Enhanced Visible-Light Driven Photocatalytic Activity of Ag@TiO2 Photocatalyst Prepared in Chitosan Matrix
Abstract
:1. Introduction
2. Results and Discussion
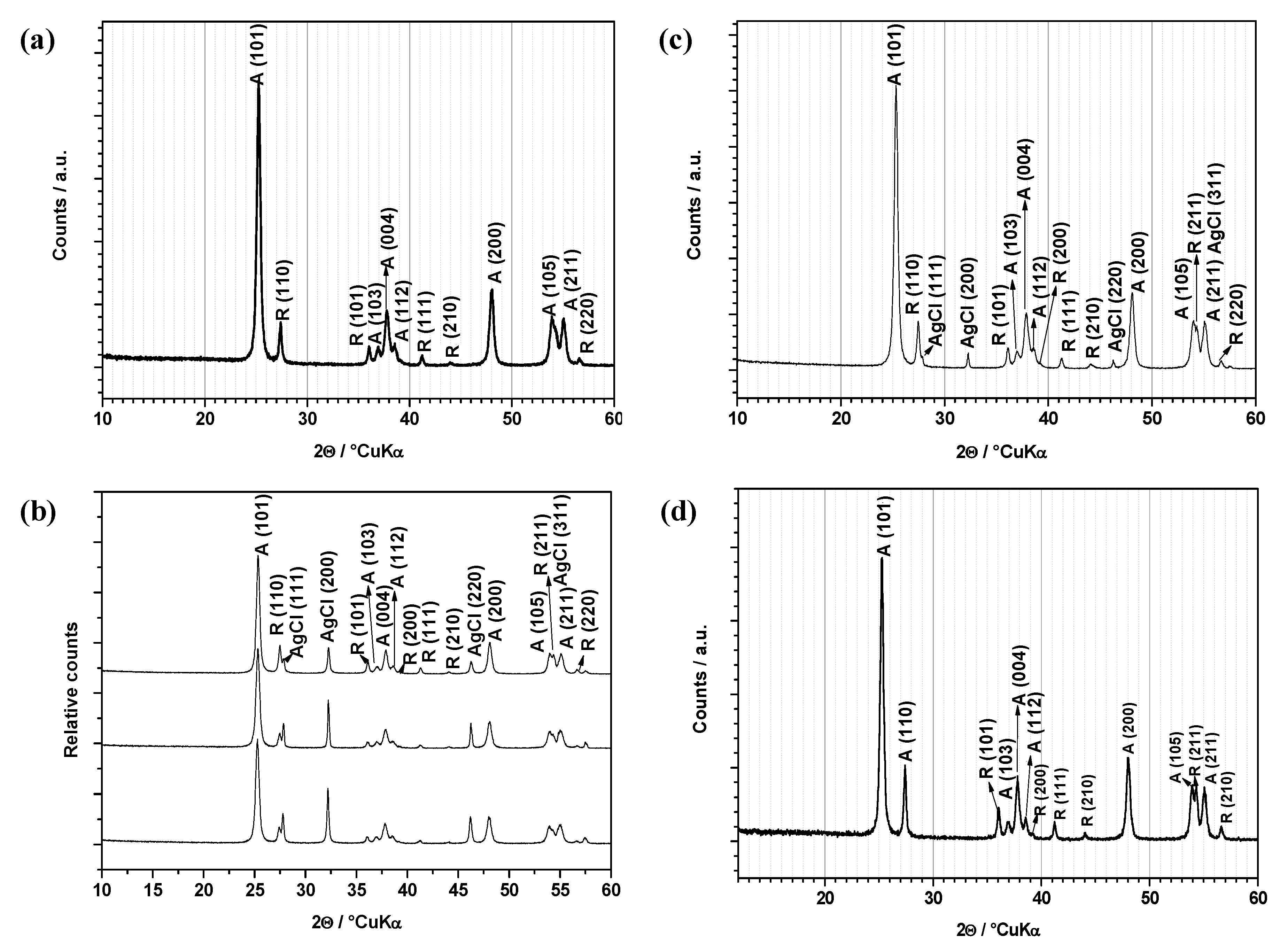
2.1. X-ray Diffraction (XRD) Analysis
2.2. Raman Spectroscopy Measurements
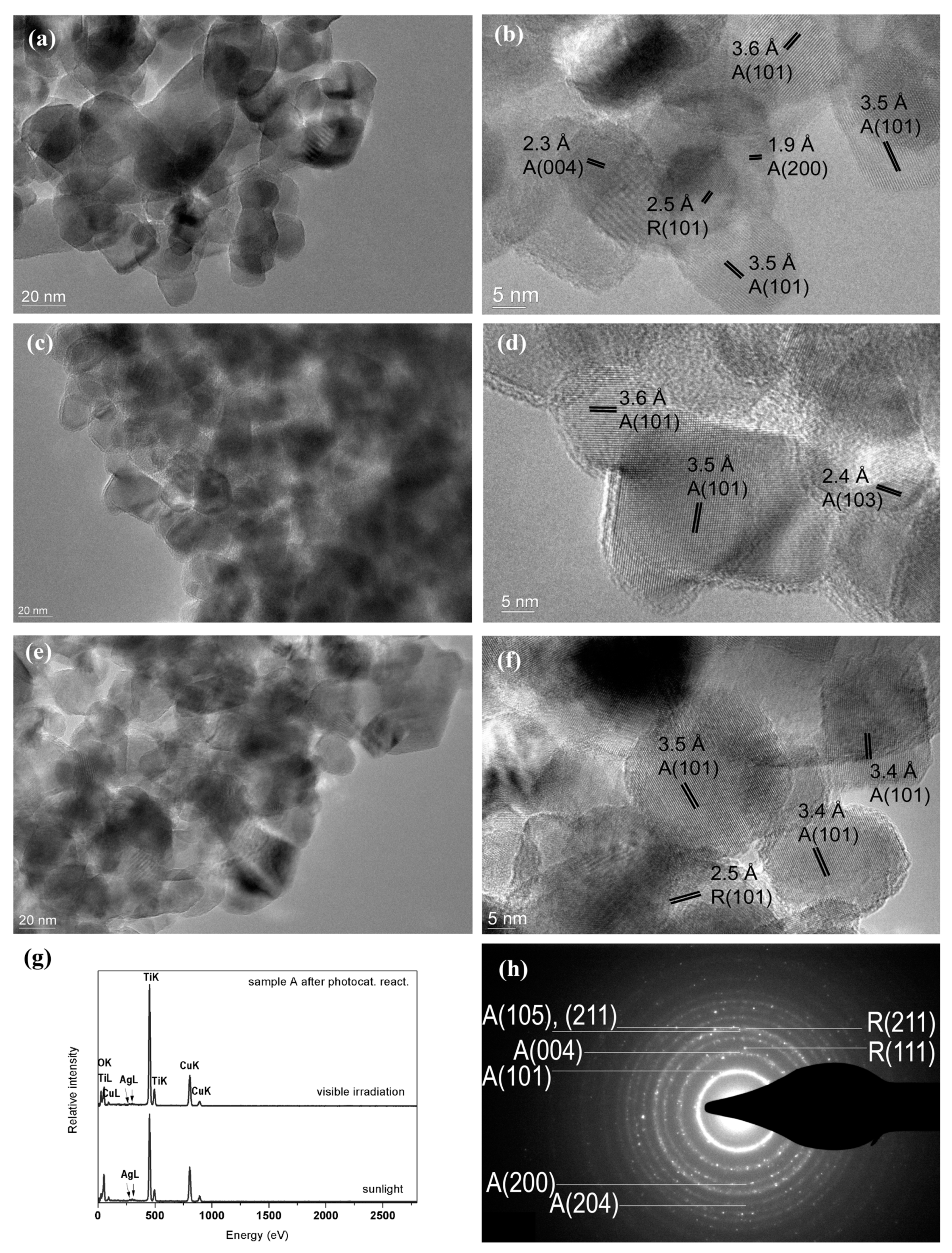
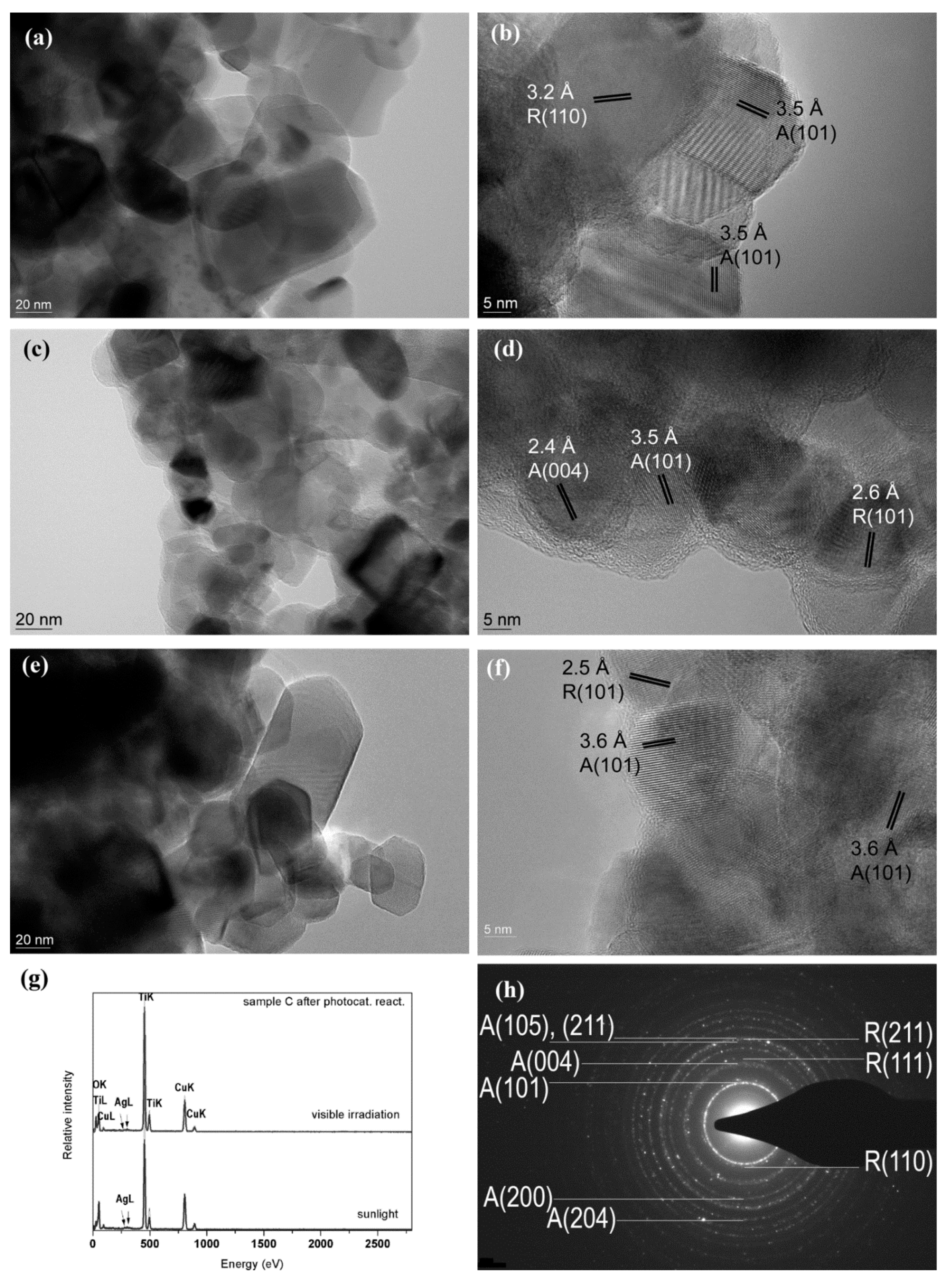
2.3. Study by TEM Techniques
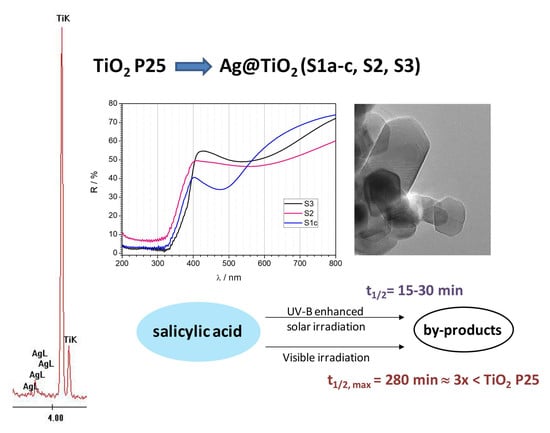
2.4. UV-Vis Diffuse-Reflectance Spectral Analysis
2.5. Detection of Ag Nanoparticles
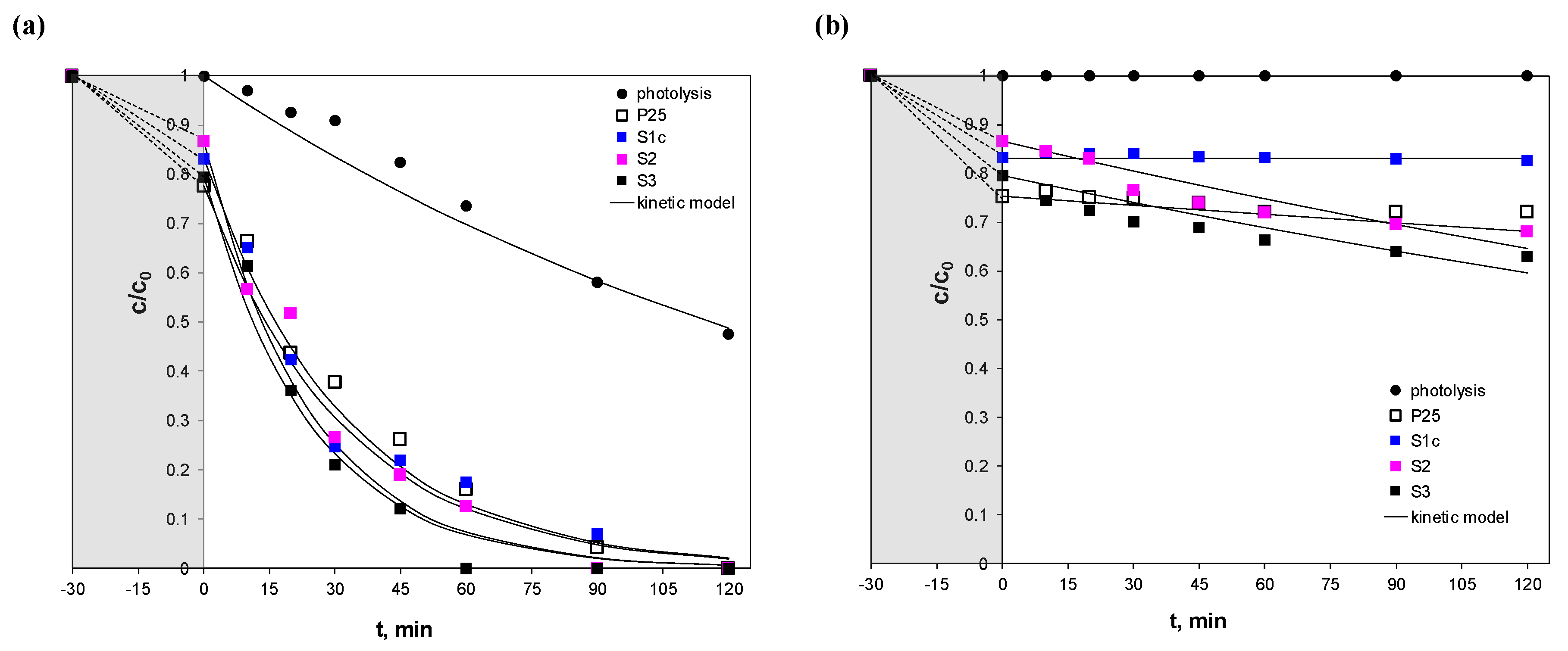
2.6. Photocatalytic Activity
3. Materials and Methods
3.1. Preparation of Ag/TiO2 Photocatalysts
3.1.1. Preparation of S1a
3.1.2. Preparation of S1b
3.1.3. Preparation of S1c
3.1.4. Preparation of S2
3.1.5. Preparation of S3
3.2. Catalysts Characterization
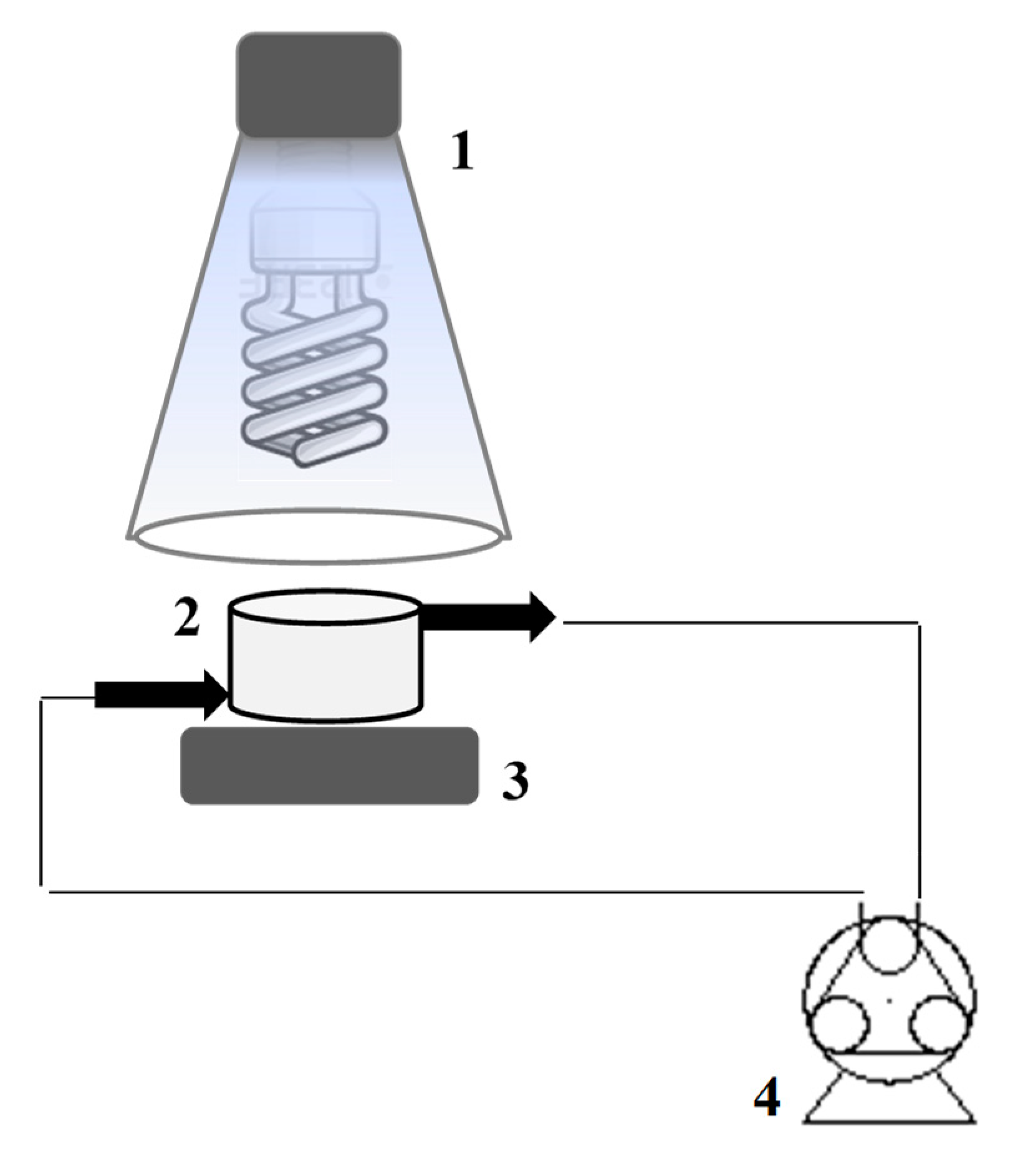
3.3. Photocatalytic Experiments
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Horáková, M.; Klementová, Š.; Kříž, P.; Balakrishna, S.K.; Špatenka, P.; Golovko, O.; Hájková, P.; Exnar, P. The synergistic effect of advanced oxidation processes to eliminate resistant chemical compounds. Surf. Coat. Technol. 2014, 241, 154–158. [Google Scholar] [CrossRef]
- Thiruvenkatachari, R.; Vigneswaran, S.; Moon, I.S. A review on UV/TiO2 photocatalytic oxidation process. Korean J. Chem. Eng. 2008, 25, 64–72. [Google Scholar] [CrossRef]
- Wols, B.A.; Hofman-Caris, C.H.M. Review of photochemical reaction constants of organic micropollutants required for UV advanced oxidation processes in water. Water Res. 2012, 46, 2815–2827. [Google Scholar] [CrossRef] [PubMed]
- Chong, M.N.; Jin, B.; Chow, C.W.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef]
- Nageswara Rao, A.; Sivasankar, B.; Sadasivam, V. Kinetic study on the photocatalytic degradation of salicylic acid using ZnO catalyst. J. Hazard. Mater. 2009, 166, 1357–1361. [Google Scholar] [CrossRef]
- Lu, S.; Wu, D.; Wang, Q.L.; Yan, J.; Buekens, A.G.; Cen, K.F. Photocatalytic decomposition on nano-TiO2: Destruction of chloroaromatic compounds. Chemosphere 2011, 82, 1215–1224. [Google Scholar] [CrossRef]
- Malato, S.; Fernández-Ibáñez, P.; Maldonado, M.I.; Blanco, J.; Gernjak, W. Decontamination and disinfection of water by solar photocatalysis: Recent overview and trends. Catal. Today 2009, 147, 1–59. [Google Scholar] [CrossRef]
- Parsons, S. Advanced Oxidation Processes for Water and Wastewater Treatment; IWA Publishing: London, UK, 2005. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Aazam, E.S. Visible light photocatalytic degradation of thiophene using Ag–TiO2/multi-walled carbon nanotubes nanocomposite. Ceram. Int. 2014, 40, 6705–6711. [Google Scholar] [CrossRef]
- Mohamed, R.M.; Baeissa, E.S. Preparation and characterisation of Pd–TiO2–hydroxyapatite nanoparticles for the photocatalytic degradation of cyanide under visible light. Appl. Catal. A Gen. 2013, 464–465, 218–224. [Google Scholar] [CrossRef]
- Suwanchawalit, C.; Wongnawa, S.; Sriprang, P.; Meanha, P. Enhancement of the photocatalytic performance of Ag-modified TiO2 photocatalyst under visible light. Ceram. Int. 2012, 38, 5201–5207. [Google Scholar] [CrossRef]
- Guo, J.-F.; Ma, B.; Yin, A.; Fan, K.; Dai, W.L. Highly stable and efficient Ag/AgCl@TiO2 photocatalyst: Preparation, characterization, and application in the treatment of aqueous hazardous pollutants. J. Hazard. Mater. 2012, 211–212, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, R.R.; Pujari, S.R.; Muley, G.G.; Patil, S.H.; Patil, K.R.; Shaikh, M.F.; Gambhire, A.B. Solar photocatalytic degradation of methylene blue using doped TiO2 nanoparticles. Solar Energy 2014, 103, 473–479. [Google Scholar] [CrossRef]
- Deng, Q.R.; Xia, X.H.; Guo, M.L.; Gao, Y.; Shao, G. Mn-doped TiO2 nanopowders with remarkable visible light photocatalytic activity. Mater. Lett. 2011, 65, 2051–2054. [Google Scholar] [CrossRef]
- Chang, S.; Liu, W. The roles of surface-doped metal ions (V, Mn, Fe, Cu, Ce, and W) in the interfacial behavior of TiO2 photocatalysts. Appl. Catal. B Environ. 2014, 156–157, 466–475. [Google Scholar] [CrossRef]
- McManamon, C.; Delaney, P.; Morris, M.A. Photocatalytic properties of metal and non-metal doped novel sub 10nm titanium dioxide nanoparticles on methyl orange. J. Colloid Interface Sci. 2013, 411, 169–172. [Google Scholar] [CrossRef]
- Ghasemi, S.; Esfandiar, A.; Setayesh, S.R.; Habibi-Yangjeh, A.; Gholami, M.R. Synthesis and characterization of TiO2–graphene nanocomposites modified with noble metals as a photocatalyst for degradation of pollutants. Appl. Catal. A Gen. 2013, 462–463, 82–90. [Google Scholar] [CrossRef]
- Suwarnkar, M.B.; Dhabbe, R.S.; Kadam, A.N.; Garadkar, K.M. Enhanced photocatalytic activity of Ag doped TiO2 nanoparticles synthesized by a microwave assisted method. Ceram. Int. 2014, 40, 5489–5496. [Google Scholar] [CrossRef]
- Vargas, E.; Vargas, R.; Núñez, O. A TiO2 surface modified with copper(II) phthalocyanine-tetrasulfonic acid tetrasodium salt as a catalyst during photoinduced dichlorvos mineralization by visible solar light. Appl. Catal. B Environ. 2014, 156–157, 8–14. [Google Scholar] [CrossRef]
- Hu, C.; Lan, Y.; Qu, J.; Hu, X.; Wang, A. Ag/AgBr/TiO2 Visible Light Photocatalyst for Destruction of Azodyes and Bacteria. J. Phys. Chem. B 2006, 110, 4066–4072. [Google Scholar] [CrossRef]
- Twu, Y.-K.; Chen, Y.-W.; Shih, C.-M. Preparation of silver nanoparticles using chitosan suspensions. Powder Technol. 2008, 185, 251–257. [Google Scholar] [CrossRef]
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Salicylic-acid (accessed on 15 May 2020).
- Gajović, A.; Furić, K.; Tomašić, N.; Popović, S.; Skoko, Ž.; Musić, S. Mechanochemical preparation of nanocrystalline TiO2 powders and their behavior at high temperatures. J. Alloys Compd. 2005, 398, 188–199. [Google Scholar] [CrossRef]
- Kortüm, G. Reflectance Spectroscopy; Springer: Berlin, Germany, 1969. [Google Scholar]
- Yu, J.; Kudo, A. Effects of Structural Variation on the Photocatalytic Performance of Hydrothermally Synthesized BiVO4. Adv. Funct. Mater. 2006, 16, 2163–2169. [Google Scholar] [CrossRef]
- Ohko, Y.; Tatsuma, T.; Fujii, T.; Naoi, K.; Niwa, C.; Kubota, Y.; Fujishima, A. Multicolour photochromism of TiO2 films loaded with silver nanoparticles. Nat. Mater. 2003, 2, 29–31. [Google Scholar] [CrossRef]
- Kazuma, E.; Tatsuma, T. Photoinduced reversible changes in morphology of plasmonic Ag nanorods on TiO2 and application to versatile photochromism. Chem. Commun. R. Soc. Chem. 2012, 48, 1733–1735. [Google Scholar] [CrossRef]
- Gong, H.M.; Xiao, S.; Su, X.R.; Han, J.B.; Wang, Q.Q. Photochromism and two-photon luminescence of Ag-TiO2 granular composite films activated by near infrared ps/fs pulses. Opt. Express 2007, 15, 13924–13929. [Google Scholar] [CrossRef]
- Herrmann, J.-M.; Tahiri, H.; Ait-Ichou, Y.; Lassaletta, G.; Gonzalez-Elipe, A.R.; Fernandez, A. Characterization and photocatalytic activity in aqueous medium of TiO2 and Ag-TiO2 coatings on quartz. Appl. Catal. B Environ. 1997, 13, 219–228. [Google Scholar] [CrossRef]
- Temgire, M.K.; Joshi, S.S. Optical and structural studies of silver nanoparticles. Radiat. Phys. Chem. 2004, 71, 1039–1044. [Google Scholar] [CrossRef]
- Hasmath Farzana, M.; Meenakshi, S. Photocatalytic aptitude of titanium dioxide impregnated chitosan beads for the reduction of Cr(VI). Int. J. Biol. Macromol. 2015, 72, 1265–1271. [Google Scholar] [CrossRef]
- Kochuveedu, S.T.; Jang, Y.H.; Kim, D.H. A study on the mechanism for the interaction of light with noble metal-metal oxide semiconductor nanostructures for various photophysical applications. Chem. Soc. Rev. 2013, 42, 8467–8493. [Google Scholar] [CrossRef]
- DAmato, C.A.; Giovannetti, R.; Zannotti, M.; Rommozzi, E.; Ferraro, S.; Seghetti, C.; Minicucci, M.; Gunnella, R.; Di Cicco, A. Enhancement of visible-light photoactivity by polypropylene coated plasmonic Au/TiO2 for dye degradation in water solution. Appl. Surf. Sci. 2018, 441, 575–587. [Google Scholar] [CrossRef]
- Grčić, I.; Papić, S.; Brnardić, I. Photocatalytic Activity of TiO2 Thin Films: Kinetic and Efficiency Study. Int. J. Chem. React. Eng. 2017, 16, 20160153. [Google Scholar] [CrossRef]

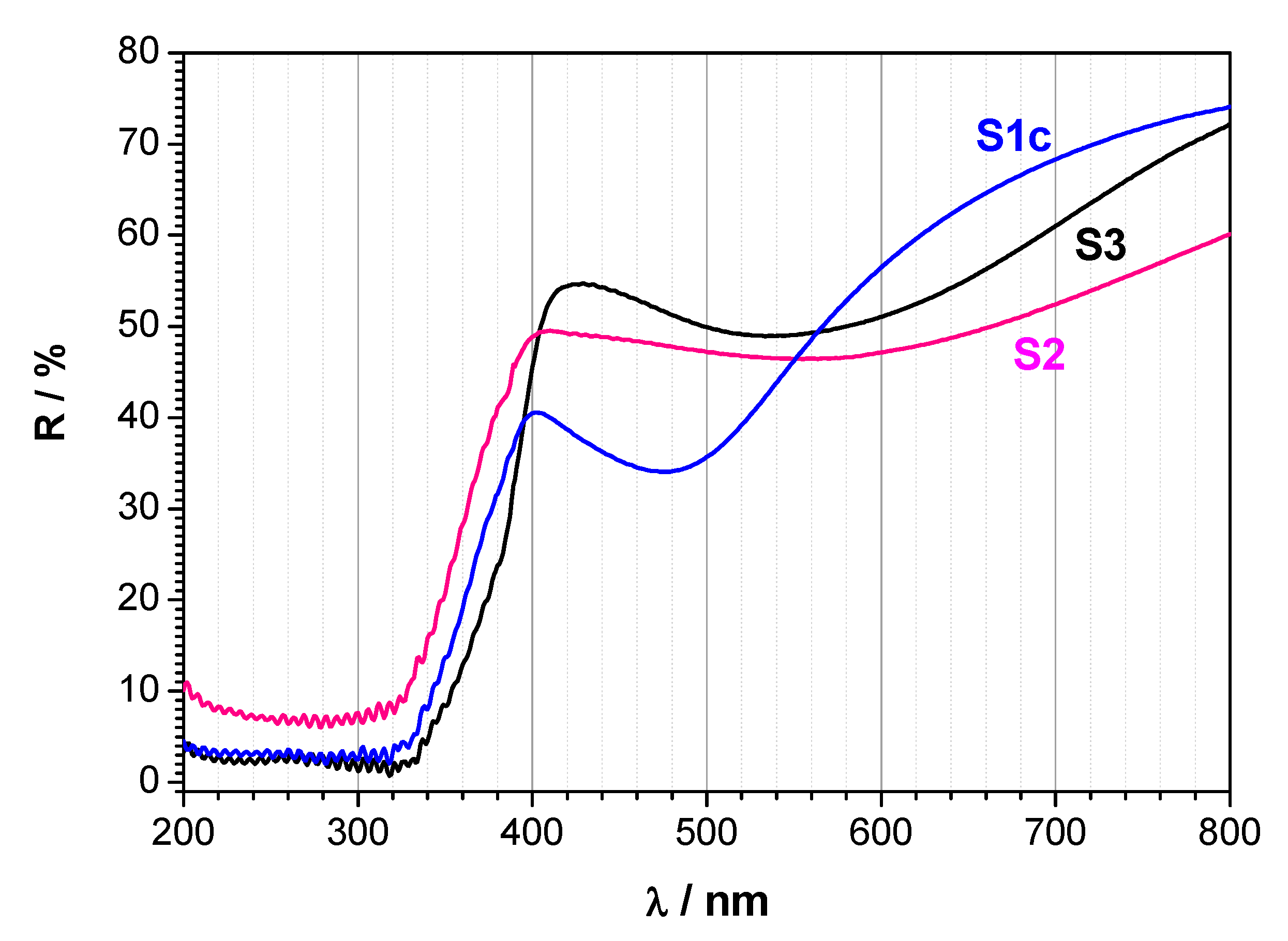

| Sample | Eg1 (eV) | Eg2 (eV) |
|---|---|---|
| S1c | 2.86 | 1.19 |
| S2 | 2.86 | 1.18 |
| S3 | 2.98 | 1.19 |
| Sample | Full-Spectrum Solar Irradiation | Visible Irradiation | ||||
|---|---|---|---|---|---|---|
| kSA,obs (× 103 min−1) | t1/2 (min) | R2 | kSA,obs (× 103 min−1) | t1/2 (min) | R2 | |
| TiO2P25 | 22.2 | 15–30 | 0.9718 | 0.83 | 835 | 0.9680 |
| S1c | 25.3 | 0.9646 | 0.003 | 231049 | 0.9168 | |
| S2 | 33.4 | 0.9125 | 2.43 | 285 | 0.9568 | |
| S3 | 35.0 | 0.9330 | 2.41 | 287 | 0.9127 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grčić, I.; Gajović, A.; Plodinec, M.; Šimunković, K.; Ivanković, H.; Willinger, M.-G. Enhanced Visible-Light Driven Photocatalytic Activity of Ag@TiO2 Photocatalyst Prepared in Chitosan Matrix. Catalysts 2020, 10, 763. https://doi.org/10.3390/catal10070763
Grčić I, Gajović A, Plodinec M, Šimunković K, Ivanković H, Willinger M-G. Enhanced Visible-Light Driven Photocatalytic Activity of Ag@TiO2 Photocatalyst Prepared in Chitosan Matrix. Catalysts. 2020; 10(7):763. https://doi.org/10.3390/catal10070763
Chicago/Turabian StyleGrčić, Ivana, Andreja Gajović, Milivoj Plodinec, Kristina Šimunković, Hrvoje Ivanković, and Marc-Georg Willinger. 2020. "Enhanced Visible-Light Driven Photocatalytic Activity of Ag@TiO2 Photocatalyst Prepared in Chitosan Matrix" Catalysts 10, no. 7: 763. https://doi.org/10.3390/catal10070763
APA StyleGrčić, I., Gajović, A., Plodinec, M., Šimunković, K., Ivanković, H., & Willinger, M.-G. (2020). Enhanced Visible-Light Driven Photocatalytic Activity of Ag@TiO2 Photocatalyst Prepared in Chitosan Matrix. Catalysts, 10(7), 763. https://doi.org/10.3390/catal10070763