Abstract
An increase of carrier concentration is one of the most important routes for enhancing the catalytic performance of semiconductor photocatalysts. In this study, the Sillén–Aurivillius oxychloride Bi4NbO8Cl with hole doping was successfully prepared by a solid-state reaction method. X-ray powder diffraction (XRD), scanning electron microscopy (SEM), ultraviolet–visible diffuse reflectance spectra (UV–vis DRS), X-ray photoelectron spectrometry (XPS) and photoluminescence spectra (PL) were used to characterize and analyze the prepared samples. The experimental results and density functional theory calculations demonstrate that hole doping can be formed in Bi4NbO8Cl by inserting zinc into the niobium site, and the photocatalytic activity can be improved by introducing additional holes into Bi4NbO8Cl. The photogenerated hole (h+) is considered to be the main active species to degrade trypan blue (TB) through trapping experiments. The optimal photocatalyst of Bi4Nb0.8Zn0.2O8Cl exhibits excellent photocatalytic activity in degradation of trypan blue under visible light irritation. Moreover, a possible photocatalytic degradation mechanism is discussed according the experimental and analytical results.
1. Introduction
Recently, environmental pollution and energy shortages have become two main challenges for human beings. In particular, the discharge of various organic wastewaters has seriously caused irreversible damage to the environment and human beings [1,2,3]. Among the reported remediation methods, semiconductor photocatalysis has been regarded as an effective route to eliminate contaminants. In particular, the development of visible light-driven photocatalysts has attracted increasing attention from the perspective of solar energy conversion [4,5].
In recent years, a novel bismuth-based photocatalyst Bi4NbO8Clhas has attracted much attention. It has a layered Sillén–Aurivillius perovskite structure and consists of single-layer NbO4 perovskite blocks that are separated by (Bi2O2)2Cl blocks, which is beneficial for the efficient separation and migration of the photogenerated charge carriers. Moreover, the valence band maxima (VBM) of Bi4NbO8Cl is mainly composed of O-2p orbital rather than Cl-3p orbits, so its VBM level is more negative than that of typical oxides [6,7,8]. Therefore, Bi4NbO8Cl is considered to be a stable visible light-response photocatalyst with narrow bandgap and which is usually applied to degrade organic pollutants. Shi et al. [9] prepared a layered Bi-based oxychloride Bi4NbO8Cl, and found that the visible light-driven photocatalytic activities for degrading methyl orange (MO) over different catalysts follow the decreasing order of Bi4NbO8Cl > Bi3O4Cl > anatase TiO2; Sundaram et al. [10] synthesized Bi4NbO8Cl nanoparticles by a solution combustion method and the mineralization efficiency of Congo red dye can reach 75% in 80 min.
However, in order to further improve the photocatalytic performance of Bi4NbO8Cl, many methods have been developed such as semiconductor recombination [11,12], deposition of noble metals [13,14], deposition of non-noble metals [15] and metal ions doping [16]. Among these methods, transition metal ions doping is considered to be one of the most promising, which can increase carrier concentration and improve the charge carrier transport [17]. Thus, transition ion doping can effectively improve the photocatalytic performance of Bi4NbO8Cl. Shangguan et al. [16] utilized yttrium-doped Bi4NbO8Cl to synthetize Bi4−xYxNbO8Cl and enhance photocatalytic activity. As common dopants, doping of transition metals tungsten (W) and zinc (Zn) is considered to be an effective method to improve the photocatalytic activity of semiconductors [18,19].
However, through our investigation, there are few reports preparing tungsten- or zinc-doped Bi4NbO8Cl material and studying their photocatalytic performance. In this work, a series of W-doped and Zn-doped Bi4NbO8Cl materials with different dopant concentrations were synthesized via a solid-state reaction method. The photocatalytic activities of the W-doped and Zn-doped Bi4NbO8Cl materials were investigated by degradation of trypan blue (TB) under visible light. Finally, the possible hole-doping mechanism of photocatalytic degradation of TB was also discussed.
2. Results
2.1. Synthesis of Bi4Nb1−xWxO8Cl and Bi4Nb1−xZnxO8Cl (x = 0.1, 0.2, 0.3) Powder
The photocatalysts were synthesized by a solid-state reaction. Firstly, 2 mM Bi(NO3)3·5H2O was dissolved in 20 mL ethylene glycol, subsequently 10 mL KCl solution (0.2 mol/L) gradually added to obtain the precursor. After filtration and washing, the precursor was dried at 333 K for 12 h to obtain BiOCl. Secondly, the stoichiometric amount of prepared BiOCl, Bi2O3 (Aladdin, 99.9%), Nb2O5 (Aladdin, 99.9%) and WO3 or ZnO powders (Aladdin, 99.9%) were adequately ground and calcined in a muffle furnace at 973 k for 24 h. Finally, Bi4Nb1−xWxO8Cl and Bi4Nb1−xZnxO8Cl (x = 0.1, 0.2, 0.3) series powders were prepared. WO3 or ZnO was used to replace the component of Nb2O5, the x value of the very small part being 0, 0.1, 0.2, 0.3, respectively. The samples with the atomic ratio of Zn to Nb (0, 1:9, 2:8, 3:7) were labeled as BNO, BNZ-1, BNZ-2 and BNZ-3, respectively. Those of W to Nb (1:9, 2:8, 3:7) were labeled as BWZ-1, BWZ-2, BWZ-3, respectively.
2.2. Characterization
An X-ray diffractometer (XRD) with Cu Kα radiation (X’Pert3 Powder, PANalytical, Almelo, the Netherlands, λ = 0.15406 nm) was used to carry out of the phase identification. The morphologies of the samples were studied by Nova a Nano 450 (FEI, Hillsboro, OR, USA) field-emission scanning electron microscope (FESEM), X-ray photoelectron spectroscopy (XPS) was performed by an ESCALAB 250xi XPS system (Thermo Fisher Scientific, Carlsbad, CA, USA). A Shimadzu UV-2550 ultraviolet UV–visible diffuse reflectance spectrum (Shimadzu, Kyoto, Japan) was used for the test of the spectral absorption curves of the samples, with a wavelength scanning range of 200–800 nm. The photoluminescence (PL) spectra were collected by a Hitachi F-4600 spectrometer (Hitachi, Tokyo, Japan).
2.3. Photocatalytic Activity
Photocatalytic activities of Bi4Nb1−xWxO8Cl and Bi4Nb1−xZnxO8Cl series were evaluated by degrading TB under a 500 W Xe Lamp (420 nm cut off filter) illumination. In the study of degradation, 30 mg prepared photocatalyst was dispersed evenly in 30 mL TB solution (10 mg/mL) at room temperature. The mixture was magnetically stirred in the dark for 30 min before illumination. Then the TB solution was exposed to visible light illumination with stirring, and 3 mL suspension was collected every 15 min, and then centrifuged at 12,000 rpm for 5 min to remove the photocatalyst. Finally, the concentration of TB was determined by a spectrophotometer.
2.4. Density Functional Theory (DFT) Calculation
Here, in this paper, we studied the electronic structures of pure and doped Bi4NbO8Cl systems by performing first-principles calculations based on density functional theory (DFT). The Wien2k program package [20] was employed and the Perdew-Burke-Ernzerh (PBE) version of the generalised gradient approximation (GGA) [21] was adopted. We used a 13 × 13 × 2 momentum grid, and R_MT*K_max = 7.0 with a muffin-tin radius R_MT =1.84, 2.30, 1.67 and 2.5 a.u. for Nb, Bi, O and Cl atoms, respectively.
The crystal structure presented in Section 3.6 is adopted for calculations. Electron and hole doping are approximately achieved by applying virtual crystal approximation (VCA) implemented in Wien2k. We treat the valence states of W and Zn as 6s25d4 and 4s2, respectively. Considering the valence state of Nb is 5d14d4, hence 20% W and 20% Zn doping induces 0.2 electron and 0.6 hole doping per Nb, respectively.
3. Discussion
3.1. X-Ray Diffractogram (XRD) Patterns Analysis
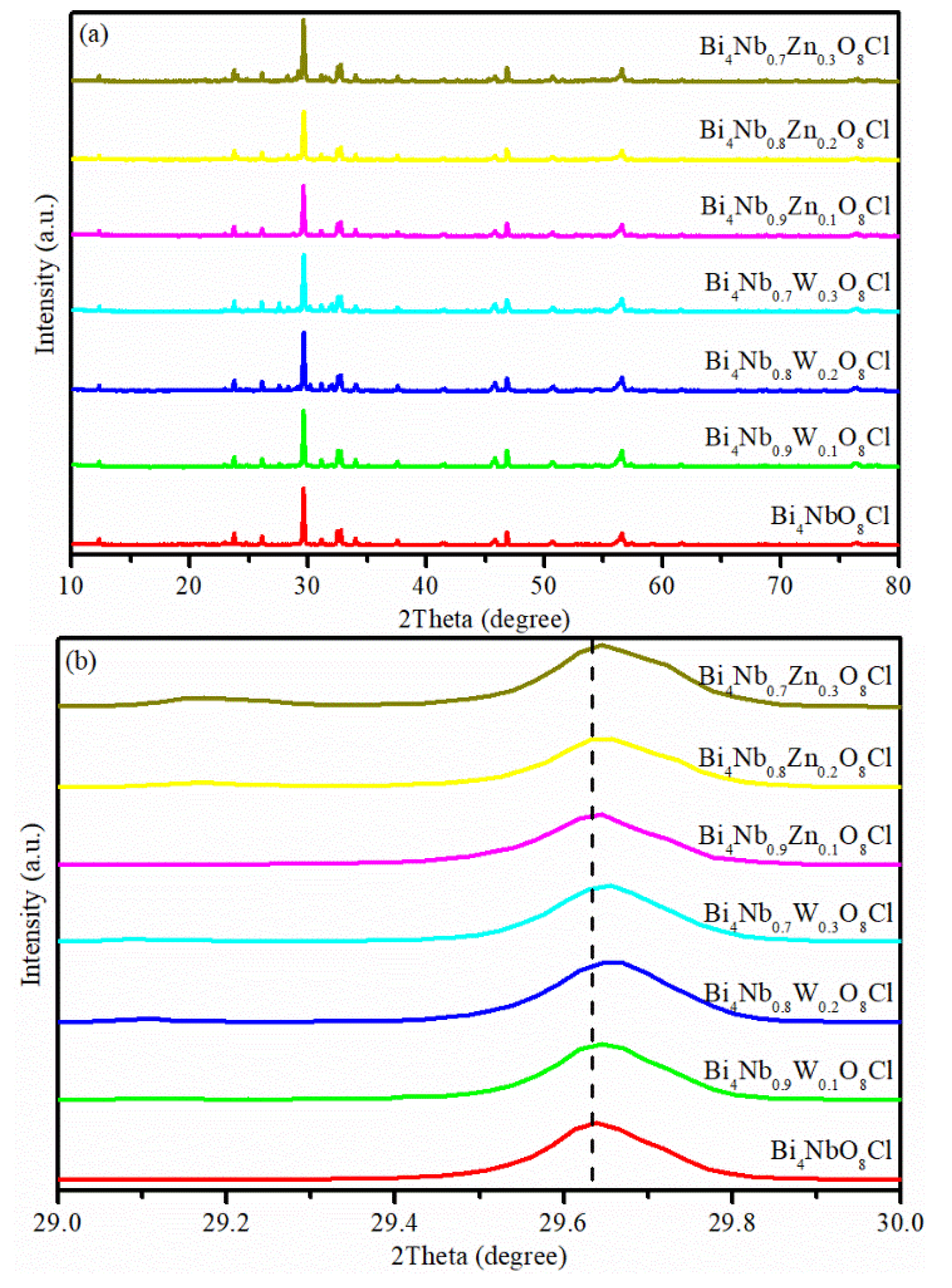
The XRD patterns of the Bi4NbO8Cl, Bi4Nb1−xWxO8Cl and Bi4Nb1−xZnxO8Cl series are shown in Figure 1. As shown in Figure 1a, the diffraction peaks observed are well indexed to the Bi4NbO8Cl (JCPDS NO.84-0843), and no other diffraction peaks can be found. Which indicates that either W or Zn doping does not affect the crystal structure of Bi4NbO8Cl. It is noteworthy that the diffraction peak at about 29.6° slightly shifts to higher angles with the increase of dopant content (Figure 1b). This phenomenon can be attributed to the ionic radius of Zn2+ (0.74 Å) and W6+ (0.62 Å) both being smaller than that of Nb5+ (0.78 Å), the incorporation of W or Zn ions in Bi4NbO8Cl lattice via substituting Nb ions consequently induce distortion in the crystal lattice of Bi4NbO8Cl [18,22,23]. This means that W or Zn elements were successfully doped to the Bi4NbO8Cl lattice.
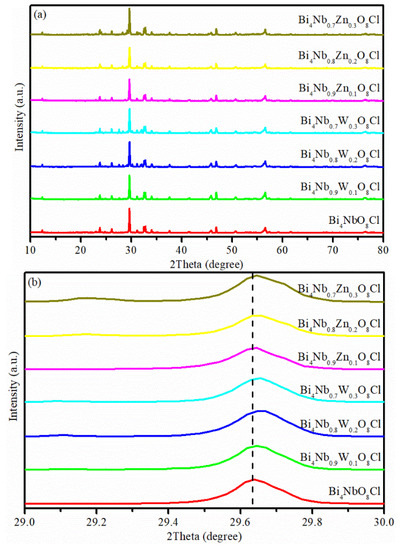
Figure 1.
(a) X-ray diffraction (XRD) patterns of Bi4NbO8Cl, Bi4Nb1−xWxO8Cl and Bi4Nb1−xZnxO8Cl series, (b) diffraction peak positions in the range of 2 θ = 29–30°.
3.2. Scanning Electron Microscopy (SEM)
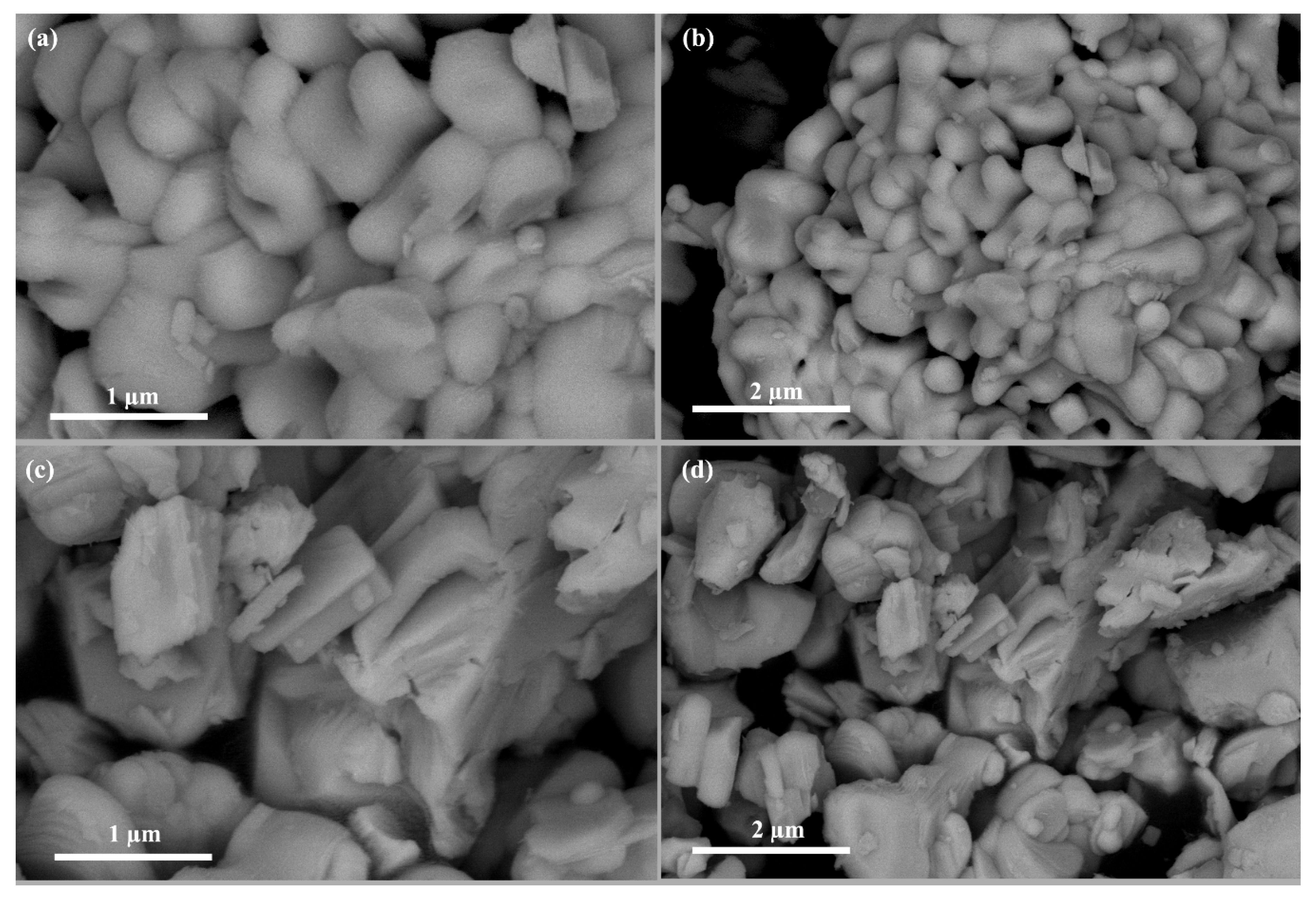
The morphologies of Bi4NbO8Cl and Bi4Nb0.8Zn0.2O8Cl are shown in Figure 2. It can be seen that Bi4NbO8Cl is composed of a large number of nanoplates (Figure 2a,b); After Zinc doping, the morphology of the sample has changed. Bi4Nb0.8Zn0.2O8Cl has a lamellar stacking structure with the thickness of about 100 nm (Figure 2a,b). The morphology of Bi4Nb0.9Zn0.1O8Cl and Bi4Nb0.7Zn0.3O8Cl are presented in Figure S1. It can be seen that the morphologies of the catalysts change little with the different amount of zinc doping.
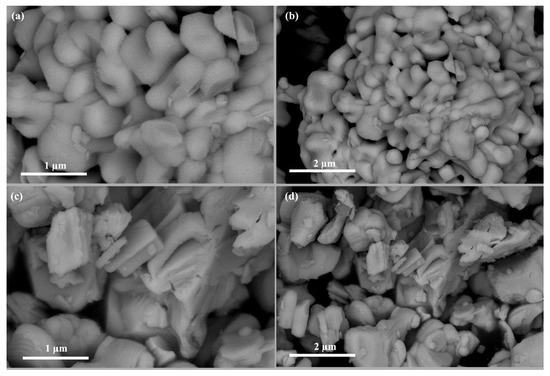
Figure 2.
The scanning electron microscope (SEM) images of Bi4NbO8Cl (a,b) andBi4Nb0.8Zn0.2O8Cl (c,d).
3.3. X-Ray Photoelectron Spectroscopy
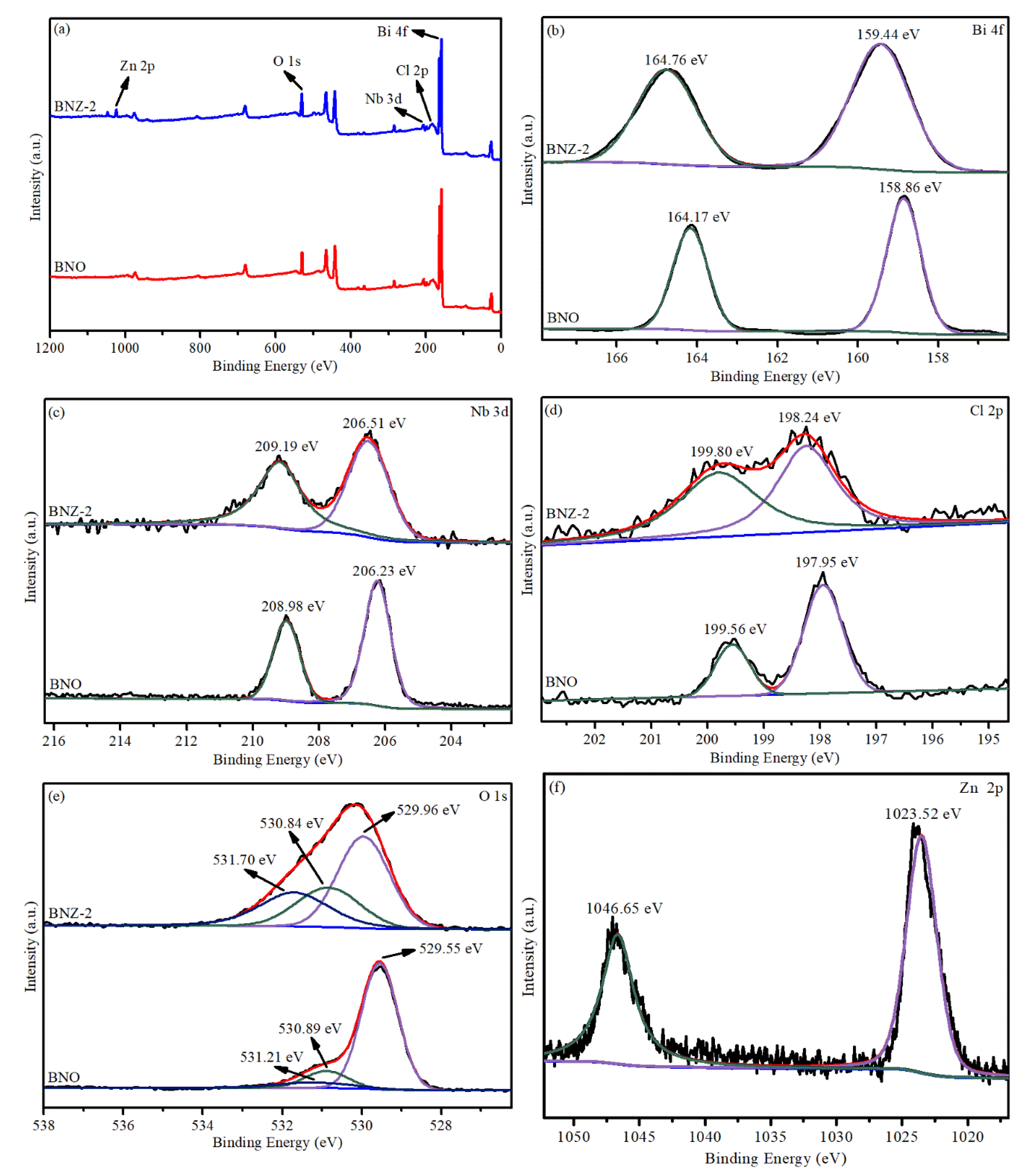
The surface chemical element states of Bi4NbO8Cl and Bi4Nb0.8Zn0.2O8Cl were investigated by X-ray photoelectron spectroscopy. Figure 3a shows the existence of C, N, Bi, Nb and Cl element in Bi4NbO8Cl. Compared with Bi4NbO8Cl, the characteristic peak of Zn is added in the Bi4Nb0.8Zn0.2O8Cl spectrum, indicating that the Zn element has been successfully doped in Bi4NbO8Cl. As shown in Figure 3b,d, the Bi 4f spectrum of Bi4NbO8Cl with the peaks at 164.17 eV and 158.86 eV are indexed to Bi 4f7/2 and Bi 4f5/2, respectively. This indicates that the valence state of Bi is trivalent [24,25]. The peaks of Nb 3d5/2 and Nb 3d3/2 appear at 206.23 eV and 208.98 eV for Bi4NbO8Cl, indicating the existence of Nb5+ state (Figure 3b). However, the two peaks for Bi4NbO8Cl located at 198.3 eV and 199.9 eV, belonging to Cl 2p3/2 and Cl 2p1/2, respectively (Figure 3c). Notably, the Bi 4f, Nb 3d and Cl 2p peaks for Bi4Nb0.8Zn0.2O8Cl all display a slight migration towards lower binding energy compared to Bi4NbO8Cl, suggesting that the chemical circumstances of Bi, Nb and Cl elements have changed since the zinc is doped [16,26]. The O 1s peaks for Bi4NbO8Cl (Figure 2d) can be divided into three peaks at 529.55 eV, 530.89eV, and 531.32 eV, which separately belong to the crystal lattice oxygen, Bi–O bonds and Nb–O bonds. However, when the zinc is doped, the characteristic O 1s peaks change. This may be attributed to the formation of Nb–O–Zn–O bond [27]. Zinc signals are detected after Zn2+ doping, as shown in Figure 3b, the peaks located at 1023.52 eV and 1046.65 eV are contributed to Zn 2p3/2 and Zn 2p1/2, respectively [28].
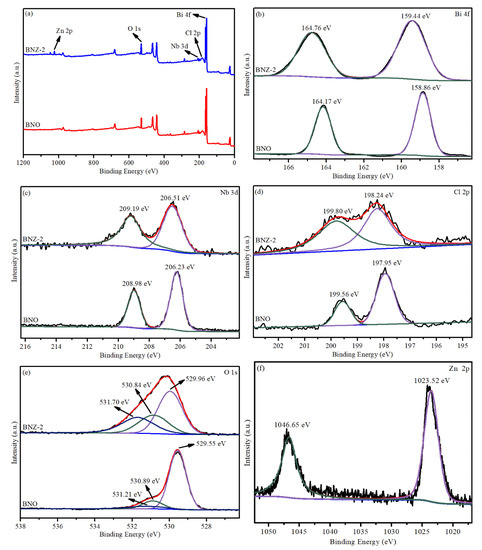
Figure 3.
X-ray photoelectron spectrometry (XPS) spectra of Bi4NbO8Cl and Bi4Nb0.8Zn0.2O8Cl: (a) Survey, (b) Bi 4f, (c) Nb 3d, (d) Cl 2p (e) O 1s and (f) Zn 2p.
3.4. Ultraviolet–Visible (UV–Vis) Diffuse Reflectance Spectrum
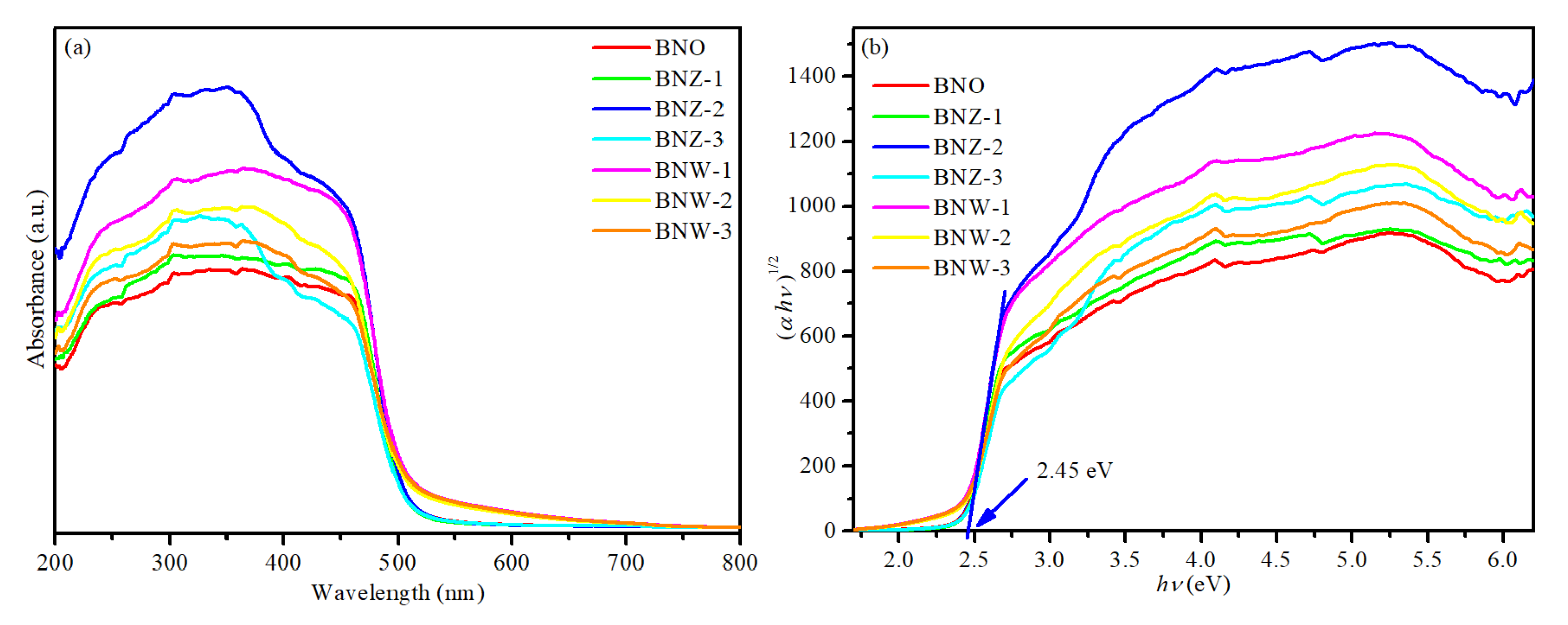
Figure 4a shows the ultraviolet–visible (UV–vis) diffuse reflectance spectrum of Bi4NbO8Cl, Bi4Nb1−xWxO8Cl and Bi4Nb1−xZnxO8Cl series. It can be seen that all the photocatalysts have strong absorption in the visible light region and exhibit almost the same absorption edges with either W or Zn doping. The band gap energy (Eg) is determined by the following equation using the data of optical absorption vs. wavelength near the band edge [29,30]:
where A indicates a constant, α, hν, and Eg are respective to absorption coefficient, photon energy and band gap energy, respectively. In this work, n is 1 due to the BiNbO8Cl being a kind of indirect gap semiconductor [31]. The band gap energy (Eg) value can be obtained by extrapolating the linear portion of the hν − (αhν)1/2 curve. As shown in Figure 4b, the band gap of BNO is about 2.45 eV and there are no significant changes after either W or Zn doping, indicating that the absorption of light is caused by the band gap transition rather than the transition of the impurity energy level. The results show that a new photocatalyst with visible light response has been successfully prepared, which may provide the excellent photocatalytic activity.
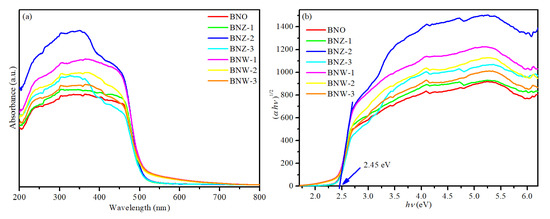
Figure 4.
(a) Ultraviolet–visible (UV–vis) absorption spectra (b) plots of (αhv)1/2 vs. hv of Bi4NbO8Cl, Bi4Nb1−xWxO8Cl and Bi4Nb1−xZnxO8Cl series.
3.5. Photocatalytic Activity
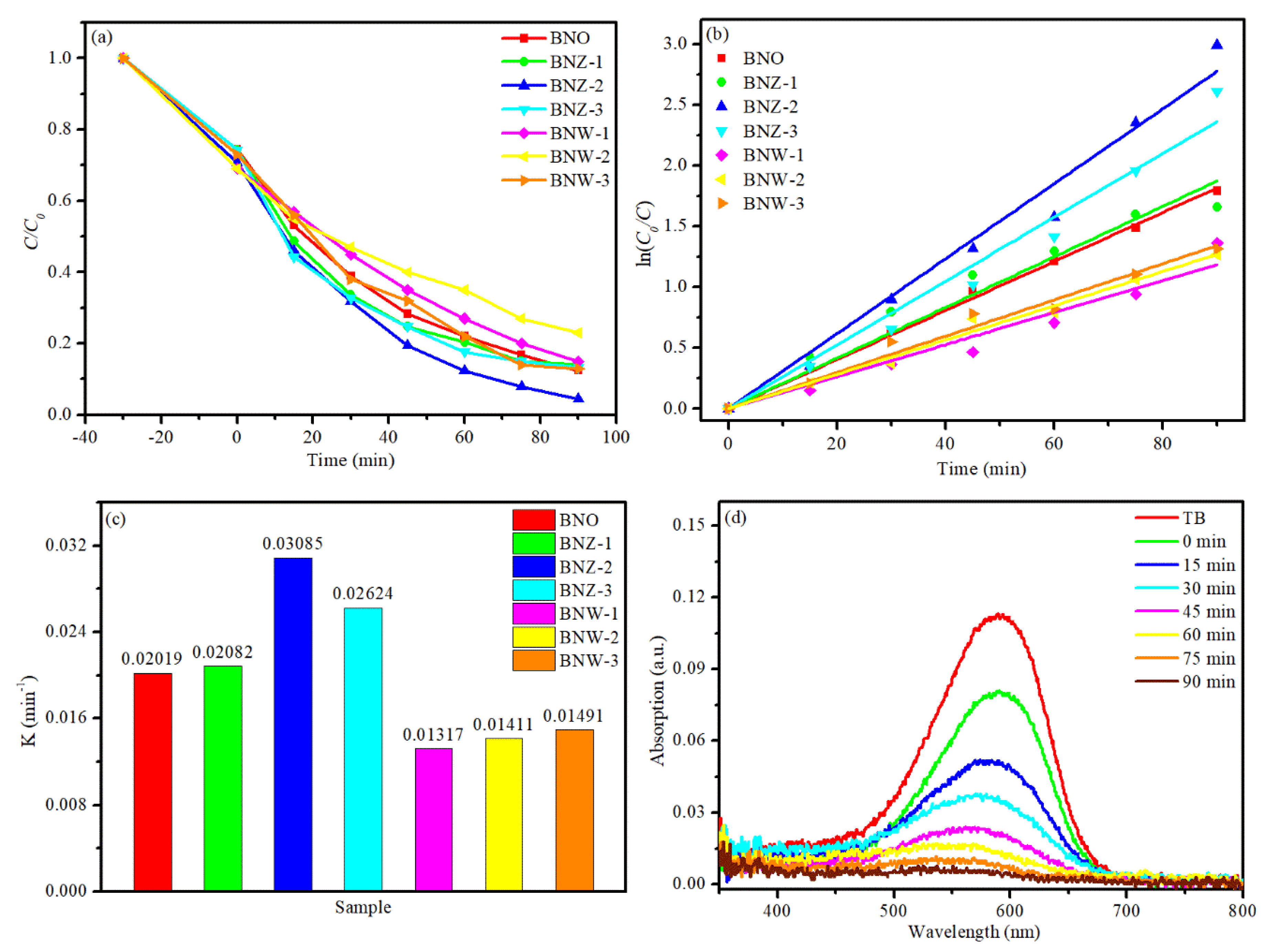
In the dye degradation experiment, TB was chosen as the dye model under visible irradiation at pH ≈ 7. Figure 5a shows the degradation profiles under visible irradiation. It can be seen that the degradation ratio of BNO is about 83.5% within 90 min. However, the photocatalytic degradation efficiency of BNW-1, BNW-2 and BNW-2 are lower than that of BNO indicating that W doping could not effectively improve the degradation efficiency of TB. In contrast, it can be found that all of the Zn-doped samples show higher photocatalytic degradation abilities than the pure Bi4NbO8Cl photocatalyst. The BNZ-2 photocatalyst displays the highest photocatalytic activity and the removal rate can reach 96% in 90 min. The results confirm that the introduction of Zinc in Bi4NbO8Cl could effectively enhance photocatalytic activity.
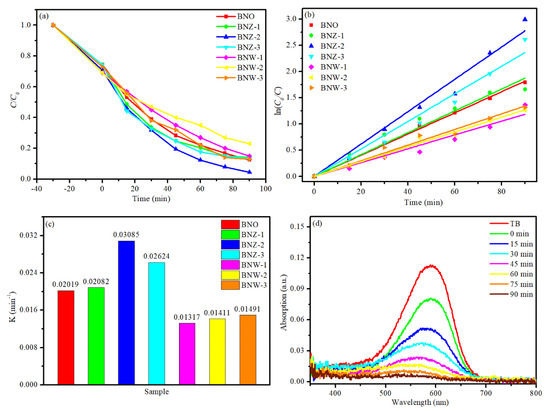
Figure 5.
(a) Photocatalytic activity, (b) kinetic curves, (c) apparent rate constant (K), (d) UV–vis absorption spectra changes with Bi4Nb0.8Zn0.2O8Cl.
In order to quantitatively study the reaction kinetics of the degradation process, a first-order model is used to fit the reaction data. The formula is as follows [32,33]: ln (C0/C) = kt + b, where k is the apparent first-order rate constant, and ln (C0/C) has a linear relationship with catalytic reaction time. As can be seen in Figure 5c, the pseudo first-order rate constants (k) for BNZ-2 reaches 0.03085 min−1, which is about 1.5 times as high as that of BNO (k = 0.02019) and about 3 times that of BNW-2, respectively. Figure 5d shows the temporal evolution of the UV–visible absorption spectral changes during TB degradation process by BNZ-2 photocatalyst. As time goes on, the characteristic absorption peaks between 500 and 800 nm region have reduced gradually, which indicates that the molecular structure of organic pollutants is destroyed.
3.6. Computational Details
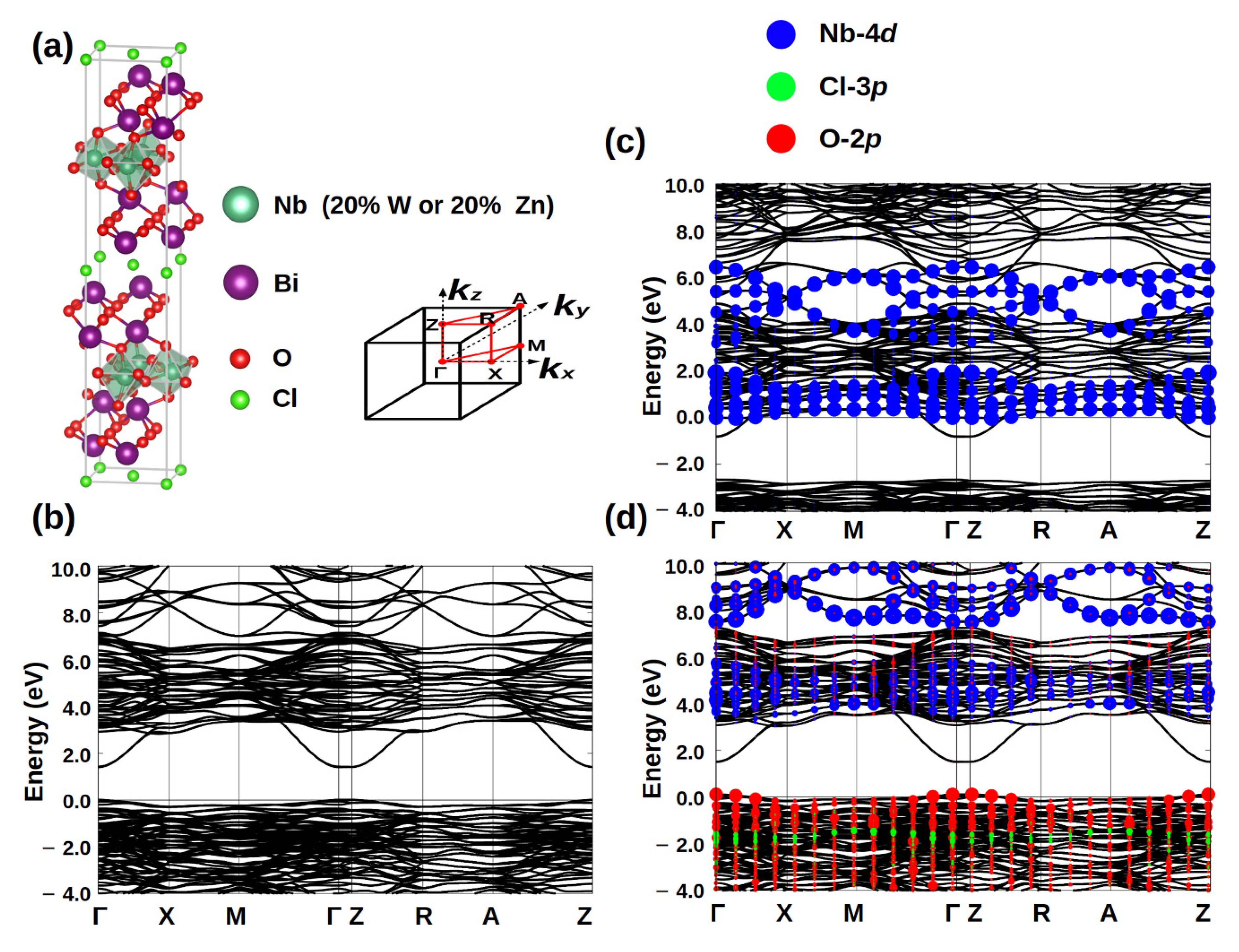
Using the first-principles methods, we calculated the band structures of Bi4NbO8Cl and doped systems. As the chemical valence is two for zinc and six for tungsten, this means hole doping for Bi4Nb0.8Zn0.2O8Cl and electron doping for Bi4Nb0.8W0.2O8Cl system. From the band structures in Figure 6, we can clearly see the tungsten doping will introduce the additional electron carriers, while the hole carriers will increase by zinc doping. Therefore, it is not too difficult to understand the reason why enhancement of photodegradation properties of Bi4Nb0.8Zn0.2O8Cl, in which the hole carriers play a role for the photocatalytic mechanism.
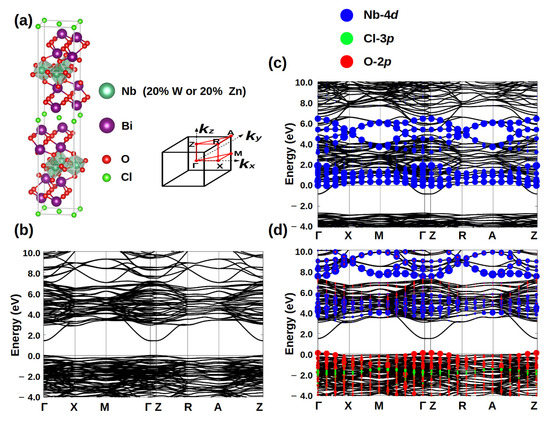
Figure 6.
(a) Crystal structure of Bi4NbO8Cl, and density functional theory (DFT) band structure of undoped (b) Bi4NbO8Cl, 20% W doping on Nb (c), and (d) 20% Zn doping on Nb. The adopted Brillouin zone and band plotting k-path are also shown in (a). In (c,d), the orbital characters of Nb-4d, O-2p and Cl-3p are labeled by blue, red and green dots, respectively. The Fermi energy is set as 0 eV.
3.7. Possible Photocatalysis Mechanism
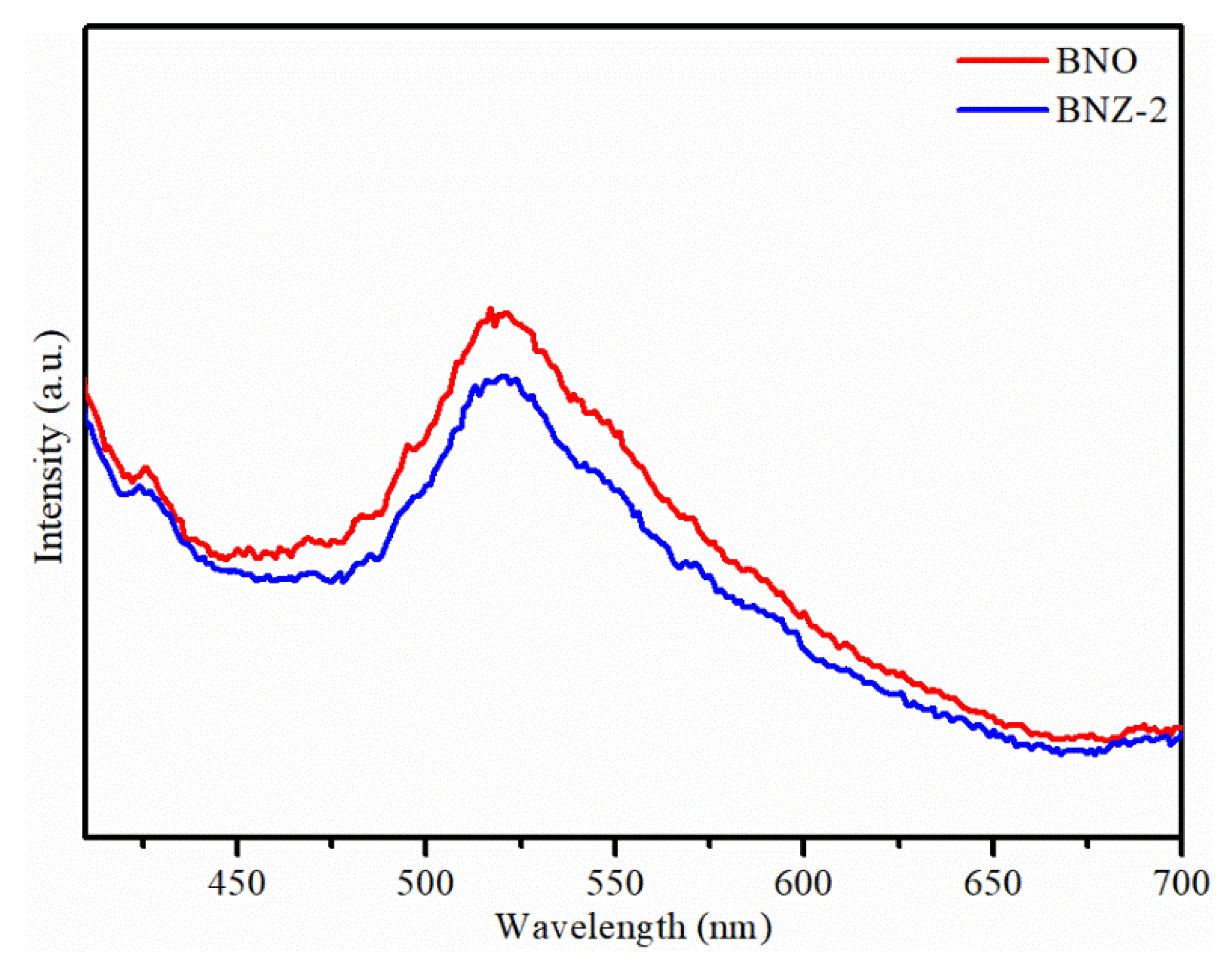
It is noteworthy that all of the W-doped Bi4NbO8Cl photocatalysts have lower photocatalytic activity than that of pure Bi4NbO8Cl. In contrast, the Zn-doped Bi4NbO8Cl photocatalysts have higher photocatalytic activities than that of pure Bi4NbO8Cl. This phenomenon can be attributed to the Zn2+ ions being incorporated into the Bi4NbO8Cl via replacing the Nb5+ lattice sites, and the positive charge of Zn2+ ion being less than that of Nb5+, thus forming a negative charge center at the position of Zn2+. In order to maintain the charge balance, Zn2+ ions will fetter the hole (h+) and make it difficult to move freely [34]. Therefore, the increase of the concentration of holes (h+) around zinc ion leads to the formation of hole doping in Bi4NbO8Cl, which has strong oxidation performance and is beneficial to photocatalytic degradation. In addition, hole fettering limit the effective recombination of photogenerated electrons and holes. The photoluminescence technique is used to analysis the separation efficiency of photoelectron-hole pairs. As shown in Figure 7, BNO exhibits higher emission peak than BNZ-2, indicating that the recombination of photoelectron-hole pairs is faster [35,36]. Therefore, the doping of zinc can promote the separation of photogenerated electrons and holes, improving the photocatalytic efficiency.
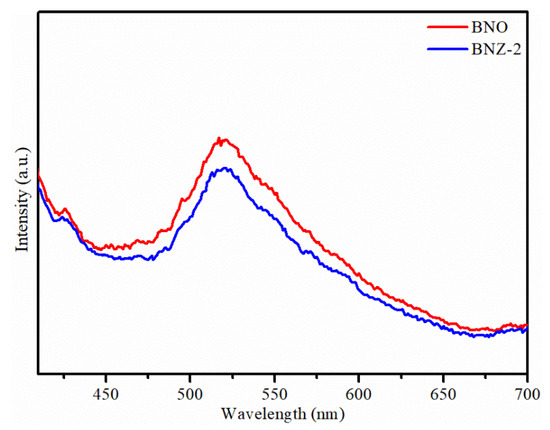
Figure 7.
Photoluminescence spectra of Bi4NbO8Cl and Bi4Nb0.8Zn0.2O8Cl photocatalysts.
By contrast, the positive charge of the W6+ ion is greater than that of the Nb5+, the W6+ will capture the electrons to maintain the charge balance, and forming the electrons (e−) doping in Bi4NbO8Cl. However, the W6+ can capture the conduction band electrons (e−) and hinder the formation of super oxide radicals (•O2−), which leads to the decrease of photocatalytic degradation efficiency [37]. Therefore, the doping of W in Bi4NbO8Cl leads to the decrease of photocatalytic activity. The above mechanism can be also used to understand the photocatalytic activity in Bi4Ti0.5W0.5O8Cl system, which is almost the same with Bi4NbO8Cl [38].
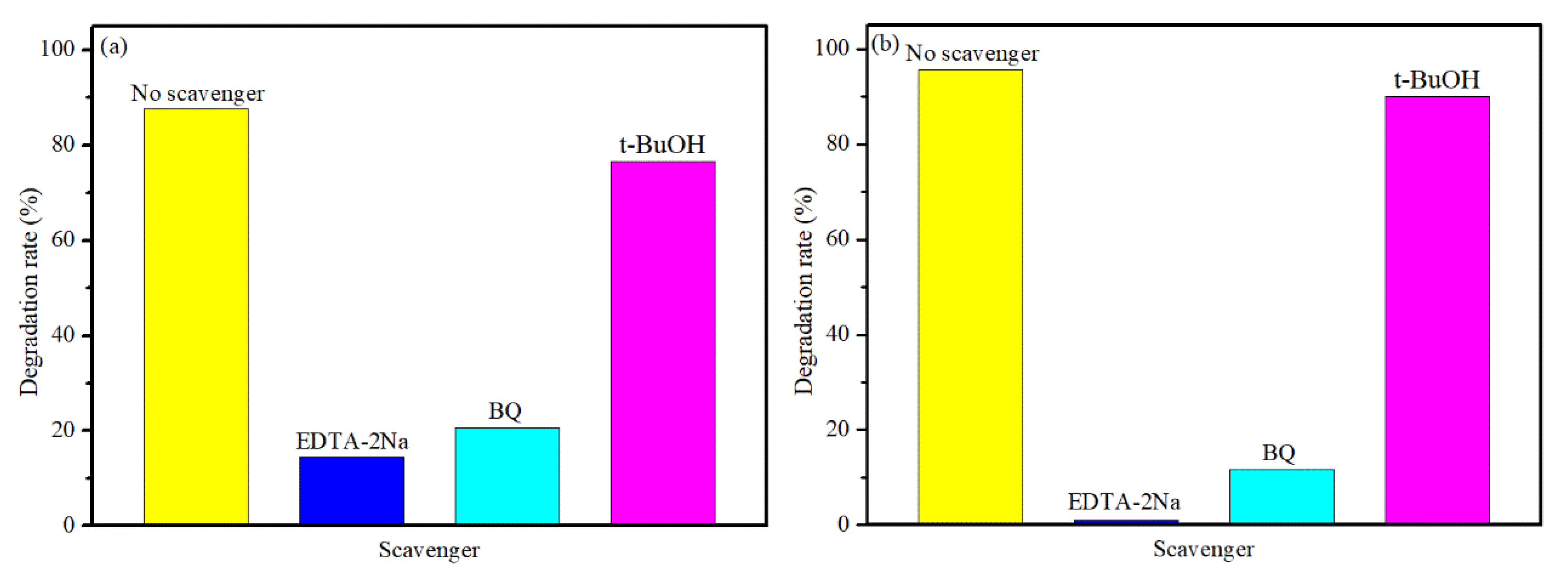
The trapping experiments were used to detect the main active species in the process of photocatalytic degradation. The Tert-butanol (t-BuOH, an •OH scavenger), p-benzoquinone (BQ; an •O2− scavenger), and disodium ethylenediamine tetraacetate (EDTA-2Na, h+ scavenger) were used as quenchers [39]. As shown in Figure 8a,b, after adding the t-BuOH, the photocatalytic degradation of TB is slightly decreased for both BNO and BNZ-2, indicating hydroxyl radicals (•OH) have little impact on the photocatalytic degradation process. When the BQ and EDTA-2Na are added, the photocatalytic degradation rate of TB decreased sharply, suggesting that the hole (h+) and super oxide radicals (•O2−) are the main active species in the TB degradation process. In contrast, it suggests that the effect of hole (h+) on BT degradation is more critical than that of super oxide radicals (•O2−) over BNZ-2 (Figure 8b).
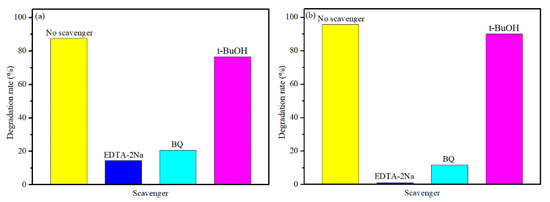
Figure 8.
Photodegradation of trypan blue (TB) by different quenchers over Bi4NbO8Cl (a) and Bi4Nb0.8Zn0.2O8Cl (b).
Based on the above experiments and analysis, a mechanism of TB degradation by Zn-doped Bi4NbO8Cl photocatalysts under visible light is proposed. As mentioned, the Zn-doped Bi4NbO8Cl photocatalysts can be excited and generate the photogenerated electrons (e−) and holes (h+) pairs under visible light. The electrons(e−) react with adsorbed O2 to generate superoxide radical (•O2−), which is a strong oxidant and can degrade TB. Because of the hole (h+) doping in Bi4NbO8Cl, the hole is far more oxidative than •OH radical. Therefore, holes (h+) play a main role in the photocatalytic degradation of TB. The photogenerated holes (h+) of Zn-doped Bi4NbO8Cl can directly transform TB molecules into H2O, CO2 and mineral [40]. Finally, the organic pollutants are photocatalytically degraded by this novel hole-doped photocatalyst. The possible charge carrier transfer path is as follows:
4. Conclusions
A novel layered photocatalyst of Bi4NbO8Cl doped with zinc was successfully synthesized by a solid-state reaction method. It was found that the introduction of zinc ions into Bi4NbO8Cl can construct hole (h+) doping in Bi4NbO8Cl and promote the separation of photogenerated electrons. Compared to Bi4NbO8Cl, Bi4Nb1−xZnxO8Cl (x = 0.1, 0.2, 0.3) series photocatalysts show significantly higher photocatalytic activities for trypan blue degradation. In particular, when the atomic ratio of Zn to Nb is 2:8, the photocatalyst showed the highest photocatalytic activity and the removal ratio of trypan blue could reach 96% in 90 min. Furthermore, hole (h+) was considered as the effective species in the photocatalytic degradation process. On the basis of the experimental results and density functional theory calculations, the hole carriers play a role in the photocatalytic mechanism. This material may have potential application prospects in the degradation of organic pollutants from wastewater.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4344/10/12/1425/s1, Figure S1. SEM images of Bi4 Nb0.9Zn0.1O8Cl (a) (b); Bi4Nb0.7Zn0.3O8Cl (c) (d).
Author Contributions
Conceptualization, X.L. and Q.Z.; Methodology, L.S. and Q.Z.; Validation, J.S., N.H. and Y.G.; Formal Analysis, N.H.; Investigation, Q.Z.; Resources, X.L. and L.S.; Data Curation, J.S. and N.H.; Writing—Original Draft Preparation, X.L. and Q.Z.; Writing—Review and Editing, L.S. and Q.Z.; Visualization, J.S., Q.Z. and N.H.; Supervision, Q.Z.; Project Administration, Q.Z.; Funding Acquisition, X.L. and Q.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Natural Science Foundation of Jiangsu Province (20KJA430004) and the Postgraduate Research and Practice Innovation Program of Jiangsu Province (SJCX20_1239).
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Zangeneh, H.; Zinatizadeh, A.A.; Zinadini, S.; Feyzi, M.; Rafiee, E.; Bahnemann, D.W. A novel L-Histidine (C, N) codoped-TiO2-CdS nanocomposite for efficient visible photo-degradation of recalcitrant compounds from wastewater. J. Hazard. Mater. 2019, 369, 384–397. [Google Scholar] [CrossRef] [PubMed]
- Li, T.T.; Zhao, L.H.; He, Y.M.; Cai, J.; Luo, M.F.; Lin, J.J. Synthesis of g-C3N4/SmVO4 composite photocatalyst with improved visible light photocatalytic activities in RhB degradation. Appl. Catal. B 2013, 129, 255–263. [Google Scholar] [CrossRef]
- Padervand, M.; Jalilian, E.; Majdani, R.; Goshadezehn, M. BiOCl/AgCl-BiOI/AgI quaternary nanocomposite for the efficient photodegradation of organic wastewaters and pathogenic bacteria under visible light. J. Water Process. Eng. 2019, 29, 100789–100796. [Google Scholar] [CrossRef]
- Pirhashemi, M.; Habibi-Yangjeh, A.; Pouran, S.R. Review on the criteria anticipated for the fabrication of highly efficient ZnO-based visible-light-driven photocatalysts. J. Ind. Eng. Chem. 2018, 62, 1–25. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, Y. A review of controllable synthesis and enhancement of performances of bismuth tungstate visible-light-driven photocatalysts. Catal. Sci. Technol. 2012, 2, 694–706. [Google Scholar] [CrossRef]
- Fujito, H.; Kunioku, H.; Kato, D.; Suzuki, H.; Higashi, M.; Kageyama, H.; Abe, R. Layered Perovskite Oxychloride Bi4NbO8Cl: A Stable Visible Light Responsive Photocatalyst for Water Splitting. J. Am. Chem. Soc. 2016, 138, 2082–2085. [Google Scholar] [CrossRef]
- You, Y.; Wang, S.; Xiao, K.; Ma, T.; Zhang, Y.; Huang, H. Z-Scheme g-C3N4/Bi4NbO8Cl Heterojunction for Enhanced Photocatalytic Hydrogen Production. ACS Sustain. Chem. Eng. 2018, 6, 16219–16227. [Google Scholar] [CrossRef]
- Ogawa, K.; Nakada, A.; Suzuki, H.; Tomita, O.; Higashi, M.; Saeki, A.; Kageyama, H.; Abe, R. Flux Synthesis of Layered Oxyhalide Bi4NbO8Cl Photocatalyst for Efficient Z-Scheme Water Splitting under Visible Light. ACS Appl. Mater. Interfaces 2019, 11, 5642–5650. [Google Scholar] [CrossRef]
- Lin, X.; Huang, T.; Huang, F.; Wang, W.; Shi, J. Photocatalytic activity of a Bi-based oxychloride Bi4NbO8Cl. J. Mater. Chem. 2007, 17, 2145–2150. [Google Scholar] [CrossRef]
- Bhat, S.S.M.; Sundaram, N.G. Photocatalysis of Bi4NbO8Cl hierarchical nanostructure for degradation of dye under Solar/UV irradiation. New J. Chem. 2015, 39, 3956–3966. [Google Scholar] [CrossRef]
- Xu, Y.; You, Y.; Huang, H.; Guo, Y.; Zhang, Y. Bi4NbO8Cl {001} nanosheets coupled with g-C3N4 as 2D/2D heterojunction for photocatalytic degradation and CO2 reduction. J. Hazard. Mater. 2019, 381, 121159. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Gao, Z.; Zhao, X.; Shi, L.; Du, F.; Song, H. Construction of p-n type Bi2O3/Bi4NbO8Cl 0D/2D heterojunction with enhanced photodegradation performance for organic pollutants. Appl. Surf. Sci. 2020, 529, 147248. [Google Scholar] [CrossRef]
- Qu, X.; Liu, M.; Zhai, H.; Zhao, X.; Shi, L.; Du, F. Plasmonic Ag-promoted layered perovskite oxyhalide Bi4NbO8Cl for enhanced photocatalytic performance towards water decontamination. J. Alloy. Compd. 2019, 810, 151919. [Google Scholar] [CrossRef]
- Ruan, Y.; Zhang, N.; Zhu, Y.; Zhao, W.; Xu, J.; Chen, H. Photoelectrochemical Bioanalysis Platform of Gold Nanoparticles Equipped Perovskite Bi4NbO8Cl. Anal. Chem. 2017, 89, 7869–7875. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhang, Y.; Wang, K.; Zhang, S.; Qu, X.; Shi, L.; Du, F. In-situ construction of Bi/defective Bi4NbO8Cl for non-noble metal based Mott-Schottky photocatalysts towards organic pollutants removal. J. Hazard. Mater. 2020, 393, 122408. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Liu, J.; Fang, W.; Qin, Z.; Jiang, Z.; Shangguan, W. Enhanced photocatalytic hydrogen evolution using a novel in situ heterojunction yttrium-doped Bi4NbO8Cl@Nb2O5. Int. J. Hydrog. Energy 2018, 43, 14281–14292. [Google Scholar] [CrossRef]
- Medhi, R.; Marquez, M.D.; Lee, T.R. Visible-Light-Active Doped Metal Oxide Nanoparticles: Review of their Synthesis, Properties, and Applications. ACS Appl. Nano Mater. 2020, 3, 6156–6185. [Google Scholar] [CrossRef]
- Guo, G.; Yan, H. Zn-doped Bi2O2CO3: Synthesis, characterization and photocatalytic properties. Chem. Phys. 2020, 538, 110920. [Google Scholar] [CrossRef]
- Xu, K.; Shen, J.; Xu, D.; Li, Z.; Zhang, S.; Wu, Z.; Feng, W.; Xiao, X.; Zhang, S.; Liu, J. Molten-salt-mediated synthesis of bulk W doped BiOCl with highly enhanced visible-light photocatalytic performances. Appl. Surf. Sci. 2019, 459, 143595. [Google Scholar] [CrossRef]
- Blaha, P.; Schwarz, K.; Madsen, G.; Kvasnicka, D.; Luitz, J. WIEN2k, An Augmented Plane Wave Plus Local Orbitals Program forCalculating Crystal Properties; Vienna University of Technology: Vienna, Austria, 2001. [Google Scholar]
- Perdew, J.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef]
- Guo, J.; Liao, X.; Lee, M.; Hyett, G.; Huang, C.; Hewak, D.W.; Mailis, S.; Zhou, W.; Jiang, Z. Experimental and DFT insights of the Zn-doping effects on the visible-light photocatalytic water splitting and dye decomposition over Zn-doped BiOBr photocatalysts. Appl. Catal. B 2019, 243, 502–512. [Google Scholar] [CrossRef]
- Truc, N.T.T.; Bach, L.G.; Hanh, N.T.; Pham, T.D.; Chi, N.T.P.L.; Tran, D.T.; Nguyen, M.V.; Nguyen, V.N. The superior photocatalytic activity of Nb doped TiO2/g-C3N4 direct Z-scheme system for efficient conversion of CO2 into valuable fuels. J. Colloid Interface Sci. 2019, 540, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Zhang, K.; Wu, D.; Ye, X.; Huang, W.; Zhou, B. Enhanced photocatalytic degradation of levofloxacin by Fe-doped BiOCl nanosheets under LED light irradiation. Chem. Eng. J. 2020, 383, 123148. [Google Scholar] [CrossRef]
- Zhong, X.; Cai, Y.; Bai, H.; Huang, W.; Zhou, B. Visible Light Driven Spherical CuBi2O4 with Surface Oxygen Vacancy Enhanced Photocatalytic Activity: Catalyst Fabrication, Performance, and Reaction Mechanism. Catalysts 2020, 10, 945. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Y.; Wang, W.; Pei, D.; Huang, G.; Chen, J.; Zhang, X.; Yu, H. Enhanced photocatalytic degradation of bisphenol A by Co-doped BiOCl nanosheets under visible light irradiation. Appl. Catal. B 2018, 121, 320–328. [Google Scholar] [CrossRef]
- Qu, X.; Liu, M.; Gao, Z.; Zhai, H.; Ren, W.; Shi, L.; Du, F. A novel ternary Bi4NbO8Cl/BiOCl/Nb2O5 architecture via in-situ solvothermal-induced electron-trap with enhanced photocatalytic activities. Appl. Surf. Sci. 2020, 506, 1446788. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, J.; Zhou, H.; Zhang, X. Facile synthesis of Zn(II)-doped g-C3N4 and their enhanced photocatalytic activity under visible light irradiation. Rare Met. 2019, 38, 459–467. [Google Scholar] [CrossRef]
- Lu, X.; Li, X.; Chen, F.; Chen, Z.; Qian, J.; Zhang, Q. Biotemplating synthesis of N-doped two-dimensional CeO2-TiO2 nanosheets with enhanced visible light photocatalytic desulfurization performance. J. Alloy. Compd. 2020, 815, 152326. [Google Scholar] [CrossRef]
- Lu, X.; Li, X.; Qian, J.; Miao, N.; Yao, C.; Chen, Z. Synthesis and characterization of CeO2/TiO2 nanotube arrays and enhanced photocatalytic oxidative desulfurization performance. J. Alloy. Compd. 2016, 661, 363–371. [Google Scholar] [CrossRef]
- Gao, Z.; Qu, X. Construction of ZnTiO3/Bi4NbO8Cl heterojunction with enhanced photocatalytic performance. Nanoscale Res. Lett. 2020, 15, 64. [Google Scholar] [CrossRef]
- Li, X.; Zhu, W.; Lu, X.; Zuo, S.; Yao, C.; Ni, C. Integrated nanostructures of CeO2/attapulgite/g-C3N4 as efficient catalyst for photocatalytic desulfurization: Mechanism, kinetics and influencing factors. Chem. Eng. J. 2017, 326, 87–98. [Google Scholar] [CrossRef]
- Wang, Y.; Jung, D.W. Synthesis of novel BiOCl/LiBiO3 p-n heterojunction photocatalysts and their enhanced photocatalytic performance. Solid State Sci. 2019, 91, 42–48. [Google Scholar] [CrossRef]
- Li, W.; Huang, W.; Zhou, H.; Yin, H.; Zheng, Y.; Song, X. Synthesis of Zn2+ doped BiOCl hierarchical nanostructures and their exceptional visible light photocatalytic properties. J. Alloy. Compd. 2015, 638, 148–154. [Google Scholar] [CrossRef]
- Jiang, D.; Wang, T.; Xu, Q.; Li, D.; Meng, S.; Chen, M. Perovskite oxide ultrathin nanosheets/g-C3N4 2D-2D heterojunction photocatalysts with significantly enhanced photocatalytic activity towards the photodegradation of tetracycline. Appl. Catal. B 2017, 201, 617–628. [Google Scholar] [CrossRef]
- Gao, C.; Xue, J.; Zhang, L.; Cui, K.; Li, H.; Yu, J. Paper-Based Origami Photoelectrochemical Sensing Platform with TiO2/Bi4NbO8Cl/Co-Pi Cascade Structure Enabling of Bidirectional Modulation of Charge Carrier Separation. Anal. Chem. 2018, 90, 14116–14120. [Google Scholar] [CrossRef]
- Zou, Y.; Gong, Y.; Lin, B.; Mellott, N.P. Photodegradation of methylene blue in the visible spectrum: An efficient W6+ ion doped anatase titania photocatalyst via a solvothermal method. Vacuum 2016, 126, 63–69. [Google Scholar] [CrossRef]
- Zhen, S.; Zhu, L.; Dong, Z.; Fan, L.; Wang, B.; Zhang, Q. A New Bi-Based Oxychloride Bi4Ti0.5W0.5O8Cl as a Photocatalyst. Catal. Lett. 2018, 148, 2480–2486. [Google Scholar] [CrossRef]
- Han, N.; Xu, Q.; Beyenea, G.; Zhang, Q. Enhanced photocatalytic activity over g-C3N4/(BiO)2(OH)xCl2−x Z-scheme heterojunction. Appl. Surf. Sci. 2020, 521, 146464–146475. [Google Scholar] [CrossRef]
- Majumdar, A.; Ghosh, U.; Pal, A. Novel 2D/2D g-C3N4/Bi4NbO8Cl nano-composite for enhanced photocatalytic degradation of oxytetracycline under visible LED light irradiation. J. Colloid Interface Sci. 2021, 584, 320–331. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).