1. Introduction
There is a long history of speculation that catalytic reactions depend on structure and shape, and thus the coordination of the catalyst [
1]. Indeed, in 1985, Falicov and Somorjai proposed that coordination-sensitive edge and kink sites would be most likely to be active for structure-sensitive reactions [
2]. Nanocluster synthesis has reached the stage where narrow particle size distributions of desired shapes of many elements and alloys may be achieved [
3]. This allows experimentalists to fine-tune catalytic reactions so that specific shapes, sizes, and thus the coordination of the catalyst is known. We model the kinetics (turnover frequency (TOF)) of surface sites showing that they strongly depend on the shape and structure of nanoclusters.
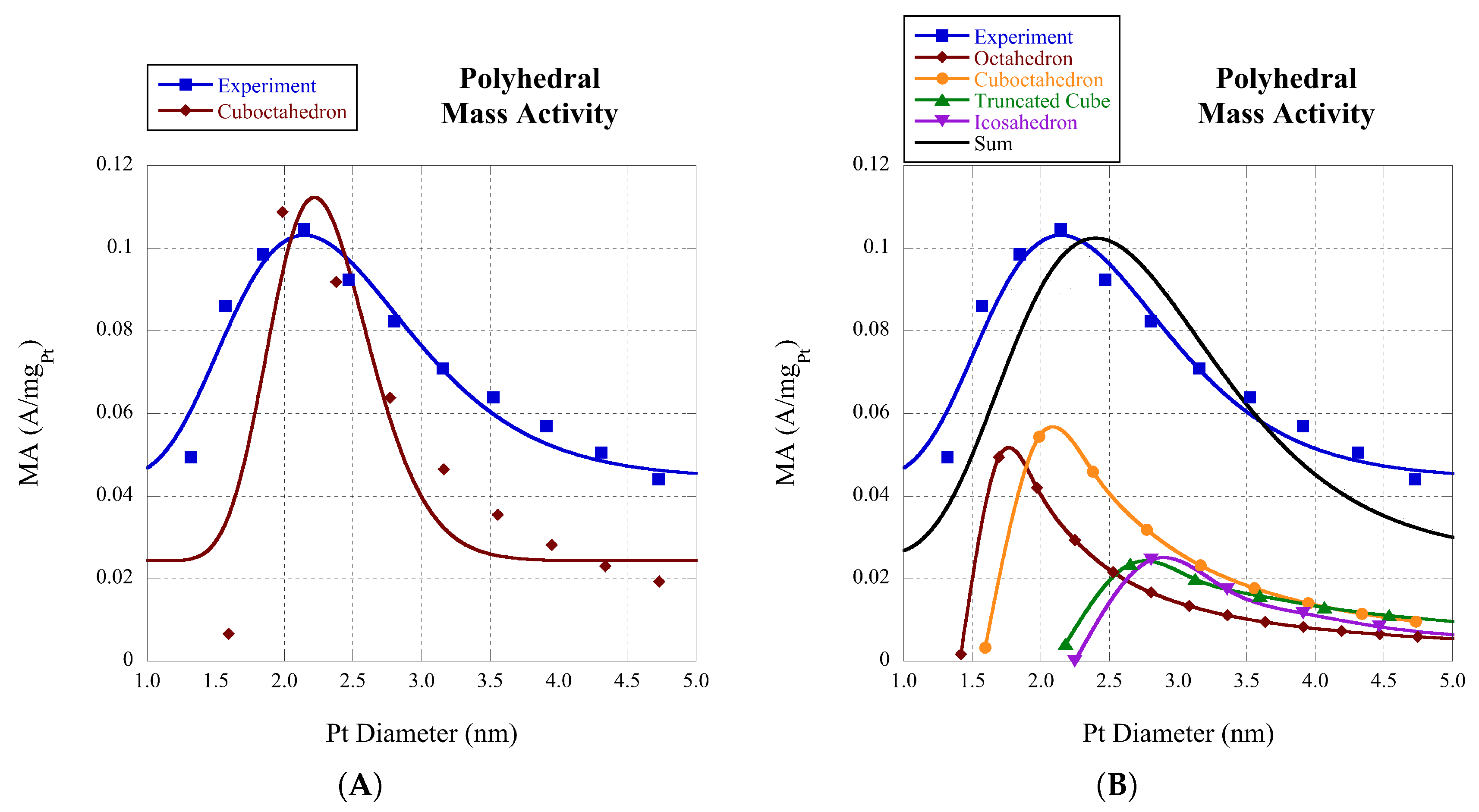
The oxygen reduction reaction (ORR) has much application to fuel cell technology. There is previous work on the size dependence of the ORR. Shao et al. showed experimentally in 2011 that platinum nanoparticles have a maximum in the mass activity around 2.2 nm [
4]. Likewise, there have been modeling efforts by Nesselberger et al. [
5], Tritsaris et al. [
6], and Tripkovic, et al. [
7]. Somewhat more recently, Rück, et al. have determined the platinum ORR size dependence via density functional theory (DFT) [
8]. Furthermore, DFT has been combined with kinetic Monte Carlo methods to look at the TOF on platinum nanoclusters [
9]. We calculate the Gibbs energy using an established procedure [
10] for the ORR reactions and incorporate it into the mass activity of the ORR via the TOF. This may lend insight into experimental needs of cluster morphology and its impact on the TOF.
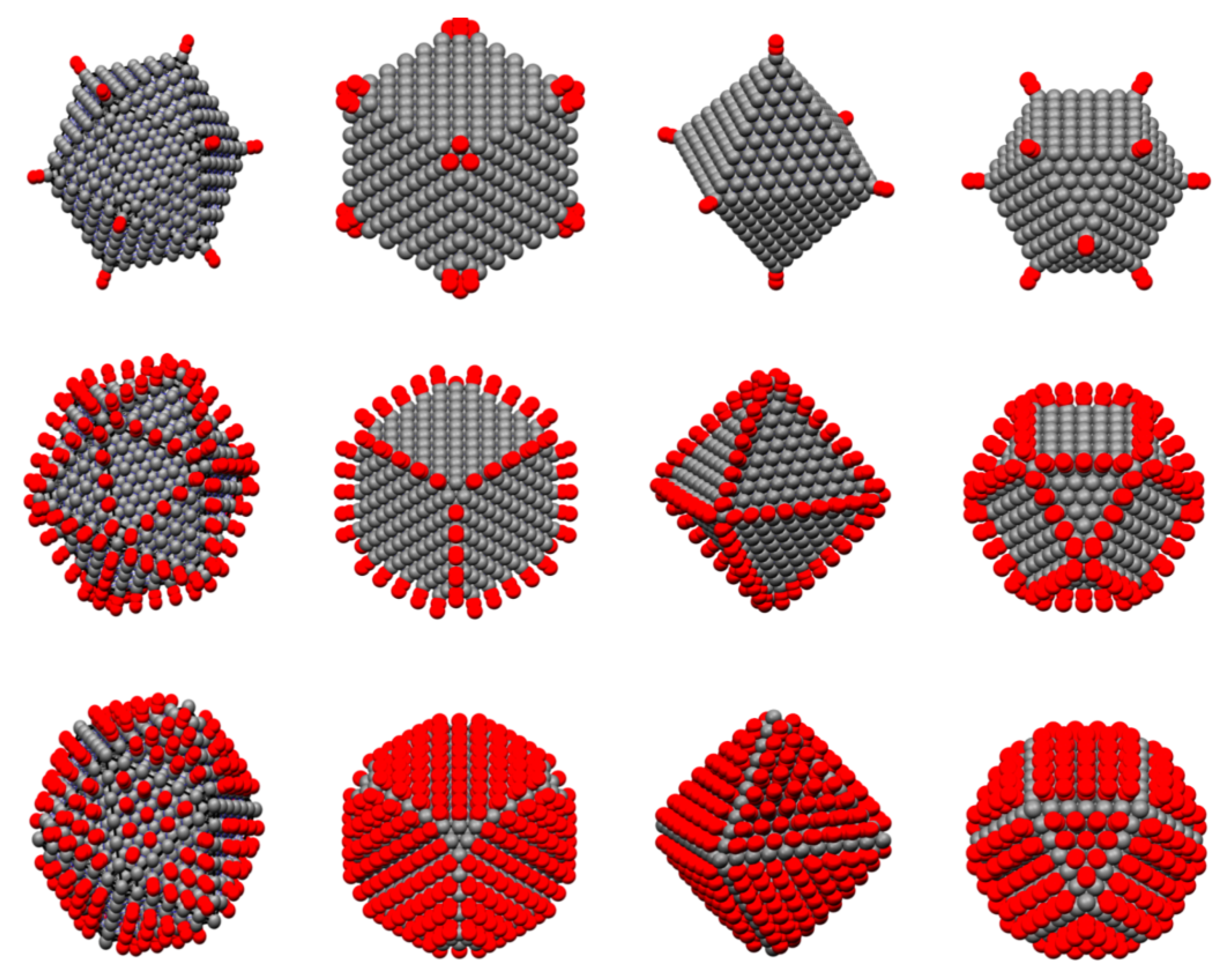
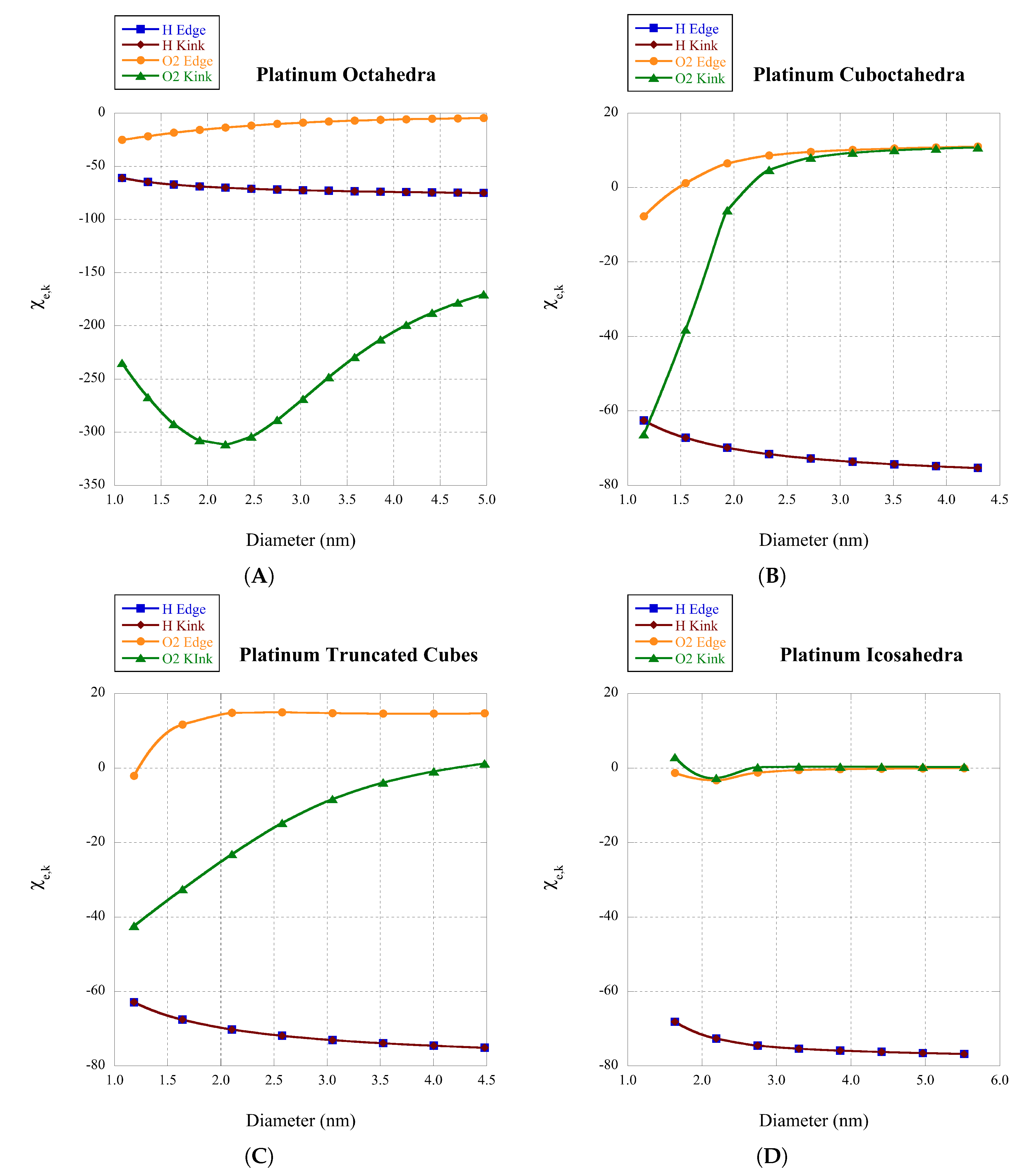
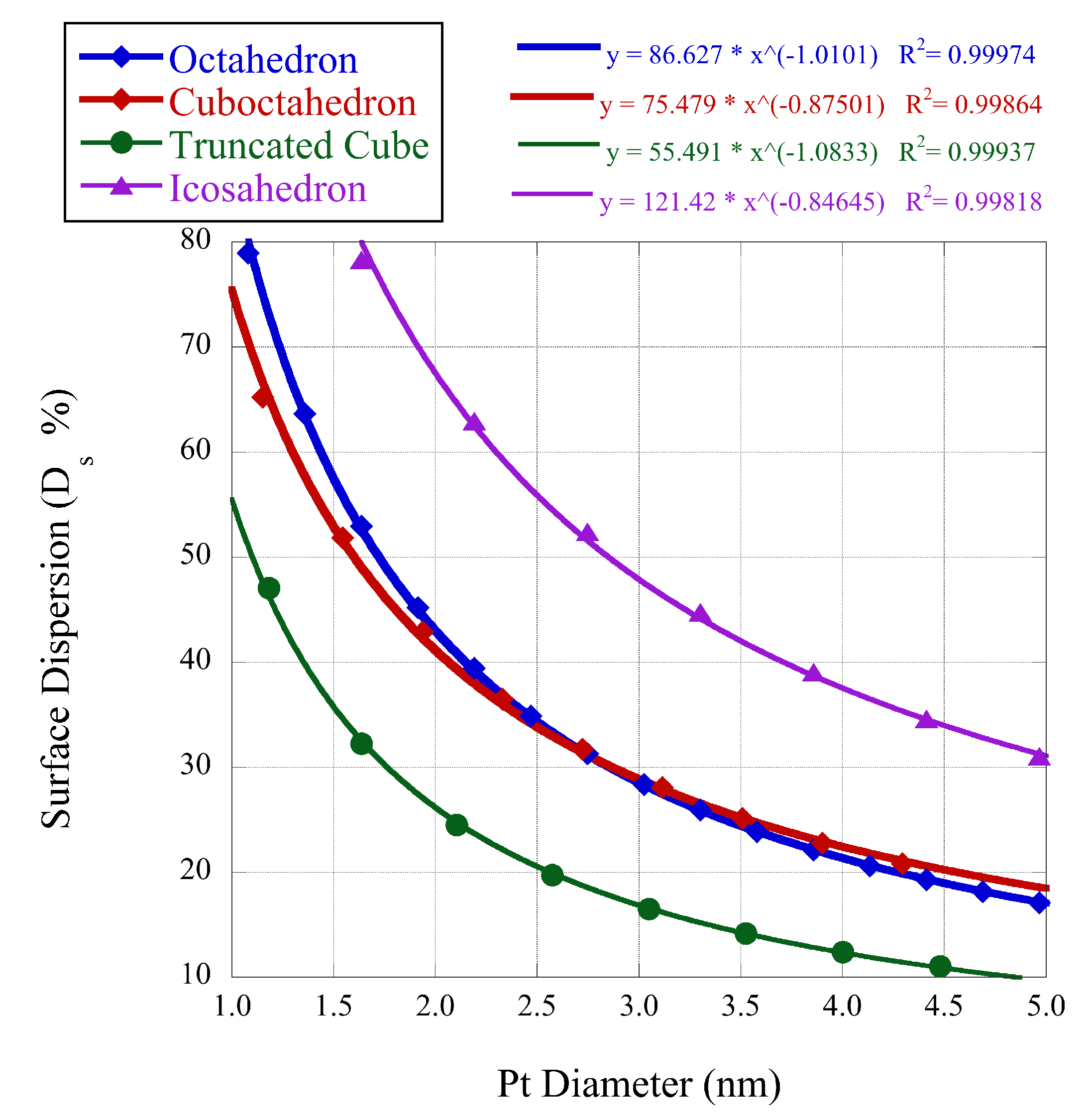
Recently, we have determined coordination specific magic formulas for the surface sites of 19 different nanoclusters [
11]. These magic formulas can assist our understanding of the coordination and size-dependent surface behavior of clusters. In the size dimension of the mesoscale, neither the atomic nor bulk apply, and much activity relies on the surface coordination of the specific cluster being studied. While the exact dimensions of the mesoscale are ambiguous, a good approximation is clusters with diameter
D ~5–100 nm. DFT studies on the surface states of nanoclusters show that for
D ~3 nm for gold [
12], and
D ~2 nm, for platinum [
13], the transition from the atomic to bulk has occurred. In addition, Hoover, et al. calculate that the difference in entropy between the thermodynamic prediction and the exact entropy is less than
(Boltzmann’s constant) when the number of atoms,
for classical close packed harmonic cubes [
14]. This shows that quite small clusters may be modeled with thermodynamics. Similarly, an estimate of
D as a lower limit of the mesoscale can be made from thermal fluctuations [
15]. For a cube of dimension
D and volume
, with
atoms per unit volume, the thermal fluctuations are about
. For solids and liquids,
. If we accept fluctuations of
, this gives
.
The Gibbs adsorption energy has been calculated by a few authors using a DFT approach [
16,
17]. Unfortunately, the size range of interest for the TOF is rather large for DFT, and a range of sizes is required. We provide a coordination approach using an empirical energy model combined with DFT, and add statistical mechanics along with thermodynamics to complete the Gibbs adsorption energy calculation. This enables us to determine the Gibbs adsorption energy,
, as a function of size and coordination. By modeling clusters using a coordination approach, we are able to avoid the need for supercomputer calculations, as the computational demands are modest (only desktop models required). This is a different approach to calculating the Gibbs energy.
2. Methods
We define
G as the Gibbs energy of the cluster (which depends on the size), then, because of adatoms being bonded to the outer shell atoms, there is an increase in
G that is called the adsorption energy and is denoted as
. This can be split up over different coordination types of the atoms on the outer shell bonding to adatoms. We suppose a kink atom adds to the adsorption energy with an amount
. Similarly an edge atom adds
, while a facet atom contributes
then [
18]
where
is the number of atoms in the outer shell of the indicated type. If
is the total number of atoms in the outer shell bonded to adatoms, then this can be rewritten as
where the edge and kink sites which have explicit coordinations for specific structures (see
Table 1). Note that Equation (
2) applies to adsorption to on-top sites; otherwise, not all adatoms will be bonded to atoms in the outer shell.
We have a fundamental relationship for the Gibbs energy and adsorption constant,
:
where
R is the gas constant and
T is the temperature in Kelvin. Brønsted–Evans–Polanyi relationships are widely used in homogeneous and heterogeneous catalysis [
18,
19], using a relationship for reaction constants
k and equilibrium constants
K as follows,
where
g and
(Polanyi parameter) are constants. The Polanyi parameter is unitless and a proper fraction, as given originally by Brønsted [
20]. We then have
where
and
We now proceed with the explanation of the method to calculate .
In the early 1990s, DFT studies on metal clusters gave
, the bonding energy of atom
i, as a function of its coordination
, and that led to the following empirical relationship [
21],
where
,
A, and
B are constants of the fit [
10], see
Table 2. By definition, we then have the bond energies,
, as a function of coordination:
where
is the bulk coordination, or 12 for fcc metals. For details on this model, we refer the reader to reference [
10].
To allow for small deviations from the average bond length, we define
i and
j as nearest neighbors, and separate them from the rest by requiring that
, where
is a threshold value, appropriate for the nanocluster. The value for
must be less than the distance for second nearest neighbors and varies with the crystal structure. For fcc crystals,
, where
is the smallest bond length [
11]. Thus,
describes the adjacency matrix for the cluster, and
describes the Euclidean matrix for the cluster. We use an adjacency matrix and Euclidean matrix to determine the diameter,
D, of the nanoclusters [
11], where the bond length in the clusters depends on the average coordination [
22]:
Here,
is the bulk bond length and
is the average coordination of the cluster. We find a linear relationship between
D and
n, the number of cluster shells, as shown in
Table 3:
This relationship is derived from MATLAB code for calculating the cluster diameter.
The energies of adsorbates have been shown to scale linearly with generalized coordination [
23,
24]. We use a DFT model for energies of adsorbates [
23]:
where the sum is over all nearest neighbors of
i,
for top sites, 18 for bridge sites, and has other values for other types of sites [
23]. In this equation,
is the number of nearest neighbors, thus each neighbor
j of atom
i has a weight of
associated with the site. Adsorbate energy,
, is linear with generalized coordination [
23]
Table 4 shows the values of the linear fits as determined by Equation (
15).
We construct our cluster-adatom Hamiltonian as follows,
with only nearest neighbor non-zero entries, i.e., a sparse matrix. In the Hamiltonian approach, the partition function is [
10,
26,
27]
and
is the inverse temperature, where
is Boltzmann’s constant,
T is the temperature in Kelvin (we use
T = 300 K), Tr is the trace of the following matrix, and
is the Hamiltonian matrix, as given by Equation (
16) [
10]. The probability of each state is then
, where
Z is the partition function, Equation (
17), and
is an eigenvalue of the Hamiltonian matrix [
27].
The Gibbs energy of adsorption in the nanoscale systems we examine is in joules (J), if we neglect the small
(pressure–volume) term, [
10,
26]
where
Z is the partition function, Equation (
17), and where
, with
the number of atoms in the cluster and
the number of adatoms at coverage theta. The Gibbs adsorption energy here is converted from more standard units by dividing by
and using a conversion factor (
) to get units of (J/mol). We average several times over the nanocluster system, as especially for the kink sites, the statistics are low.