Abstract
Here, a spherical CuBi2O4 catalyst with surface oxygen vacancy was fabricated through a facile hydrothermal method, which exhibited remarkable enhanced photocatalytic activity of refractory chemicals in the heterogeneous sulfate radical-based Fenton-like reaction under visible light emitting diode (LED) light irradiation. The property of the catalysts was systematically characterized by scanning electron microscopy (SEM)/high resolution transmission electron microscopy (HRTEM), X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), and UV/vis methods. The effects of parameters of solution pH, potassium peroxymonosulfate (PMS) concentration, catalyst dosage, and catalyst reusability on Rhodamine B (RhB) degradation were investigated. In the interface reaction, the improved photodegradation efficiency could be attributed to the decomposition of PMS, which produced sulfate radicals and hydroxyl radicals owing to the transmission of photo-generated electron/hole pairs. Herein, the introduction of surface oxygen vacancy as well as the cycling of copper valence states (Cu(II)/Cu(I) pairs) can facilitate the production of free reactive radicals, leading to the high degradation efficiency. The catalyst showed high removal efficiency and presented good cycle stability in the reaction. Additionally, the free radical quencher experiment and electron spin resonance (EPR) experiments were conducted, and a proposed photocatalytic mechanism was also illustrated.
1. Introduction
Owing to the rapid development of industry and fast growth of the population, the increased water pollution problem has attracted much more attention. To get the solution for these deteriorated water situations, it was really urgent to develop effective and economic technologies to eliminate these problems [1,2,3]. In recent years, advanced oxidation processes (AOPs) have brought more concern in the degradation of organic pollutants, which was considered really difficult to degrade by traditional methods [4,5,6]. Among these various AOPs, sulfate radical-based advanced oxidation processes (SR-based AOPs) possessed high removal efficiency of theses concomitants, leading to the formation of sulfate radical (SO4−), which had similar redox potential with hydroxyl radicals [7,8,9]. Additionally, heterogenous catalyst was employed for the activation of persulfate (PS) or potassium peroxymonosulfate (PMS, trade name Oxone) in SR-Fenton like oxidation process to achieve degradation and mineralization of contaminants in the wastewater. By introducing the visible light in the heterogeneous SR-Fenton like system (i.e., heterogeneous SR-photo-Fenton like process), the reaction rate was improved in the reaction, which was also widely studied.
In the heterogeneous SR-photo-Fenton like process, the heterogeneous catalyst played a great role for the degradation efficiency. Firstly, the heterogeneous catalysts were effective for the PMS activation, leading to the decomposition of PMS and the formation of sulfate radicals [10,11,12]. On the other hand, the catalyst could also be stimulated by the visible light, while the generated hole/electron pairs could participant in the degradation process. Many kinds of heterogeneous catalysts have been investigated and applied in the heterogeneous SR-photo-Fenton like process, such as hybrid metal-organic framework (MIL53(Fe)) [13], inorganic oxide heterojunction (CuBi2O4/Bi2O3) [14], metal oxides (Co3O4) [15], and perovskite structure (BiFeO3) [16]. In the heterogeneous SR-photo-Fenton like process, PMS was not only an oxidant, but also an electron acceptor, which could prevent the recombination of photo-generated holes and electrons [17,18,19]. The formed holes and electrons could react with PMS, facilitating the generation of sulfate radicals and hydroxyl radicals. Thus, it could improve the reduction between the high valence state and the low valence state of metal ions, facilitating the cycling of transition metal ions. It was considered that the synergistic effect existed between the catalyst/visible light process and heterogeneous SR-Fenton like reaction [20,21,22,23]. Unfortunately, the heterogeneous catalyst possessed high efficiency and stability was still rare, and the reaction mechanism needs further exploration in the heterogeneous SR-photo-Fenton like process.
In recent years, Bi-based catalysts, which acted as the photocatalyst for the degradation of contaminants owing to their unique properties and narrow band gap, have received more attention, facilitating the application of visible light. CuBi2O4, as one of p-type Bi-based semiconductor, have aroused widespread concern as it took advantage of the low band gap (1.3–3.7 eV) and high stability [24,25,26]. The low band gap provided much convenience in the absorption of low energy photons and a better response to the visible light, which has shown notable photocatalytic performance in heterogeneous SR-Fenton like systems [27,28,29]. By the introduction of visible light, the degradation efficiency was enhanced owing to the reduction of transition Cu ions and photo-generated holes/electrons pairs on the surface of catalyst. Moreover, the photocatalytic performance by single usage of CuBi2O4 was unsatisfied, which suffered quick recombination of photogenerated holes and electrons. The degradation efficiency of the target pollutant was assumed to be further improved by using pure CuBi2O4 catalyst alone. On the other hand, to achieve better degradation efficiency, a large amount of photocatalyst was utilized in these systems. It was reported that, in the presence of PMS/PS, which acted as the electron acceptor, the photocatalytic activity was much improved, while the comparison between the absence and presence of PMS/PS by the application of Bi-based catalyst is listed in Table S1. Moreover, the photocatalysis performance of CuBi2O4 could also be improved by coupling with other semiconductors, such as Ag2S/CQDs [30], Bi2MoO6 [31], Ag3PO4 [32], and WO3 [33]. On the other hand, the introduction of surface oxygen vacancies on catalyst was considered an effective way to improve its photocatalytic activity, which can grip the photo-generated holes and electrons and inhibit the recombination of the hole/electron pairs. Many studies investigated the good catalytic activity of catalyst with surface oxygen vacancies, such as Fe(III)/Bi2MoO6 [34], BiOBr [35], and TiO2 [36]. With the presence of surface oxygen vacancy on the catalyst, the results proved that the band gap was narrowed and the separation and migration of photo-generated electron/holes was facilitated, resulting in better degradation efficiency.
In this study, CuBi2O4 with surface oxygen vacancy (CuBi2O4-OVs) was synthesized by the one-pot hydrothermal method, which was used in the heterogeneous SR-photo-Fenton like reaction for the removal of RhB. Visible LED light was chosen as the light source instead of the high-power xenon lamp or gold halide/tungsten lamp owing to its long working life, broad emission spectrum, and cost-effectiveness, and was considered as an efficient light source for removal of pollutants in wastewater. The cycling catalytic activity and copper ion leaching was carefully evaluated. The effects of initial pH, PMS concentration, and catalyst dosage were investigated. Therefore, on the basis of the various characterization analyses, a possible catalytic mechanism for the heterogeneous SR-photo-Fenton like reaction by the usage of CuBi2O4-OVs catalyst was proposed. Radical quenching experiments and EPR tests were conducted to evaluated the main reactive free radicals. Overall, these results showed a novel strategy for the heterogeneous SR-photo-Fenton like reactions in wastewater treatment.
2. Results
2.1. Synthesis of Catalysts
A hydrothermal method was used to synthesize the CuBi2O4 catalyst with surface oxygen vacancy. Here, 2 mM Bi(NO3)3·5H2O and 1 mM Cu(NO3)2·3H2O were dissolved in 10 mL of ethylene glycol, respectively. Then, both solutions were mixed under stirring. Afterward, 30 mL ethanol and 0.3 g of glucose were added to the mixed solution. After being completely dissolved, the mixture was transferred into the 100 mL Teflon-lined stainless-steel autoclave and kept at 160 °C for 12 h. The solid was centrifuged and washed with deionized water and ethanol, which was dried at 70 °C for 24 h, which denoted as CuBi2O4-OVs. CuBi2O4 was also prepared without the addition of glucose. The glucose was used in the synthesis procedure, which played a great role on the morphology of the nanocomposites owing to the reduction effect, thus further improving the photocatalytic performance of the catalyst. Pure CuO and Bi2O3 were also prepared by the above method with the addition of Cu(NO3)2·3H2O or Bi(NO3)3·5H2O, respectively.
2.2. Material Characterization
X-ray diffraction (XRD) pattern was performed using a Bruker D8ADVANCE X-ray diffractometer with a graphite monochromatic Cu Kα radiation (λ = 1.54 Å) at the accelerating voltage 40 kV and the current 30 mA over the 2θ scanning range of 10–80°. Scanning electron microscopy (SEM) was characterized on a Hitach SU8220 field emission with X-ray energy dispersive spectra (EDS) instrument, which would find out the element distribution. High resolution transmission electron microscopy (TEM) images were obtained using FEI Talos F200S with high-resolution at 200 kV. The X-ray photoelectron spectroscopy (XPS) was carried out on Thermo Fisher ESCALAB250Xi. The diffuse reflectance spectrum (DRS) was operated on ultraviolet visible near infrared spectrophotometer with Shimadzu UV-3600 Plus. The Brunauer-Emmett-Teller (BET) specific surface area, pore size, and volumes were analyzed by N2 adsorption–desorption technology using automatic micromeritics instrument corporation TriStar II 3020.
2.3. Photocatalytic Performance Experiments
In the experimental setup, a 30 W LED lamp (30 W, 460 nm, Xujia Company, Shanghai, China) was employed as the light source. Further, 0.1 g photocatalyst was added to 200 mL of RhB solution (20 mg/L). Before the light turned on, the mixture was magnetically stirred in the dark for 60 min in order to obtain the absorption–desorption equilibrium. Then, 0.049 g PMS was added in the system and LED lights were turned on. The sample was taken out and filtered through 0.22 μm membrane filters; the concentrations of RhB and total organic carbon (TOC) were measured by UV/vis spectrophotometer (UV 3600 II, Shanghai, China) and TOC (Elementar vario) analyzer, respectively. Each sample was measured three times and the average values are shown in the figures. Electron spin resonance (ESR, JES FA200, JEOL) was used to measure the intensity of free radicals in methanol and deionized water. The copper leaching measurements were performed using the inductively coupled plasma mass spectrometry (ICP-MS) method (Agilent 7000). In the batch experiments, the degradation of RhB was fitted well with the pseudo first order kinetics model, which can be expressed as Equation (1).
where C refers to the concentration of RhB at time t, C0 refers to the initial RhB concentration, kapp refers to the kinetic rate constant, and t refers to the reaction time.
ln C/C0= −kapp × t
3. Discussion
3.1. Characterization of Prepared Samples
The morphology and microstructure of the as-prepared CuBi2O4 and CuBi2O4-OVs were characterized by SEM and HRTEM. Figure 1 presented that the CuBi2O4 sample was composed of a sphere-like morphology, which was made of nanocrystals of 20–40 nm thickness. However, it was also observed that large bulks were presented on the surface with agglomeration, owing to the anisotropic growth of crystals on the surface. The diameter of the sphere was in the width of ~400 nm. On the other hand, the morphology of CuBi2O4-OVs synthesized after the addition of glucose showed smaller diameter of ~350 nm with a wool-ball like morphology, which was different with the CuBi2O4. The results can be attributed to the reductant of glucose, which prevent the growth of crystallization, resulting in the decreased crystallinity of the CuBi2O4 sample. Furthermore, the elemental distribution of the CuBi2O4-OVs composite was determined by energy dispersive spectroscopy (EDS). It was observed that there were three elements detected in the as-prepared samples, that is, Cu, Bi, and O, where the atomic ratio of Cu and Bi was close to 1:2.
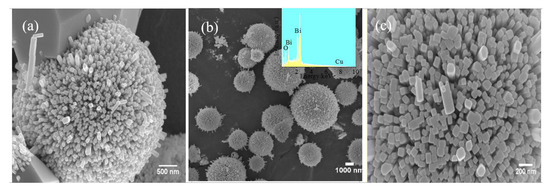
Figure 1.
Scanning electron microscopy (SEM) images of as-prepared (a) CuBi2O4 and (b,c) CuBi2O4-OVs; the inset is the energy dispersive spectroscopy (EDS) spectra of CuBi2O4-OVs.
The HRTEM images clearly showed the lattice spacing of 0.245 nm and 0.253 nm, which was attributed to the (310) plane of the CuBi2O4 and CuBi2O4-OVs samples, respectively, which was shown in Figure 2 [37]. Moreover, the EDS element mapping image of Cu, Bi, and O demonstrated the homogeneous distribution of these elements, which were presented on the surface of catalyst. However, the lattice edges of the CuBi2O4-OVs sample were not distinct, found with disordered and blurred boundaries, which could be attributed to the introduction of surface oxygen vacancies. The TEM image of CuBi2O4-OVs showed that the average size of the particles was close to 64 nm, where the particles presented partial agglomeration. The results of HRTEM images were consistent with the XRD data.
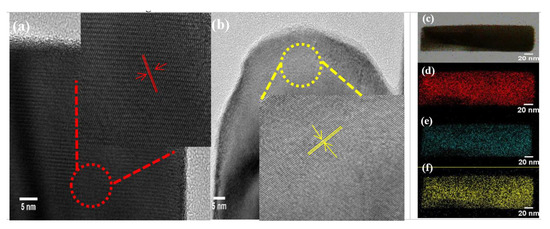
Figure 2.
Transmission electron microscopy (TEM) images of as-prepared (a) CuBi2O4 and (b) CuBi2O4-OVs, and (c) all elements EDS mapping images of CuBi2O-OVs, (d) Bi 4f, (e) Cu 2p, and (f) O 1s. The inset image is magnified image of the encircled area.
The crystal properties of the prepared CuBi2O4-OVs, Bi2O3, and CuO samples were characterized by XRD, which is shown in Figure 3. All the diffraction peaks of CuBi2O4-OVs corresponded well to the phase of CuBi2O4 (JCPDS No. 48-1886). No impurities were found, indicating the preparation procedure was successful and the crystal structure of CuBi2O4 was unchanged during the synthesis [38,39]. The diffraction peaks were narrow and strong, which showed the good crystallization of the CuBi2O4 catalyst. With the introduction of surface oxygen vacancy, the intensity of diffraction peaks was slightly decreased. Diffraction peaks of CuO (JCPDS 44-0706) and Bi2O3 (JCPDS 27-0050) were also identified, demonstrating the pure crystal structure of synthesized catalysts. Moreover, the prepared samples possessed an average size of around 53.2 nm according to the Debye–Scherrer formula, which was in good agreement with SEM and TEM results.
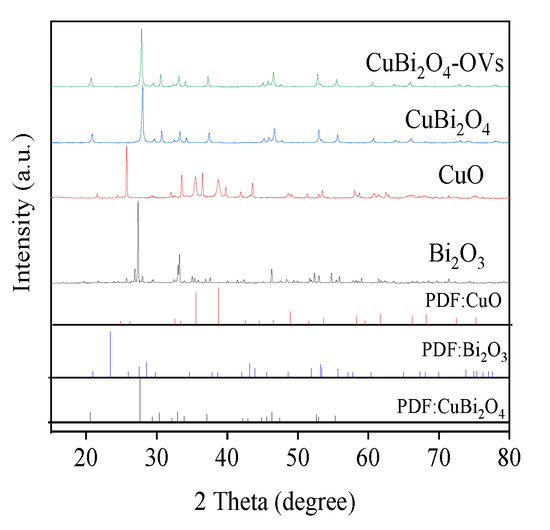
Figure 3.
X-ray diffraction (XRD) patterns of as-prepared catalysts.
The chemical composition and element state of the prepared samples were carried out by the XPS method. As shown in Figure 4, the peaks of Bi, O, Cu, and trace C were found on the surface of as-prepared samples, which were sharp and clear. The characteristic peaks of Bi 4f were centered at 158.6 eV and 163.9 eV, corresponding to Bi 4f5/2 and Bi 4f7/2, which indicated the existence of Bi3+ [40]. It was observed that the peaks were slightly shifted to higher binding energy owing to the introduction of surface oxygen vacancy. The peak observed at 955.5 eV with a satellite peak at 964.8 eV in the XPS spectra of Cu 2p was assigned to Cu 2p1/2, and the peak of Cu 2p3/2 centered at 934.3 eV would be deconvoluted to 935.6 eV and 932.8 eV, respectively, which was considered as Cu(II) and reduced copper species [41]. The presence of reduced copper species on the surface of sample could be attributed to the reduction of Cu(II), which facilitated the heterogeneous SR-photo-Fenton like reaction by the cycling reaction between Cu(II) and Cu(I). The peak centered around 530 eV can be denoted as O 1s, which shifted to a higher binding energy owing to the electron attraction effect of oxygen vacancy. The characteristic peak of O 1s could be deconvoluted into three internal peaks at 529.7 eV, 531.5 eV, and 532.5 eV in the fresh catalyst, representing the lattice oxygen, metal-O bonds (Bi-O, Cu-O), hydroxyl groups, and absorbed H2O on the surface, respectively [42]. The peak of hydroxyl groups and absorbed H2O in CuBi2O4-OVs took a much higher proportion than that of the CuBi2O4 samples. Additionally, the metal-O bonds were also different, which further proved the formation of surface oxygen vacancy on the catalyst. The proportion of the three peaks was changed after the heterogeneous SR-photo-Fenton like reaction, demonstrating the participation of the H2O and oxygen vacancy in the reactions, leading to the formation of reactive oxygen radicals. The results also proved that there was a redox transition of Cu ions under LED light irradiation.
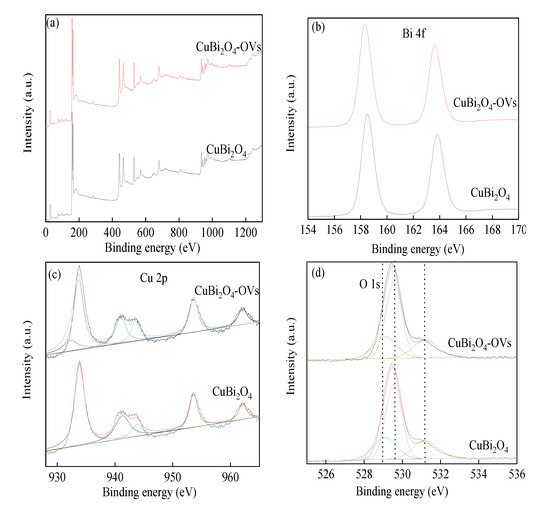
Figure 4.
X-ray photoelectron spectroscopy (XPS) spectra of as-prepared catalysts. (a) full-scale XPS spectra, (b) Bi 4f, (c) Cu 2p, and (d) O 1s.
The UV/vis diffusion spectra of the CuBi2O4 and CuBi2O4-OVs catalyst were shown in Figure 5. It was observed that both the CuBi2O4 and CuBi2O4-OVs catalyst showed a long band absorption in the visible light region, implying good response to the visible light. However, the absorption area in the visible light region was higher in the CuBi2O4-OVs than in the CuBi2O4 catalyst owing to the introduction of surface oxygen vacancy. The results proved that the enhanced absorption in visible light region would result in better catalytic activity. The band gap energy (Eg) of CuBi2O4 and CuBi2O4-OVs was determined by the Tauc model, which is commonly used in the semiconductor catalyst [43].
where a, hv, A and Eg, are the absorbance, photon energy, a constant, and the band gap, respectively. The band gap energy of the CuBi2O4 and CuBi2O4-OVs catalyst was calculated to be fixed at 1.83 eV and 1.72 eV, respectively, which was assumed to perform good photocatalytic activity under visible LED light irradiation. In the presence of surface oxygen vacancy, the narrowed band gap showed enhanced adsorption ability to visible light, promoting the separation of photo-generated holes and electrons pairs, causing the enhancement of photocatalytic activity. The N2 absorption/desorption isotherms of the CuBi2O4 and CuBi2O4-OVs catalyst are shown in Figure S1. The isotherms of both the CuBi2O4 and CuBi2O4-OVs catalyst presented type H3 isotherms. The BET surface area of CuBi2O4 was 4.76 m2/g and the pore size was around 10.9 nm, as the BET surface area of CuBi2O4-OVs was 5.01 m2/g and the pore size was 11.1 nm. With the presence of surface oxygen vacancy, the BET surface area and pore size was slightly increased, which is listed in Table S2.
ahv = A(hv − Eg)n/2
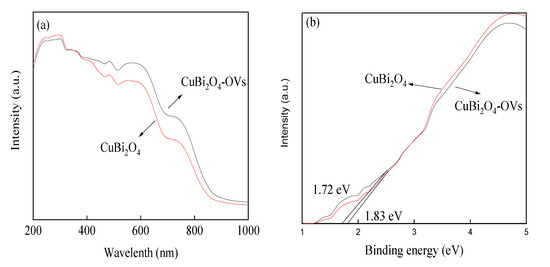
Figure 5.
(a,b) UV/vis diffusion reflectance spectra of as-prepared catalysts.
3.2. Heterogeneous SR-Photo Degradation of RhB
The prepared catalysts have no adsorption removal efficiency on RhB in the reaction time owing to the limited BET surface area. Negligible removal of RhB was obtained by using PMS alone and using LED light alone, owing to hardly any production of reactive free radicals. Figure 6 shows the degradation efficiency of photocatalysis, heterogeneous SR-Fenton reaction, and heterogeneous SR-photo-Fenton like reaction was 29.4%, 56.8%, and 87.9%, respectively. The value of reaction constant kapp for heterogeneous SR-photo-Fenton like process was higher than the sum of photocatalysis and heterogeneous SR-Fenton like reaction, which demonstrated there was a synergistic effect for RhB degradation in heterogeneous SR-photo-Fenton like reaction with CuBi2O4-OVs as catalyst. Herein, compared with the CuBi2O4-OVs catalyst, the removal efficiency of RhB was 100% and 40.9% for CuO and Bi2O3, respectively. On the other hand, with the introduction of surface oxygen vacancy, the synergistic effect between the heterogeneous SR-Fenton like reaction and photocatalysis could improve the degradation activity of RhB as well as the degradation rate constant [44]. The results might be attributed to the increased hydroxyl group on the surface, which formed more reactive oxygen species in the heterogeneous SR-photo-Fenton like reaction. The separation and transmission of photo-generated holes and electrons was also improved owing to the presence of surface oxygen vacancy, leading to better degradation efficiency. The addition of visible LED light to the heterogeneous SR-Fenton like systems enhanced the RhB removal degradation efficiency and rate constant. Consequently, the heterogeneous SR-photo-Fenton like system showed the highest removal efficiency for RhB by using CuBi2O4-OVs catalyst combined with surface oxygen vacancy [45].
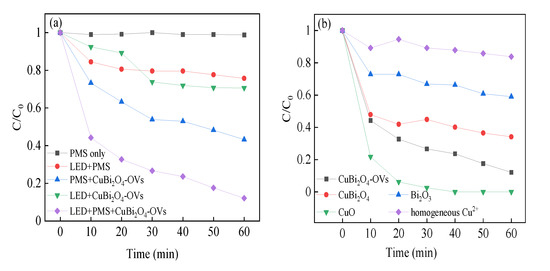
Figure 6.
(a) Comparison of RhB degradation activity in different systems; (b) effect of different catalyst on RhB degradation. Reaction conditions: RhB (20 mg/L), PMS (0.4 mM), catalyst (0.5 g/L), neutral pH.
The pseudo-first-order kinetic model was applied to calculate the degradation rate constants, which fitted well with the results. The kapp values were 0.0064, 0.0126, and 0.0304 h−1 for photocatalysis, heterogeneous SR-Fenton like reaction, and heterogeneous SR-photo-Fenton like reaction, respectively, which demonstrated the existence of a synergistic effect. Moreover, the metal leaching problem was very important in the heterogeneous reaction, which directly affected the stability of the heterogeneous catalyst. The leaching copper was very low and ranged from 0.020 to 0.0895 mg/L as the pH value declined from 10.7 to 3.2. The SR-photo-Fenton like reaction with 0.0895 mg/L copper ions in the system was also carried out and showed a poor contribution to PMS activation, evidencing that the catalyst played a significant role in the system. Therefore, photo-generated holes and electrons would participate in the redox transmission of copper ions in the heterogeneous SR-photo-Fenton like reaction, improving the formation of reactive oxygen species, leading to the enhancement of removal efficiency [46].
3.3. Effect of Reaction Conditions
Then, the reaction conditions were explored to get the best removal efficiency. As shown in Figure 7a, the removal efficiency of RhB declined from 86.2% to 37.9% as the solution pH climbed from 3.2 to 4.9. When the solution pH further climbed from 4.9 to 8.8, the degradation efficiency and rate constant were almost at the same level. Moreover, the removal efficiency of RhB was enhanced to 96.6% as the solution pH was raised to 10.7. In the acidic solution, the formation of SO52− ions was obtained and showed no catalytic activity [47]. The results showed that PMS possessed more stability rather than decomposed to sulfate radicals, thus retarding the degradation efficiency in highly acidic conditions [48]. When the solution pH came to neutral and basic condition, the hydroxyl radicals formed, which showed similar redox potential with sulfate radicals, resulting in better RhB removal efficiency. The removal rate remained unchanged in the pH range of 4.9–8.8, which proved that the CuBi2O4-OVs catalyst would be effective in a wide range of pH in the heterogeneous SR-photo-Fenton like process.
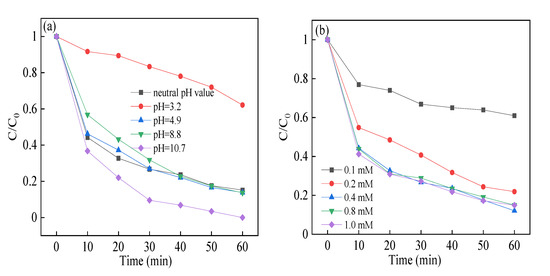
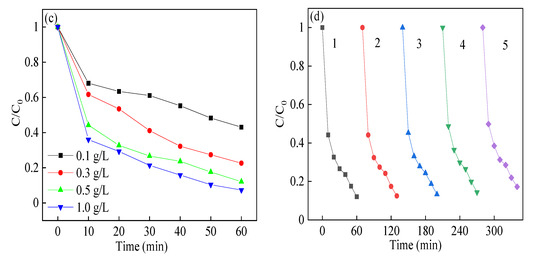
Figure 7.
Effect of (a) reaction initial pH value; (b) PMS concentration; (c) catalyst dosage; and (d) reuse on RhB degradation in heterogeneous sulfate radical (SR)-photo-Fenton like reaction. Reaction conditions: RhB (20 mg/L), PMS (0.1–1.0 mM), catalyst (0.1–1.0 g/L), pH in the range of 3.2–10.7 in (a–c); PMS (0.4 mM), catalyst (0.5 g/L), neutral pH in (d).
SO4− + OH− → SO42− + ·OH
HSO5− → SO52− + H+
The removal efficiency of RhB with different PMS concentrations in the range of 0.1–1.0 mM in the heterogeneous SR-photo-Fenton like reaction was shown in Figure 7b. The degradation efficiency of RhB increased from 39.1% to 87.9% with the increased PMS concentration, and the degradation rate constant varied from 0.0071 to 0.0304 h−1. However, the degradation efficiency of RhB was not further increased; as the PMS concentration rose higher than 0.4 mM, the rate constant remained nearly unchanged. The results could be attributed to the reactive sites on the surface of catalyst, which participated in the activation of PMS, leading to greater generation of oxidant species in the increment of PMS concentrations; on the other hand, overdosed oxidants will compete with other reactive radicals, resulting in the consumption of free radicals and declined removal efficiency of RhB [49]. When the PMS concentration was too high, it would inhibit the reaction between the reactive species and the contaminants, resulting in the decreased removal rate constant, which was a waste of PMS.
The removal efficiency of RhB in heterogeneous SR-photo-Fenton like reaction with different catalyst dosages was shown in Figure 7c. When the catalyst dosage increased from 0.1 g/L to 1.0 g/L, the removal efficiency climbed from 57.1% to 92.7% in the reaction time, where the degradation rate changed from 0.012 to 0.0391 h−1. As the catalyst dosage was over 0.5 g/L, the increasing rate of kapp was not fast enough when the catalyst dosage was fixed at 1.0 g/L. Firstly, with the increment of the catalyst addition, more reactive sites were employed to generate more reactive oxygen species, where more surface area was provided in the reaction. Secondly, the excessive catalyst would be an obstacle to the adsorption and transmission of visible light [50]. This phenomenon would give a negative effect on the excitation of electrons and lead to the reduction of activation efficiency. All the above results indicated that the prepared CuBi2O4-OVs catalyst showed high and effective activity for RhB removal in the heterogeneous SR-photo-Fenton like reaction.
The recycling experiments of CuBi2O4-OVs were evaluated under the optimized reaction conditions, which are shown in Figure 7d. The RhB degradation efficiency remained high in five successive reactions, and only showed slightly decreased efficiency in the fifth cycling experiment with 82.7% RhB removal. This might be attributed to the occupancy of reactive sites on the surface of catalyst in the successive reactions, where the leaching copper also affected the removal efficiency. Moreover, the rate constant also remained in the range of 0.0304 to 0.0258 h−1. The leached copper element was no more than 0.0895 mg/L, demonstrating the stability of synthesized CuBi2O4-OVs in heterogeneous SR-photo-Fenton like reaction. Compared with the fresh composite, the XRD diffraction peaks of CuBi2O4-OVs had no obvious change after the reaction, which are shown in Fig. S2. The major phase of the CuBi2O4-OVs catalyst showed no obvious difference between the fresh and used catalyst, indicating the stability of the CuBi2O4-OVs structure. The XPS peak of O 1s in the used catalyst showed slightly increased owing to the increment of oxygen or the water adsorption on the surface of catalyst. All the above results showed the catalyst had good reusability and stability in the heterogeneous SR-photo-Fenton like reaction.
TOC experiments were also used to evaluate the mineralization of RhB under optimal reaction conditions, which were shown in Figure 8. The TOC degradation was 13.3%, 32.8%, and 63.2% in the photocatalysis reaction, heterogeneous SR-Fenton like reaction, and heterogeneous SR-photo-Fenton like reaction, respectively. The results showed the mineralization of RhB was satisfied owing to the synergism effect, and were in good agreement with the degradation efficiency of RhB. Moreover, the CuBi2O4-OVs was also employed for the removal of other organic contaminants in the heterogeneous SR-photo-Fenton reaction, such as Orange II, methyl red (MR), ciprofloxacin (CIP), and levofloxacin (LVF). The degradation efficiency of Orange II and MR was 85.1% and 83.7% in the reaction time, whereas the degradation of CIP and LVF was about 10.4% and 16.2%, respectively. The results showed that the heterogeneous SR-photo-Fenton like reaction was effective for mostly common organic pollutants, especially for the removal of dyes.
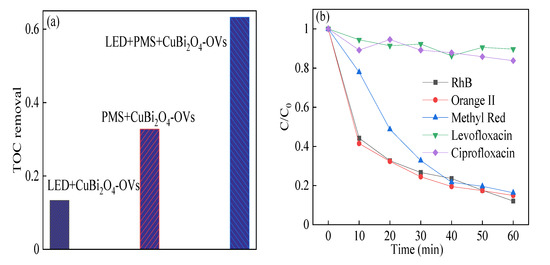
Figure 8.
(a) Total organic carbon (TOC) removal of RhB; (b) degradation of various contaminants in heterogeneous SR-photo-Fenton like reaction. Reaction conditions: contaminants (20 mg/L), PMS (0.4 mM), catalyst (0.5 g/L), neutral pH.
3.4. Reaction Mechanism
It was known that the reactive radical species dominated the reaction rate and efficiency in the heterogeneous SR-photo-Fenton like reaction. In order to identify the reactive radicals, radical quencher chemicals were applied in the system to find the possible free radicals. Compared with the reaction with sulfate radicals, the reaction between tert-Butyl alcohol (TBA) and hydroxyl radicals was really fast. In this case, TBA was usually considered as the trapping agent of ·OH. Methanol (MeOH) could react with sulfate and hydroxyl radicals in the meantime, which was used as the trapping agent for sulfate radicals and hydroxyl radicals. It can be seen in Figure 9a that the degradation efficiency of RhB was 79.5% and 70.6%, which slightly decreased in the presence of TBA and MeOH compared with the control experiment. Herein, it was proposed that both sulfate radicals and hydroxyl radicals were formed in the reaction. However, the two free radicals took a little contribution for the reaction, implying the two scavenging chemicals took a moderate inhibition in the reaction. Moreover, in order to identify the existence of photogenerated holes and superoxide radicals, p-benzoquinone (BQ) and sodium oxalate (SO, Na2C2O4) were used as quenching chemicals for the reactive species. When BQ and SO chemicals were added in the solution, the degradation efficiency of RhB was declined to 15.8% and 40.2%, which demonstrated the superoxide radicals and holes were also generated and dominated in the reaction. It was assumed that the superoxide, photogenerated holes, sulfate radicals, and hydroxyl radicals were all produced in the reaction. The sulfate radicals, holes, and superoxide radicals played a dominant role in the RhB removal process, leading to enhancement of the photocatalytic reaction.
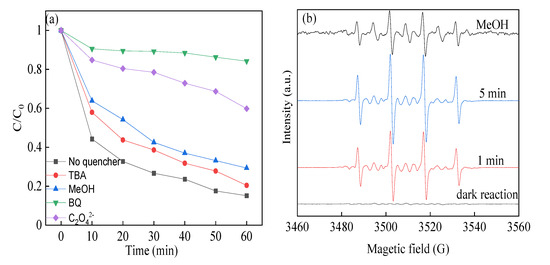
Figure 9.
(a) Different quenching chemicals to remove RhB; (b) DMPO spin-trapping EPR spectra in heterogeneous SR-photo-Fenton like reaction. BQ, p-benzoquinone.
In order to evidence the existence of free reactive radicals, EPR experiments were also conducted using 5, 5-Dimethyl-1-Pyrrolidine-N-oxide (DMPO) as a spin trapping agent, as shown in Figure 9b. Weak typical peaks of DMPO-OH were identified under dark condition, indicating certain number of radicals were produced as a result of the activation of PMS. However, when the heterogeneous SR-Fenton like system was irradiated by visible LED light, strong signals of DMPO-OH peaks were observed. The EPR signals of DMPO-SO4 were found with weak intensity owing to the quick reaction with H2O, leading to the generation of hydroxyl radicals, which made it difficult to capture the signal in the solution [51]. The signal remained sharp after 5 min reaction, evidencing the persistent production of these free radicals. Moreover, the signal of DMPO-O2 radicals was also observed, which proved the presence of superoxide radicals in the systems. Thus, the CuBi2O4-OVs catalyst could produce lots of free reactive radicals in the heterogeneous SR-photo-Fenton like reaction, which agreed well with the results of the quenching experiments.
3.5. Proposed Reaction Mechanism
Figure 10 illustrates the mechanism in the heterogeneous SR-photo-Fenton like reaction using the CuBi2O4-OVs catalyst under LED light irradiation on the basis of the above results. In the XPS spectra, the peaks for Cu element could be attributed to Cu(II) and Cu(I). The redox pair of Cu(I)/Cu(II) could participate in the decomposition of PMS to generate SO4− and ·OH, which was much higher after the reaction than that of fresh catalyst. The continuous redox of Cu(II) occurred through the reduction caused by generated electrons, leading to the production of reduced Cu species. Meanwhile, the electrons could jump from CB to VB on the catalyst surface, which was occupied with rich surface oxygen vacancy, facilitating the rapid conversion of Cu(II)/Cu(I) pairs. With the presence of surface oxygen vacancy, the free radicals were formed more quickly, leading to better degradation efficiency. Moreover, surface oxygen vacancy could be considered as a defect on the catalyst surface, acting as the reactive sites, which was effective to prevent the recombination of photo-generated holes/electrons, leading to better catalytic activity [52]. Additionally, the visible region of catalyst was also extended, which was helpful for the visible light adsorption on the surface.
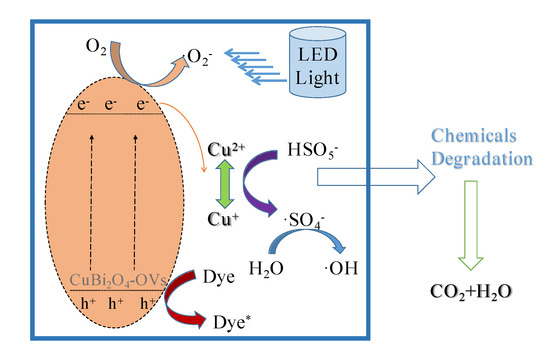
Figure 10.
Mechanism of heterogeneous SR-photo-Fenton like degradation of RhB using the CuBi2O4-OVs catalyst.
In conclusion, as the CuBi2O4-OVs catalyst was irradiated by visible LED light, the production of photo-generated hole and electrons occurred, which would react with PMS to generate free radicals. PMS was applied as the electron acceptor and the oxidant in the heterogeneous SR-photo-Fenton like reaction, which suppressed the recombination of holes and electrons, leading to better degradation efficiency [53]. As the surface oxygen vacancy was presented on the surface of catalyst, the free radicals were quickly formed owing to the quick transmission of photo-generated carriers and good separation efficiency of the pairs. The extended response to the visible light would also be attributed to the introduction of surface oxygen vacancy. Then, with the persistent attack of superoxide, holes, sulfate radicals, and hydroxyl radicals, the target pollutant RhB was gradually degraded in the reaction and mineralized to CO2 and H2O.
CuBi2O4 + hv → h+ + e−
Cu(I) + HSO5− → SO4− +Cu(II) + OH−
Cu(II) + HSO5− → SO5− + Cu(I) + H+
Cu(II) + e− → Cu(I)
HSO5− + e− → SO4− + OH−
HSO5− + h+ → SO5− + H+
SO5− + SO5− → 2SO4− + O2
HSO5− → SO4− + OH (under light irradiation)
(radicals) + RhB → intermediates → CO2 + H2O
4. Conclusions
In this study, the CuBi2O4-OVs catalyst was prepared and applied in a heterogeneous SR-photo-Fenton like system. The removal of RhB fitted well with the pseudo first-order kinetics model. The optimal experiment condition was obtained such that the catalyst dosage was fixed at 0.5 g/L and PMS concentration was fixed at 0.4 mM under neutral pH, which showed a synergism effect of the photocatalysis and heterogeneous SR-Fenton like reaction. The introduction of surface oxygen vacancy could prevent the recombination of photogenerated holes and electrons and narrow the band gap of CuBi2O4, leading to higher degradation efficiency. With the increased dosage of PMS and catalyst, the removal efficiency was improved, but retarded with overdosed addition owing to side competitive reactions. The CuBi2O4-OVs catalyst exhibited good photocatalytic performance. Moreover, the reusability of catalyst was excellent in five successive experiments with low leaching of Cu ions. The roles of superoxide radicals, holes, sulfate radicals, and hydroxyl radicals were identified by quencher chemicals and EPR experiments. The reaction mechanism was also proposed in the heterogeneous SR-photo-Fenton like reaction. Therefore, the heterogeneous SR-photo-Fenton like system has good aspect for the treatment of organic pollutant in wastewater.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4344/10/8/945/s1, Figure S1: N2 adsorption–desorption isotherm of CuBi2O4 and CuBi2O4-OVs; Figure S2: XRD patterns of the CuBi2O4-OVs catalyst before and after the reaction; Table S1: Studies on of Bi-based catalysts in the photocatalytic reactions; Table S2: Surface area, pore volume, pore size of CuBi2O4, and CuBi2O4-OVs samples.
Author Contributions
Methodology, X.Z.; investigation, Y.C., H.B., and X.Z.; resources, W.H. and B.Z.; writing—original draft preparation, X.Z.; writing—review and editing, X.Z.; project administration, X.Z., W.H., and B.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work is financially supported by the Project of Youth Innovative Foundation of Guangdong Province of China (Grant No. 201912017QX); Provincial undergraduate innovation and Entrepreneurship Project (S202013177014); Promotion Project Funds of Beijing Normal University, Zhuhai (Grant No. 201850001); Quality Engineering Project of Beijing Normal University, Zhuhai (Grant No. 201832); and Plan of Youth Teachers of Guangdong Higher Education Association (Grant No. 19GYB060).
Acknowledgments
This work is financially supported by Project of Youth Innovative Foundation of Guangdong Province of China for help in XRD, SEM, TEM, and XPS analysis.
Conflicts of Interest
The authors declare that there is no conflict of interests.
References
- Frank, S.N.; Bard, A.J. Heterogeneous photocatalytic oxidation of cyanide and sulfite in aqueous solutions at semiconductor powders. J. Phys. Chem. 1977, 81, 1484–1488. [Google Scholar] [CrossRef]
- Li, R.; Li, T.; Zhou, Q. Impact of titanium Dioxide (TiO2) modification on its application to pollution treatment—A Review. Catalyst 2020, 10, 804. [Google Scholar] [CrossRef]
- Karthikeyan, C.; Arunachalam, P.; Ramachandran, K.; Al-Mayouf, A.M.; Karuppuchamy, S. Recent advances in semiconductor metal oxides with enhanced methods for solar photocatalytic applications. J. Alloy. Compd. 2020, 828, 15. [Google Scholar] [CrossRef]
- Gray, H.E.; Powell, T.; Choi, S.; Smith, D.S.; Parker, W.J. Organic phosphorus removal using an integrated advanced oxidation-ultrafiltration process. Water Res. 2020, 1821, 115968. [Google Scholar]
- Gonzalez, T.; Dominguez, J.R.; Correia, S. Neonicotinoids removal by associated binary, tertiary and quaternary advanced oxidation processes: Synergistic effects, kinetics and mineralization. J. Environ. Manag. 2020, 2611, 110156. [Google Scholar] [CrossRef]
- Wang, J.; Zhuan, R. Degradation of antibiotics by advanced oxidation processes: An overview. Sci. Total Environ. 2020, 701, 135023. [Google Scholar] [CrossRef]
- Zhao, Q.; Mao, Q.; Zhou, Y.; Wei, J.; Liu, X.; Yang, J.; Luo, L.; Zhang, J.; Chen, H.; Chen, H.; et al. Metal-free carbon materials-catalyzed sulfate radical-based advanced oxidation processes: A review on heterogeneous catalysts and applications. Chemosphere 2017, 189, 224–238. [Google Scholar] [CrossRef]
- Oh, W.D.; Dong, Z.; Lim, T.T. Generation of sulfate radical through heterogeneous catalysis for organic contaminants removal: Current development, challenges and prospects. Appl. Catal. B 2016, 1945, 169–201. [Google Scholar] [CrossRef]
- Hu, P.; Long, M. Cobalt-catalyzed sulfate radical-based advanced oxidation: A review on heterogeneous catalysts and applications. Appl. Catal. B 2016, 181, 103–117. [Google Scholar] [CrossRef]
- Shukla, P.; Sun, H.; Wang, S.; Ang, H.M.; Tade, M.O. Co-SBA-15 for heterogeneous oxidation of phenol with sulfate radical for wastewater treatment. Catal. Today 2011, 175, 380–385. [Google Scholar] [CrossRef]
- Wang, Y.; Indrawirawan, S.; Duan, X.; Sun, H.; Ang, H.M.; Tade, M.O.; Wang, S. New insights into heterogeneous generation and evolution processes of sulfate radicals for phenol degradation over one-dimensional α-MnO2 nanostructures. Chem. Eng. J. 2015, 266, 12–20. [Google Scholar] [CrossRef]
- Wu, C.H.; Yang, M.T.; Lin, K.Y.A. Magnetic Co/Fe nanohybrid supported on carbonaceous marcosphere as a heterogeneous catalyst for sulfate radical-based chemical oxidation. J. Environ. Chem. Eng. 2018, 6, 426–434. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, J.; Chen, J.; Feng, X.; Cai, W. Rapid degradation of tetracycline hydrochloride by heterogeneous photocatalysis coupling persulfate oxidation with MIL-53(Fe) under visible light irradiation. J. Hazard. Mater. 2020, 392, 122315. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, C.; Zhang, Y.; Meng, W.; Yu, B.; Pu, S.; Yuan, D.; Qi, F.; Xu, B.; Chu, W. Sulfate radical-based photo-Fenton reaction derived by CuBi2O4 and its composites with α-Bi2O3 under visible light irradiation: Catalyst fabrication, performance and reaction mechanism. Appl. Catal. B 2018, 235, 264–273. [Google Scholar] [CrossRef]
- Lv, C.; Liang, H.; Chen, H.; Wu, L. Hydroxyapatite supported Co3O4 catalyst for enhanced degradation of organic contaminants in aqueous solution: Synergistic visible-light photo-catalysis and sulfate radical oxidation process. Microchem. J. 2019, 149, 103959. [Google Scholar] [CrossRef]
- Soltani, T.; Lee, B.K. Enhanced formation of sulfate radicals by metal-doped BiFeO3 under visible light for improving photo-Fenton catalytic degradation of 2-chlorophenol. Chem. Eng. J. 2017, 313, 1258–1268. [Google Scholar] [CrossRef]
- Zhang, M.W.; Lin, K.Y.A.; Huang, C.F.; Tong, S. Enhanced degradation of toxic azo dye, amaranth, in water using Oxone catalyzed by MIL-101-NH2 under visible light irradiation. Sep. Purif. Tech. 2019, 227, 115632. [Google Scholar] [CrossRef]
- Karbasi, M.; Karimzadeh, F.; Raeissi, K.; Giannakis, S.; Pulgarin, C. Improving visible light photocatalytic inactivation of E. coli by inducing highly efficient radical pathways through peroxymonosulfate activation using 3-D, surface-enhanced, reduced graphene oxide (rGO) aerogels. Chem. Eng. J. 2020, 396, 125189. [Google Scholar] [CrossRef]
- Bi, W.; Wu, Y.; Dong, W. The degradation of oxytetracycline with low concentration of persulfate sodium motivated by copper sulphate under simulated solar light. Chem. Eng. J. 2020, 393, 122782. [Google Scholar] [CrossRef]
- Wang, A.; Chen, Z.; Zheng, Z.; Xu, H.; Yan, K. Remarkably enhanced sulfate radical-based photo-Fenton-like degradation of levofloxacin using the reduced mesoporous MnO@MnOx microspheres. Chem. Eng. J. 2020, 379, 122340. [Google Scholar] [CrossRef]
- Feng, Q.; Zhou, J.; Luo, W.; Ding, L.; Cai, W. Photo-Fenton removal of tetracycline hydrochloride using LaFeO3 as a persulfate activator under visible light. Ecotoxicol. Environ. Saf. 2020, 198, 110661. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Ji, F.; Guo, Q.; Fan, J.; Xu, X. Combination of heterogeneous Fenton-like reaction and photocatalysis using Co-TiO2 nanocatalyst for activation of KHSO5 with visible light irradiation at ambient conditions. J. Environ. Sci. 2014, 26, 2440–2450. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Cheng, H.; Ma, J.; Qin, Y.; Kong, Y.; Komarneni, S. Bi2MoO6 microspheres for the degradation of orange II by heterogeneous activation of persulfate under visible light. Mater. Lett. 2020, 261, 127099. [Google Scholar] [CrossRef]
- Li, M.; Tian, X.; Zou, X.; Han, X.; Du, C.; Shan, B. Promoting photoelectrochemical hydrogen evolution activity of CuBi2O4 photocathode through ramping rate control. Int. J. Hydrogen Energy 2020, 45, 15121–15128. [Google Scholar] [CrossRef]
- Ma, C.; Ma, D.K.; Yu, W.; Chen, W.; Huang, S. Ag and N-doped graphene quantum dots co-modified CuBi2O4 submicron rod photocathodes with enhanced photoelectrochemical activity. Appl. Surf. Sci. 2019, 481, 661–668. [Google Scholar] [CrossRef]
- Chen, X.; Dai, Y.; Guo, J. Hydrothermal synthesis of well-distributed spherical CuBi2O4 with enhanced photocatalytic activity under visible light irradiation. Mater. Lett. 2015, 161, 251–254. [Google Scholar] [CrossRef]
- Sabri, M.; Habibi-Yangjeh, A.; Ghosh, S. Novel ZnO/CuBi2O4 heterostructures for persulfate-assisted photocatalytic degradation of dye contaminants under visible light. J. Photoch. Photobiol. A 2020, 391, 112397. [Google Scholar] [CrossRef]
- Zhang, H.; Nengzi, L.; Li, X.; Wang, Z.; Cheng, X. Construction of CuBi2O4/MnO2 composite as Z-scheme photoactivator of peroxymonosulfate for degradation of antibiotics. Chem. Eng. J. 2020, 386, 124011. [Google Scholar] [CrossRef]
- Oh, W.D.; Dong, Z.; Lim, T.T. Hierarchically-structured Co-CuBi2O4 and Cu-CuBi2O4 for sulfanilamide removal via peroxymonosulfate activation. Catal. Today 2017, 280, 2–7. [Google Scholar] [CrossRef]
- Gao, H.; Wang, F.; Wang, S.; Wang, X.; Yi, Z.; Yang, H. Photocatalytic activity tuning in a novel Ag2S/CQDs/CuBi2O4 composite: Synthesis and photocatalytic mechanism. Mater. Res. Bull. 2019, 115, 140–149. [Google Scholar] [CrossRef]
- Shi, W.; Li, M.; Huang, X.; Ren, H.; Guo, F.; Tang, Y.; Lu, C. Construction of CuBi2O4/Bi2MoO6 p-n heterojunction with nanosheets-on-microrods structure for improved photocatalytic activity towards broad-spectrum antibiotics degradation. Chem. Eng. J. 2020, 394, 125009. [Google Scholar] [CrossRef]
- Chen, X.; Yu, C.; Zhu, R.; Li, N.; Chen, M.; Lin, Q.; Xu, S.; Chen, X.; Wang, H. Photocatalytic performance and mechanism of Z-Scheme CuBi2O4/Ag3PO4 in the degradation of diclofenac sodium under visible light irradiation: Effects of pH, H2O2, and S2O82−. Sci. Total Environ. 2020, 711, 134643. [Google Scholar] [CrossRef]
- Wang, L.; Huang, T.; Yang, G.; Lu, C.; Dong, F.; Li, Y.; Guan, W. The precursor-guided hydrothermal synthesis of CuBi2O4/WO3 heterostructure with enhanced photoactivity under simulated solar light irradiation and mechanism insight. J. Hazard. Mater. 2020, 381, 120956. [Google Scholar] [CrossRef]
- Fu, F.; Shen, H.; Sun, X.; Xue, W.; Shoneye, A.; Ma, J.; Luo, L.; Wang, D.; Wang, J.; Tang, J. Synergistic effect of surface oxygen vacancies and interfacial charge transfer on Fe(III)/Bi2MoO6 for efficient photocatalysis. Appl. Catal. B 2019, 247, 150–162. [Google Scholar] [CrossRef]
- Lyu, J.; Hu, Z.; Li, Z.; Ge, M. Removal of tetracycline by BiOBr microspheres with oxygen vacancies: Combination of adsorption and photocatalysis. J. Phys. Chem. Solids 2019, 129, 61–70. [Google Scholar] [CrossRef]
- Li, D.; Haneda, H.; Labhsetwar, N.K.; Hishita, S.; Ohashi, N. Visible-light-driven photocatalysis on fluorine-doped TiO2 powders by the creation of surface oxygen vacancies. Chem. Phys. Lett. 2005, 401, 579–584. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, Y.; Yang, G.; Liu, C.; Wang, J. Hydrothermal synthesis of CuBi2O4 nanosheets and their photocatalytic behavior under visible light irradiation. Mater. Lett. 2013, 107, 291–294. [Google Scholar] [CrossRef]
- Shan, W.; Hu, Y.; Bai, Z.; Zheng, M.; Wei, C. In situ preparation of g-C3N4/bismuth-based oxide nanocomposites with enhanced photocatalytic activity. Appl. Catal. B 2016, 188, 1–12. [Google Scholar] [CrossRef]
- Hossain, M.K.; Samu, G.F.; Gandha, K.; Santhanagopalan, S.; Liu, J.P.; Janaky, C.; Rajeshwar, K. Solution combustion synthesis, characterization, and photocatalytic activity of CuBi2O4 and its nanocomposites with CuO and α-Bi2O3. J. Phys. Chem. C 2017, 121, 8252–8261. [Google Scholar] [CrossRef]
- Brezesinski, K.; Ostermann, R.; Hartmann, P.; Perlich, J.; Brezesinski, T. Exceptional Photocatalytic Activity of Ordered Mesoporous β-Bi2O3 Thin Films and Electrospun Nanofiber Mats. Chem. Mater. 2010, 22, 3079–3085. [Google Scholar] [CrossRef]
- Zhang, L.; Lyu, L.; Nie, Y.; Hu, C. Cu-doped Bi2O3/Bi0 composite as an efficient Fenton-like catalyst for degradation of 2-chlorophenol. Sep. Purif. Tech. 2016, 157, 203–208. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, C.; Xu, B.; Qi, F.; Chu, W. Degradation of benzotriazole by a novel Fenton-like reaction with mesoporous Cu/MnO2: Combination of adsorption and catalysis oxidation. Appl. Catal. B 2016, 199, 447–457. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Chai, J.; Xue, S.; Wang, R.; Jiang, L.; Wang, J.; Zhang, Z.; Dionysiou, D.D. Construction of novel symmetric double Z-scheme BiFeO3/CuBi2O4/BaTiO3 photocatalyst with enhanced solar-light-driven photocatalytic performance for degradation of norfloxacin. Appl. Catal. B 2020, 272, 119017. [Google Scholar] [CrossRef]
- Sun, Q.; Wu, S.; Li, K.; Han, B.; Chen, Y.; Pang, B.; Yu, L.; Dong, L. The favourable synergistic operation of photocatalysis and catalytic oxygen reduction reaction by a novel heterogeneous CoFe2O4-TiO2 nanocomposite. Appl. Surf. Sci. 2020, 516, 146142. [Google Scholar] [CrossRef]
- Hu, J.; Li, J.; Cui, J.; An, W.; Liu, L.; Liang, Y.; Cui, W. Surface oxygen vacancies enriched FeOOH/Bi2MoO6 photocatalysis-fenton synergy degradation of organic pollutants. J. Hazard. Mater. 2020, 384, 121399. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhou, J.; Zhang, T.; Ding, L. Enhanced degradation of tetracycline hydrochloride using photocatalysis and sulfate radical-based oxidation processes by Co/BiVO4 composites. J. Water Process. Eng. 2019, 32, 100918. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, H. Mn-based catalysts for sulfate radical-based advanced oxidation processes: A review. Environ. Int. 2019, 133, 105141. [Google Scholar] [CrossRef]
- Chen, Z.; Bi, S.; Zhao, G.; Chen, Y.; Hu, Y. Enhanced degradation of triclosan by cobalt manganese spinel-type oxide activated peroxymonosulfate oxidation process via sulfate radicals and singlet oxygen: Mechanisms and intermediates identification. Sci. Total Environ. 2020, 711, 134715. [Google Scholar] [CrossRef]
- Hao, S.M.; Yu, M.Y.; Zhang, Y.J.; Abdelkrim, Y.; Qu, J. Hierarchical mesoporous cobalt silicate architectures as high-performance sulfate-radical-based advanced oxidization catalysts. J. Colloid. Interf. Sci. 2019, 545, 128–137. [Google Scholar] [CrossRef]
- Baran, N.Y.; Baran, T.; Menteş, A. Design of highly robust halloysite nanoclay supported palladium complex as a highly active heterogeneous catalyst for construction of biaryls. Appl. Clay Sci. 2019, 18115, 105225. [Google Scholar] [CrossRef]
- Hu, L.; Wang, P.; Shen, T.; Wang, Q.; Zhang, G. The application of microwaves in sulfate radical-based advanced oxidation processes for environmental remediation: A review. Sci. Total. Environ. 2020, 722, 137831. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Sun, W.; Su, S.; Ao, Z.; Liu, C.; Yao, G.; Lai, B. Degradation of bisphenol A by peroxymonosulfate activated with oxygen vacancy modified nano-NiO-ZnO composite oxides: A typical surface-bound radical system. Chem. Eng. J. 2020, 400, 125915. [Google Scholar] [CrossRef]
- Huang, Z.; Wu, P.; Liu, J.; Yang, S.; Chen, M.; Li, Y.; Niu, W.; Ye, Q. Defect-rich carbon based bimetallic oxides with abundant oxygen vacancies as highly active catalysts for enhanced 4-aminobenzoic acid ethyl ester (ABEE) degradation toward peroxymonosulfate activation. Chem. Eng. J. 2020, 395, 124936. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).