Abstract
Dielectric barrier discharge (DBD) could generate non-thermal plasma (NTP) with the advantage of fast reactivity and high energy under atmosphere pressure and low-temperature. The presented work investigated the selective catalytic reduction (SCR) of nitric oxide (NO) using a combination of NTP and an Mn-Cu/ZSM5 catalyst with ammonia (NH3) as a reductant. The experimental results illustrate that the plasma-assisted SCR process enhances the low-temperature catalytic performance of the Mn-Cu/ZSM5 catalyst significantly, and it exhibits an obvious improvement in the NO removal efficiency. The reaction temperature is maintained at 200 °C in order to simulate the exhaust temperature of diesel engine, and the 10% Mn-8% Cu/ZSM5 catalyst shows the highest NO removal performance with about 93.89% at an energy density of 500 J L−1 and the selectivity to N2 is almost 99%. The voltage, frequency and energy density have a positive correlation to NO removal efficiency, which is positively correlated with the power of NTP system. In contrast, the O2 concentration has a negative correlation to the NO removal, and the NO removal efficiency cannot be improved when the NO removal process reaches reaction equilibrium in the NTP system.
1. Introduction
The environmental hazards of nitric oxides (NOx) have been proved to be related to the photochemical smog and greenhouse effects closely in the last several decades. The wanton emission of NOx poses great threats to the ecosystems, which not only causes a series of environmental problems, but also endangers human health [1,2,3,4]. The mobile sources emission is the main source of NOx, especially the high-power diesel engines, and nitric oxide (NO) constitutes a great majority of NOx. The selective catalytic reduction (SCR) of NOx with ammonia (NH3) is regarded as one of the most effective strategies for NOx emission control owing to its reliable stability, which has been widely used in diesel engine [5,6,7,8]. However, the NH3-SCR has a high operating temperature window (>350 °C), and the exhaust temperature of the diesel engine is usually 200 °C. The increasingly stringent emission regulations demand the development of high-performance NH3-SCR available for low-temperature conditions [9,10].
The SCR catalyst is the core of NH3-SCR technology, and the V2O5-WO3/TiO2 catalyst shows excellent reactive activity, which possess desirable tolerance of SO2 and high catalytic performance from 300 to 400 °C. However, a number of obstacles haunt this technology, such as a narrow work temperature window and poor N2 selectivity [11,12,13]. Therefore, it is necessary to develop a low-temperature SCR catalyst with the wide temperature window. Manganese (Mn)-based SCR catalysts have been widely investigated for several years due to its high catalytic activity and superior low-temperature performance. In addition, it is generally believed that copper (Cu) has excellent catalytic performance as a promoter at low temperature, and it could strengthen the overall mechanical properties of the catalyst [14,15,16,17,18,19,20]. Wang et al. synthesized a lot of Mn-Cu/ZSM5 catalysts using the wetness impregnation method, and the result indicated that the incorporation of Mn improved the low-temperature activity of the SCR catalyst effectively [21]. Yan et al. designed a series of novel low-temperature CuwMnyTi1-yOx catalysts, in which CuO and MnOx were as the active components and TiO2 was as the support. The results suggested that the amount of acid sites increased significantly after adding Cu, and the NOx removal efficiency of the Cu1Mn0.5Ti0.5Ox catalyst reached 90% at 200 °C [22]. The ZSM5 has been widely used as the support material of catalysts on account of its unique crystal structure, thermal stability, and excellent catalytic activity, and it has been reported that the framework of ZSM5 plays an important role in the selective catalytic reduction of NO [23,24].
Recently, the non-thermal plasma (NTP) technology has been proposed as a promising and effective in the treatment of gaseous pollutants [25,26,27,28]. The dielectric barrier discharges (DBD) appear well adapted to remove NOx with high purification efficiency and low energy cost compared with other non-thermal gas discharges [29]. It is reported that there are two methods for NTP to remove NO. One method is to form higher valence oxides by oxidation, such as NO2, N2O5, etc. The other method is to form N2 and O2 by reduction [30,31,32]. Wang et al. prepared a lot of bimetal oxide catalysts (M-Cu, M = Mn, Ce, Cr, Co, and Fe), and then he combined catalysts with NTP to evaluate the NOx removal at various temperatures. The result showed that the bimetal oxide catalysts combined with NTP exhibited excellent NOx removal efficiency [33]. Rajanikanth et al. used the barrier discharge corona assisted by V2O5/TiO2 catalyst to remove NOx, and he found that the plasma-assisted catalytic process exhibited a remarkable improvement in NO removal efficiency [34].
NTP appears a wide foreground in the removal of NOx form diesel engine due to its high conversion efficiency and easy installation, which could generate lots of free radicals like hydroxyl radical (·OH), nitrogen radical (·N), and oxygen radical (·O). However, these active species are usually short-lived, which would disappear before entering the second catalyst stage. Therefore, the synergistic effect of a one-stage plasma-over-catalyst (POC) system is better than the two-stage plasma-followed-by-catalyst (PFC) hybrid system [35,36]. This research reports observations of an synergistic effect between NTP and Mn-Cu/ZSM5 catalysts in a one-stage POC reactor for NH3-selective-reduction of NO at 200 °C and investigates the influence parameters of NO removal in order to provide references for practical applications.
2. Results and Discussion
2.1. Characterizations of Mn-Cu/ZSM5 Catalysts
The characterizations of Mn-Cu/ZSM5 catalysts were carried out in this section, which could reveal the composition of catalysts concretely. In order to observe the micromorphology of the catalyst in detail, the 10% Mn-8% Cu/ZSM5 was selected as the representative to get SEM, HRTEM and EDS images, and the results are shown in Figure 1. It shows that the 10% Mn-8% Cu/ZSM5 catalyst is mainly constituted by pseudospherical particle. The HRTEM micrograph reveals that the lattice fringe of 10% Mn-8% Cu/ZSM5 catalyst was 2.5 Å, which could be assigned to the (111) crystal planes of crystalline CuO in accordance with the standard CuO card (JCPDS 01-1117). It illustrates that the target Cu was loaded on the surface of the 10% Mn-8% Cu/ZSM5 catalyst. Further, the EDS images of O, Al, Si, Mn and Cu elements were performed on the 10% Mn-8% Cu/ZSM5 catalyst, which indicates that the active metal elements Mn and Cu were uniformly dispersed on the surface of the ZSM5 carrier. The Al and Si elements were derived from the ZSM5 carrier, and the O element was derived from the active metal oxides of the 10% Mn-8% Cu/ZSM5 catalyst in addition to the ZSM5 carrier.
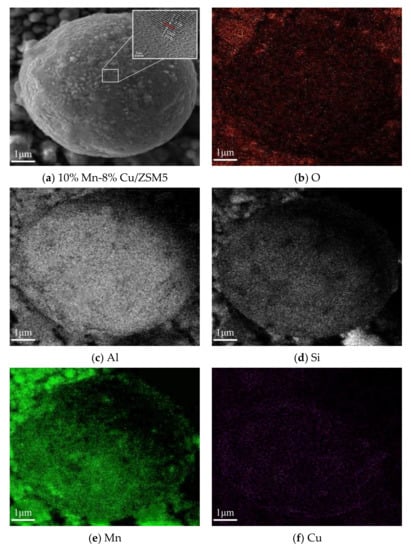
Figure 1.
SEM, HRTEM and EDS images of the 10% Mn-8% Cu/ZSM5 catalyst.
The Brunauer–Emmett–Teller (BET) surface area, pore volume, and pore diameter of ZSM5 and Mn-Cu/ZSM5 catalysts are summarized in Table 1. Obviously, the BET surface area of the prepared catalysts decreased because the ZSM5 was impregnated with Mn and Cu metal elements, and the pore volume was reduced while the pore diameter increased. Li et al. investigated the relationship between the catalytic activity and specific surface areas, and he discovered that the larger specific surface area promoted the NOx adsorption significantly because it could provide more acid sites for the catalytic reaction [37].

Table 1.
Physical characteristics of ZSM5 and Mn-Cu/ZSM5 catalysts.
The XRD pattern of Mn-Cu/ZSM5 catalysts with different loadings of 4%, 6%, 8%, and 10% Cu are shown in Figure 2. The Mn was not found in the XRD pattern. On the one hand, a small amount of Mn-based metal oxide catalysts were present in the amorphous state. On the other hand, the Mnn+ ions were incorporated into the Cu oxide lattice, and then it tends to form a solid solution during the calcinations based on related research [38,39]. Tang et al. found that the amorphous MnOx played an important role in the removal of NOx [40]. Boningari et al. revealed that amorphous MnO2 was of higher activity if compared with crystalline MnO2 for NO reduction [41]. Fang et al. reported that some Mn oxides are strongly associated with Cu oxides to form CuMn2O4, which was an active oxidation catalyst because of the easy electron transfer between Mn and Cu ions without a change in lattice, improving the lattice oxygen mobility and redox quality [42]. The (111) crystal planes of crystalline CuO were detected at 37°. Zhao et al. reported that the chemical forms of Cu species were present nearly exclusively in the form of oligomeric species and mononuclear ions [43].
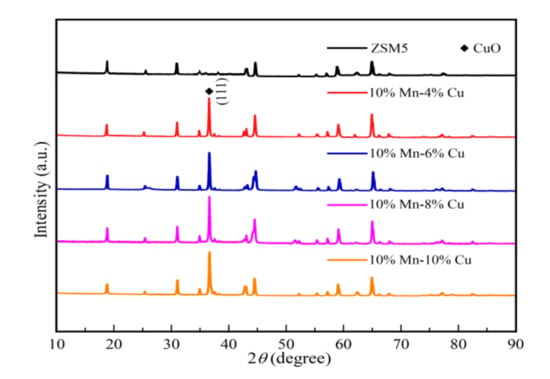
Figure 2.
The XRD pattern of Mn-Cu/ZSM5 catalysts with different loadings of 4%, 6%, 8%, and 10% Cu, respectively.
Figure 3 shows the XPS pattern of Mn-Cu/ZSM5 catalysts with different loadings of 4%, 6%, 8%, and 10% Cu, respectively. As depicted in the picture, the Mn 2p3/2 spectra could be separated into three peaks, including Mn2+, Mn3+, and Mn4+. Table 2 displays the Mn valence binding energy distribution of Mn-Cu/ZSM5 catalysts, which indicates that Mn oxides are mainly in Mn3+ and Mn4+. The ion concentration of Mn3+ and Mn4+ reached maximum when the loading amount of Cu was 8%. Fang et al. revealed that every kind of pure MnOx had its characteristic peak, and different MnOx species played important roles in the SCR process. Furthermore, Mn2O3 and Mn3O4 species were active for an NOx reduction based on the SCR performance [44]. Stanciulescu et al. reported that the SCR catalysts containing Mn3+ appeared to be more active for NOx reduction than those with Mn4+ [43]. According to the relevant research, the redox cycle between Mn and Cu with different chemical states and NO oxidation mechanism could be described by the following reactions [45,46,47,48,49].
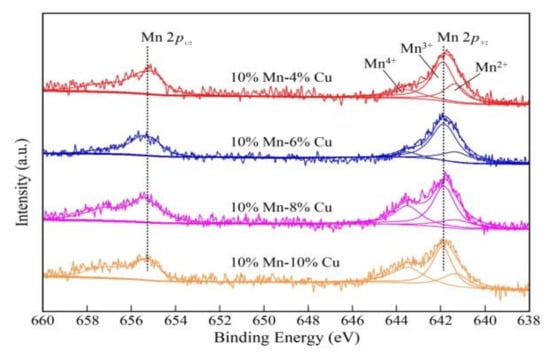
Figure 3.
The XPS pattern of Mn-Cu/ZSM5 catalysts with different loadings of 4%, 6%, 8%, and 10% Cu, respectively.

Table 2.
The Mn valence binding energy distribution of Mn-Cu/ZSM5 catalysts.
2.2. Effect of Voltage on NO Removal Efficiency
Figure 4 displays that the voltage was positively correlated with the NO removal efficiency in range of 10-20 kV at a frequency of 1000 Hz. The tested concentration of NO and NH3 were 300 ppm and 60 ppm, respectively. The NO removal efficiency was just 39.16% at a voltage of 20 kV only in the presence of NTP, and NO removal efficiency was 92.08% in combination of NTP and 10% Mn-8% Cu/ZSM5 catalyst, increased by 52.92%. The N2 selectivity remained stable at nearly 99%. Du et al. found that more energy would be injected into the NTP system with the increase of voltage, and a lot of active species, free radicals, and high-energy electrons could generate under this condition [50]. On one hand, high-energy electrons could collide with H2O and O2 in the air. The ultimate result is that lots of active species and free radicals would generate in the NTP system, such as ·OH, ·O, and so on. On the other hand, high-energy electrons could collide with pollutant molecules directly or indirectly, and then break the chemical bonds. Clearly, the electric field strength was gradually increased with the voltage increasing. Therefore, the electric field intensity and quantity of plasma generated per unit time increased to the NO removal efficiency.
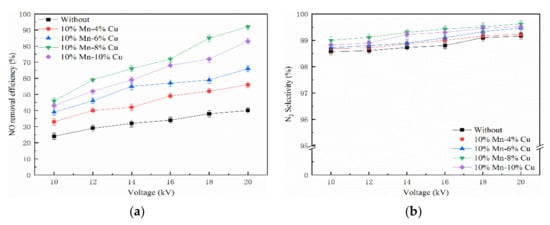
Figure 4.
Effect of voltage on NO removal efficiency. (a) NO removal efficiency and (b) N2 selectivity.
2.3. Effect of Frequency on NO Removal Efficiency
Figure 5 illustrates the effect of frequency on NO removal efficiency at a voltage of 20 kV. The tested concentration of NO and NH3 were 300 ppm and 60 ppm, respectively. Obviously, the selectivity to N2 was almost 99%, and the NO removal efficiency was proportional to the discharge frequency, which indicates that the NO removal efficiency was greatly affected by frequency. Zhu et al. found that the energy injected into the NTP system was positively correlated with frequency [51]. Compared with only plasma, the NO removal efficiency increased in the plasma–catalyst system. Among all the catalysts, the 10% Mn-8% Cu/ZSM5 catalyst shows the highest activity for NO removal. Meanwhile, it suggests that there was a significant synergistic effect between the NTP and Mn-Cu/ZSM5 catalyst. However, the NO removal efficiency tended to be gentle at high frequency, which indicates that the energy consumption of NO decreased compared to a low frequency. Yan et al. revealed that the gaseous benzene removal efficiency would increase with the increase of frequency because the energy imported to the reactor in unit time increased [52].
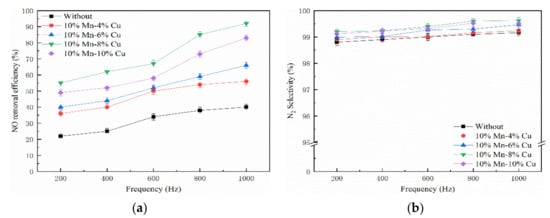
Figure 5.
Effect of frequency on NO removal efficiency. (a) NO removal efficiency and (b) N2 selectivity.
2.4. Effect of Energy Density on NO Removal
The discharge power is measured according to the V-Q Lissajous figure method [53], which is shown in Figure 6.
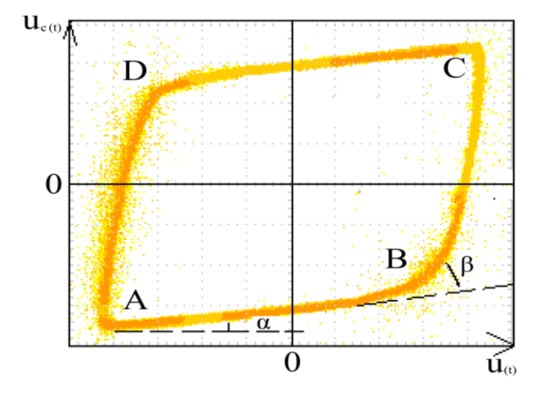
Figure 6.
V-Q Lissajous graph.
The discharge power was given by Equation (5), and the energy density was calculated using Equation (6).
In the equation, T is the period of discharge cycle, in s; u(t) represents the high voltage applied between electrodes, in kV; i(t) is the current changes with time, in A; f represents power frequency, in Hz; uc(t) is the voltage at both ends of Cm, in kV; Cm represents the measuring capacitance, which is 0.12 μF; S is the area of Lissajous graphics. ε is the energy density, in J L−1; and Q is the gas flow, in L min−1.
Figure 7 shows the variation of energy density and the NO removal efficiency. The tested concentration of NO and NH3 was 300 ppm and 60 ppm, respectively. The NO removal efficiency was climbing up with the increase of energy density, and Mn-Cu/ZSM5 catalysts enhanced the NO removal efficiencies in the presence of NTP, which presents a straight line that approximated the proportional increase. The NO removal efficiency was only 41.66% without the catalyst at an energy density of 500 J L−1, and NO removal efficiency was 93.89% in combination of NTP and 10% Mn-8% Cu/ZSM5 catalyst, increased by 52.23%, and the selectivity to N2 is almost 99%. The results indicate that the 10% Mn-8% Cu catalyst with plasma exhibits the highest catalytic activity.
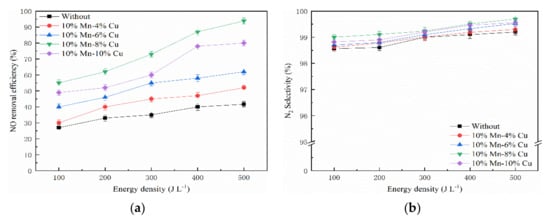
Figure 7.
Effect of energy density on NO removal efficiency. (a) NO removal efficiency and (b) N2 selectivity.
2.5. Effect of O2 Concentration on NO Removal
The relationship between energy density and NO removal efficiency under different O2 concentrations (0, 2%, 4%, 6%, and 8%) is shown as Figure 8, which is in combination with NTP and the 10% Mn-8% Cu/ZSM5 catalyst. The tested concentration of NO and NH3 was 300 ppm and 60 ppm, respectively. The energy density varied from 100 to 500 J L−1. The result indicates that the NO removal efficiency increased gradually with the energy density increasing, and then decreased with the increase of O2 concentration under the same energy density. The N2 selectivity shows a similar trend. This result is consistent with Zhao’s results, and Zhao et al. revealed that the NOx removal efficiency decreased with the O2 concentration increasing, and he found that there was a critical O2 concentration to make the NOx removal efficiency zero [54]. According to relevant research, large quantities of active species would be generated when the O2 concentration increased in the NTP system, such as ozone (O3) and so on. Then, lots of NH3 would be oxidized to NO molecules, which play a negative role in the removal of NO. As a final result, the NO and NH3 were partially oxidized to NO2 [55,56]. Therefore, the NO removal process is suppressed and the NO removal efficiency decreased. In the presence of O2 and O3, the following reactions would occur in the NTP system based on the relevant research [57,58,59].
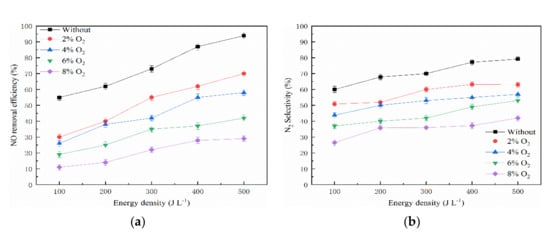
Figure 8.
Effect of O2 concentration on NO removal efficiency. (a) NO removal efficiency and (b) N2 selectivity.
The relationship between energy density and NO2 concentration is presented in Figure 9. In the presence of O2 molecules, the NO2 concentration increased slightly when the energy density was over 100 J L−1, and then the NO2 concentration remained stable with the energy density continually increasing. Ruggeri et al. revealed that NO2 was a key reaction product in the catalytic reaction and the SCR reaction dominated the NO reduction with a catalyst below 200 °C [60]. The electric field of the reaction system was strengthened with the increase of energy density, and more NH3 molecules participated in the reactions with O2 and ·O, which led to new NO2 molecule production. On the one hand, the active species O3, ·O, and ·N formed in the plasma–catalyst system reacted with NO to form different NOx. On the other hand, O3 and ·O could react with NO to oxidize NO to NO2. Koebel et al. found that the NO would be oxidized to higher valence oxides because O3 was more oxidative than O2. So, the NO2 was the main product in the presence of O2 [61]. The oxidation of NO to NO2 could be summarized as the following reactions [62,63,64,65].
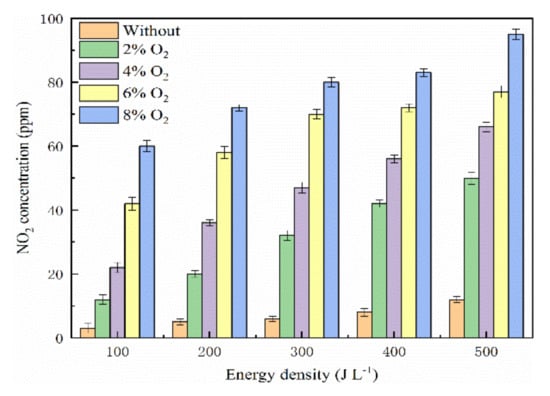
Figure 9.
Effect of O2 concentration on NO2 concentration.
2.6. Effect of NH3 Concentration on NO Removal
The effect of NH3 concentration and energy density on NO removal efficiency is presented in Figure 10. The tested NO concentration was 300 ppm and the NH3 concentration varied from 20 ppm to 100 ppm. The result indicates that the NO removal efficiency was affected by the addition of NH3 significantly.
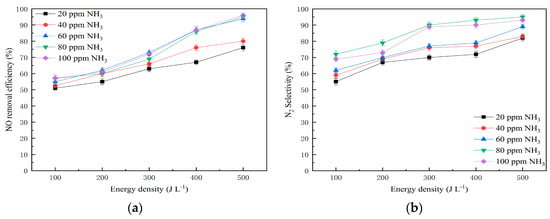
Figure 10.
Effect of NH3 concentration on NO removal efficiency. (a) NO removal efficiency and (b) N2 selectivity.
In the presence of NH3, there was higher probability of collision between NO molecules and high-energy electrons in the NTP system. As a result, the numbers of active species and free radicals were improved with the increase of energy density. When the NH3 concentration increased in the NTP system, it is generally believed that the ammonia radical (·NH2) plays an important role in the NOx removal. Wang et al. reported that the collision reactions of NO molecules were enhanced with the increase of NH3 concentration, and this is because an enormous amount of free radicals would generate in the NTP system, such as NH2·, NH·, and H· [66]. As a result, more NO molecules are removed under the same energy density. Therefore, the energy consumption decreased in the presence of NH3 in the NTP system. However, the NO removal efficiency tends to stabilize at high NH3 concentrations, which indicates that there is an optimal NH3 concentration in combination of NTP and the Mn-Cu/ZSM5 catalyst. On the whole, the NO removal efficiency cannot be improved when the NO removal process reaches reaction equilibrium in the NTP system, even if the NH3 concentration increased continuously. The main chemical reactions could be concluded as follows [67,68].
2.7. The Catalytic Long-Term Stability of the 10% Mn-8% Cu/ZSM5 Catalyst
The long-time stability is an important index to evaluate the NO removal efficiency of the 10% Mn-8% Cu/ZSM5 catalyst in the NTP system, which could provide basic research data for the practical applications. The tested concentration of NO and NH3 was 300 ppm and 60 ppm, respectively. The reaction temperature was maintained at 200 °C, and the O2 concentration was 0 and the energy density of plasma was 500 J L−1. As can be seen from Figure 11, a 25 h continuous test was performed in this section, and the long-term stability of the 10% Mn-8% Cu/ZSM5 catalyst at an energy density of 500 J L−1 was explored. The NO removal efficiency of the 10% Mn-8% Cu/ZSM5 catalyst was rather stable with a slight fluctuation around ± 1% after the 25 h of continuous testing, and the NO removal efficiency remained at nearly 93%. At the same time, the N2 selectivity remained stable at nearly 99%, which illustrates that there was almost no deactivation that occurred during the 25 h test at an energy density of 500 J L−1, and further demonstrates that the 10% Mn-8% Cu/ZSM5 catalyst had excellent long-term stability in the NTP system.
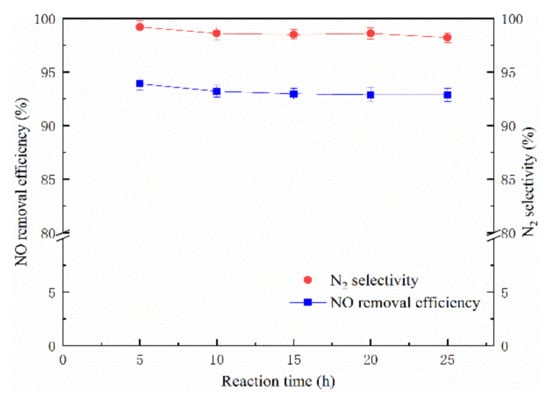
Figure 11.
The catalytic long-term stability of the 10% Mn-8% Cu/ZSM5 catalyst.
3. Materials and Methods
3.1. Catalyst Preparation
ZSM5 (Si/Al = 25) was purchased from Nankai Catal. Corp. The Mn-Cu/ZSM5 catalysts were synthesized using the incipient wetness impregnation method. The Mn(NO3)2 and Cu(NO3)2 were dissolved in deionized water, and the corresponding proportion ZSM5 was added in solution. The active component Mn loading was 10 wt % and the target Cu loadings were 4 wt %, 6 wt %, 8 wt %, and 10 wt %, respectively. The ZSM5 and mixture of metal nitrates was heated for 2 h in the ohmic heating equipment, and then stirred for 5 h continuously. The obtained solid samples were dried at 100 °C, and then calcined for 5 h at 500 °C in air. Immediately, these catalysts were cooled down to room temperature under N2 atmosphere. Finally, the Mn-Cu/ZSM5 catalysts were pressed, crushed, and sieved to 40–60 mesh to obtain suitable particles for the following experiments, which were noted as 10% Mn-4% Cu/ZSM5, 10% Mn-6% Cu/ZSM5, 10% Mn-8% Cu/ZSM5, and 10% Mn-10% Cu/ZSM5, respectively.
3.2. Catalyst Characterization
The structural and morphological properties of synthesized solid catalyst samples were characterized using scanning electron microscopy (SEM), high-resolution transmission electron microscopy (HRTEM), energy dispersive spectroscopy (EDS), X-ray diffraction (XRD), and X-ray photoelectron spectroscopy (XPS), respectively. The S80 JEOL (SEM, S80 JEOL, Tokyo Japan) was used to examine the microscopy of Mn-Cu/ZSM5 catalysts surface, and the HRTEM images were recorded on a Tecnai G2 F20 S-TWIN microscope (FEI, Hillsboro, OR, USA) at an accelerating voltage of 200 kV. The specific surface area and pore size of Mn-Cu/ZSM5 catalysts were calculated by the Brunauer–Emmett–Teller (BET) method and the Barrett–Joyner–Halenda (BJH) method. Prior to the measurements, these catalysts were outgassed overnight under vacuum. The EDS for the O, Al, Si, Mn, and Cu elements of Mn-Cu/ZSM5 catalysts were measured using an electric refrigeration X-ray energy spectrometer connected to SEM. The XRD measurement was carried out by an XRD-7000 S system (Shimadzu Corporation, Kyoto, Japan). The XPS was implemented on the surface analysis system (VG Multilab2000) with Al Κα radiation (hv = 1486.6 eV) as the excitation source at 150 W, and the C1 s line at 284.6 eV was taken as the standard.
3.3. Experimental Setup
The schematic of the DBD plasma experiment is shown as Figure 12. The Mn-Cu/ZSM5 catalyst (2 mL, 40–60 mesh) was placed at the center of the DBD plasma reactor. The concentrations of Ar, O2, NO, and NH3 in the experimental system were regulated by the mass flow controller (MFC), and then the gases flowed into the buffer tank. After they were mixed thoroughly, the gases were tested with the DBD plasma reactor, which could generate a NTP with the advantage of fast reactivity and high energy at atmosphere pressure and low-temperature. The total gas flow was kept constant at 2 L min−1, and the Ar was used as a carrier gas. The gas hourly space velocity (GHSV) was 60,000 h−1. The temperature of the mixed gas was maintained at 200 °C in the NTP system. The plasma reactor was connected to an AC power, and the output waveform of DBD plasma was measured by Tektronix-TBS1102 digital oscilloscope, which was equipped with the current loop and P6015A high voltage probe. The concentrations of NOx (NO + NO2) and N2O in the outlet gas were analyzed by an FT-IR gas analyzer (GASM ET DX 4000, Finland).
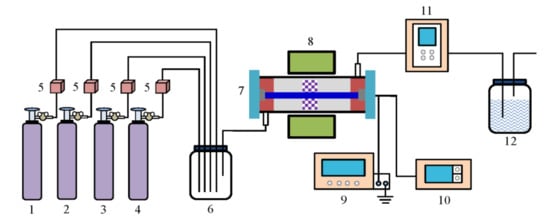
Figure 12.
Schematic of the dielectric barrier discharge (DBD) plasma experiment. 1 Ar, 2 O2, 3 NO, 4 NH3, 5 Mass flow controller, 6 Buffer tank, 7 Plasma reactor, 8 Resistance furnace, 9 Power supply, 10 Digital oscilloscope, 11 Gas analyzer, and 12 Alkali absorption.
Figure 13 presents the DBD plasma reactor, which adopts the coaxial line structure. The dielectric tube was made of quartz and the sealing gaskets at both ends were made of polytetrafluoroethylene. The copper wire mesh was covered in the quartz, and it was used as a ground electrode. The diameter of dielectric tube was 20 mm, and the thickness of the dielectric tube was 2 mm. The length of the DBD plasma reactor was 500 mm.
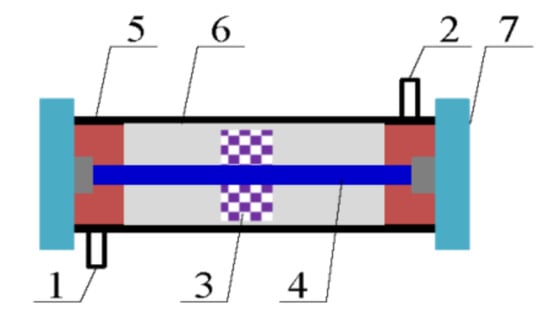
Figure 13.
Schematic diagram of the plasma reactor structure. 1 Inlet, 2 Outlet, 3 Mn-Cu/ZSM5 catalyst, 4 Internal electrode, 5 Dielectric tube, 6 Ground electrode, and 7 Sealing gaskets.
The NO removal efficiency and N2 selectivity were calculated by the following Equations (29) and (30). The analysis of experimental results was made in triplicate, and the error bar was used as an index to evaluate the difference between the average and measurement. The following Equations (31) and (32) were the average and error bar, respectively.
where is the concentration of NO at the inlet of the DBD plasma reactor, is the concentration of N2O at the outlet of the DBD plasma reactor, and is the concentration of NOx (NO + NO2) at the outlet of the DBD plasma reactor. represents the average of three experimental results, , and are the result of three experiments, respectively. and are the measured and averaged result, respectively.
4. Conclusions
The presented work proved that the plasma-assisted catalytic for NO removal was an effective method, which could provide a new strategy for the removal of NOx from the diesel engine and it has huge potential for industrial applications in the future. The NO removal efficiency in the NTP system over Mn-Cu/ZSM5 catalysts was investigated, and the results indicate that the combination of NTP and Mn-Cu/ZSM5 catalyst promoted NO removal efficiency significantly. In addition, the influence parameters of voltage, frequency, energy density, and O2 and NH3 concentration on the NO removal efficiency were explored. The detailed experimental results are shown as follows:
- (1)
- The plasma-assisted SCR process exhibited an obvious improvement in NO removal efficiency, and 10% Mn-8% Cu/ZSM5 shows the highest catalytic activity with about 93.89% at an energy density of 500 J L−1, and the selectivity to N2 was almost 99%.
- (2)
- The voltage and frequency were positively correlated with the power of the NTP system. The NO removal efficiency increased with the voltage, frequency, and energy density increasing.
- (3)
- The O2 concentration was negatively correlated with the NO removal in the NTP system. With the O2 concentration increasing, the NO removal efficiency decreased significantly under the same energy density.
- (4)
- The NH3 concentration was related to the NO removal closely, and the NO removal efficiency could not be improved when the NO removal process reached reaction equilibrium in the NTP system.
Author Contributions
All authors have contributed in various degrees to the analytical methods used, to the research concept, to the experiment design, to the acquisition of data, or analysis and interpretation of data, to draft the manuscript or to revise it critically for important intellectual content. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Engineering Laboratory for Mobile Source Emission Control Technology (NELMS2019A13), and the Major Science and Technology Projects of Shanxi Province (No. 20181102017), and the Shanxi Province Unveiled the List of Bidding Projects (No. 20191101007), and the Central Guide Local Science and Technology Development (No. 19943816G) and the Fundamental Research Funds for the Central Universities (No. 2009QH03).
Conflicts of Interest
The authors confirm that this article content has no conflict of interest.
References
- Yang, Y.; Liu, J.; Liu, F.; Wang, Z.; Ding, J.; Huang, H. Reaction mechanism for NH3-SCR of NOx over CuMn2O4 catalyst. Chem. Eng. J. 2019, 361, 578–587. [Google Scholar] [CrossRef]
- Xu, L.; Yang, Q.; Hu, L.; Wang, D.; Peng, Y.; Shao, Z.; Lu, C.; Li, J. Insights over Titanium Modified FeMgOx Catalysts for Selective Catalytic Reduction of NOx with NH3: Influence of Precursors and Crystalline Structures. Catalysts 2019, 9, 560. [Google Scholar] [CrossRef]
- An, D.H.; Zhang, X.Y.; Cheng, X.X.; Dong, Y. Performance of Mn-Fe-Ce/GOx for Catalytic Oxidation of Hg0 and Selective Catalytic Reduction of NOx in the Same Temperature Range. Catalysts 2018, 8, 399. [Google Scholar] [CrossRef]
- Han, L.P.; Gao, M.; Hasegawa, J.; Li, S.X.; Shen, Y.J.; Li, H.R.; Shi, L.Y.; Zhang, D.S. SO2-Tolerant Selective Catalytic Reduction of NOx over Meso-TiO2@Fe2O3@Al2O3 Metal-Based Monolith Catalysts. Environ. Sci. Technol. 2019, 53, 6462–6473. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.Y.; Tang, X.L.; Yi, H.H.; Zhao, S.Z.; Li, C.L.; Li, J.Y.; Shi, Y.R.; Meng, X.M. A Review on Selective Catalytic Reduction of NOx by NH3 over Mn-Based Catalysts at Low Temperatures: Catalysts, Mechanisms, Kinetics and DFT Calculations. Catalysts 2017, 7, 199. [Google Scholar] [CrossRef]
- Gholami, R.; Stere, C.E.; Goguet, A.; Hardacre, C. Non-thermal-plasma-activated de-NO x catalysis. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2017, 376, 0054. [Google Scholar] [CrossRef] [PubMed]
- Mohapatro, S.; Rajanikanth, B.S. Dielectric barrier discharge cascaded with red mud waste to enhance NO x removal from diesel engine exhaust. IEEE Trans. Dielectr. Electr. Insul. 2012, 19, 641–647. [Google Scholar] [CrossRef]
- Miessner, H.; Francke, K.P.; Rudolph, R.; Hammer, T. NOx removal in excess oxygen by plasma-enhanced selective catalytic reduction. Catal. Today 2002, 75, 325–330. [Google Scholar] [CrossRef]
- Zhou, X.; Yu, F.; Sun, R.B.; Tian, J.Q.; Wang, Q.; Dai, B.; Dan, J.M.; Pfeiffer, H. Two-dimensional MnFeCo layered double oxide as catalyst for enhanced selective catalytic reduction of NOx with NH3 at low temperature (25–150 °C). Appl. Catal. A Gen. 2020, 592, 117432. [Google Scholar] [CrossRef]
- Damma, D.; Ettireddy, P.R.; Reddy, B.M.; Smirniotis, P.G. A Review of Low Temperature NH3-SCR for Removal of NOx. Catalysts 2019, 9, 349. [Google Scholar] [CrossRef]
- Xin, Y.; Li, H.; Zhang, N.N.; Li, Q.; Zhang, Z.L.; Cao, X.M.; Hu, P.; Zheng, L.R.; Anderson, J.A. Molecular-Level Insight into Selective Catalytic Reduction of NOx with NH3 to N2 over a Highly Efficient Bifunctional V-alpha-MnOx Catalyst at Low Temperature. ACS Catal. 2018, 8, 4937–4949. [Google Scholar] [CrossRef]
- Lopez-Hernandez, I.; Mengual, J.; Palomares, A.E. The Influence of the Support on the Activity of Mn-Fe Catalysts Used for the Selective Catalytic Reduction of NOx with Ammonia. Catalysts 2020, 10, 63. [Google Scholar] [CrossRef]
- Wang, R.H.; Hao, Z.F.; Li, Y.; Liu, G.Q.; Zhang, H.; Wang, H.T.; Xia, Y.G.; Zhan, S.H. Relationship between structure and performance of a novel highly dispersed MnOx on Co-Al layered double oxide for low temperature NH3-SCR. Appl. Catal. B Environ. 2019, 258, 117983. [Google Scholar] [CrossRef]
- Li, L.C.; Wang, Y.S.; Zhang, L.; Yu, Y.X.; He, H.B. Low-Temperature Selective Catalytic Reduction of NOx on MnO2 Octahedral Molecular Sieves (OMS-2) Doped with Co. Catalysts 2020, 10, 396. [Google Scholar] [CrossRef]
- Han, L.P.; Cai, S.X.; Gao, M.; Hasegawa, J.; Wang, P.L.; Zhang, J.P.; Shi, L.Y.; Zhang, D.S. Selective Catalytic Reduction of NOx with NH3 by Using Novel Catalysts: State of the Art and Future Prospects. Chem. Rev. 2019, 119, 10916–10976. [Google Scholar] [CrossRef]
- You, Y.C.; Chen, S.Y.; Li, J.Y.; Zeng, J.; Chang, H.Z.; Ma, L.; Li, J.H. Low-temperature selective catalytic reduction of N2O by CO over Fe-ZSM5 catalysts in the presence of O2. J. Hazard. Mater. 2020, 383, 121117. [Google Scholar] [CrossRef]
- Bozbag, S.E.; Sanli, D.; Ozener, B.; Hisar, G.; Erkey, C. An Aging Model of NH3 Storage Sites for Predicting Kinetics of NH3 Adsorption, Desorption and Oxidation over Hydrothermally Aged Cu-Chabazite. Catalysts 2020, 10, 411. [Google Scholar] [CrossRef]
- Zhu, T.; Zhang, X.; Bian, W.J.; Han, Y.W.; Liu, T.S.; Liu, H.B. DeNO(x) of Nano-Catalyst of Selective Catalytic Reduction Using Active Carbon Loading MnOx-Cu at Low Temperature. Catalysts 2020, 10, 135. [Google Scholar] [CrossRef]
- Husnain, N.; Wang, E.L.; Fareed, S. Low-Temperature Selective Catalytic Reduction of NO with NH3 over Natural Iron Ore Catalyst. Catalysts 2019, 9, 956. [Google Scholar] [CrossRef]
- Wang, C.; Wang, J.; Wang, J.Q.; Wang, Z.X.; Chen, Z.X.; Li, X.L.; Shen, M.Q.; Yan, W.J.; Kang, X. The Role of Impregnated Sodium Ions in Cu/SSZ-13 NH3-SCR Catalysts. Catalysts 2018, 8, 593. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, X.Y.; Liu, J.; Liu, H.Z.; Wang, Y.; Sun, B.M. Effects of temperature on NOx removal with Mn-Cu/ZSM5 catalysts assisted by plasma. Appl. Therm. Eng. 2018, 130, 1224–1232. [Google Scholar] [CrossRef]
- Yan, Q.H.; Chen, S.N.; Zhang, C.; Wang, Q.; Louis, B. Synthesis and catalytic performance of Cu1Mn0.5Ti0.5Ox mixed oxide as low-temperature NH3-SCR catalyst with enhanced SO2 resistance. Appl. Catal. B Environ. 2018, 238, 236–247. [Google Scholar] [CrossRef]
- Wen, Z.C.; Du, M.M.; Li, Y.; Wang, Z.H.; Xu, J.R.; Cen, K.F. Quantum chemistry study on the oxidation of NO catalyzed by ZSM5 supported Mn/Co-Al/Ce. J. Theor. Comput. Chem. 2017, 16, 1750044. [Google Scholar] [CrossRef]
- Jodlowski, P.J.; Kuterasinski, L.; Jedrzejczyk, R.J.; Chlebda, D.; Gancarczyk, A.; Basag, S.; Chmielarz, L. DeNO(x) Abatement Modelling over Sonically Prepared Copper USY and ZSM5 Structured Catalysts. Catalysts 2017, 7, 205. [Google Scholar] [CrossRef]
- Zhu, T.; Wang, R.N.; Bian, W.J.; Chen, Y.; Jing, W.D. Advanced oxidation technology for H2S odor gas using non-thermal plasma. Plasma Sci. Technol. 2018, 20, 054007. [Google Scholar] [CrossRef]
- Pan, H.; Qiang, Y. Promotion of Non-thermal Plasma on Catalytic Reduction of NOx by C3H8 Over Co/BEA Catalyst at Low Temperature. Plasma Chem. Plasma P. 2014, 34, 811–824. [Google Scholar] [CrossRef]
- Nguyen, D.B.; Nguyen, V.T.; Heo, I.J.; Mok, Y.S. Removal of NOx by selective catalytic reduction coupled with plasma under temperature fluctuation condition. J. Ind. Eng. Chem. 2019, 72, 400–407. [Google Scholar] [CrossRef]
- Zhu, T.; Bian, W.J.; Ma, M.F.; Ye, W.L.; Wang, R.N.; Zhang, X. Influence of gas atmosphere on synergistic control of mercury and dioxin by non-thermal plasma. Plasma Sci. Technol. 2019, 21, 044006. [Google Scholar] [CrossRef]
- Broer, S.; Hammer, T. Selective catalytic reduction of nitrogen oxides by combining a non-thermal plasma and a V2O5-WO3/TiO2 catalyst. Appl. Catal. B Environ. 2000, 28, 101–111. [Google Scholar] [CrossRef]
- Guan, B.; Lin, H.; Cheng, Q.; Huang, Z. Removal of NOx with Selective Catalytic Reduction Based on Nonthermal Plasma Preoxidation. Ind. Eng. Chem. Res. 2011, 50, 5401–5413. [Google Scholar] [CrossRef]
- Chen, J.X.; Pan, K.L.; Yu, S.J.; Yen, S.Y.; Chang, M.B. Combined fast selective reduction using Mn-based catalysts and nonthermal plasma for NOx removal. Environ. Sci. Pollut. Res. 2017, 24, 21496–21508. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Liu, H.Z.; Zhang, X.Y.; Guo, Y.H.; Zhang, Y.S.; Wang, Y.; Sun, B.M. A plasma-assisted catalytic system for NO removal over CuCe/ZSM-5 catalysts at ambient temperature. Fuel Process. Technol. 2017, 158, 199–205. [Google Scholar] [CrossRef]
- Wang, T.; Liu, H.Z.; Yang, B.; Sun, B.M.; Xiao, H.P.; Zhang, Y.S. Effect of plasma-catalyst system on NO removal using M-Cu (M=Mn, Ce, Cr, Co, and Fe) catalysts. Jpn. J. Appl. Phys. 2016, 55, 116202. [Google Scholar] [CrossRef]
- Rajanikanth, B.S.; Ravi, V. DeNOx study in diesel engine exhaust using barrier discharge corona assisted by V2O5/TiO2 catalyst. Plasma Sci. Technol. 2004, 6, 2411–2415. [Google Scholar] [CrossRef]
- Sun, Q.; Zhu, A.M.; Yang, X.F.; Niu, J.H.; Xu, Y.; Song, Z.M.; Liu, J. Selective catalytic reduction of NOx in dielectric barrier discharge plasmas. Eur. Phys. J. Appl. Phys. 2005, 30, 129–133. [Google Scholar] [CrossRef]
- Kwak, J.H.; Peden, C.H.F.; Szanyi, J. Non-thermal plasma-assisted NOx reduction over Na-Y zeolites: The promotional effect of acid sites. Catal. Lett. 2006, 109, 1–6. [Google Scholar] [CrossRef]
- Li, L.; Wang, L.S.; Pan, S.W.; Wei, Z.L.; Huang, B.C. Effects of cerium on the selective catalytic reduction activity and structural properties of manganese oxides supported on multi-walled carbon nanotubes catalysts. Chin. J. Catal. 2013, 34, 1087–1097. [Google Scholar] [CrossRef]
- Fan, X.Y.; Qiu, F.M.; Yang, H.S.; Tian, W.; Hou, T.F.; Zhang, X.B. Selective catalytic reduction of NOx with ammonia over Mn-CeOx/TiO2-carbon nanotube composites. Catal. Commun. 2011, 12, 1298–1301. [Google Scholar] [CrossRef]
- Jiang, L.J.; Liu, Q.C.; Zhao, Q.; Ren, S.; Kong, M.; Yao, L.; Meng, F. Promotional effect of Ce on the SCR of NO with NH3 at low temperature over MnO(x) supported by nitric acid-modified activated carbon. Res. Chem. Intermed. 2018, 44, 1729–1744. [Google Scholar] [CrossRef]
- Tang, X.L.; Hao, J.M.; Xu, W.G.; Li, J.H. Low temperature selective catalytic reduction of NOx with NH3 over amorphous MnOx catalysts prepared by three methods. Catal. Commun. 2006, 8, 329–334. [Google Scholar] [CrossRef]
- Boningari, T.; Ettireddy, P.R.; Somogyvari, A.; Liu, Y.; Vorontsov, A.; McDonald, C.A.; Smirniotis, P.G. Influence of elevated surface texture hydrated titania on Ce-doped Mn/TiO2 catalysts for the low-temperature SCR of NOx under oxygen-rich conditions. J. Catal. 2015, 325, 145–155. [Google Scholar] [CrossRef]
- Fang, D.; Xie, J.L.; Mei, D.; Zhang, Y.M.; He, F.; Liu, X.Q.; Li, Y.M. Effect of CuMn2O4 spinel in Cu–Mn oxide catalysts on selective catalytic reduction of NOx with NH3 at low temperature. RSC Adv. 2014, 4, 25540. [Google Scholar] [CrossRef]
- Zhao, Z.W.; Li, E.W.; Qin, Y.; Liu, X.L.; Zou, Y.; Wu, H.; Zhu, T.Y. Density functional theory (DFT) studies of vanadium-titanium based selective catalytic reduction (SCR) catalysts. J. Environ. Sci. China 2020, 90, 119–137. [Google Scholar] [CrossRef] [PubMed]
- Fang, D.; Xie, J.L.; Hu, H.; Yang, H.; He, F.; Fu, Z.B. Identification of MnOx species and Mn valence states in MnOx/TiO2 catalysts for low temperature SCR. Chem. Eng. J. 2015, 271, 23–30. [Google Scholar] [CrossRef]
- Stanciulescu, M.; Caravaggio, G.; Dobri, A.; Moir, J.; Burich, R.; Charland, J.P.; Bulsink, P. Low-temperature selective catalytic reduction of NOx with NH3 over Mn-containing catalysts. Appl. Catal. B Environ. 2012, 123, 229–240. [Google Scholar] [CrossRef]
- Jiang, B.Q.; Liu, Y.; Wu, Z.B. Low-temperature selective catalytic reduction of NO on MnOx/TiO2 prepared by different methods. J. Hazard. Mater. 2009, 162, 1249–1254. [Google Scholar] [CrossRef]
- Brookshear, D.W.; Nam, J.G.; Nguyen, K.; Toops, T.J.; Binder, A. Impact of sulfation and desulfation on NOx reduction using Cu-chabazite SCR catalysts. Catal. Today 2015, 258, 359–366. [Google Scholar] [CrossRef]
- Samojeden, B.; Grzybek, T. The influence of the promotion of N-modified activated carbon with iron on NO removal by NH3-SCR (Selective catalytic reduction). Energy 2016, 116, 1484–1491. [Google Scholar] [CrossRef]
- Salazar, M.; Hoffmann, S.; Singer, V.; Becker, R.; Grunert, W. Hybrid catalysts for the selective catalytic reduction (SCR) of NO by NH3 center dot On the role of fast SCR in the reaction network. Appl. Catal. B Environ. 2016, 199, 433–438. [Google Scholar] [CrossRef]
- Du, C.M.; Wang, J.; Zhang, L.; Li, H.X.; Liu, H.; Xiong, Y. The application of a non-thermal plasma generated by gas-liquid gliding arc discharge in sterilization. New J. Phys. 2012, 14, 013010. [Google Scholar] [CrossRef]
- Zhu, T.; Zhang, X.; Ma, M.F.; Wang, L.F.; Xue, Z.Y.; Hou, Y.M.; Ye, Z.F.; Liu, T.S. 1,2,4-trichlorobenzene decomposition using non-thermal plasma technology. Plasma Sci. Technol. 2020, 22, 034011. [Google Scholar] [CrossRef]
- Yan, X.F.; Hu, Z. Experiment and analysis on the treatment of gaseous benzene using pulsed corona discharge plasma. Plasma Sci. Technol. 2004, 6, 2241–2246. [Google Scholar]
- Liang, W.J.; Ma, L.; Li, J.; Li, J.X.; Zheng, F. Control of Hydrogen Sulfide by a Wire-Tube Dielectric Barrier Discharge AC Plasma Reactor. CLEAN-Soil Air Water 2012, 40, 586–591. [Google Scholar] [CrossRef]
- Zhao, G.B.; Garikipati, S.V.B.; Hu, X.D.; Argyle, M.D.; Radosz, M. Effect of oxygen on nonthermal plasma reactions of nitrogen oxides in nitrogen. AIChE J. 2005, 51, 1800–1812. [Google Scholar] [CrossRef]
- Liu, Y.H.C.; Zhang, R.X.; Hou, H.Q.; Chen, S.P.; Zhang, R.N. NO reduction using low-temperature SCR assisted by a DBD method. Plasma Sci. Technol. 2018, 20, 014002. [Google Scholar] [CrossRef]
- Kuwahara, T.; Yoshida, K.; Kuroki, T.; Hanamoto, K.; Sato, K.; Okubo, M. High Reduction Efficiencies of Adsorbed NOx in Pilot-Scale Aftertreatment Using Nonthermal Plasma in Marine Diesel-Engine Exhaust Gas. Energies 2019, 12, 3800. [Google Scholar] [CrossRef]
- Babaie, M.; Kishi, T.; Arai, M.; Zama, Y.; Furuhata, T.; Ristovski, Z.; Rahimzadeh, H.; Brown, R. Influence of non-thermal plasma after-treatment technology on diesel engine particulate matter composition and NOx concentration. Int. J. Environ. Sci. Technol. 2015, 13, 221–230. [Google Scholar] [CrossRef]
- Hammer, T.; Kappes, T.; Baldauf, M. Plasma catalytic hybrid processes: Gas discharge initiation and plasma activation of catalytic processes. Catal. Today 2004, 89, 5–14. [Google Scholar] [CrossRef]
- Li, J.H.; Ke, R.; Li, W.; Hao, J.M. Mechanism of selective catalytic reduction of NO over Ag/Al2O3 with the aid of non-thermal plasma. Catal. Today 2008, 139, 49–58. [Google Scholar] [CrossRef]
- Ruggeri, M.P.; Nova, I.; Tronconi, E.; Pihl, J.A.; Toops, T.J.; Partridge, W.P. In-situ DRIFTS measurements for the mechanistic study of NO oxidation over a commercial Cu-CHA catalyst. Appl. Catal. B Environ. 2015, 166, 181–192. [Google Scholar] [CrossRef]
- Koebel, M.; Madia, G.; Elsener, M. Selective catalytic reduction of NO and NO2 at low temperatures. Catal. Today 2002, 73, 239–247. [Google Scholar] [CrossRef]
- Guo, X.R.; Ha, K.H.; Du, D.F. New Experiment of Diesel Exhaust Treatment by Atmospheric Pressure Plasma-Wood Fiber Combination. Catalysts 2020, 10, 577. [Google Scholar] [CrossRef]
- Miessner, H.; Francke, K.P.; Rudolph, R. Plasma-enhanced HC-SCR of NOx in the presence of excess oxygen. Appl. Catal. B Environ. 2002, 36, 53–62. [Google Scholar] [CrossRef]
- McAdams, R.; Beech, P.; Shawcross, J.T. Low Temperature Plasma Assisted Catalytic Reduction of NOx in Simulated Marine Diesel Exhaust. Plasma Chem. Plasma P. 2008, 28, 159–171. [Google Scholar] [CrossRef]
- Hammer, T. Non-thermal plasma application to the abatement of noxious emissions in automotive exhaust gases. Plasma Sources Sci. T. 2002, 11, A196. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Kuang, H.L.; Zhang, J.F.; Chu, L.L.; Ji, Y.L. Nitrogen oxide removal by non-thermal plasma for marine diesel engines. RSC Adv. 2019, 9, 5402–5416. [Google Scholar] [CrossRef]
- Park, S.Y.; Deshwal, B.R.; Moon, S.H. NOx removal from the flue gas of oil-fired boiler using a multistage plasma-catalyst hybrid system. Fuel Process. Technol. 2008, 89, 540–548. [Google Scholar] [CrossRef]
- Talebizadeh, P.; Babaie, M.; Brown, R.; Rahimzadeh, H.; Ristovski, Z.; Arai, M. The role of non-thermal plasma technique in NOx treatment: A review. Renew. Sustain. Energy Rev. 2014, 40, 886–901. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).