Asymmetric Friedel–Crafts Alkylation of Indoles Catalyzed by Chiral Aziridine-Phosphines
Abstract
1. Introduction
2. Results and Discussion
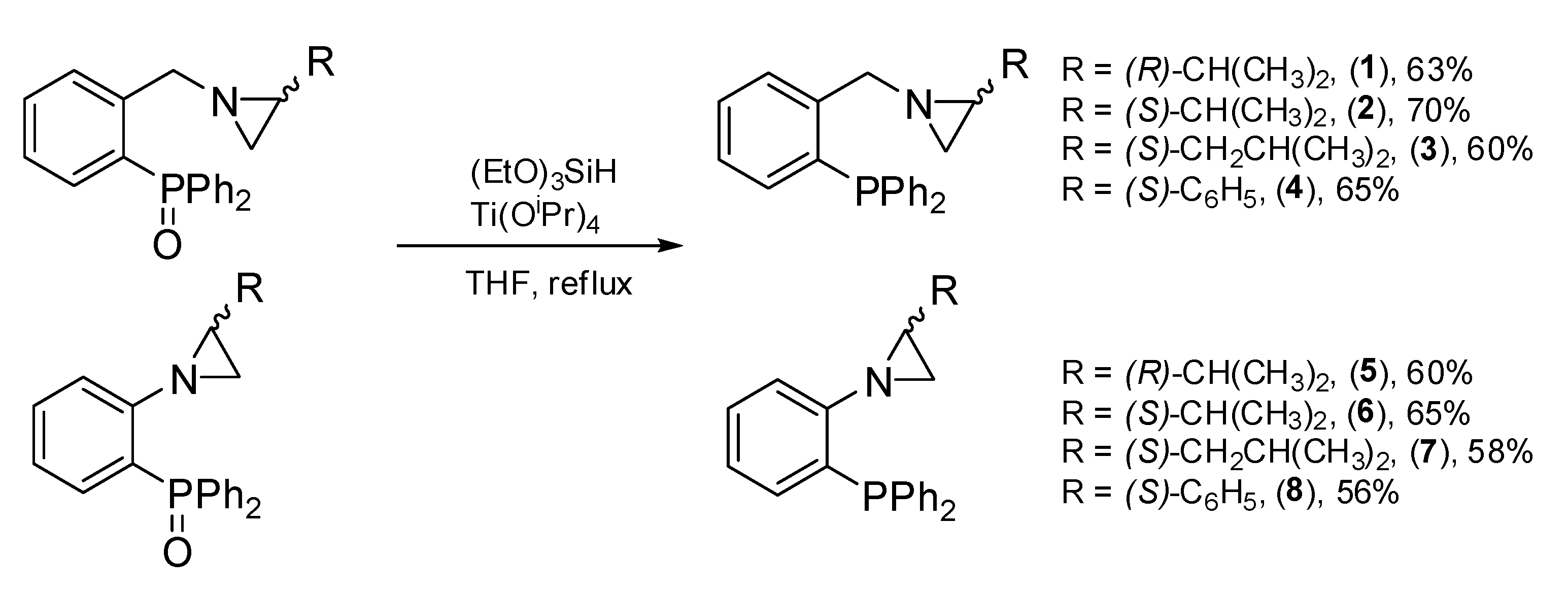
2.1. Synthesis of the Aziridine-Phosphines 1–8
2.2. Asymmetric Friedel–Crafts Alkylation of Indole Catalyzed by Aziridine Phosphines 1–8
2.3. Asymmetric Friedel–Crafts Reaction Catalyzed by Aziridine-Phosphine 6
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Reduction of Phosphinoyl-Aziridines to Aziridine-Phosphines 1–8—General Procedure (Coumbe, et al., 1994)
3.2.2. Asymmetric Friedel–Crafts Alkylation of Indoles—General Procedure (Kim, et al., 2010)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Da Gama Oliveira, V.; do Carmo Cardoso, M.F.; da Silva Magalhães Forezi, L. Organocatalysis: A brief overview on its evolution and application. Catalysts 2018, 8, 605. [Google Scholar] [CrossRef]
- Pellissier, H. Asymmetric organocatalysis. Tetrahedron 2007, 63, 9267–9331. [Google Scholar] [CrossRef]
- Kagan, H.B.; Gopalaiah, K. Early history of asymmetric synthesis: Who are the scientists who set up the basic principles and the first experiments. New J. Chem. 2011, 35, 1933–1937. [Google Scholar] [CrossRef]
- Li, L.; Chen, Z.; Zhang, X.; Jia, Y. Divergent strategy in natural product total synthesis. Chem. Rev. 2018, 118, 3752–3832. [Google Scholar] [CrossRef]
- Shimokawa, J. Divergent strategy in natural product total synthesis. Tetrahedron Lett. 2014, 55, 6156–6162. [Google Scholar] [CrossRef]
- Krautwald, S.; Carreira, E.M. Stereodivergence in asymmetric catalysis. J. Am. Chem. Soc. 2017, 139, 5627–5639. [Google Scholar] [CrossRef]
- Choi, J.; Fu, G.C. Catalytic asymmetric synthesis of secondary nitriles via stereoconvergent Negishi arylations and alkenylations of racemic α-bromonitriles. J. Am. Chem. Soc. 2012, 134, 9102–9105. [Google Scholar] [CrossRef]
- Kalek, M.; Fu, G.C. Phosphine-catalyzed doubly stereoconvergent γ-additions of racemic heterocycles to racemic allenoates: The catalytic enantioselective synthesis of protected α,α-disubstituted α-amino acid derivatives. J. Am. Chem. Soc. 2015, 137, 9438–9442. [Google Scholar] [CrossRef] [PubMed]
- Park, J.K.; Lackey, H.H.; Ondrusek, B.A.; McQuade, D.T. Stereoconvergent synthesis of chiral allylboronates from an E/Z mixture of allylic aryl ethers using a 6-NHC-Cu(I) catalyst. J. Am. Chem. Soc. 2011, 133, 2410–2413. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, T.B.; Jørgensen, K.A. Catalytic asymmetric Friedel-Crafts alkylation reactions—Copper showed the way. Chem. Rev. 2008, 108, 2903–2915. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Bisai, A.; Singh, V.K. Enantioselective Friedel-Crafts alkylation of indoles with nitroalkanes catalyzed by a bis(oxazoline)-Cu(II) complex. Tetrahedron Lett. 2007, 48, 1127–1129. [Google Scholar] [CrossRef]
- Li, W. Chiral bis(oxazolinyl)thiophenes for enantioselective Cu(II)-catalyzed Friedel-Crafts alkylation of indole derivatives with nitroalkenes. Catal. Lett. 2014, 144, 943–948. [Google Scholar] [CrossRef]
- Liu, T.-Y.; Cui, H.-L.; Chai, Q.; Long, J.; Li, B.-J.; Wu, Y.; Ding, L.-S.; Chen, Y.-C. Organocatalytic asymmetric Friedel-Crafts alkylation/cascade reactions of naphthols and nitroolefins. Chem. Commun. 2007, 2228–2230. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Q.; Song, J.; Hong, R.; Li, H.; Deng, L. Asymmetric Friedel-Crafts reaction of indoles with imines by an organic catalyst. J. Am. Chem. Soc. 2006, 128, 8156–8157. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, H.S.; Şahin, E.; Çakici, M.; Kiliç, H. Asymmetric Friedel-Crafts alkylation of pyrrole with nitroalkenes catalyzed by a copper complex of a bisphenol A-derived Schiff base. Tetrahedron 2015, 71, 2882–2890. [Google Scholar] [CrossRef]
- Kim, H.Y.; Kim, S.; Oh, K. Orthogonal enantioselectivity approaches using homogeneous and heterogeneous catalyst systems: Friedel-Crafts alkylation of indole. Angew. Chem. Int. Ed. 2010, 49, 4476–4478. [Google Scholar] [CrossRef]
- Liu, J.; Gong, L.; Meggers, E. Asymmetric Friedel-Crafts alkylation of indoles with 2-nitro-3-arylacrylates catalyzed by a metal-templated hydrogen bonding catalyst. Tetrahedron Lett. 2015, 56, 4653–4656. [Google Scholar] [CrossRef]
- Kaushik, N.K.; Kaushik, N.; Attri, P.; Kumar, N.; Kim, C.H.; Verma, A.K.; Choi, E.H. Biomedical importance of indoles. Molecules 2013, 18, 6620–6662. [Google Scholar] [CrossRef]
- Sakamoto, T.; Itoh, J.; Mori, K.; Akiyama, T. Chiral Brønsted acid catalyzed Friedel-Crafts alkylation reaction of indoles with α,β-unsaturated ketones: Short access to optically active 2- and 3-substituted indole derivatives. Org. Biomol. Chem. 2010, 8, 5448–5454. [Google Scholar] [CrossRef]
- Ali, S.; Wisal, A.; Tahir, M.N.; Ali, A.; Hameed, S.; Ahmed, M.N. One-pot synthesis, crystal structure and antimicrobial activity of 6-benzyl-11-(p-tolyl)-6H-indolo[2,3-b]quinoline. J. Mol. Struct. 2020, 1210, 128035. [Google Scholar] [CrossRef]
- Sansinenea, E.; Martínez, E.F.; Ortiz, A. Organocatalytic synthesis of chiral spirooxindoles with quaternary stereogenic centers. Eur. J. Org. Chem. 2020. [Google Scholar] [CrossRef]
- Hong, S.K.; Park, W.; Park, Y.S. Asymmetric synthesis of 4-aryl dihydroisoquinolin-3-ones and 2-aryl morpholin-3-ones using AgOTf-activated α-bromo arylacetate. Tetrahedron 2020, 76, 130841. [Google Scholar] [CrossRef]
- Wang, Z.; Zu, L. Organocatalytic enantioselective direct alkylation of phloroglucinol derivatives: Asymmetric total synthesis of (+)-aflatoxin B2. Chem. Commun. 2019, 5171–5174. [Google Scholar] [CrossRef]
- Yang, H.; Tang, W. Efficient enantioselective syntheses of chiral natural products facilitated by ligand design. Chem. Rec. 2020, 20, 23–40. [Google Scholar] [CrossRef]
- Khatri, H.R.; Carney, N.; Rutkoski, R.; Bhattarai, B.; Nagorny, P. Recent progress in steroid synthesis triggered by the emergence of new catalytic methods. Eur. J. Org. Chem. 2020, 7, 755–776. [Google Scholar] [CrossRef]
- Zhao, Y.-L.; Lou, Q.-X.; Wang, L.-S.; Hu, W.-H.; Zhao, J.-L. Organocatalytic Friedel-Crafts alkylation/Lactonization reaction of naphthols with 3-trifluoroethylidene oxindoles: The asymmetric synthesis of dihydrocoumarins. Angew. Chem. Int. Ed. 2017, 56, 338–342. [Google Scholar] [CrossRef]
- Roemer, M.; Wild, D.A.; Sobolev, A.N.; Skelton, B.W.; Nealon, G.L.; Piggott, M.J.; Koutsantonis, G.A. Carbon-rich trinuclear octamethylferrocenophanes. Inorg. Chem. 2019, 58, 3789–3799. [Google Scholar] [CrossRef]
- Doğan, Ö.; Çağli, E. PFAM catalyzed enantioselective diethylzinc addition to imines. Turk. J. Chem. 2015, 39, 290–296. [Google Scholar] [CrossRef]
- Dogan, Ö.; Tan, D. Enantioselective direct aldol reactions promoted by phosphine oxide aziridinyl phosphonate organocatalysts. Tetrahedron Asymmetry 2015, 26, 1348–1353. [Google Scholar] [CrossRef]
- Eröksüz, S.; Dogan, Ö.; Garner, P.P. A new chiral phosphine oxide ligand for enantioselective 1,3-dipolar cycloaddition reactions of azomethine ylides. Tetrahedron Asymmetry 2010, 21, 2535–2541. [Google Scholar] [CrossRef]
- Dogan, Ö.; Isci, M.; Aygun, M. New phosphine oxide aziridinyl phosphonates as chiral Lewis bases for the Abramov-type phosphonylation of aldehydes. Tetrahedron Asymmetry 2013, 24, 562–567. [Google Scholar] [CrossRef]
- Dogan, Ö.; Bulut, A.; Tecimer, M.A. Chiral phosphine oxide aziridinyl phosphonate as a Lewis base catalyst for enantioselective allylsilane addition to aldehydes. Tetrahedron Asymmetry 2015, 26, 966–969. [Google Scholar] [CrossRef]
- Wujkowska, Z.; Zawisza, A.; Leśniak, S.; Rachwalski, M. Phosphinoyl-aziridines as a new class of chiral catalysts for enantioselective Michael addition. Tetrahedron 2019, 75, 230–235. [Google Scholar] [CrossRef]
- Buchcic, A.; Zawisza, A.; Leśniak, S.; Adamczyk, J.; Pieczonka, A.M.; Rachwalski, M. Enantioselective Mannich reaction promoted by chiral phosphinoyl-aziridines. Catalysts 2019, 9, 837. [Google Scholar] [CrossRef]
- Leśniak, S.; Rachwalski, M.; Jarzyński, S.; Obijalska, E. Lactic acid derived aziridinyl alcohols as highly effective catalysts for asymmetric additions of an organozinc species to aldehydes. Tetrahedron Asymmetry 2013, 24, 1336–1340. [Google Scholar] [CrossRef]
- Pieczonka, A.M.; Leśniak, S.; Rachwalski, M. Direct asymmetric aldol condensation catalyzed by aziridine semicarbazide zinc(II) complexes. Tetrahedron Lett. 2014, 55, 2373–2375. [Google Scholar] [CrossRef]
- Pieczonka, A.M.; Marciniak, L.; Rachwalski, M.; Leśniak, S. Enantiodivergent aldol condensation in the presence of aziridine/acid/water systems. Symmetry 2020, 12, 930. [Google Scholar] [CrossRef]
- Coumbe, T.; Lawrence, N.J.; Muhammad, F. Titanium (IV) catalysis in the reduction of phosphine oxides. Tetrahedron Lett. 1994, 35, 625–628. [Google Scholar] [CrossRef]
- Zhang, T.-X.; Zhang, W.-X.; Luo, M.-M. Metal-free reduction of tertiary phosphine oxides with Hantzsch ester. Chin. Chem. Lett. 2014, 25, 176–178. [Google Scholar] [CrossRef]
- Busacca, C.A.; Raju, R.; Grinberg, N.; Haddad, N.; James-Jones, P.; Lee, H.; Lorenz, J.C.; Saha, A.; Senanayake, C.H. Reduction of tertiary phosphine oxides with DIBAL-H. J. Org. Chem. 2008, 73, 1524–1531. [Google Scholar] [CrossRef] [PubMed]
- Provis-Evans, C.B.; Emanuelsson, E.A.C.; Webster, R.L. Rapid metal-free formation of free phosphines from phosphine oxides. Adv. Synth. Catal. 2018, 360, 3999–4004. [Google Scholar] [CrossRef]
- Sowa, S.; Stankevič, M.; Szmigielska, A.; Małuszyńska, H.; Kozioł, A.E.; Pietrusiewicz, K.M. Reduction of functionalized tertiary phosphine oxides with BH3. J. Org. Chem. 2015, 80, 1672–1688. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Sun, J. Triflimide (HNTf2) in organic synthesis. Chem. Rev. 2018, 118, 10349–10392. [Google Scholar] [CrossRef]
- Shi, H.; Herron, A.N.; Shao, Y.; Shao, Q.; Yu, J.-Q. Enantioselective remote meta-C–H arylation and alkylation via a chiral transient mediator. Nature 2018, 558, 581–586. [Google Scholar] [CrossRef] [PubMed]


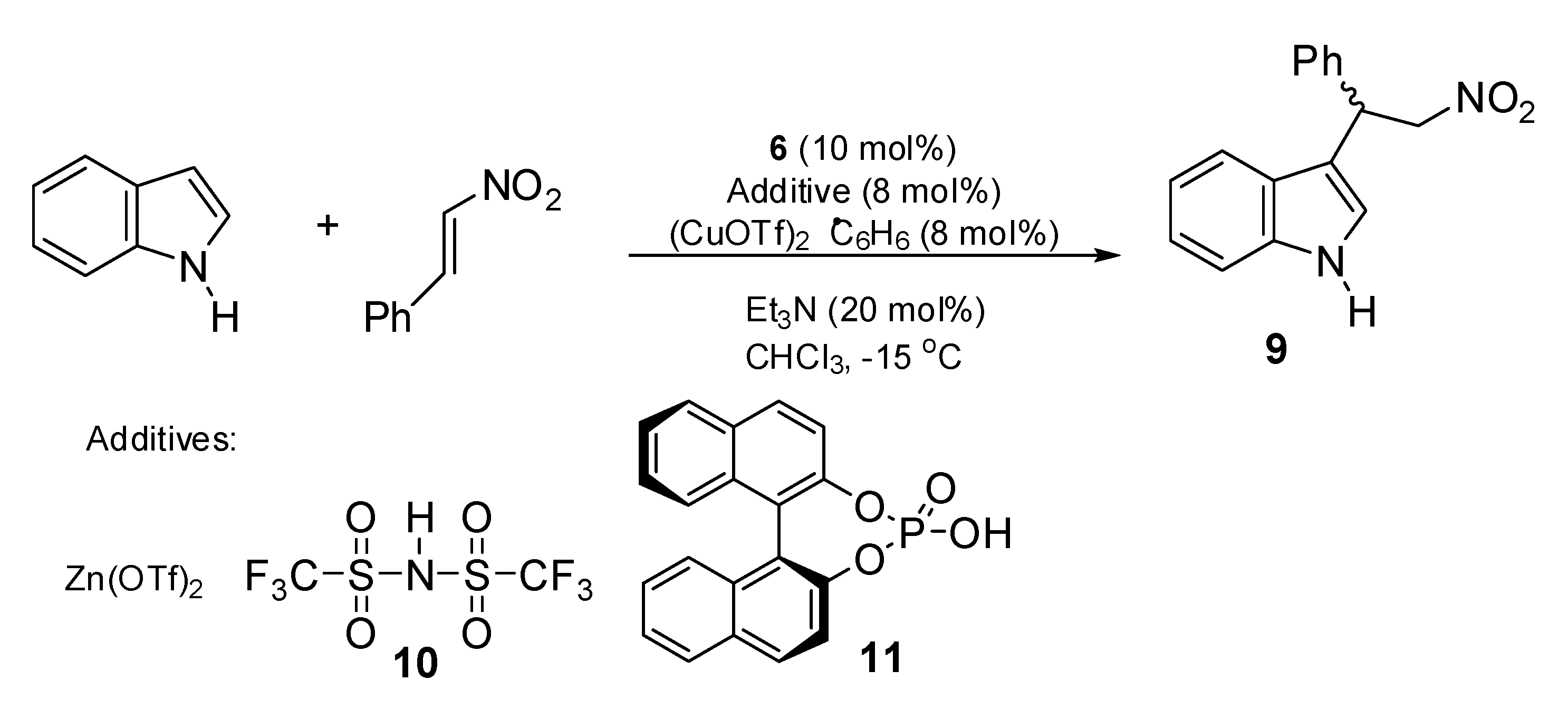


| Entry | Catalyst | Yield [%] | ee [%] a | Abs. Conf. b |
|---|---|---|---|---|
| 1 | 1 | 40 | 30 | (R) |
| 2 | 2 | 39 | 30 | (S) |
| 3 | 3 | 36 | 27 | (S) |
| 4 | 4 | 33 | 24 | (S) |
| 5 | 5 | 69 | 84 | (R) |
| 6 | 6 | 75 | 80 | (S) |
| 7 | 7 | 68 | 68 | (S) |
| 8 | 8 | 65 | 68 | (S) |
| Entry | Additive | Yield [%] | ee [%] a | Abs. Conf. b |
|---|---|---|---|---|
| 1 | Zn(OTf)2 c | 60 | 56 | (S) |
| 2 | 10 | 62 | 84 | (S) |
| 3 | 11 | 63 | 84 | (S) |
| Entry | R1 | R2 | Product | Yield [%] | ee [%] a | Abs. Conf. b |
|---|---|---|---|---|---|---|
| 1 | H | 4-MeC6H4 | 12 | 77 | 80 | (S) |
| 2 | H | 4-ClC6H4 | 13 | 75 | 80 | (S) |
| 3 | H | 4-OMeC6H4 | 14 | 80 | 84 | (S) |
| 4 | H | 3-ClC6H4 | 15 | 72 | 80 | (S) |
| 5 | OMe | Ph | 16 | 85 | 88 | (S) |
| 6 | Br | Ph | 17 | 88 | 92 | (S) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buchcic, A.; Zawisza, A.; Leśniak, S.; Rachwalski, M. Asymmetric Friedel–Crafts Alkylation of Indoles Catalyzed by Chiral Aziridine-Phosphines. Catalysts 2020, 10, 971. https://doi.org/10.3390/catal10090971
Buchcic A, Zawisza A, Leśniak S, Rachwalski M. Asymmetric Friedel–Crafts Alkylation of Indoles Catalyzed by Chiral Aziridine-Phosphines. Catalysts. 2020; 10(9):971. https://doi.org/10.3390/catal10090971
Chicago/Turabian StyleBuchcic, Aleksandra, Anna Zawisza, Stanisław Leśniak, and Michał Rachwalski. 2020. "Asymmetric Friedel–Crafts Alkylation of Indoles Catalyzed by Chiral Aziridine-Phosphines" Catalysts 10, no. 9: 971. https://doi.org/10.3390/catal10090971
APA StyleBuchcic, A., Zawisza, A., Leśniak, S., & Rachwalski, M. (2020). Asymmetric Friedel–Crafts Alkylation of Indoles Catalyzed by Chiral Aziridine-Phosphines. Catalysts, 10(9), 971. https://doi.org/10.3390/catal10090971







