatons [1]. Carbon and hydrogen are the principal building blocks needed to synthesize hydrocarbons. It is envisioned that these hydrocarbons may one day be used to produce operational fuel. Synthesizing “drop-in” replacement fuel at or near the point of use, translates into “Freedom of Action for the Warfighter” and potential long-term cost savings and strategic advantages for the Department of Defense (DOD) [2,3,4]. If the energy required for the process is nuclear or renewable, the entire low carbon fuel process could be considered CO2 neutral [5,6,7].
The future capability of producing fuel from inorganic carbon (CO2) and H2 in seawater is dependent on the development of processes and technologies specifically designed for such applications. The U.S. Navy has recently patented a process and an apparatus for the simultaneous extraction of CO2 and production of H2 from seawater [8,9,10]. However, the primary limitations in using the CO2 and H2 as building blocks for the synthesis of hydrocarbons are the high energy barrier for the redox and polymerization reactions necessary to synthesize longer chain molecules to be used as fuel [5,6,7,11]. While electrochemical and photochemical CO2 conversion processes in water continue to improve in efficiency, challenges remain for these fuel synthesis approaches. These challenges include low hydrocarbon yields, catalyst stability, and difficulty in scaling-up the processes [5,7,11]. Two-step thermochemical approaches are one of the few proven scalable methods for the production of liquid hydrocarbons ranging from C6–C17 from CO2 and H2 [6,12,13,14]. Step 1 involves the conversion of CO2 and H2 to intermediates (methanol, olefins, CO) [6,12,13,14,15,16,17]. Step 2 processes these intermediates to C6–C17 hydrocarbons. Commercially, methanol and CO intermediates have both been successfully utilized in this two-step thermochemical approach [13,14], whereas the synthesis of olefin intermediates has only been extensively studied and demonstrated at the laboratory scale [6,17]. In order to evaluate the feasibility of directly synthesizing olefin intermediates as the first step towards operational fuel production for military and commercial applications, the process has to be scaled-up and demonstrated in thermochemical reactor platforms that will be relevant to off-shore and remote synthetic fuel production applications [18,19]. An additional advantage to scaling the chemical conversion of CO2 to light olefin intermediates is that these intermediates serve as key building blocks in the chemical industry [6,12,17].
Commercial-scale, low-cost, modular fixed-bed reactors are being designed and evaluated for remote Fischer-Tropsch synthesis (FTS) processes that use natural gas as the starting material [18]. These advantages could also make commercial-scale fixed-bed reactors ideal for the scale-up of CO2 hydrogenation technologies. Since catalyst physical properties and the reactor type are known to influence the product selectivity, mass transfer, and conversion of hydrogenation reactions [17,18,19,20,21], they are important parameters to consider upon transitioning from the laboratory to commercial scale.
In previous work, highly active Fe-Mn-K/supported CO2 hydrogenation catalysts were characterized and evaluated at the laboratory scale in both a continuously stirred tank/thermal reactor (CSTR) [22] and a fixed-bed reactor [17]. The catalyst materials were demonstrated to be capable of functioning as an effective catalyst to convert CO2 to short-chain olefins. In the present work, the synthesis of Fe-Mn-K-based catalyst is scaled-up 300 times and operated in a commercial-scale prototype modular fixed-bed reactor that is 176 times larger by volume than previous laboratory scale studies. The findings of this paper show how catalyst and reactor scale-up along with recycling a portion of the product stream significantly enhance CO2 conversion efficiency and dramatically change product selectivity. These results are used to expand modeling efforts to bridge the gap between bench-scale research and the development and implementation of a commercial process.
2. Results and Discussion
Commercial tubular fixed-bed reactors for FTS offer scalable solutions for off-shore and remote synthetic fuel production applications [18,19]. The potential challenges associated with using the reactors for FTS are the likely high-pressure drops, low catalyst utilization, and insufficient removal of the heat generated during the exothermic reaction [18]. OxEon Energy developed the reactor used in this study (Figure 1) along with the cooling fin (Figure 2) after having several years of experience working with the hydrogenation of syngas (CO and H2) to hydrocarbons using cobalt- and iron-based catalysts [18]. Industry standard FT tube size is typically under 1″. OxEon Energy is able to use a larger tube size because of the thermal management structure shown in Figure 1 and Figure 2 that distributes the heat throughout the catalyst bed produced by the exothermic FT reaction. Optimizing the hydrogenation reactions in the larger reactor tube size will drastically reduce the number of tubes required for a process. This was intended to reduce long-term fabrication and catalyst servicing costs [18]. The reactor and the skid that supports the hydrogenation reactions in this test series are pictured in Figure 3.
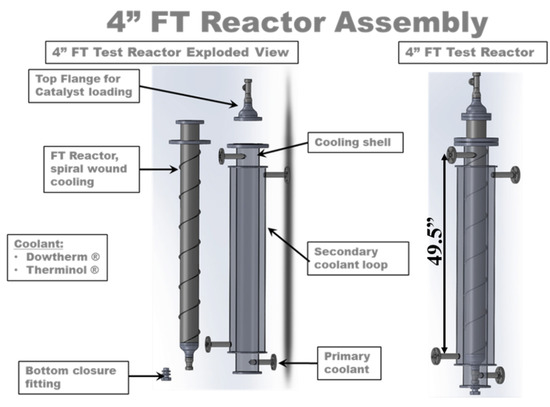
Figure 1.
Schematic of OxEon Energy commercial-scale fixed-bed reactor prototype and dimensions.
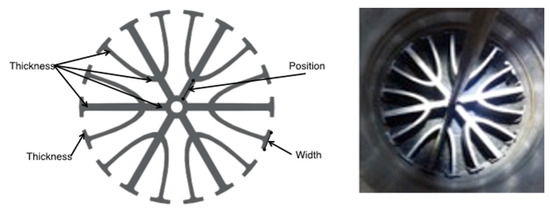
Figure 2.
Schematic and picture of internal cooling fin.
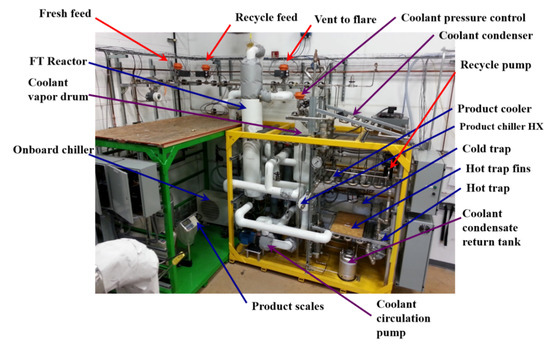
Figure 3.
The OxEon Energy commercial-scale fixed-bed reactor prototype and skid that supports the reactor assembly.
The synthesis of light olefins from CO2 in the fixed-bed environment presents even more challenges than traditional FTS. Equation (1) describes the reverse water–gas shift reaction (RWGS), which is endothermic and produces carbon monoxide (CO). Modeling and kinetic analysis of this reaction on the laboratory scale indicates the RWGS reaction rate is highest at the top of the reactor bed and decreases over the length of the catalyst bed [17]. The CO produced is carried forward in an exothermic FT step (Equation (2)) to produce predominantly monounsaturated hydrocarbons (Equation (3)).
nCO2 + nH2 ⇄ nCO + nH2O ∆RH300 °C = + 38 kJ/mol
nCO + 2nH2 → (CH2)n + nH2O ∆RH300 °C = −166 kJ/mol
nCO2 + 3nH2 → (CH2)n + 2nH2O
A thermodynamically favorable side reaction associated with CO2 hydrogenation is the highly competitive methanation reaction (Equation (4)) [23].
CO2 + 4H2 → CH4 + 2H2O
Another competing side reaction is the Boudouard reaction (Equation (5)) [23].
2CO ⇄ C + CO2
The water formed in the primary reactions shown in Equations (1)–(3) is twice the amount of water produced in traditional FTS. As this water accumulates along the fixed catalyst bed during the hydrogenation reaction, the catalyst will likely be more susceptible to re-oxidizing [17]. Even though the overall reaction is exothermic in nature, the role the RWGS reaction has on the overall process and temperatures along the catalyst bed can be better elucidated at the commercial-scale. The larger scale allows for more precise monitoring of the reaction conditions across the reactor bed.
Five K profile probe thermocouples purchased from Omega (31 Stainless steel, 72) were positioned approximately 12 inches from one another along the catalyst bed starting from the top of the bed. The thermocouples were measured by Labview to monitor the exothermic behavior of the reaction as it proceeds down the reactor bed. During a typical commercial-scale reaction, the top of the catalyst bed measured an average temperature of 555 K, while the remainder of the catalyst bed was measured at 574 K and never rose above 575 K in a single pass of the feedstock. Since the feedstock gases are preheated before they enter the top of the reactor bed, the 19 K difference in temperature is attributed to the endothermic nature of the RWGS reaction (Equation (1)) and slow heat transfer during preheating the feedstock entering the bed. This temperature difference is not common in the FTS process that is highly exothermic (Equation (2)) and requires the thermal management structure shown in Figure 2 to distribute the heat throughout the catalyst bed, preventing any risk of thermal runaway. The thermocouple data supports findings from a three-dimensional fixed-bed computational model that shows how the RWGS reaction rate is high near the entrance of the catalyst bed and is always more than the FT reaction rate throughout the length of the catalyst bed [17]. This explains why the temperature should be cooler at the beginning of the reaction and suggests there is a lower risk of thermal runaway or the need for additional thermal management structures for these types of CO2 hydrogenation reactions.
Prior to choosing a catalyst to measure performance in the commercial-scale fixed-bed reactor environment, the composition of Fe:Mn:K-based catalysts on gamma alumina and Macrolite supports were well characterized in a laboratory scale fixed-bed environment [17]. Since gamma alumina supports are susceptible to hydroxylation in fixed-bed environments [17], there was motivation to replace the gamma alumina support of the iron-based catalysts with the engineered chemically inert ceramic aluminosilicate material, Macrolite®, (M2). After the successful demonstration of commercially prepared 100:3.93:2.36:3.23 M2: Fe:Mn:K catalyst (Fe:M2-1, Table 1) at the laboratory scale, Water Star Inc. (Newbury, OH, USA) prepared a small batch (~500 to 1000 g) of higher concentrated iron-based catalyst on the Macrolite® support (support 100:17:12:16.5 ratio of M2:Fe:Mn:K) (Fe:M2-2, Table 1). The Fe:M2-2 catalyst composition closely resembled previously published iron-based catalyst compositions loaded on gamma alumina support [22]. Fe:M2-2 was characterized and tested at the laboratory scale to ensure that the CO2 hydrogenation performance properties were similar or better than those measured for the gamma alumina supported catalyst [17] under similar reaction conditions. Table 1 (Row 2) shows a CO2 conversion of 41% and an O/P ratio of almost 5. Based on the laboratory-scale results, Water Star Inc. scaled up the catalyst synthesis of the 100:17:12:16.5 support M2: iron: manganese: potassium catalyst (Fe:M2-3) to kilogram quantities for testing in the commercial-scale fixed-bed reactor environment that are presented in this study.

Table 1.
Summary of product selectivity, olefin/paraffin ratio, CO2 conversion, and ASF values over Fe-based CO2 hydrogenation catalysts in both laboratory and commercial single-channel scalable modular thermochemical reactor units.
The single pass of the feedstock over the commercial-scale catalyst bed is the closest reaction conditions to those run at the laboratory scale, as there is currently no mechanism in place to recycle the product stream at the laboratory scale. Data were collected on the conversion of CO2 and H2 over the Fe:M2-3 catalyst for a 96 h period. CO2 conversion, product selectivity, and olefin/paraffin ratio as a function of GHSV, product recycling, and catalyst regeneration are reported in Table 1, along with data for other catalyst blends and reactor scales. When the Fe:M2-3 catalyst was reacted in the commercial-scale fixed-bed reactor at the lowest total GHSV of 4.6 × 10−4 L/s-g for 96 h under single pass feedstock conditions (Table 1, Rows 4–7), the average CO2 conversion was 26% with a CO selectivity of 46% and methane selectivity of 6%. The olefin/paraffin (O/P) ratio increased from 3.1 to 4.4 in the first 48 h of operation. The hydrocarbon selectivity (percent conversion of CO2 to C2–C5+ hydrocarbons) was 48% on average. The percent yield values in Table 1 were calculated by multiplying the C2–C5+ selectivity values by the CO2 conversion given in the Table. The average C2–C5+ yield measured is approximately 12% by weight on a carbon basis at a GHSV of 4.6 × 10−4 L/s-g.
Loosely comparing the average commercial-scale hydrogenation results of Fe:M2-3 (Table 1, Rows 4–7) to the laboratory-scale operating conditions performed with the small-scale commercially prepared catalyst, Fe:M2-2, at a higher GHSV of 9.6 × 10−4 L/s-g (Table 1, Row 2), a 37% decrease in CO2 conversion and an 86% increase in CO selectivity is observed on the commercial scale for the Fe:M2-3 catalyst. This was an unexpected result, as the lower GHSV tested at the commercial scale was anticipated to yield higher CO2 conversions. This significant difference in conversion and selectivity at the two reactor scales prompted the implementation of a regeneration process before changing the commercial-scale reactor operating conditions and studying the catalyst performance. Mechanisms for catalyst regeneration and the long-term catalyst stability are important for the commercial feasibility of any catalyst process [24]. Fe:M2-3 was regenerated by flowing hydrogen over the catalyst bed at until methane was not detected by the inline GC equipped with a TCD detector. Then nitrogen with air to make 1% O2 was flowed over the catalyst bed. The O2 content was gradually increased until no further CO2 was measured by the GC. This method was used to remove all hydrocarbon and carbon remaining on the catalyst and also dried the catalyst bed.
After catalyst regeneration, the reaction was resumed at a total GHSV of 1.3 × 10−3 L/s-g with the recycling feature applied to the experimental runs. The water in the recycle feed was condensed and removed from the feed. The dry recycle feed at 9.2 × 10−4 L/s-g was blended with fresh feed at 3.5 × 10−4 L/s-g back into the reactor at a 2.6:1 recycle feed to fresh feed Table 1, Rows 8–11). It is worth noting that the top of the catalyst bed measured an average temperature of 540 K. This is, on average, 15 K cooler than the single pass temperatures measured at the top of the reactor. This additional drop in temperature is believed to be the result of the recycle feed being treated for removal of liquid and water by passing it through a cold trap (Figure 4). The temperatures in the remainder of the catalyst bed reached an average of 575 K, which is similar to those measured during the single pass conditions.
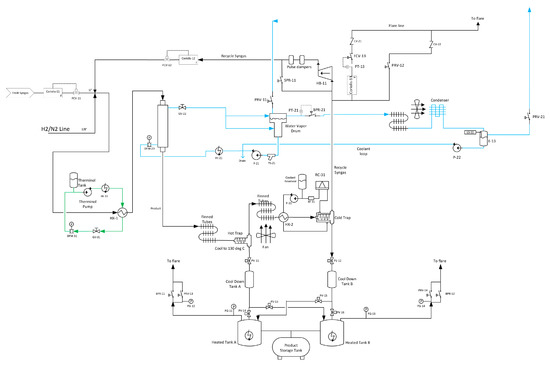
Figure 4.
OxEon Energy commercial-scale fixed-bed reactor prototype process flow diagram.
After 96 h on stream, under these recycling conditions, the average CO2 conversion was measured to be 62% (Table 1, Rows 8–11), far greater than the 26% CO2 conversion originally measured under the single pass conditions (Table 1, Rows 4–7). Under the recycling conditions, methane selectivity increased from 6% to 9% and C2–C5+ selectivity increased from 48% to 76%. CO selectivity was reduced from 45% to 15%, while the O/P remained at 4. This reduction in CO selectivity also corresponds well with the 75% increase in average C2–C5+ yield, indicating the CO produced during the first pass over the catalyst is hydrogenated to higher hydrocarbons during the second pass over the catalyst bed.
Increasing the total GHSV from 1.3 × 10−3 to 1.4 × 10−3 L/s-g by the addition of 1.1 × 10−4 L/s-g of fresh feed and 2.0 × 10−5 L/s-g of recycled feed over a 72 h period significantly affects the selectivity of the catalyst (Table 1, Rows 12–14). Since the GHSV was only increased by 7.7%, the change in CO2 conversion and product selectivity can be attributed more to the lower ratio of recycle feed 2:1 to fresh feed. Reducing the amount of unreacted CO by reducing the amount of recycle feed on each pass over the catalyst bed lowered the average CO2 conversion from 62% (Table 1, Rows 8,9) to 53% (Table 1, Rows 12–14) and hydrocarbon selectivity from 78% to 69%. This is further substantiated at lower GHSV. While GHSV has been shown to have little effect on the chain growth probability of C1–C5+ at the laboratory scale, it has had a significant impact on CO2 and CO conversion. In particular, their conversion can be significantly improved by lowering the GHSV and thus increasing the residence time of the reagents CO2, CO, and H2 in contact with the active catalyst bed. When the GHSV is lowered to 8.0 × 10−4 L/s-g and the ratio of recycle feed to fresh feed is returned to 2.6:1, the highest average CO2 conversion and hydrocarbon selectivity are reported at 68.5% and 79 (Table 1, Rows 15,16). In addition, the lower GHSV reduces CO selectivity to 9% in favor of greater C2–C5+ yield at 54%.
A detailed kinetic analysis at the commercial prototype modular fixed-bed reactor scale is challenging from a cost, time, and materials perspective. In this study, the single pass feedstock conditions and the ability to recycle a portion of the product stream in the commercial-scale prototype reactor are modeled using reaction kinetics ascertained at the laboratory scale [17]. The single pass feedstock reactor conditions were modeled at a GHSV of 4.6 × 10−4 L/s-g, a gas mass fraction composition of 82.2% CO2, 10.9% H2, and 6.9%N2, inlet temperature of 553 K, and 2 Mpa (20 Bar) pressure. The single pass feedstock reactor model yielded results with a conversion of 22% CO2 and product selectivities of 52% CO, 43% C3H6, and 5% CH4 with an outlet temperature of 575 K (Table 2). The model suggests that if the reactor outlet temperatures are raised to 608 K, the CO2 conversion will increase to 26% and the CO and CH4 selectivities will decrease (45% and 6%) in favor of higher hydrocarbon formation, 49% C3H6 (Table 2). Both of these sets of model results (575 and 608 K outlet temperature) are very similar to those reported by Riedel et al. [24]. The model results also fall in a similar range as the averaged prototype reactor data measured and are shown in Table 1 and Table 2.

Table 2.
3D kinetic modeling predictions for product selectivity and CO2 conversion under single pass feedstock conditions and recycling conditions compared to the average measured for the rector prototype. Single pass feedstock conditions at GHSV of 4 × 10−4 L/s-g and recycling conditions fresh feed GHSV of 4 × 10−4 L/s-g and 9.4 × 10−4 L/s-g of recycled feed.
Recycling conditions were incorporated into the model using a feed gas consisting of a combined fresh (4.6 × 10−4 L/s-g GHSV and recycled 9.4 × 10−4 L/s-g GHSV) gas mass fraction composition of 81.0% CO2, 10.6% H2, 1.0% CO, 1.2% C3H6, 0.2% CH4, 6.0% N2, and 0.0% H2O. The model provided results indicating 53% conversion of CO2 and product selectivities of 21% CO, 65% C3H6, and 14% CH4 with an outlet temperature of 574 K (Table 2). The model results align well with the measured data for the recycled conditions as provided in Table 1 and Table 2.
Any differences in the modeling results may be attributed to the complexities of the commercial-scale reactor configuration and the axial location of the thermocouples. Temperature excursions within the prototype reactor could have risen above the 573 K without being measured by the thermocouples due to the finned nature of the reactor (Figure 2). The internally finned reactor creates separate channels of flow all separated by an aluminum wall, which causes multiple “micro” reactors within the large reactor resulting in potential differences in temperatures. The model supports this possible temperature variation by providing a better fit at reactor outlet temperatures that are 35 K higher than those measured during the reactions. Neither of the reactor models indicated a radial heat transfer limitation. However, a primary limitation in CO2 conversion is the heat required by the endothermic RWGS reaction that initially drops the temperature in the first section of the bed followed by the subsequent exothermic FT reaction that begins to release heat that needs to be removed. This further substantiates previous modeling and kinetic analysis that indicate that the FT reaction is the rate-limiting step in the use of these supported iron-based catalysts [17].
After the commercial-scale testing was completed, significant differences in catalyst activity and selectivity were observed for the commercially prepared small-batch catalyst Fe:M2-2 tested at the laboratory scale (Table 1, Row 2) and the large batch Fe:M2-3 prepared and measured at the commercial scale. Fe:M2-3 activity and selectivity with respect to CO2 conversion, O/P, and hydrocarbon yields align more closely with the Fe:M2-1 that was prepared commercially with a lower iron concentration (100:3.93:2.36:3.23 M2: Fe:Mn:K) [17]. These observations motivated further characterization of both catalysts. The activity of the Fe:M2-3 catalyst was measured at a GHSV of 4.6 × 10−4 L/s-g in the NRL laboratory scale fixed-bed reactor, while both the Fe:M2-2 and Fe:M2-3 were characterized and compared by XPS and XRD shown in Table 3. When the hydrogenation results of Fe:M2-3 are compared at the different reactor scales and GHSV of 4.6 × 10−4 L/s-g, the results in Table 1 (LS/kg) show a 57% loss in CO2 conversion at the laboratory scale to 11%. CO selectivity was reduced by 56% in favor of C2–C5+ formation at 36%, and the methane selectivity was similar at 6%.

Table 3.
Comparing XPS data for two commercially prepared catalyst formulations.
Elemental analysis using XPS (Table 3) reveals that Fe:M2-3 catalyst batch contains twice the concentration of manganese and less than half the iron concentration of Fe:M2-2 catalyst. The effects of iron loading on CO2 conversion and product selectivity have been well established [22]. Table 1 provides data taken in the laboratory-scale reactor for the well-characterized iron/manganese/potassium/Macrolite® catalysts with a relative iron loading that is 3.93% by weight of the M2 support, Fe:M2-1. The CO2 conversion observed is 26% compared to 41% for the higher iron-loaded catalyst, Fe:M2-2 under laboratory-scale reactor conditions. These results are in line with similar reports documenting the change in CO2 conversion and product selectivity as a function of increase in the iron [22] and further supports the findings that the Fe:M2-3 catalyst was not made to the specified metal loadings. Contributions of aluminum, oxygen, and carbon were factored out of the XPS analysis to reduce any potential impact of surface oxygen and advantageous carbon species. There were also various trace elements that only existed in one catalyst or the other, including silicon, calcium, nitrogen, and chlorine.
X-ray diffraction analysis in Figure 5 revealed that both Fe:M2-2 and Fe:M2-3 contain the same iron oxide crystal phase (Fe2O3 hematite), but the support phases between the two catalyst are clearly different. Yellow tick marks have been added to the pattern to indicate discrepancies between the Fe:M2-2 and Fe:M2-3 catalysts. When compared to the hematite simulated pattern, it is evident that both catalysts contain the iron oxide phase, leading to the conclusion that the support is the primary difference between the two catalysts. Unfortunately, no one alumina or aluminosilicate phase we examined accurately matches the supports in question. In summary, it is clear that these catalysts are different compositionally and crystallographically from one another. After providing further evidence to the supplier on the differences in catalyst support, the supplier revealed that different supports were in fact used between the small-scale catalyst batch Fe:M2-2 and the large-scale catalyst batch Fe:M2-3. While it was believed that little difference in chemical activity existed between the two supports, this difference ultimately led to the decrease in activity of Fe:M2-3 relative to the Fe-M2-2 small-scale catalyst test batch measured at the laboratory scale.
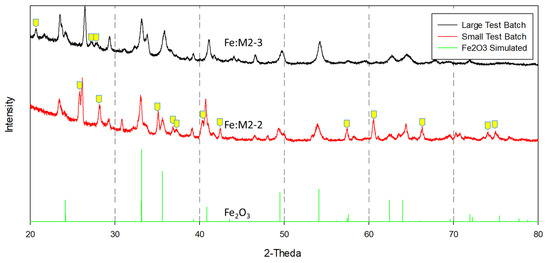
Figure 5.
X-ray diffraction analysis of commercially prepared catalyst formulations, large test batch (Fe:M2-3), and small test batch (Fe:M2-2).
3. Experimental Methods and Methodology
3.1. Commercial Large Scale Catalyst Preparation
Approximately 10 kg of iron-based metal catalyst (consisting of the following ratio: 100:17:12:16.5 of support: iron: manganese: potassium) was synthesized by Water Star Inc (Newbury, OH, USA) using typical incipient wetness impregnation (IWI) methods. The support was a Macrolite® engineered ceramic media (M2) (Fairmount Water Solutions). This catalyst is denoted in Table 1 as Fe:M2-3. The catalyst particles ranged from 0.6 mm to 2 mm in diameter. Before the catalyst was loaded into the reactor, the catalyst was reduced in hydrogen, passivated, and soaked in mineral oil for 12 h. Approximately 9 mL of mineral oil was used for every 20 g of catalyst. This is similar to the amounts of mineral oil used in the laboratory scale studies [17].
3.2. Commercial-Scale Fixed-Bed Reactor Prototype Setup
Figure 1 provides a schematic of the commercial-scale fixed-bed reactor, which was designed and assembled by OxEon Energy (formally known as Ceramatec) located in Salt Lake City, Utah, US. Approximately 6090 g of catalyst was loaded into a 4″ (100 mm) nominal tube size, single tube reactor. The reactor has a 4.5″ outer diameter (OD), 0.12″ wall, and an inner diameter (ID) of 4.26″. The catalyst occupies a length of approximately 49.5″ of the reactor tube assembly. The center of the tube contains an internal cooling fin to disseminate the heat throughout the catalyst bed that is generated during exothermic reactions (Figure 2). The reactor and the skid that supports the reactor used in this test series are pictured in Figure 3 and Figure 4 and provide the process flow diagram of the commercial-scale OxEon Energy test facility. Following the process flow diagram shown in Figure 4, a 3:1 ratio of H2/CO2 was flowed over the Fe:M2-3 catalyst at GHSV ranging between 8.0 × 10−4 L/s-g and 1.4 × 10−3 L/s-g. Nitrogen was used as the internal standard and all experiments were carried out at 300 °C and 20 bar.
The effluent fixed gases (H2, CO2, CO, N2) and CH4 from the reactor were analyzed in real time using an inline GC (Inficon) equipped with a TCD detector. Figure 4 shows that the system was designed such that when the products exit the reactor, they go to a hot trap and heavy hydrocarbons such as wax (typically seen if FT reactions using cobalt-based catalyst) are drained; the remainder of the hydrocarbons and non-converted synthesis gas goes to the fin tube to cool to room temperature. The gases then proceed through a chiller to a cold trap (cold trap is maintained at −4°C) where the light oil and water are condensed, captured, and separated periodically. The non-condensate gases pass through a tee and are split such that part of the stream goes to flare and the other part goes to the recycle compressor. The composition of both gas streams are identical and the recycled feed is then blended back into the reactor at a ratio of 2:1 recycle feed to fresh feed (CO2 and H2). The flaring/removal of 1/3 of the gas stream is important to keep methane, an unwanted byproduct of this reaction, to reasonable levels. Labview is used for the inline GC analysis to ensure that the GHSV and CO2:H2 ratio remained constant during the recycling process. Analysis provided in Table 1 occurred approximately every 24 h for a given GHSV.
The long chain effluent from the reaction (i.e., hydrocarbons greater than C-2) was analyzed separately using a GC (Bruker 456, (Billerica, MA, USA) equipped with a flame ionization detector. It is important to note that all selectivities and yields are reported on a per carbon atom consumed basis and not per mole of product (i.e., propane selectivity is weighted by three due to the fact that it accounts for three carbon atoms per molecule). Five thermocouples (type and company) were positioned along the reactor bed and monitored by Labview to monitor the exothermic behavior of the reaction as it proceeds down the reactor bed.
3.3. Computational Modeling for Fixed-Bed Reactors
The OxEon Energy commercial-scale fixed-bed reactor prototype results were modeled according to previous reaction kinetics based on Willauer et al. but factored by 100 for the reverse water–gas shift (rWGS) reaction, 6.75 for the Fischer-Tropsch reaction, and 6 for the methanation reaction [17,24]. The same reaction kinetics were used to model both the single pass and recycling capability. The single pass of the feedstock over the reactor along with the recycle capability were modeled with commercial CFD software CFX© on the Department of Defense High Performance Computing Modernization Program servers. The number of nodes used in the model was 60,975 with 305,050 elements. The k-ε turbulence model was used with the scalable wall function, compressible gas flow, and finite rate with equilibrium chemical kinetics. The reactor was modeled as a packed bed of length 1.2573 m (49.5 inch) and inner diameter of 0.1082 m (4.26 inch), a bed porosity of 40%, and a permeability of 1.9 × 10−9 m2/s. A total catalyst weight of 6090 g with 1888 g of active metal was used as the basis for the reaction kinetics. The model outlet pressures and imposed wall temperatures were variables used to converge on the reported pilot test results for outlet temperature and mass flows. For both of the reactor configurations, heat was removed from the reactor, which is similar to the porotype reactor configuration.
3.4. Laboratory-Scale Fixed-Bed Reactor Setup
The laboratory-scale fixed-bed plug flow thermochemical reactor process requires 20 g of catalyst for evaluation in a 9 to 12″ long stainless steel tube (3/8″ ID, 1/2″ OD). The catalyst bed occupies 8 to 10″ length of the stainless steel tube. The catalyst quantities required for the evaluations were synthesized commercially by Water Star Inc. Water Star prepared two small-scale iron-based metal catalysts for evaluation on the laboratory scale that differed in wt.% metal loading by IWI methods. The first catalyst, 100:3.93:2.36:3.23 M2:Fe:Mn:K, has been studied extensively [17] and is referred to as Fe:M2-1 in Table. The second catalyst was prepared at a higher catalyst loading of 100:17:12:16.5 M2:Fe:Mn:K and is listed in Table 1 as Fe:M-2. Each small-scale batch consisted of 500 g to 1000 g of catalyst. The catalysts were recovered after the hydrogenation reactions, rinsed with hexanes, vacuum-dried, and then characterized by XRD and XPS.
In a typical CO2 hydrogenation experiment, 10 mL of mineral oil is added to the catalyst and the catalyst was reduced in situ by flowing 100 mL/min H2 at 300 °C and 20 bar for 18 h. Three mass flow controllers (Brooks Instruments, Hatfield, PA, USA) were used to control the flows of CO2, H2, and N2 into the reactor. Immediately following reduction, hydrogenation of CO2 was conducted at 20 bar and 300 °C with a H2/CO2 ratio of 3:1 and a 10 mL/min N2 internal standard at 20 bar and gas hourly space velocity (GHSV) of 9.6 × 10−4 L/s-g. The GHSV is defined as standard liters per second of total CO2 and H2 flow divided by the grams of total elemental Fe, Mn, and K metals in the reactor. The effluent gases were passed through a cold trap, at 10 °C to condense the water vapor and any heavy liquid hydrocarbons formed in the reactor. The effluent gases were analyzed in real time using an inline gas chromatograph (GC) (Agilent Technologies, Fast RGA analyzer, Santa Clara, CA, USA. Hydrocarbons were separated using an HP-Al/S column (Agilent Technologies) 19091P-512, 25 μm × 320 μm × 8 μm) and detected with an FID detector. Fixed gases (H2, CO2, CO, N2) were separated on a Unibead IS column (4 ft, 60/80 mesh in UltiMetal, Agilent Technologies) and a 5Å molecular sieve column (8 ft, 60/80 mesh) and detected with a TCD detector. The GC was calibrated using a mixture of gases with known molar ratio (MESA Specialty Gas, Santa Ana, CA, USA). Time-on-stream (TOS) for the catalyst was 48 h.
3.5. Catalyst Characterization
Powder X-ray diffraction analysis was performed using a Rigaku Smartlab X-ray Diffractometer (Austin, TX, USA) using a Cu_Kα source and collected between 20 and 80 2Θ. The XPS measurements were performed using a Thermo Scientific K-alpha equipped with a monochromatic Al Kα source and 180° double focusing hemispherical analyzer with 128-channel detector was used to collect X-ray photoelectron spectroscopy data. The nominal XPS spot size and analyzer field of view were 100 µm2. Charge compensation was necessary.
4. Conclusions
The commercial large-scale synthesis of a well-characterized NRL iron-based catalyst and its performance in a commercial-scale modular fixed-bed reactor prototype was evaluated in this test series. A significant reduction in CO2 conversion and change in product selectivity at the large scale suggested the commercially scaled-up synthesized catalyst (Fe:M2-3) was significantly less active than previous catalysts synthesized and tested at the laboratory scale (Fe:M2-2). The reduced catalyst activity was further substantiated spectroscopically by the confirmation of lower iron loadings and differences in catalyst support characteristics used in the large-scale synthesis of the catalyst. The ability to recycle a portion of the product stream at this scale provided a solution to overcome challenges associated with the lower catalyst activity. CO2 conversion increased from 26% to as high as 69% and the product selectivity shifted from 45% CO to 9% CO in favor of C2–C5+ hydrocarbon production upon recycling the effluent product stream.
The results presented serve to highlight the potential challenges associated with scaling-up materials and processes for commercial implementation. They also suggest that the iron-based catalyst with the higher metal loading will have even better performance characteristics in the commercial-scale prototype reactor. The modeling and kinetic analysis using kinetics established at the laboratory support trends associated with reaction temperatures and FT reaction rates. The higher loading catalyst and the endothermic cooling associated with the RWGS reaction will be the subject of further evaluations as NRL pursues determination of the feasibility of producing olefin intermediates from CO2 and H2 as the first step of a two-step thermochemical process to produce operational fuel for the military and commercial applications.
Author Contributions
All authors assisted in the collection of the data, the data analysis, and the preparation of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Office of Naval Research both directly and through the Naval Research Laboratory and OPNAV N45.
Conflicts of Interest
The authors declare no conflict of interest.
References
- United States. DOE Genomics: GTL Roadmap: Systems Biology for Energy and Environment; Department of Energy Office of Science: Washington, DC, USA, 2005.
- Summary of the 2018 National Defense Strategy of the United States of America. Available online: https://dod.defense.gov/Portals/1/Documents/pubs/2018-National-Defense-Strategy-Summary.pdf (accessed on 10 January 2020).
- Department of Defense. Operational Energy Strategy. 2016. Available online: http://www.acq.osd.mil/eie/Downloads/OE/2016%20DoD%20Operational%20Energy%20Strategy%20WEBc.pdf (accessed on 10 January 2020).
- Naval S&T Strategy 2015, Office of Naval Research. Available online: https://www.navy.mil/strategic/2017-Naval-Strategy.pdf (accessed on 10 January 2020).
- Burkart, M.D.; Hazari, N.; Tway, C.L.; Zeitler, E.L. Opportunities and challenges for catalysis in carbon dioxide utilization. ACS Catal. 2019, 9, 7937–7956. [Google Scholar] [CrossRef]
- Choi, Y.H.; Jang, Y.J.; Park, H.; Kim, W.Y.; Lee, Y.H.; Choi, S.H.; Lee, J.S. Carbon dioxide fischer-tropsch synthesis: A new path to carbon-neutral fuels. Appl. Catal. B 2017, 202, 605–610. [Google Scholar] [CrossRef]
- Song, J.T.; Song, H.; Kim, B.; Oh, J. Towards higher rate electrochemical CO2 conversion: From liquid-phase to Gas-phase systems. Catalysts 2019, 9, 224. [Google Scholar] [CrossRef]
- DiMascio, F.; Hardy, D.R.; Lewis, M.K.; Willauer, H.D.; Williams, F.W. Extraction of Carbon Dioxide and Hydrogen from Seawater and Hydrocarbon Production Therefrom. U.S. Patent 9,303,323, 5 April 2016. [Google Scholar]
- DiMascio, F.; Willauer, H.D.; Hardy, D.R.; Williams, F.W.; Lewis, M.K. Electrochemical Module Configuration for the Continuous Acidification of Alkaline Water Sources such as Seawater and Recovery of CO2 with Continuous Hydrogen Production. U.S. Patent 9,719,178, 1 August 2017. [Google Scholar]
- DiMascio, F.; Willauer, H.D.; Hardy, D.R.; Lewis, M.K.; Williams, F.W. Electrochemical Module Configuration for the Continuous Acidification of Alkaline Water Sources such as Seawater and Recovery of CO2 with Continuous Hydrogen Gas Production. U.S. Patent 10,450,661, 22 October 2019. [Google Scholar]
- Chang, K.; Zhang, H.; Cheng, M.-J.; Lu, Q. Application of ceria in CO2 conversion catalysis. ACS Catal. 2020, 10, 613–631. [Google Scholar] [CrossRef]
- Porosoff, M.D.; Yan, B.; Chen, J.G. Catalytic reduction of CO2 by H2 for synthesis of CO, methanol and hydrocarbons: Challenges and opportunities. Energy Environ. Sci. 2016, 9, 62–73. [Google Scholar] [CrossRef]
- Olah, G.A.; Goeppert, A.; Prakash, G.K.S. Beyond Oil and Gas: The Methanol Economy; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006. [Google Scholar]
- MacDonald, F. Science Alert 27 Apr. 2015. Available online: http://www.sciencealert.com/audi-have-successfully-made-diesel-fuel-from-air-and-water (accessed on 3 June 2020).
- Porosoff, M.D.; Yang, X.; Bosocoboinik, J.A.; Chen, J.G. Molydenum carbide as alternative catalysts to precious metals for highly selective reduction of CO2 to CO. Angew. Chem. Int. Ed. 2014, 53, 6705–6709. [Google Scholar] [CrossRef] [PubMed]
- Porosoff, M.D.; Baldwin, J.W.; Peng, X.; Mpourmpakis, G.; Willauer, H.D. Potassium-promoted molybdenum carbide as a highly active and selective catalyst for CO2 conversion to CO. ChemSusChem 2017, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bradley, M.J.; Ananth, R.; Willauer, H.D.; Baldwin, J.W.; Hardy, D.R.; DiMascio, F.; Williams, F.W. The role of catalyst environment on CO2 hydrogenation in a fixed-bed reactor. J. CO2 Utiliz. 2017, 17, 1–9. [Google Scholar] [CrossRef]
- Frost, L.; Elangovan, E.; Hartvigsen, J. Production of synthetic fuels by high-temperature co-electrolysis of carbon dioxide and steam with Fischer-Tropsch synthesis. Can. J. Chem. Eng. 2016, 94, 636–641. [Google Scholar] [CrossRef]
- LeViness, S.; Deshmukh, S.R.; Richard, L.A.; Robota, H.J. Velocys Fischer-Tropsch synthesis technology-new advances on state-of-the art. Top. Catal. 2014, 57, 518–525. [Google Scholar] [CrossRef]
- Davis, B.H. Fischer-Tropsch synthesis: Overview of reactor development and future potentialities. Top. Catal. 2005, 32, 143–168. [Google Scholar] [CrossRef]
- Espinoza, R.L.; Steynberg, A.P.; Jager, B.; Vosloo, A.C. Low temperature Fischer-Tropsch synthesis from a Sasol perspective. Appl. Catal. A 1999, 186, 13–16. [Google Scholar] [CrossRef]
- Dorner, R.W.; Hardy, D.R.; Williams, F.W.; Willauer, H.D. K and Mn doped iron-based CO2 hydrogenation catalysts: Detection of KAlH4 as part of the catalyst’s active phase. Appl. Catal. A 2010, 373, 112–121. [Google Scholar] [CrossRef]
- Riedel, T.; Schaub, G.; Jun, K.-W.; Lee, K.-W. Kinetics of CO2 hydrogenation on a K-promoted Fe catalyst. Ind. Eng. Chem. Res. 2001, 40, 1355–1363. [Google Scholar] [CrossRef]
- Moulijn, J.A.; van Diepen, A.E.; Kapeteijn, F. Catalyst deactivation: Is it predictable? What to do? Appl. Catal. A 2001, 212, 3–16. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).