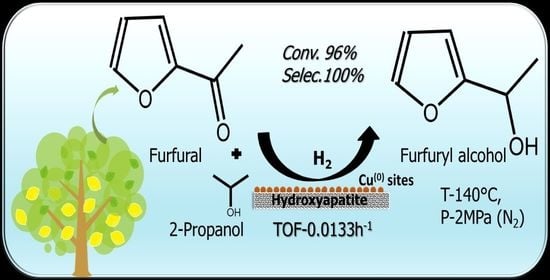
High Performance and Sustainable Copper-Modified Hydroxyapatite Catalysts for Catalytic Transfer Hydrogenation of Furfural
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterisation
2.2. Catalytic Activity
2.2.1. Effect of Cu Loading on CTH of FA
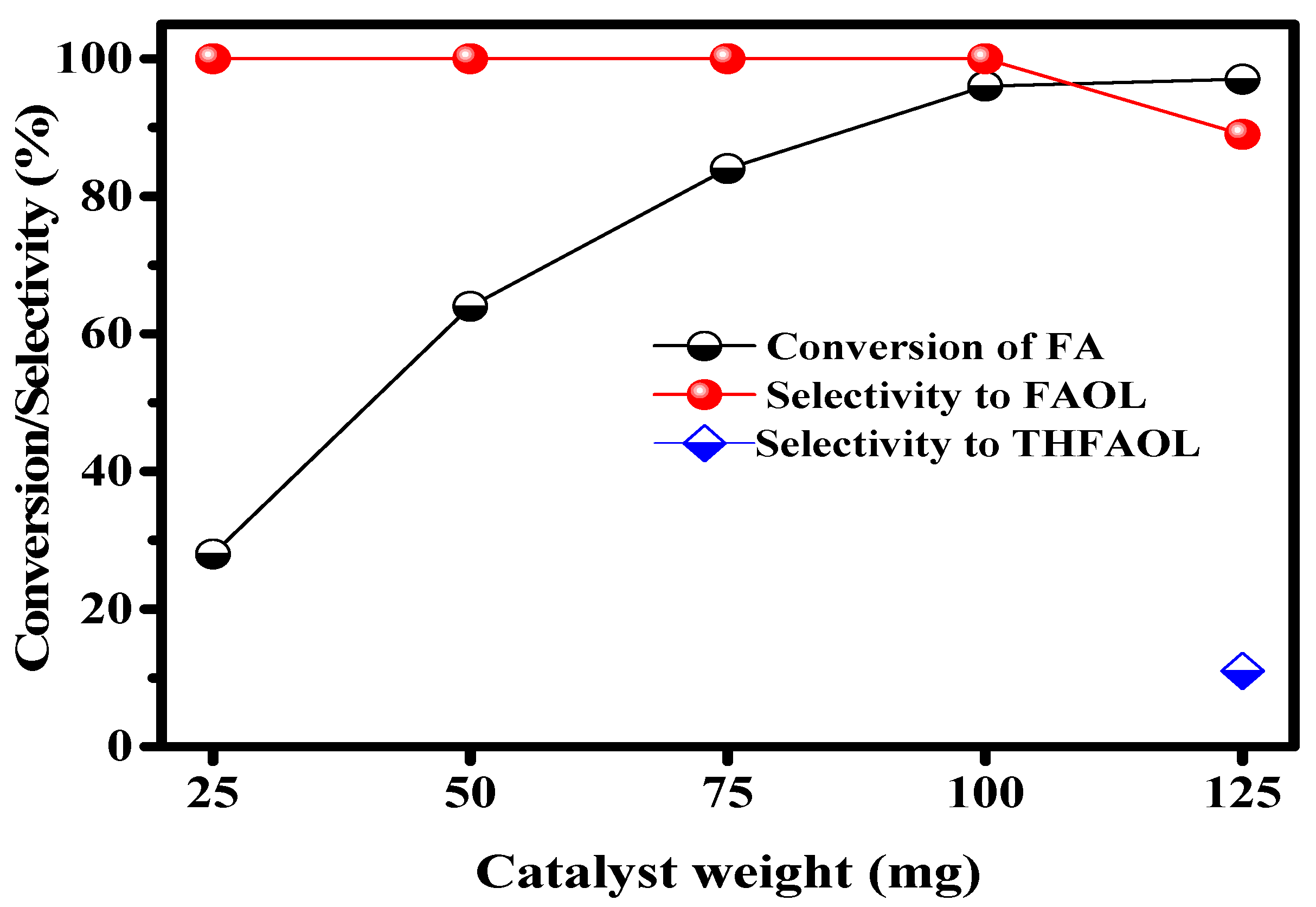
2.2.2. Effect of Catalyst Weight on CTH of FA
2.2.3. Effect of N2 Pressure on CTH of FA
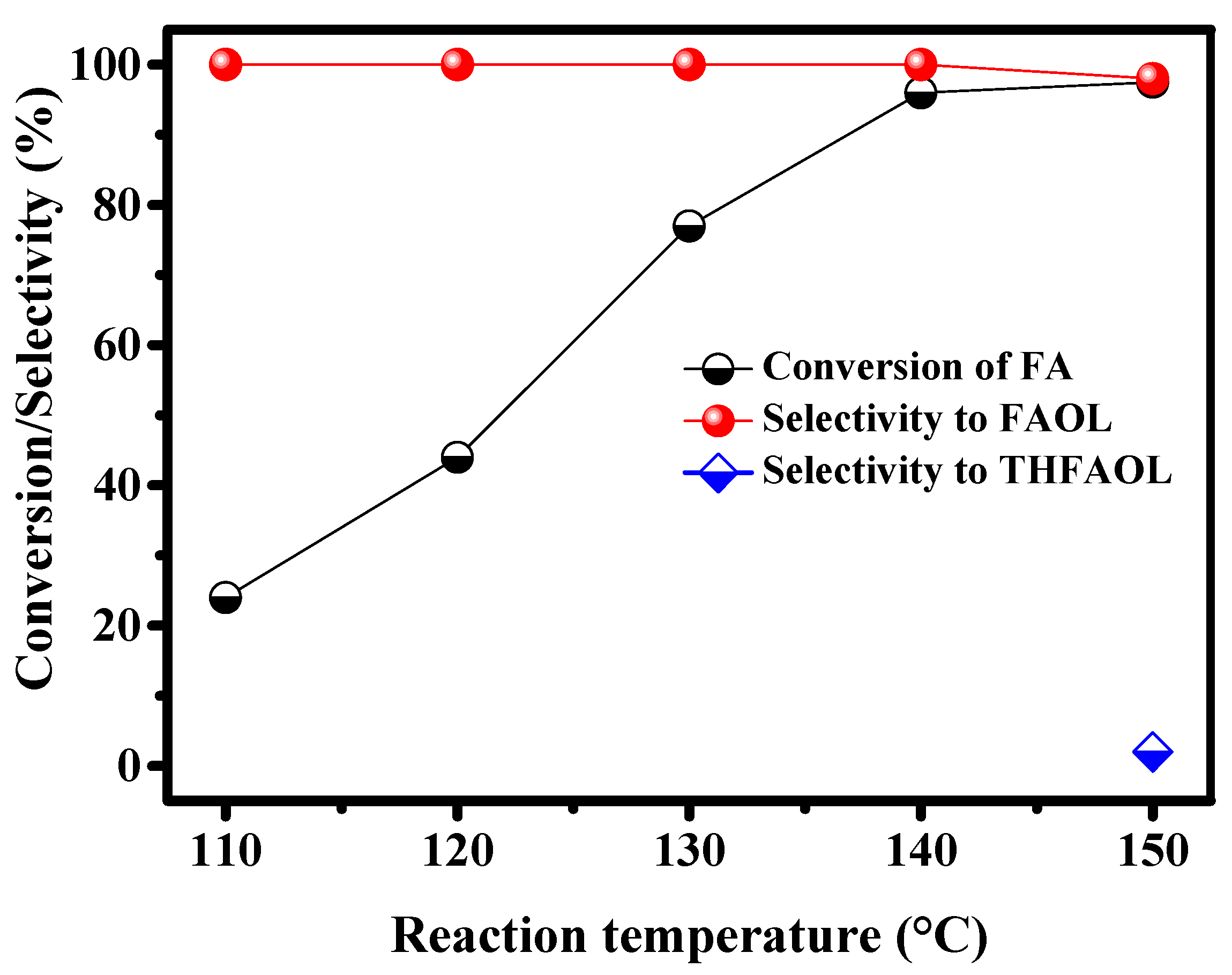
2.2.4. Effect of Reaction Temperature on CTH of FA
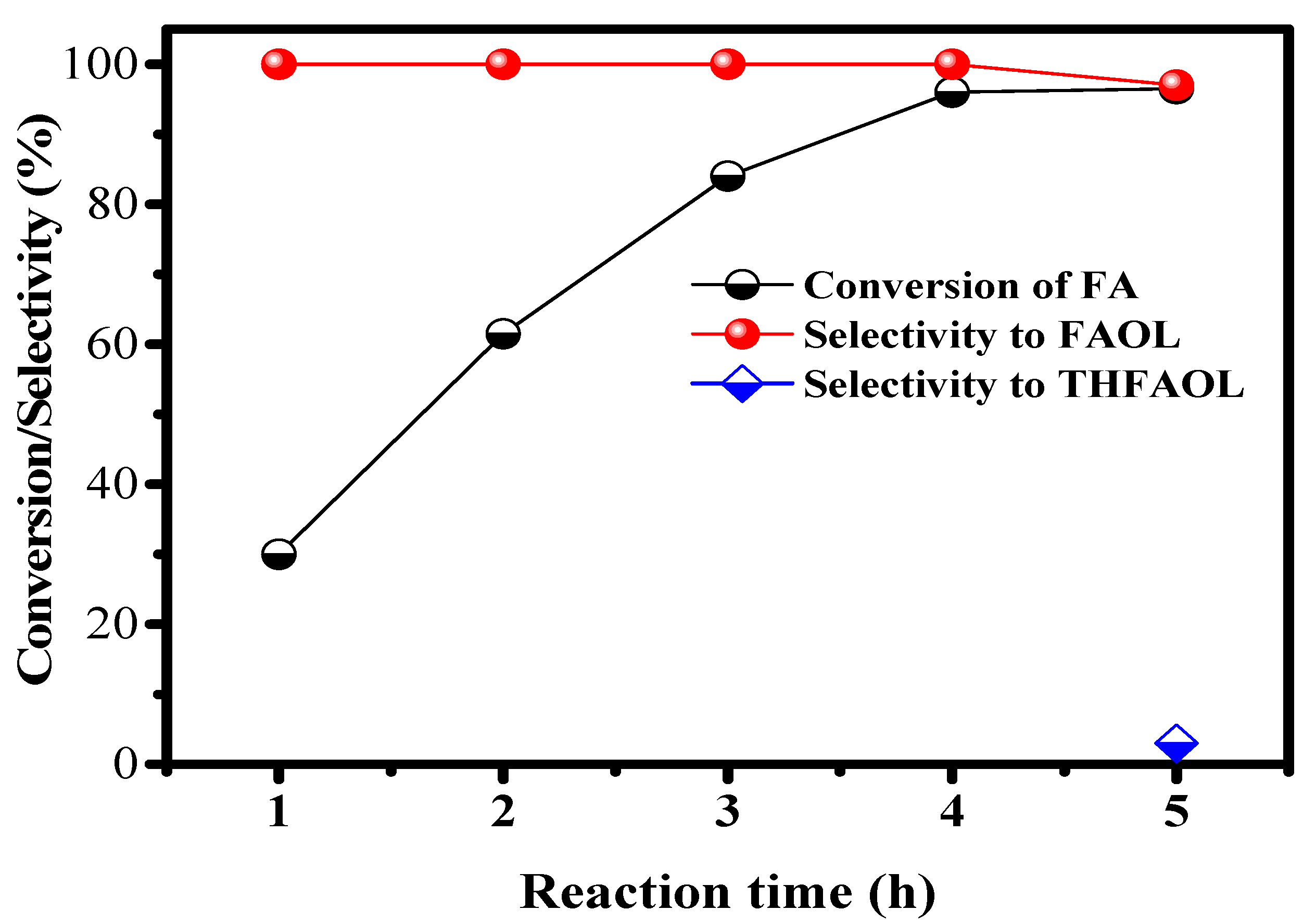
2.2.5. Effect of Reaction Time on CTH of FA
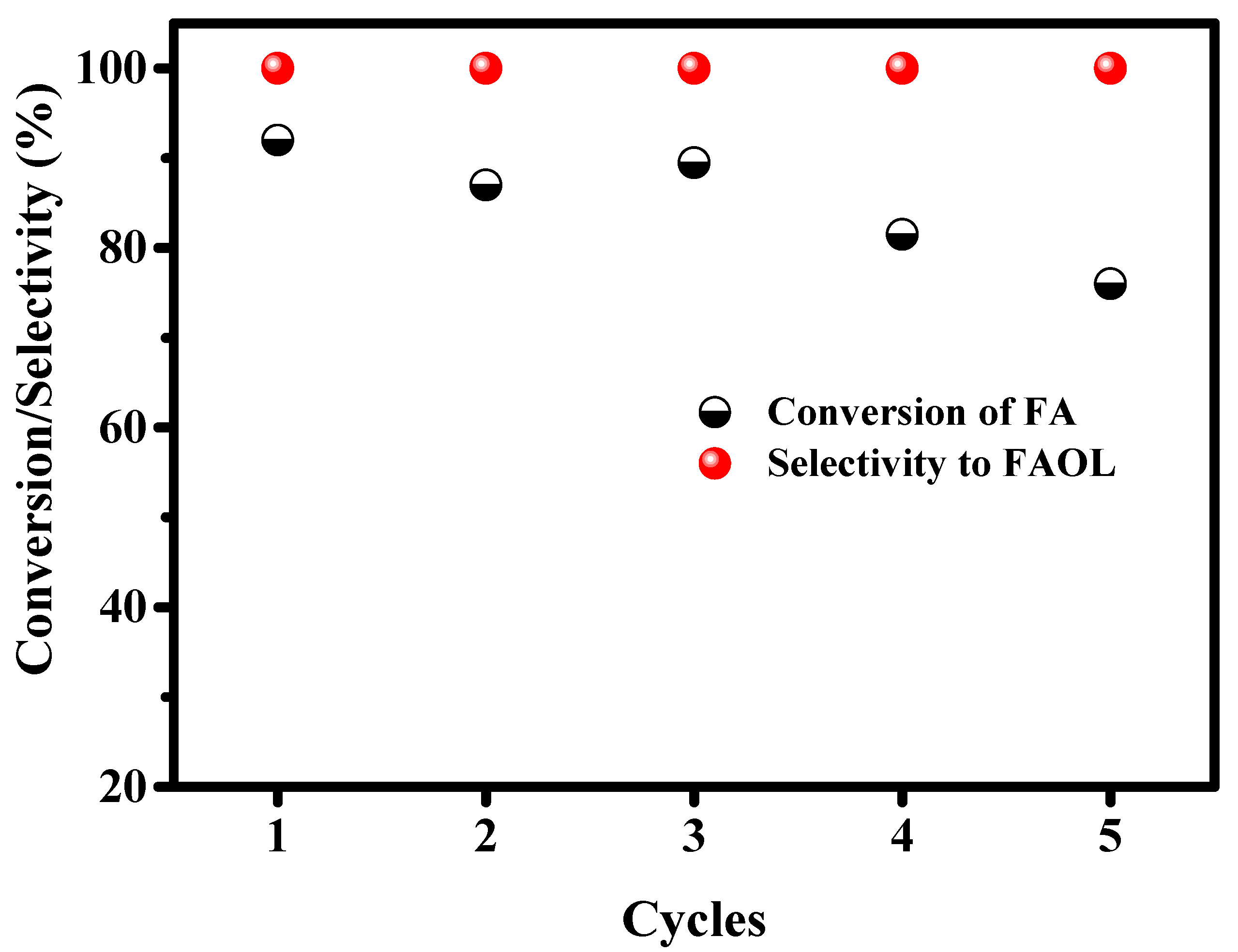
2.2.6. Recyclibity of 5CuHAp
3. Materials and Methods
3.1. Synthesis of Catalysts
3.2. Activity Tests
3.3. Catalytic Characterisation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Beims, R.F.; Hu, Y.; Shui, H.; Xu, C.C. Hydrothermal liquefaction of biomass to fuels and value-added chemicals: Products applications and challenges to develop large-scale operations. Biomass Bioenerg. 2020, 135, 105510. [Google Scholar] [CrossRef]
- Kucherov, F.A.; Romashov, L.V.; Galkin, K.I.; Ananikov, V.P. Chemical Transformations of Biomass-Derived C6-Furanic Platform Chemicals for Sustainable Energy Research, Materials Science, and Synthetic Building Blocks. ACS Sustain. Chem. Eng. 2018, 6, 8064–8092. [Google Scholar] [CrossRef]
- Wang, M.; Dewil, R.; Maniatis, K.; Wheeldon, J.; Tan, T.; Baeyens, J.; Fang, Y. Biomass-derived aviation fuels: Challenges and perspective. Prog. Energy Combust. Sci. 2019, 74, 31–49. [Google Scholar] [CrossRef]
- Audemar, M.; Wang, Y.; Zhao, D.; Royer, S.; Jérôme, F.; Len, C.; De Oliveira Vigier, K. Synthesis of Furfuryl Alcohol from Furfural: A Comparison between Batch and Continuous Flow Reactors. Energies 2020, 13, 1002. [Google Scholar] [CrossRef]
- Xu, C.; Paone, E.; Rodríguez-Padrón, D.; Luque, R.; Mauriello, F. Recent catalytic routes for the preparation and the upgrading of biomass derived furfural and 5-hydroxymethylfurfura. Chem. Soc. Rev. 2020, 49, 4273–4306. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, D.; Rodríguez-Padrón, D.; Len, C. Recent Advances in Catalytic Hydrogenation of Furfural. Catalysts 2019, 9, 796. [Google Scholar] [CrossRef]
- Zhang, Z.; Tong, X.; Zhang, H.; Li, Y. Versatile catalysis of iron: Tunable and selective transformation of biomass-derived furfural in aliphatic alcohol. Green Chem. 2018, 20, 3092–3100. [Google Scholar] [CrossRef]
- Svetlana, A.S.; Andrey, A.S.; Alexander, V.F.; Olga, A.B.; Andrey, A.S.; Maksim, Y.L.; Vadim, A.Y. Highly Active Cu FeAl-containing Catalysts for Selective Hydrogenation of Furfural to Furfuryl Alcohol. Catalysts 2019, 9, 816. [Google Scholar]
- Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sádaba, I.; Granados, M.L. Furfural: A renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy Environ. Sci. 2016, 9, 1144–1189. [Google Scholar] [CrossRef]
- Srivastava, S.; Jadeja, G.; Parikh, J.K. Copper-cobalt catalyzed liquid phase hydrogenation of furfural to 2-methylfuran: An optimization, kinetics and reaction mechanism study. Chem. Eng. Res. Des. 2018, 132, 313–324. [Google Scholar] [CrossRef]
- Gallezot, P. Conversion of biomass to selected chemical products. Chem. Soc. Rev. 2012, 41, 1538–1558. [Google Scholar] [CrossRef] [PubMed]
- Rasina, D.; Lombi, A.; Santoro, S.; Ferlin, F.; Vaccaro, L. Searching for novel reusable biomass-derived solvents: Furfuryl alcohol/water azeotrope as a medium for waste-minimised copper-catalysed azide–alkyne cycloaddition. Green Chem. 2016, 18, 6380–6386. [Google Scholar] [CrossRef]
- Yan, K.; Wu, G.; LaFleur, T.; Jarvis, C. Production, properties and catalytic hydrogenation of furfural to fuel additives and value-added chemicals. Renew. Sustain. Energy Rev. 2014, 38, 663–676. [Google Scholar] [CrossRef]
- Romano, P.N.; De Almeida, J.M.A.R.; Carvalho, Y.; Priecel, P.; Sousa-Aguiar, E.F.; Lopez-Sanchez, J.A. Microwave-Assisted Selective Hydrogenation of Furfural to Furfuryl Alcohol Employing a Green and Noble Metal-Free Copper Catalyst. ChemSusChem 2016, 9, 3387–3392. [Google Scholar] [CrossRef]
- Rodiansono, R.; Astuti, M.D.; Mujiyanti, D.R.; Santoso, U.T.; Shimazu, S. Novel preparation method of bimetallic Ni-In alloy catalysts supported on amorphous alumina for the highly selective hydrogenation of furfural. Mol. Catal. 2018, 445, 52–60. [Google Scholar] [CrossRef]
- Mironenko, R.M.; Belskaya, O.B.; Gulyaeva, T.I.; Nizovskii, A.I.; Kalinkin, A.V.; Bukhtiyarov, V.; Lavrenov, A.V.; Likholobov, V. Effect of the nature of carbon support on the formation of active sites in Pd/C and Ru/C catalysts for hydrogenation of furfural. Catal. Today 2015, 249, 145–152. [Google Scholar] [CrossRef]
- Liu, L.; Lou, H.; Chen, M. Selective hydrogenation of furfural over Pt based and Pd based bimetallic catalysts supported on modified multiwalled carbon nanotubes (MWNT). Appl. Catal. A Gen. 2018, 550, 1–10. [Google Scholar] [CrossRef]
- Aldosari, O.F.; Iqbal, S.; Miedziak, P.J.; Brett, G.L.; Jones, D.R.; Liu, X.; Edwards, J.K.; Morgan, D.J.; Knight, D.K.; Hutchings, G.J. Pd–Ru/TiO2 catalyst—An active and selective catalyst for furfural hydrogenation. Catal. Sci. Technol. 2016, 6, 234–242. [Google Scholar] [CrossRef]
- Chiara, P.; Laura, C.; Aguado-Molina, R.; Martin-Treceno, S.; Mark, A.K.; Cardenas-Lizana, F. Continuous furfuryl alcohol production via coupled dehydrogenation-hydrogenation over supported Cu and Au catalysts: A consideration of hydrogen generation and transfer. Mol. Catal. 2020, 492, 110912. [Google Scholar] [CrossRef]
- Du, J.; Zhang, J.; Sun, Y.; Jia, W.; Si, Z.; Gao, H.; Tang, X.; Zeng, X.; Lei, T.; Liu, S.; et al. Catalytic transfer hydrogenation of biomass-derived furfural to furfuryl alcohol over in-situ prepared nano Cu-Pd/C catalyst using formic acid as hydrogen source. J. Catal. 2018, 368, 69–78. [Google Scholar] [CrossRef]
- Villaverde, M.M.; Garetto, T.F.; Marchi, A.J. Liquid-phase transfer hydrogenation of furfural to furfuryl alcohol on Cu–Mg–Al catalysts. Catal. Commun. 2015, 58, 6–10. [Google Scholar] [CrossRef]
- He, J.; Yang, S.; Riisager, A. Magnetic nickel ferrite nanoparticles as highly durable catalysts for catalytic transfer hydrogenation of bio-based aldehydes. Catal. Sci. Technol. 2018, 8, 790–797. [Google Scholar] [CrossRef]
- Gong, W.; Chen, C.; Fan, R.; Zhang, H.; Wang, G.; Zhao, H. Transfer-hydrogenation of furfural and levulinic acid over supported copper catalyst. Fuel 2018, 231, 165–171. [Google Scholar] [CrossRef]
- Guangzhi, X.; Chen, L.; Aiyun, H.; Shan, W.; Haijun, W. A novel synthesis of zirconium tannate with high stability: New insight into the structure of the catalyst for hydrogenation. Appl. Catal. A Gen. 2020, 602, 117666. [Google Scholar] [CrossRef]
- Jing, G.; Zhang, J.; Yazhuo, W.; Denian, L.; Hongyu, H.; Haoran, Y.; Yong, C. Efficient transfer hydrogenation of biomass derived furfural and levulinic acid via magnetic zirconium nanoparticles: Experimental and kinetic study. Ind. Crop. Prod. 2020, 145, 112133. [Google Scholar] [CrossRef]
- Nagaiah, P.; Gidyonu, P.; Ashokraju, M.; Venkata, R.M.; Prathap, C.; David, R.B.; Seetha, R.R.K. Magnesium Aluminate Supported Cu Catalyst for Selective Transfer Hydrogenation of Biomass Derived Furfural to Furfuryl Alcohol with Formic Acid as Hydrogen Donor. Chemistry 2019, 4, 145–151. [Google Scholar] [CrossRef]
- Ping, X.; Xu, X.; Shan, W.; Junjiang, Z.; Yujun, Z. One-pot synthesis of LaFeO3@C composites for catalytic transfer hydrogenation reactions: Effects of carbon precursors. Appl. Catal. A Gen. 2020, 603, 117742. [Google Scholar] [CrossRef]
- Okamoto, Y.; Imanaka, T.; Teranishi, S. Hydrogen Transfer Reaction between Alcohols and Acetone over metal oxides. Bul. Chem. Soc. Jpn. 1972, 45, 938–939. [Google Scholar] [CrossRef]
- Maderuelo-Solera, R.; López-Asensio, R.; Cecilia, J.A.; Jiménez-Gómez, C.; García-Sancho, C.; Moreno-Tost, R.; Maireles-Torres, P. Catalytic transfer hydrogenation of furfural to furfuryl alcohol over calcined MgFe hydrotalcites. Appl. Clay Sci. 2019, 183, 105351. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, W.; Sang, S.; Gao, L.; Xiao, G. Supported Cu catalysts for the hydrogenation of furfural in aqueous phase: Effect of support. Asia Pac. J. Chem. Eng. 2017, 12, 422–431. [Google Scholar] [CrossRef]
- Fan, W.; Zehui, Z. Catalytic Transfer Hydrogenation of Furfural into Furfuryl Alcohol over Magnetic γ-Fe2O3@HAP Catalyst. ACS Sustain. Chem. Eng. 2017, 5, 942–947. [Google Scholar]
- Shi, R.; Wang, F.; Li, Y.; Huang, X.; Shen, W. A highly efficient Cu/La2O3 catalyst for transfer dehydrogenation of primary aliphatic alcohols. Green Chem. 2010, 12, 108–113. [Google Scholar] [CrossRef]
- Hu, Q.; Fan, G.; Yang, L.; Cao, X.; Zhang, P.; Wang, B.; Li, F. A gas-phase coupling process for simultaneous production of γ-butyrolactone and furfuryl alcohol without external hydrogen over bifunctional base-metal heterogeneous catalysts. Green Chem. 2016, 18, 2317–2322. [Google Scholar] [CrossRef]
- Panagiotopoulou, P.; Vlachos, D. Liquid phase catalytic transfer hydrogenation of furfural over a Ru/C catalyst. Appl. Catal. A Gen. 2014, 480, 17–24. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, J. Selective Transfer Hydrogenation of Biomass-Based Furfural and 5-Hydroxymethylfurfural over Hydrotalcite-Derived Copper Catalysts Using Methanol as a Hydrogen Donor. ACS Sustain. Chem. Eng. 2017, 5, 5982–5993. [Google Scholar] [CrossRef]
- Yang, X.; Meng, Q.; Ding, G.; Wang, Y.; Chen, H.; Zhu, Y.L.; Li, Y.W. Construction of novel Cu/ZnO-Al2O3 composites for furfural hydrogenation: The role of Al components. Appl. Catal. A Gen. 2018, 561, 78–86. [Google Scholar] [CrossRef]
- Yan, K.; Chen, A.C. Efficient hydrogenation of biomass-derived furfural and levulinic acid on the facilely synthesized noble-metal-free Cu−Cr catalyst. Energy 2013, 58, 357–363. [Google Scholar] [CrossRef]
- Elliot, J.C. Structure and Chemistry of the Apatites and Other Calcium Orthophosphates; Elsevier: Amsterdam, The Netherlands, 1994. [Google Scholar]
- Zachary, D.Y.; Robert, J.D. Hydrogen transfer reactions relevant to Guerbet coupling of alcohols over hydroxyapatite and magnesium oxide catalysts. Catal. Sci. Technol. 2018, 8, 1722–1729. [Google Scholar]
- Rodrigues, E.G.; Keller, T.C.; Mitchell, S.; Pérez-Ramírez, J. Hydroxyapatite, an exceptional catalyst for the gas-phase deoxygenation of bio-oil by aldol condensation. Green Chem. 2014, 16, 4870–4874. [Google Scholar] [CrossRef]
- Hassib, T.; Samir, D.; Carolina, P.; Gérard, D. Copper loaded hydroxyapatite catalyst for selective catalytic reduction of nitric oxide with ammonia. Appl. Catal. B Environ. 2011, 107, 158–163. [Google Scholar]
- Putrakumar, B.; Nagaraju, N.; Kumar, V.P.; Chary, K.V. Hydrogenation of levulinic acid to γ-valerolactone over copper catalysts supported on γ-Al2O3. Catal. Today 2015, 250, 209–217. [Google Scholar] [CrossRef]
- Putrakumar, B.; Vijayanand, P.; Pavan, K.V.; Chary, K.V.R. Hydrogenation of biomass-derived levulinic acid to γ-valerolactone over copper catalysts supported on ZrO2. J. Chem. Technol. Biotechnol. 2015, 91, 769–776. [Google Scholar] [CrossRef]
- Praliaud, H.; Mikhailenko, S.; Chajar, Z.; Primet, M. Surface and bulk properties of Cu–ZSM-5 and Cu/Al2O3 solids during redox treatments. Correlation with the selective reduction of nitric oxide by hydrocarbons. Appl. Catal. B Environ. 1998, 16, 359–374. [Google Scholar] [CrossRef]
- Dow, W.P.; Wang, Y.P.; Huang, T.J. Yitria-stabilized zirconia supported copper oxide catalyst: I. Effect of oxygen vacancy of support on copper oxide reduction. J. Catal. 1996, 160, 155–170. [Google Scholar] [CrossRef]
- Kim, K.-H.; Ihm, S.-K. Characteristics of titania supported copper oxide catalysts for wet air oxidation of phenol. J. Hazard. Mater. 2007, 146, 610–616. [Google Scholar] [CrossRef]
- Aziz, M.; Jalil, A.; Wongsakulphasatch, S.; Vo, D.-V.N. Understanding the role of surface basic sites of catalysts in CO2 activation in dry reforming of methane: A short review. Catal. Sci. Technol. 2020, 10, 35–45. [Google Scholar] [CrossRef]
- Gao, P.; Li, F.; Zhan, H.; Zhao, N.; Xiao, F.; Wei, W.; Zhong, L.; Wang, H.; Sun, Y. Influence of Zr on the performance of Cu/Zn/Al/Zr catalysts via hydrotalcite-like precursors for CO2 hydrogenation to methanol. J. Catal. 2013, 298, 51–60. [Google Scholar] [CrossRef]
- Isahak, W.N.R.W.; Zatil, A.C.R.; Mohamad, W.I.; Khomah, I.; Rahimi, M.Y.; Mohamed, W.M.H.; Mohd, A.Y. Adsorption–desorption of CO2 on different type of copper oxides surfaces: Physical and chemical attractions studies. J. CO2 Util. 2013, 2, 8–15. [Google Scholar] [CrossRef]
- Chen, H.; Ruan, H.; Lu, X.; Fu, J.; Langrish, T.; Lu, X. Efficient catalytic transfer hydrogenation of furfural to furfuryl alcohol in near-critical isopropanol over Cu/MgO-Al2O3 catalyst. Mol. Catal. 2018, 445, 94–101. [Google Scholar] [CrossRef]
- Chary, K.V.R.; Guggilla, V.S.; Dhachapally, N.; Kottapalli, K.S.; Bojja, S. Characterization and Reactivity of Copper Oxide Catalysts Supported on TiO2−ZrO2. J. Phys. Chem. B 2005, 109, 9437–9444. [Google Scholar] [CrossRef]
- Gong, W.; Chen, C.; Zhang, Y.; Zhou, H.; Wang, H.; Zhang, H.; Zhang, Y.; Wang, G.; Zhao, H. Efficient Synthesis of Furfuryl Alcohol from H2-Hydrogenation/Transfer Hydrogenation of Furfural Using Sulfonate Group Modified Cu Catalyst. ACS Sustain. Chem. Eng. 2017, 5, 2172–2180. [Google Scholar] [CrossRef]
- Wang, T.; Du, J.; Sun, Y.; Tang, X.; Wei, Z.-J.; Zeng, X.; Liu, S.-J.; Lin, L. Catalytic transfer hydrogenation of biomass-derived furfural to furfuryl alcohol with formic acid as hydrogen donor over CuCs-MCM catalyst. Chin. Chem. Lett. 2020. [Google Scholar] [CrossRef]
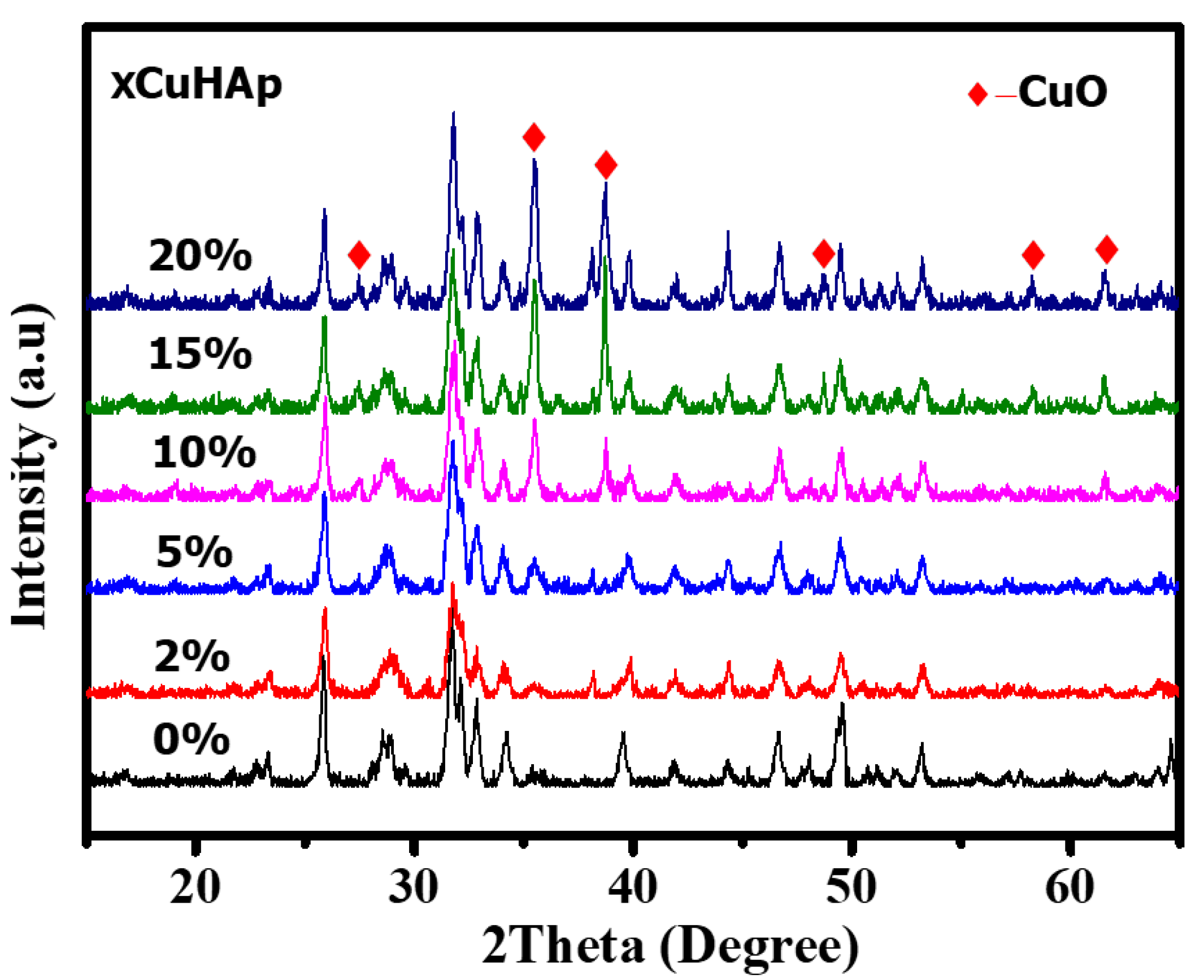

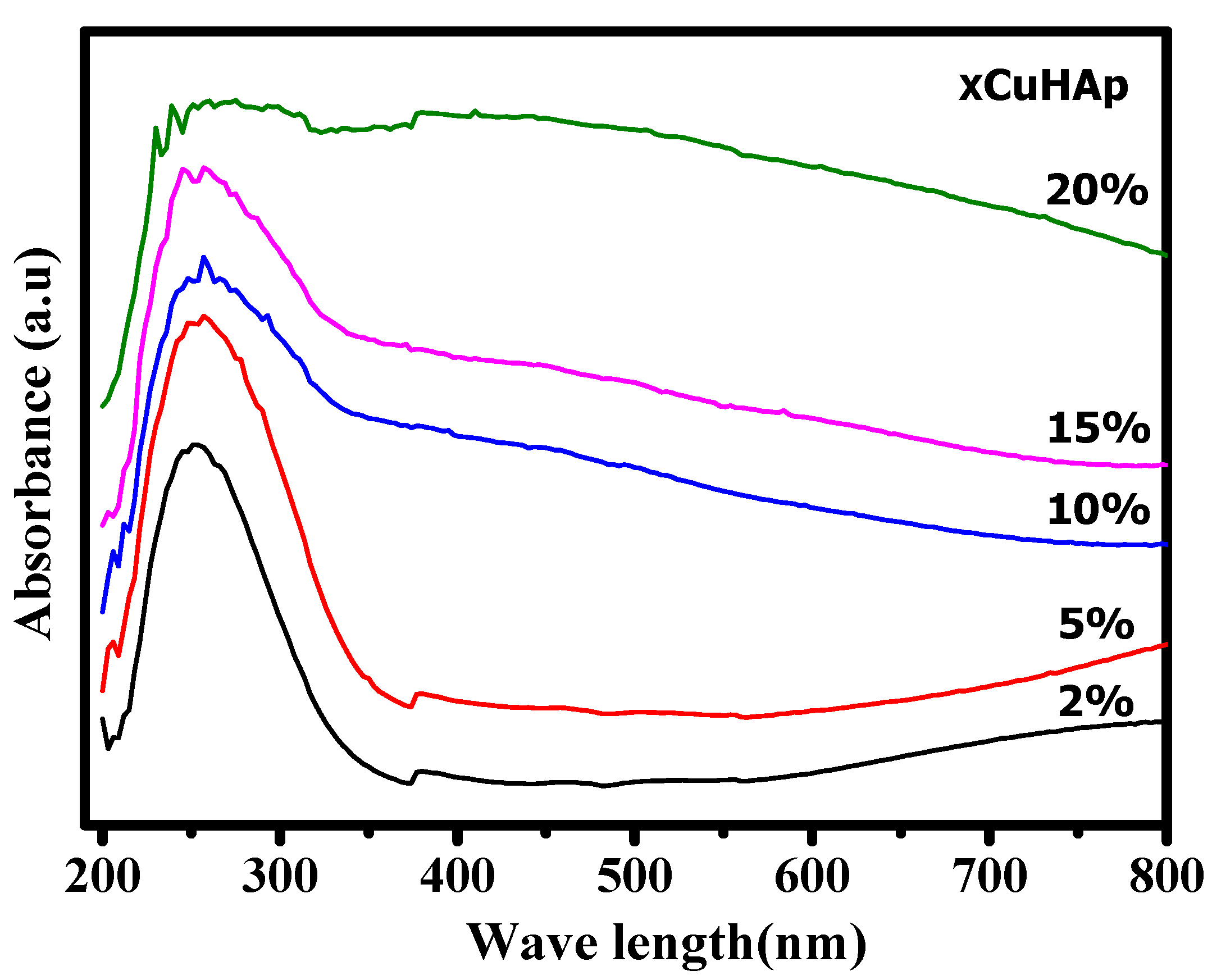
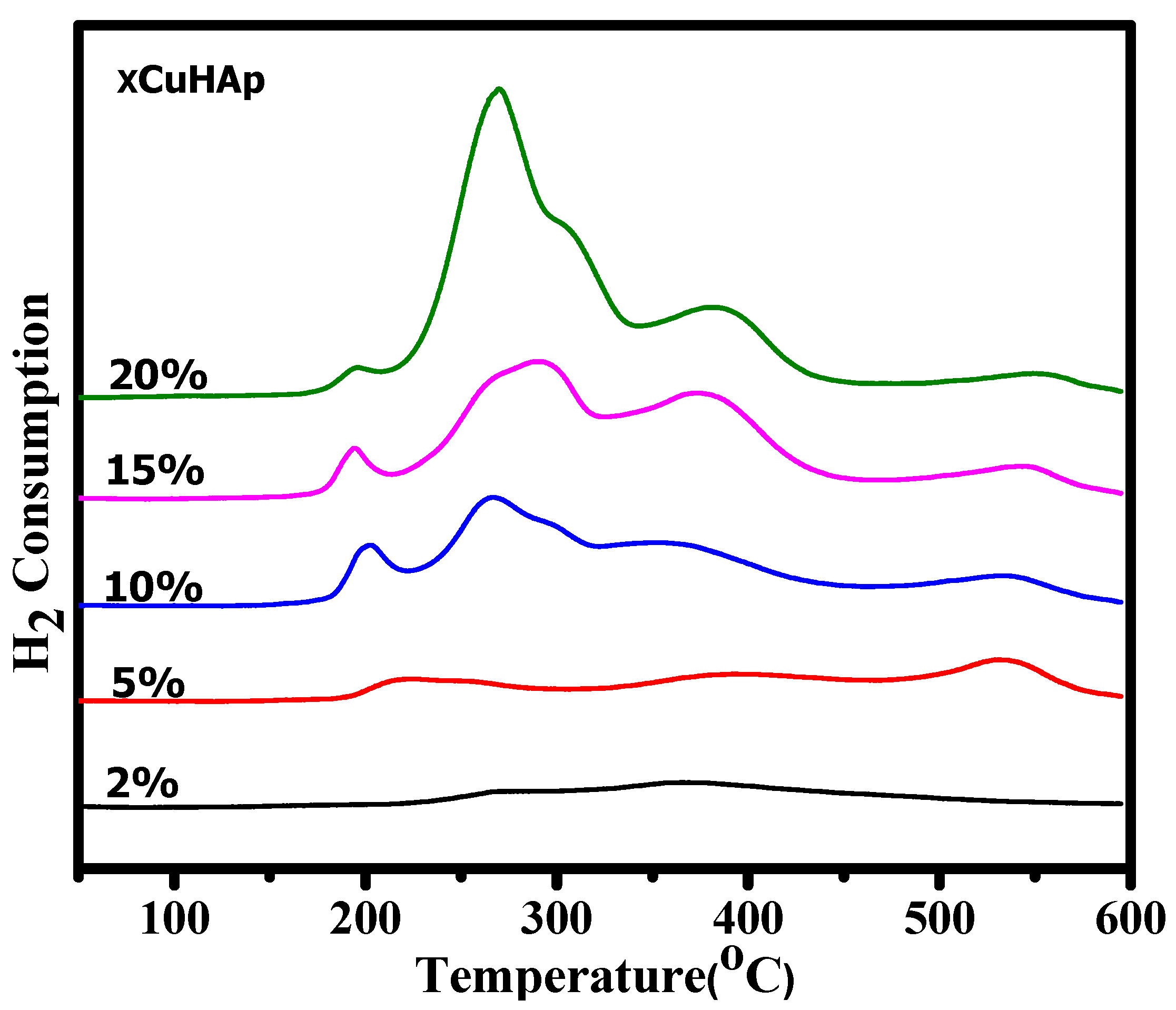
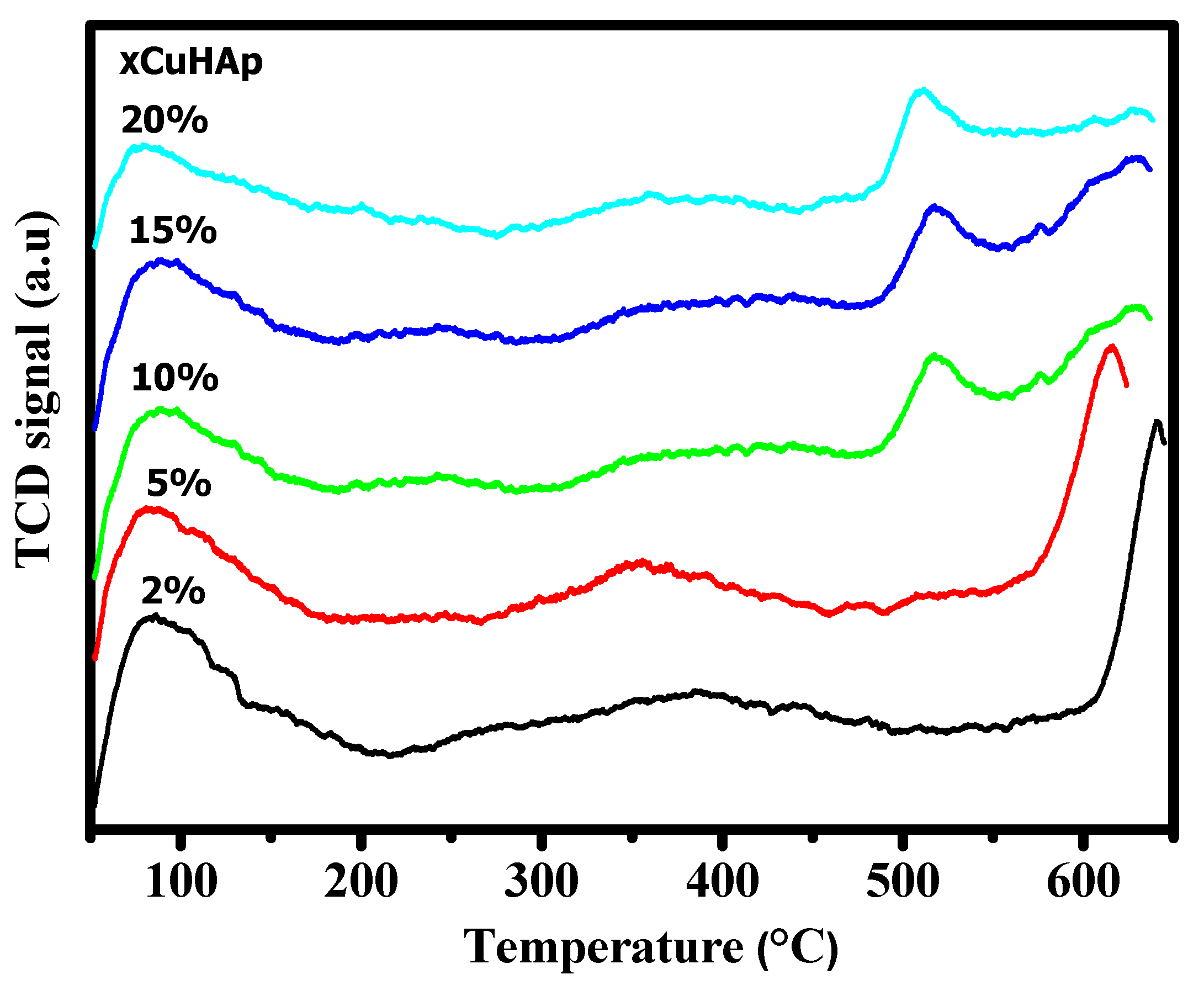
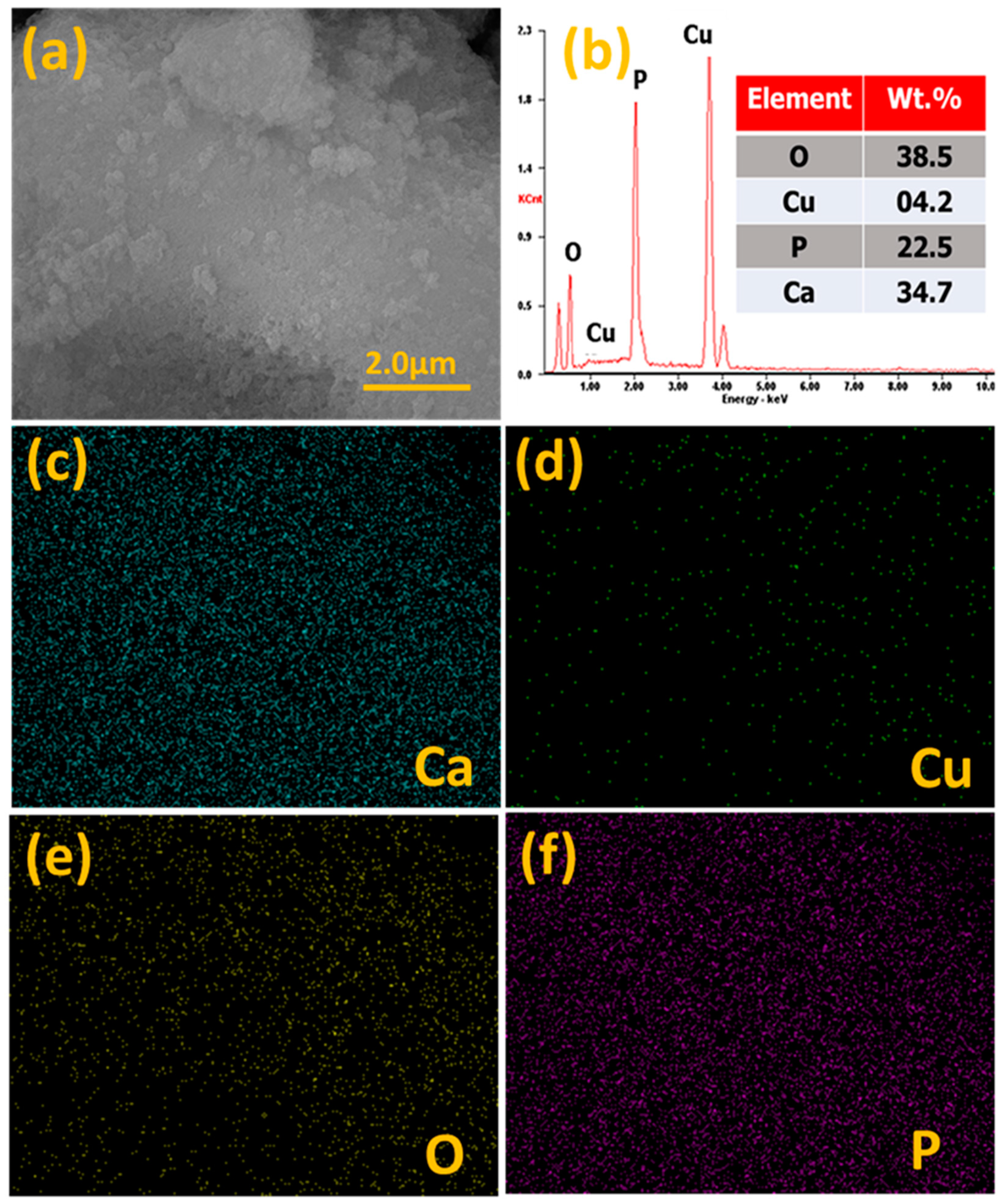
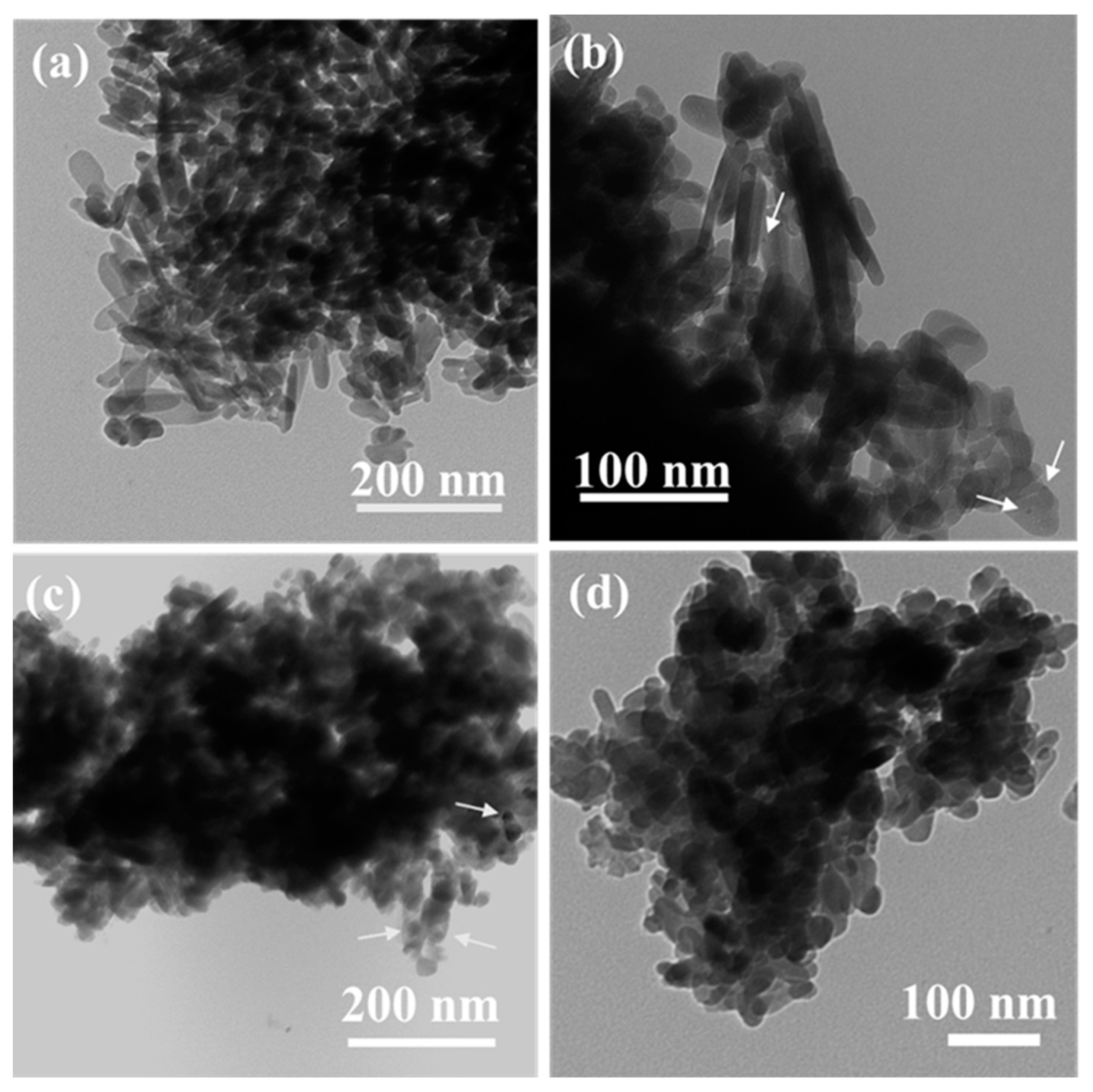
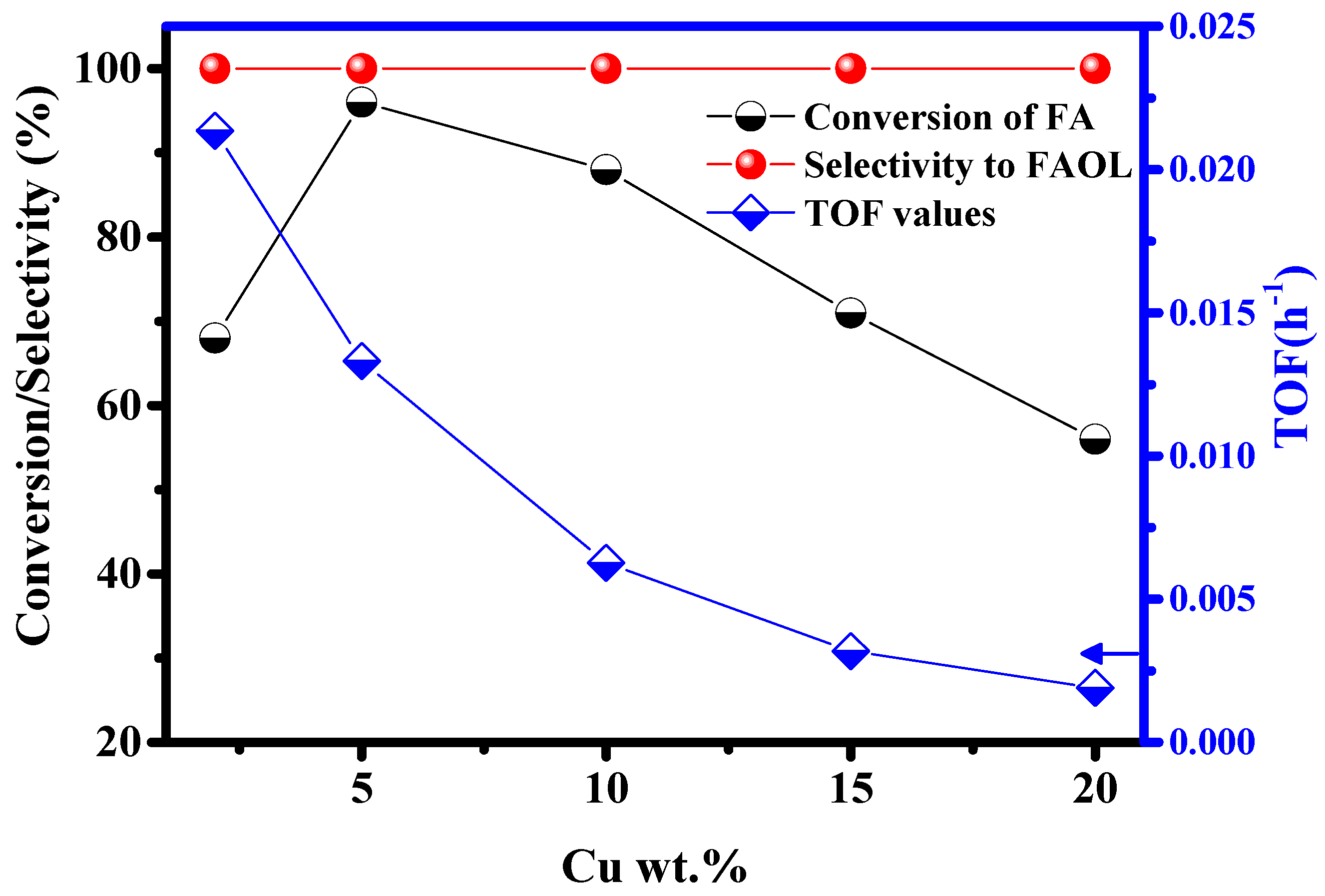

| Catalyst | Cu (wt.%) a | BET Surface Area (m2/gm) b | Cu Surface Area (m2/gm) c | Particle Size (nm) c | H2 Uptake (µmol/g) d |
|---|---|---|---|---|---|
| HAp | - | 76 | - | - | - |
| 2CuHAp | 1.81 | 62 | 204 | 3.3 | 51 |
| 5CuHAp | 4.57 | 52 | 210 | 3.20 | 127 |
| 10CuHAp | 8.9 | 46 | 143 | 4.70 | 152 |
| 15CuHAp | 14.1 | 43 | 92 | 7.30 | 160 |
| 20CuHAp | 18.7 | 37 | 62 | 10.8 | 174 |
| Catalyst | H-Donor | T (°C) | P (MPa) | Time (h) | XFA (%) | SFAOL (%) | Ref. |
|---|---|---|---|---|---|---|---|
| Cu/AC-SO3H | 2-PrOH | 150 | 4 | 5 | 100 | 100 | [52] |
| Cu-Al | MeOH | 200 | 1 | 2.5 | 100 | 94 | [35] |
| Cu-Mg-Al | 2-PrOH | 150 | - | 6 | 100 | 100 | [21] |
| Cu-Mg-Al2O3 | 2-PrOH | 210 | - | 1 | 100 | 89.3 | [50] |
| CuCs-MCM | HCOOH | 170 | - | 1 | 100 | 99.6 | [53] |
| CuHAp | 2-PrOH | 140 | 2 | 4 | 96 | 100 | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Putrakumar, B.; Seelam, P.K.; Srinivasarao, G.; Rajan, K.; Rajesh, R.; Rao, K.R.; Liang, T. High Performance and Sustainable Copper-Modified Hydroxyapatite Catalysts for Catalytic Transfer Hydrogenation of Furfural. Catalysts 2020, 10, 1045. https://doi.org/10.3390/catal10091045
Putrakumar B, Seelam PK, Srinivasarao G, Rajan K, Rajesh R, Rao KR, Liang T. High Performance and Sustainable Copper-Modified Hydroxyapatite Catalysts for Catalytic Transfer Hydrogenation of Furfural. Catalysts. 2020; 10(9):1045. https://doi.org/10.3390/catal10091045
Chicago/Turabian StylePutrakumar, Balla, Prem Kumar Seelam, Ginjupalli Srinivasarao, Karthikeyan Rajan, Rajendiran Rajesh, K. Ramachandra Rao, and Tongxiang Liang. 2020. "High Performance and Sustainable Copper-Modified Hydroxyapatite Catalysts for Catalytic Transfer Hydrogenation of Furfural" Catalysts 10, no. 9: 1045. https://doi.org/10.3390/catal10091045
APA StylePutrakumar, B., Seelam, P. K., Srinivasarao, G., Rajan, K., Rajesh, R., Rao, K. R., & Liang, T. (2020). High Performance and Sustainable Copper-Modified Hydroxyapatite Catalysts for Catalytic Transfer Hydrogenation of Furfural. Catalysts, 10(9), 1045. https://doi.org/10.3390/catal10091045