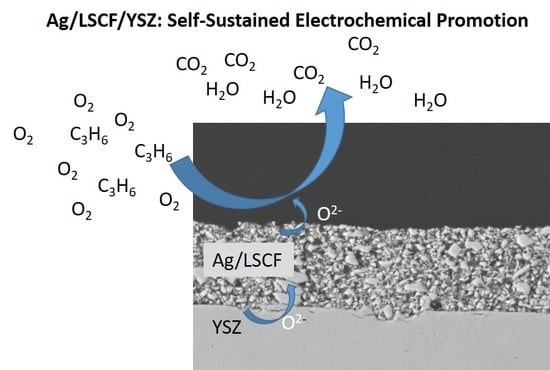
Catalytic and Electrochemical Properties of Ag Infiltrated Perovskite Coatings for Propene Deep Oxidation
Abstract
1. Introduction
2. Results and Discussions
2.1. Characterization of Initial LSCF Powders
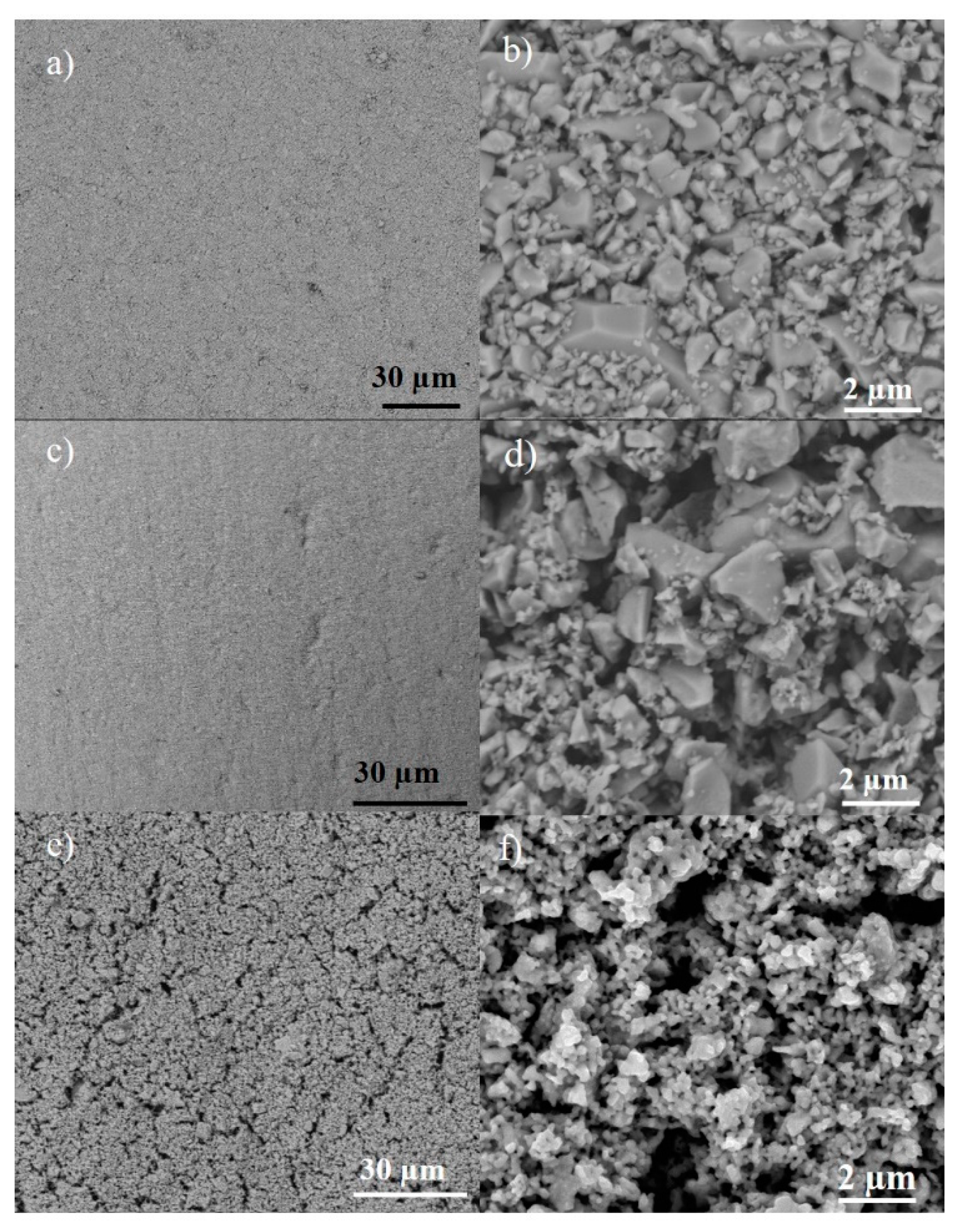
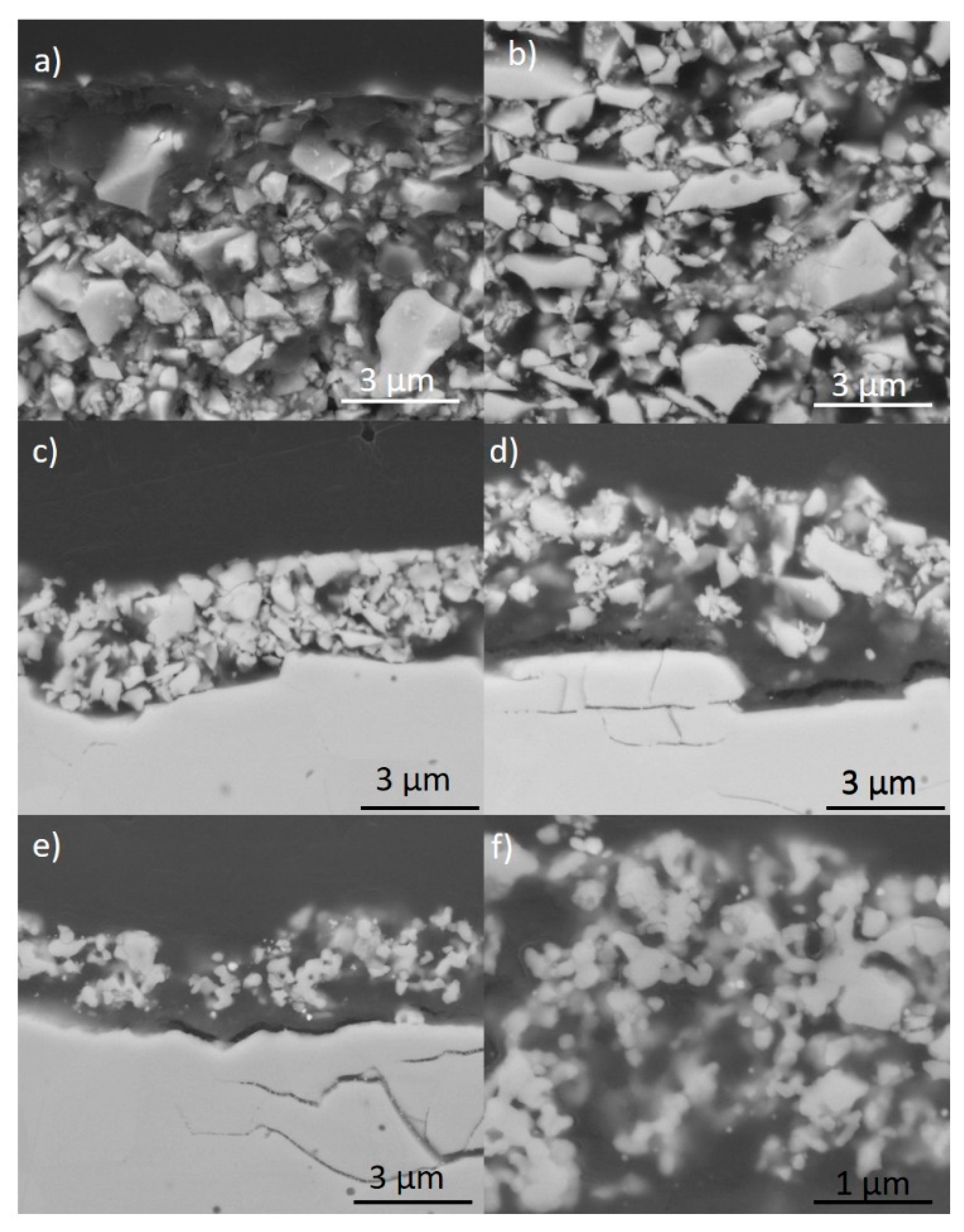
2.2. Preparation and Characterization of LSCF Layers
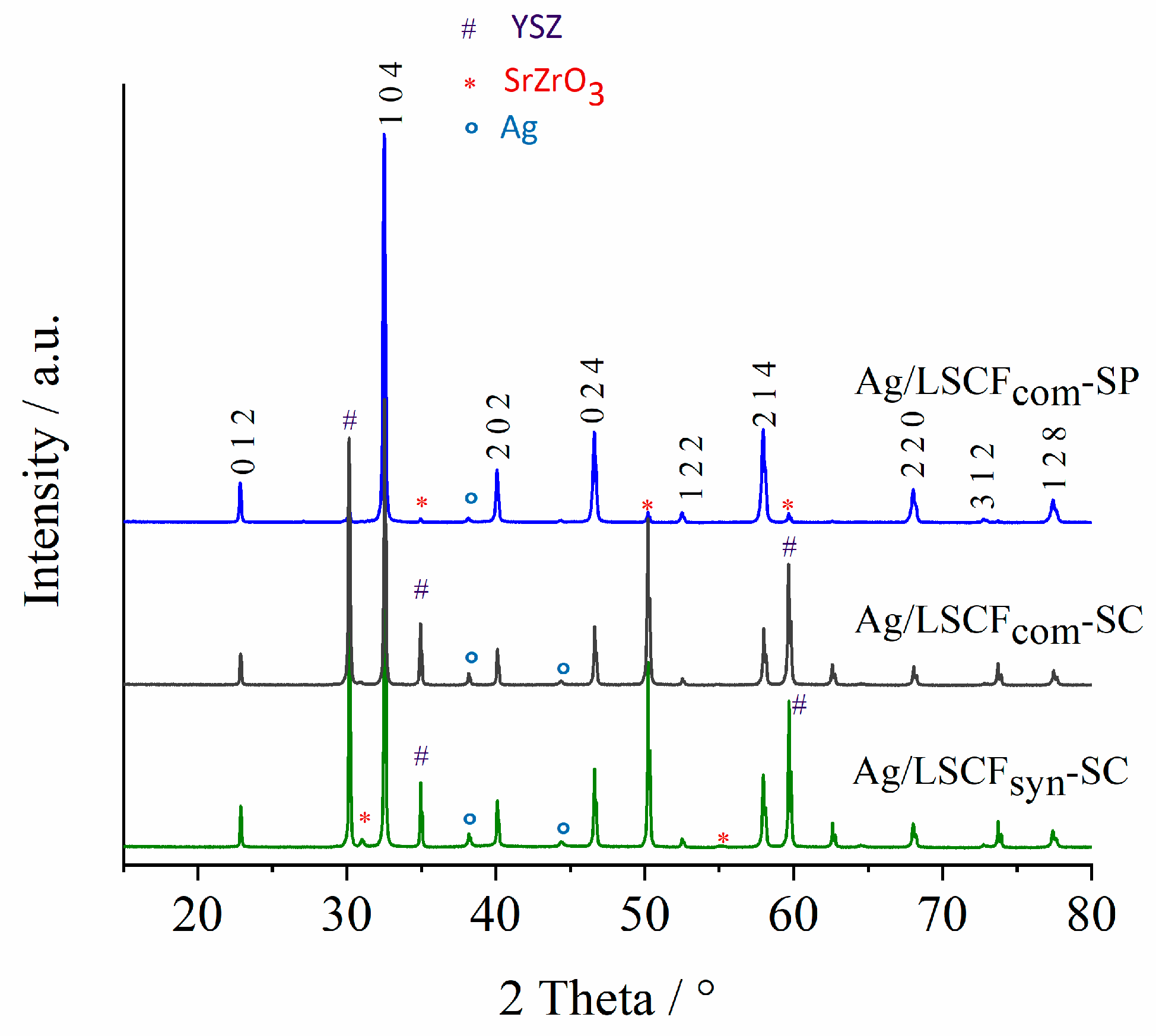
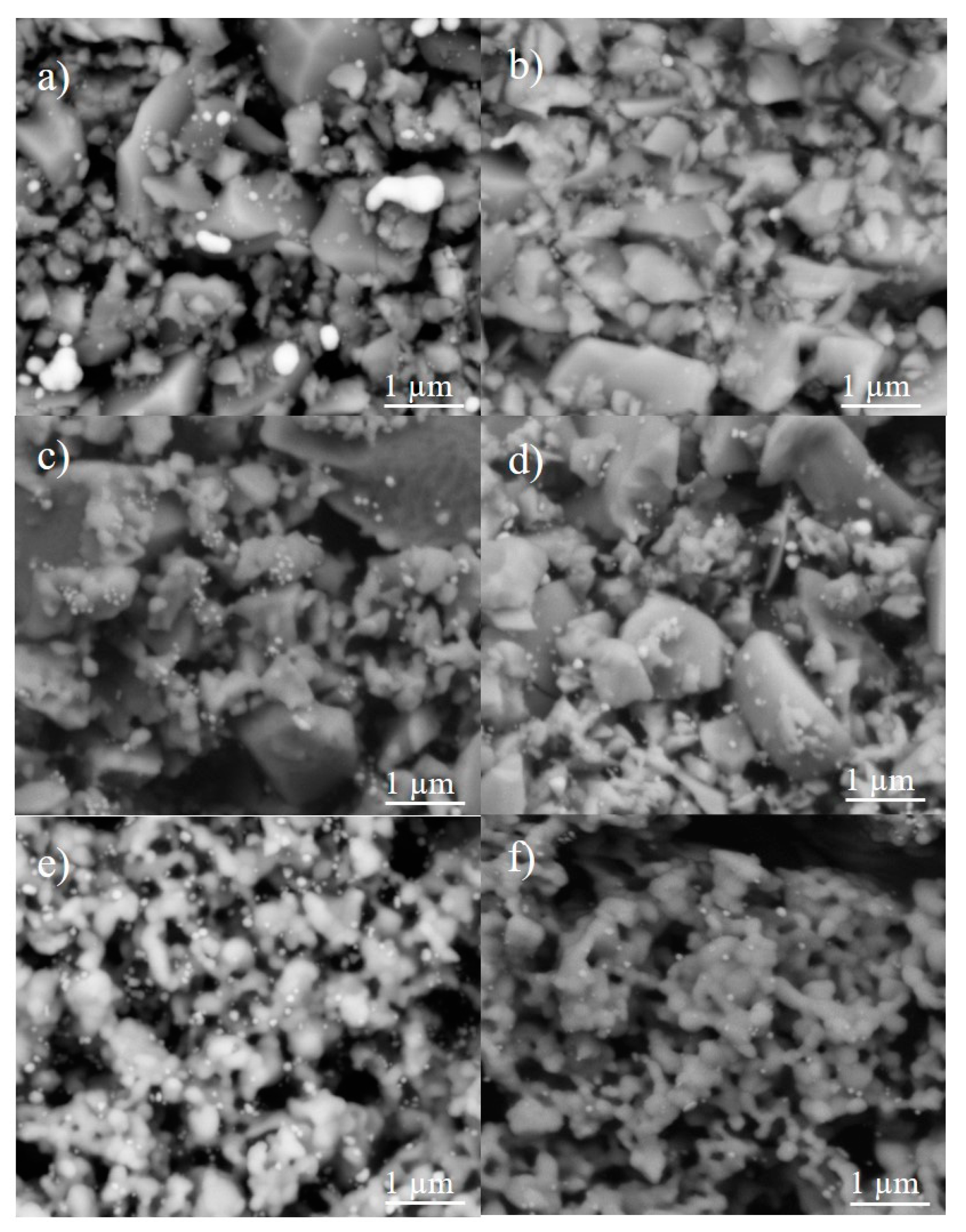
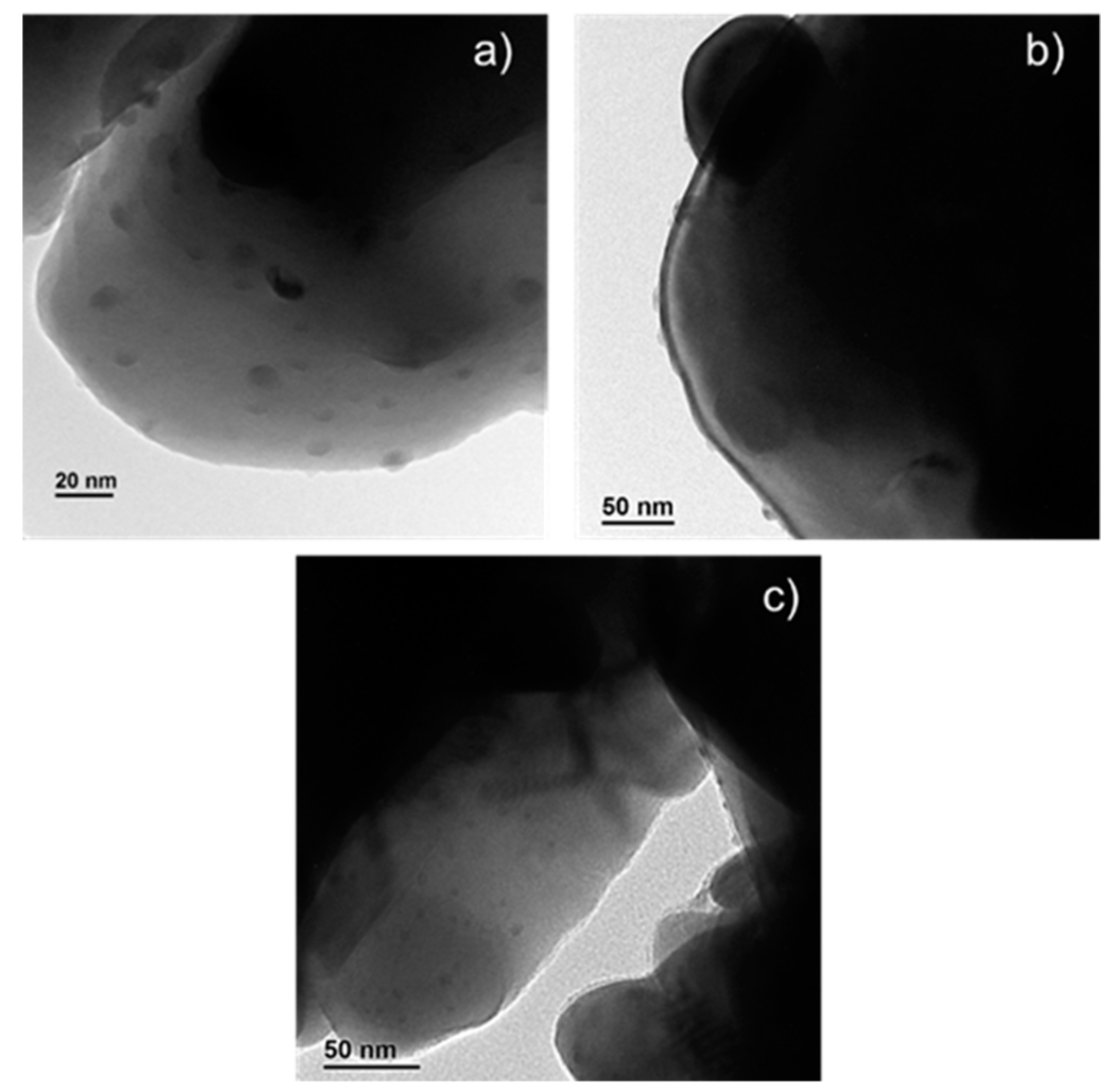
2.3. Characterization of Ag-Infiltrated LSCF Layers
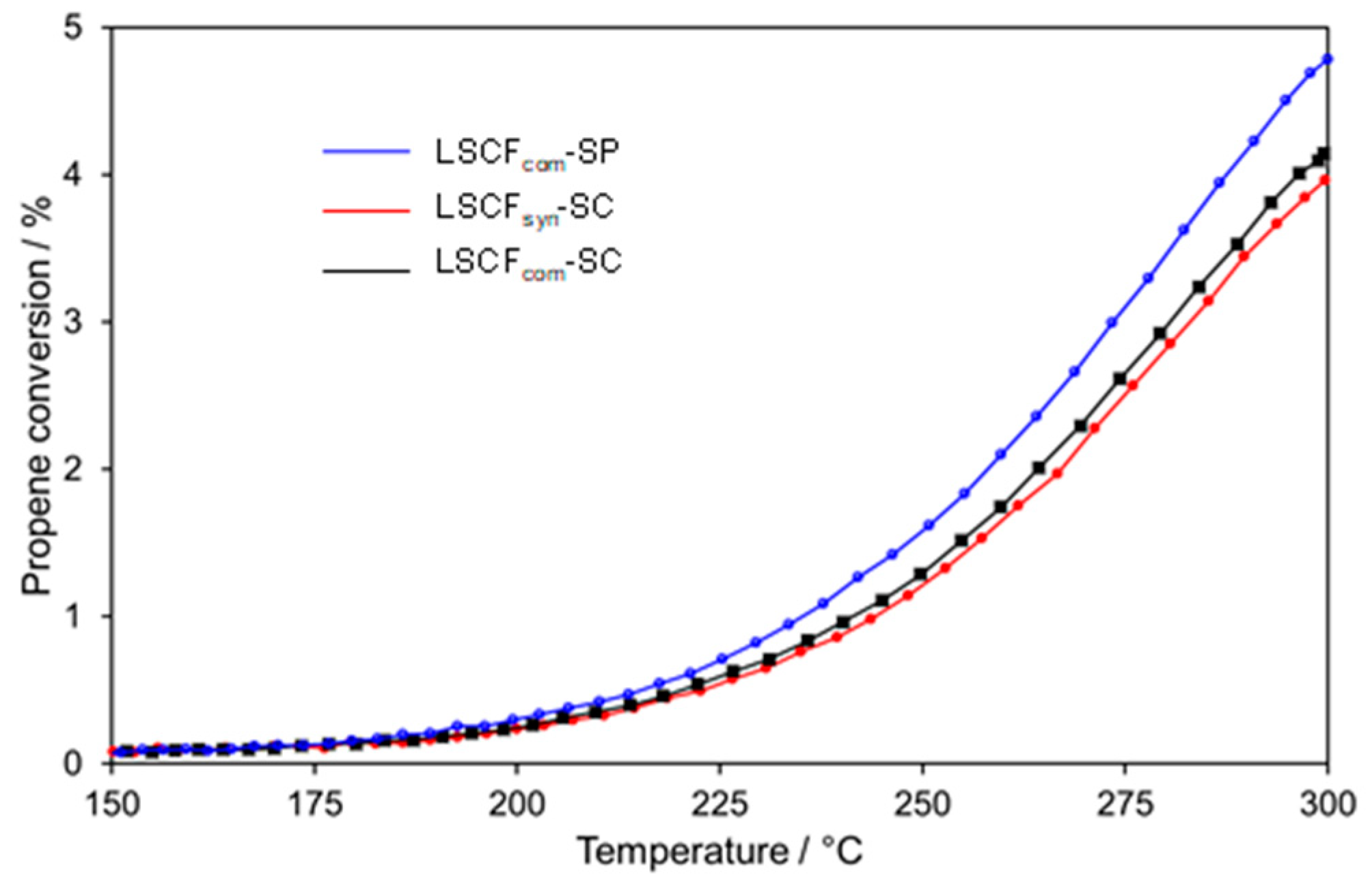
2.4. Catalytic Performances for Propene Oxidation
2.4.1. Bare LSCF Coatings
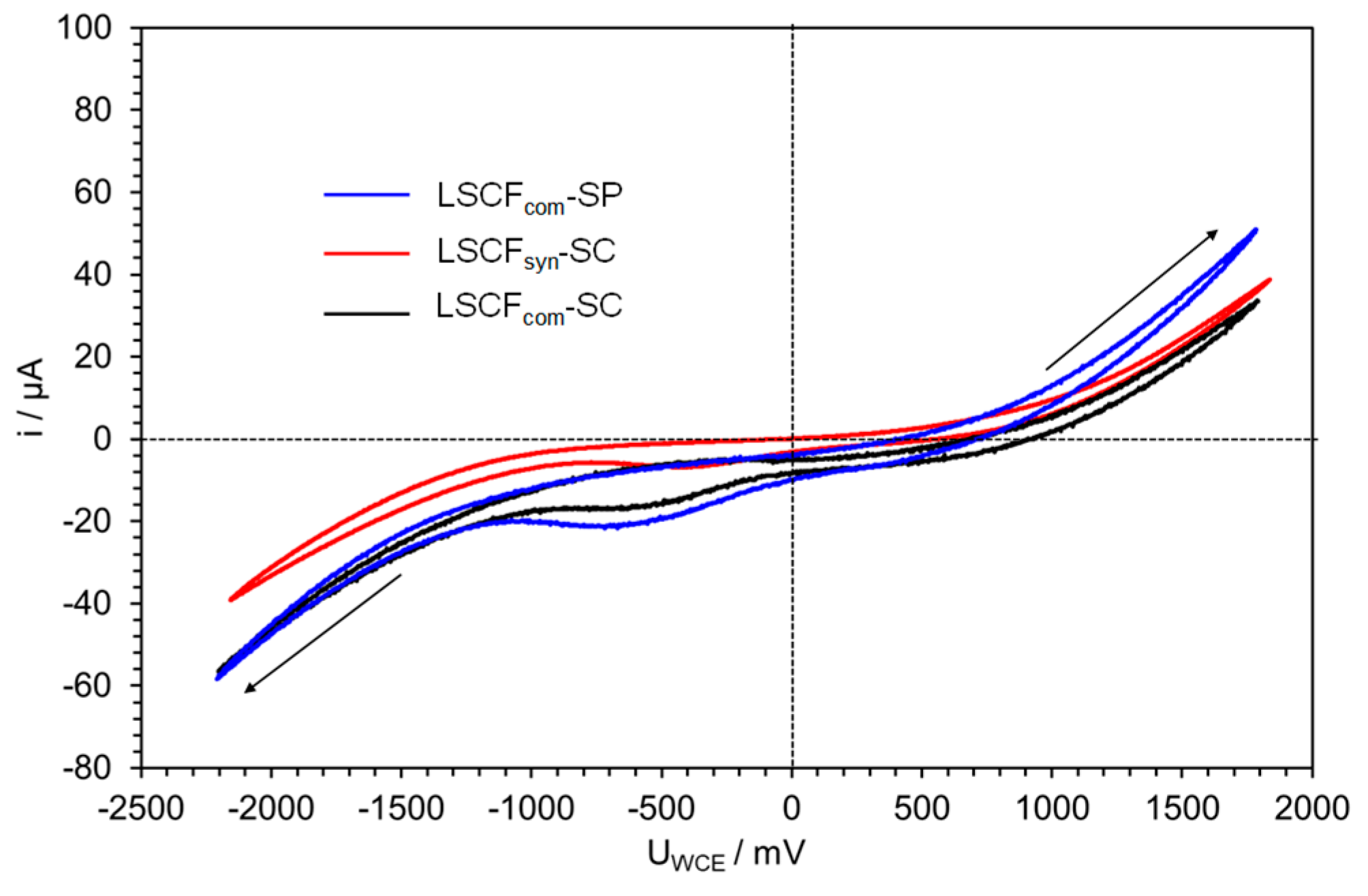
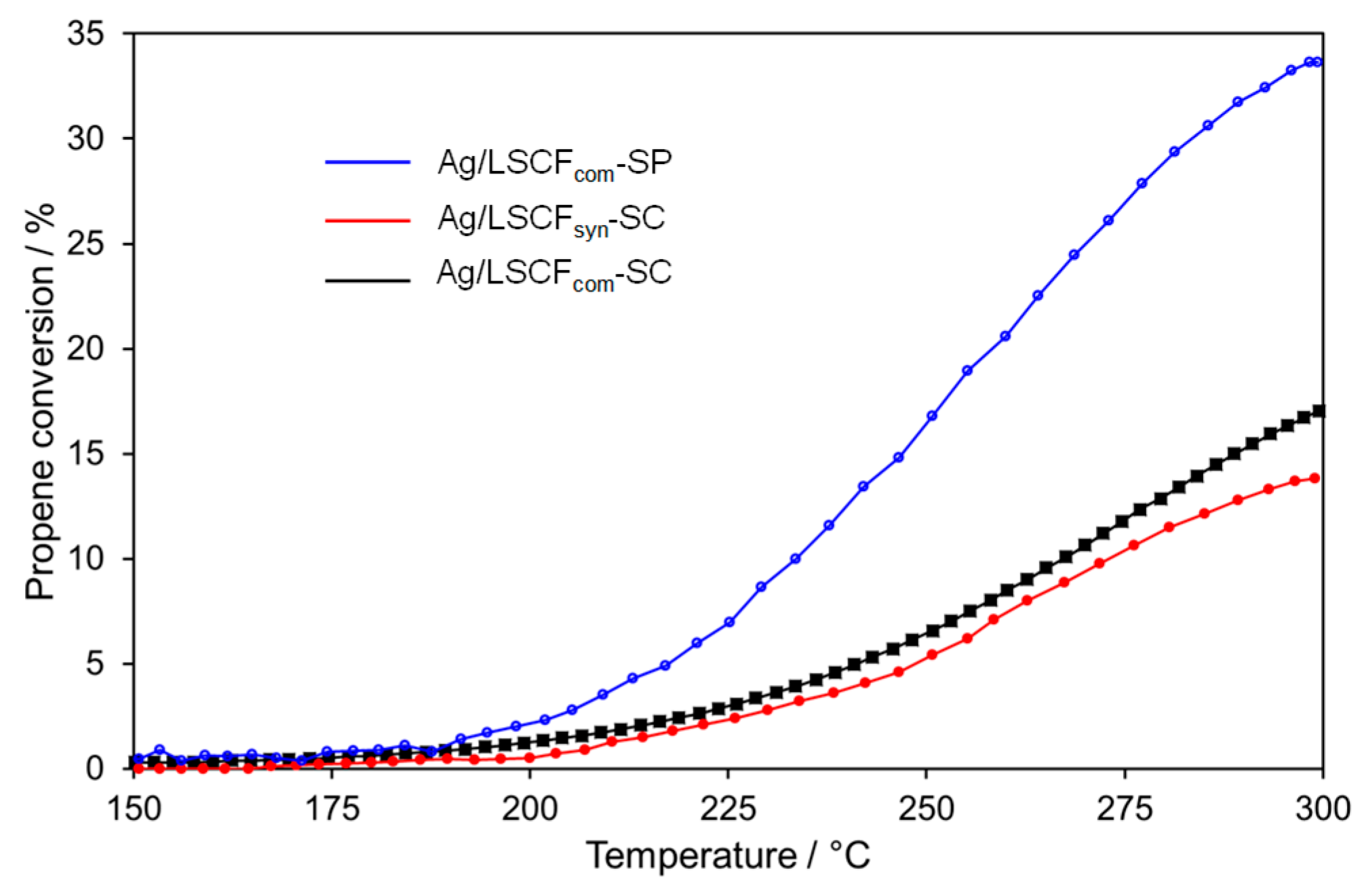
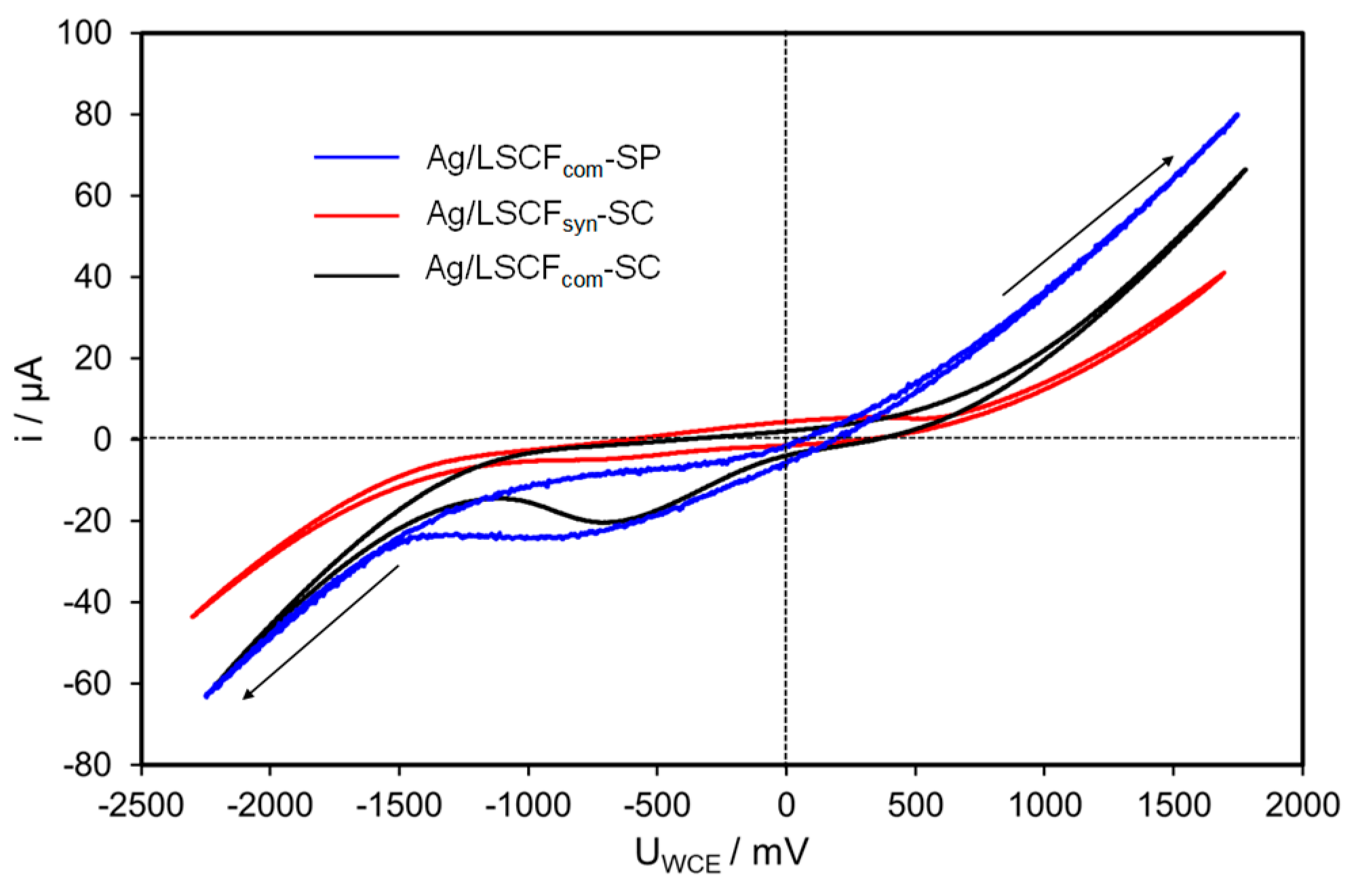
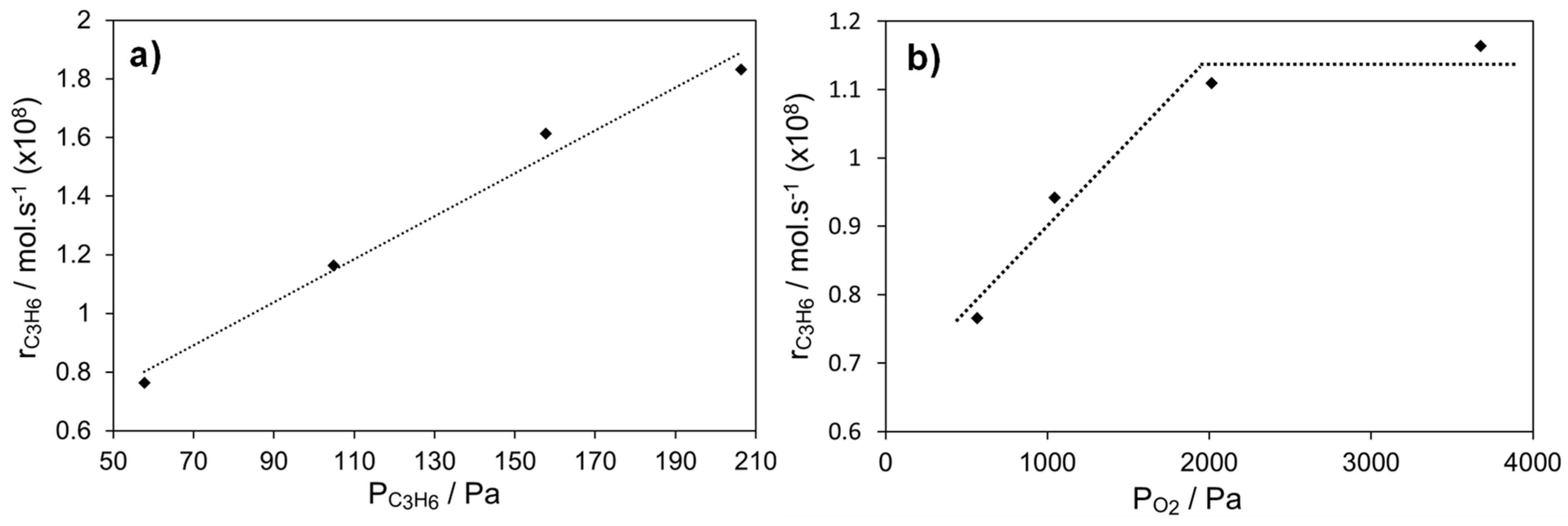
2.4.2. Ag Infiltrated LSCF Catalytic Layers
3. Materials and Methods
3.1. Preparation of LSCF Powders
3.2. LSCF Deposition on YSZ Pellets
3.2.1. Screen-Printing
3.2.2. Screen-Printing
3.2.3. Ag Infiltration
3.3. Characterization of LSCF, LSCF/YSZ, and Ag/LSCF/YSZ
3.4. Catalytic and Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Liotta, L.F. Applied Catalysis B: Environmental Catalytic oxidation of volatile organic compounds on supported noble metals. Appl. Catal. B Environ. 2010, 100, 403–412. [Google Scholar] [CrossRef]
- Yazdi, M.A.P.; Lizarraga, L.; Vernoux, P.; Billard, A.; Briois, P. Lanthanum cobaltite nanostructured coatings. Catalysts 2019, 9, 381. [Google Scholar] [CrossRef]
- Kalaitzidou, I.; Cavoue, T.; Boreave, A.; Burel, L.; Gaillard, F.; Retailleau-Mevel, L.; Baranova, E.A.; Rieu, M.; Viricelle, J.P.; Horwat, D.; et al. Electrochemical promotion of propylene combustion on Ag catalytic coatings. Catal. Commun. 2019, 104, 28–31. [Google Scholar] [CrossRef]
- Vernoux, P.; Lizarraga, L.; Tsampas, M.N.; Sapountzi, F.M.; De Lucas-Consuegra, A.; Valverde, J.; Souentie, S.; Vayenas, C.G.; Tsiplakides, D.; Balomenou, S.; et al. Ionically conducting ceramics as active catalyst supports. Chem. Rev. 2013, 113, 8192–8260. [Google Scholar] [CrossRef]
- Tsampas, M.N.; Sapountzi, F.M.; Vernoux, P. Applications of yttria stabilized zirconia (YSZ) in catalysis. Catal. Sci. Technol. 2015, 5, 4884–4900. [Google Scholar] [CrossRef]
- Nicole, J.; Tsiplakides, D.; Pliangos, C.; Verykios, X.E.; Comninellis, C.; Vayenas, C.G. Electrochemical promotion and metal-support interactions electrochemical promotion and metal-support interactions. J. Catal. 2001, 204, 23–34. [Google Scholar] [CrossRef]
- Vernoux, P.; Guth, M.; Xingang, L. Ionically conducting ceramics as alternative catalyst supports. Electrochem. Solid-State Lett. 2009, 12, 9–12. [Google Scholar] [CrossRef]
- Fortunato, M.A.; Princivalle, A.; Capdeillayre, C.; Petigny, N.; Tardivat, C.; Guizard, C.; Tsampas, M.N.; Sapountzi, F.M.; Vernoux, P. Role of lattice oxygen in the propane combustion over Pt/Yttria-stabilized zirconia: Isotopic studies. Top. Catal. 2014, 57, 1277–1286. [Google Scholar] [CrossRef]
- Isaifan, R.J.; Baranova, E.A. Effect of ionically conductive supports on the catalytic activity of platinum and ruthenium nanoparticles for ethylene complete oxidation. Catal. Today 2015, 241, 107–113. [Google Scholar] [CrossRef]
- Isaifan, R.J.; Couillard, M.; Baranova, E.A. Low temperature-high selectivity carbon monoxide methanation over yttria-stabilized zirconia-supported Pt nanoparticles. Int. J. Hydrogen Energy 2017, 42, 13754–13762. [Google Scholar] [CrossRef]
- Dole, H.A.E.; Costa, A.C.G.S.A.; Couillard, M.; Baranova, E.A. Quantifying metal support interaction in ceria-supported Pt, PtSn and Ru nanoparticles using electrochemical technique. J. Catal. 2016, 333, 40–50. [Google Scholar] [CrossRef]
- Zhou, X.; Huang, H.; Liu, H. Study of partial oxidation reforming of methane to syngas over self-sustained electrochemical promotion catalyst. Int. J. Hydrogen Energy 2013, 38, 6391–6396. [Google Scholar] [CrossRef]
- Dole, H.A.E.; Safady, L.F.; Ntais, S.; Couillard, M.; Baranova, E.A. Electrochemically enhanced metal-support interaction of highly dispersed Ru nanoparticles with a CeO2 support. J. Catal. 2014, 318, 85–94. [Google Scholar] [CrossRef]
- Hajar, Y.M.; Patel, K.D.; Tariq, U.; Baranova, E.A. Functional equivalence of electrochemical promotion and metal support interaction for Pt and RuO2 nanoparticles. J. Catal. 2017, 352, 42–51. [Google Scholar] [CrossRef]
- Hernandez, W.Y.; Hadjar, A.; Giroir-Fendler, A.; Andy, P.; Princivalle, A.; Klotz, M.; Marouf, A.; Guizard, C.; Tardivat, C.; Viazzi, C.; et al. Electrochemically-assisted NOx storage-reduction catalysts. Catal. Today 2015, 241, 143–150. [Google Scholar] [CrossRef]
- Kindermann, L.; Das, D.; Nickel, H.; Hilpert, K. Chemical compatibility of the LaFeO3 base perovskites (La0.6Sr0.4)zFe0.8M0.203-d (Z = 1, 0.9; M = Cr, Mn, Co, Ni) with yttria stabilized zirconia. Solid State Ion. 1996, 89, 215–220. [Google Scholar] [CrossRef]
- Tu, H.Y.; Takeda, Y.; Imanishi, N.; Yamamoto, O. Ln0.4Sr0.6Co0.8Fe0.203-d (Ln=La, Pr, Nd, Sm, Gd) for the electrode in solid oxide fuel cells. Solid State Ion. 1999, 117, 277–281. [Google Scholar] [CrossRef]
- Petric, A.; Huang, P.; Tietz, F. Evaluation of La-Sr-Co-Fe-O perovskites for solid oxide fuel cells and gas separation membranes. Solid State Ion. 2000, 135, 719–725. [Google Scholar] [CrossRef]
- Teraoka, Y.; Nobunaga, T.; Okamoto, K.; Miura, N.; Yamazoe, N. Influence of constituent metal cations in substituted LaCoO3 on mixed conductivity and oxygen permeability. Solid State Ion. 1991, 48, 207–212. [Google Scholar] [CrossRef]
- Tezyk, V.; Rossignol, C.; Sergent, N.; Djurado, E.; Laurencin, J.; Siebert, E. Electrochimica Acta cyclic voltammetry and high-frequency series resistance of electrode microstructure and the oxygen partial pressure. Electrochim. Acta 2019, 304, 312–322. [Google Scholar] [CrossRef]
- Vayenas, C.G.; Brosda, S.; Pliangos, C. Rules and mathematical modeling of electrochemical and chemical promotion. J. Catal. 2001, 203, 329–350. [Google Scholar] [CrossRef]
- Tsiplakides, D.; Vayenas, C.G. Temperature programmed desorption of oxygen from Ag films interfaced with Y2O3-doped ZrO2. J. Catal. 1999, 185, 237–251. [Google Scholar] [CrossRef]
- Kambolis, A.; Lizarraga, L.; Tsampas, M.N.; Burel, L.; Rieu, M.; Viricelle, J.-P.; Vernoux, P. Electrochemical promotion of catalysis with highly dispersed Pt nanoparticles. Electrochem. Commun. 2012, 19, 5–8. [Google Scholar] [CrossRef]
- Hajar, Y.; di Palma, V.; Kyriakou, V.; Verheijen, M.A.; Baranova, E.A.; Vernoux, P.; Kessels, W.M.M.; Creatore, M.; van de Sanden, M.C.M.; Tsampas, M.N. Atomic layer deposition of highly dispersed Pt nanoparticles on a high surface area electrode backbone for electrochemical promotion of catalysis. Electrochem. Commun. 2017, 84, 40–44. [Google Scholar] [CrossRef]
- Pechini, M.P. Method of Preparing Lead and Alkaline Earth Titanates and Niobates and Coating Method Using the Same to Form a Capacitor. US Patent 3 330 697, 1967. [Google Scholar]
- Penkala, B.; Gatla, S.; Aubert, D.; Ceretti, M.; Tardivat, C.; Paulus, W.; Kaper, H. In situ generated catalyst: Copper(II) oxide and copper (I) supported on Ca2Fe2O5 for CO oxidation. Catal. Sci. Technol. 2018, 8, 5236–5243. [Google Scholar] [CrossRef]
- Tsampas, M.N.; Sapountzi, F.M.; Boréave, A.; Vernoux, P. Investigation of the Electrochemical Promotion of Catalysis origins on electrochemical catalysts with oxygen ion conductive supports: Isotopic labeling mechanistic studies. Solid State Ion. 2014, 262, 257–261. [Google Scholar] [CrossRef]
| Sample | Accelerating Rate (rpm/s) | Spinning Rate (rpm) | Spinning Time (s) |
|---|---|---|---|
| LSCFcom | 500 | 2500 | 10 |
| LSCFsyn | 500 | 2750 | 20 |
| Layers | Powder | Deposition Method | Thickness (µm) (Edge–Center) | Mass of LSCF (mg) | Mass of Ag (µg) |
|---|---|---|---|---|---|
| LSCFcom-SC | Commercial | Spin-Coating | 2–5 | 2.7 ± 0.2 | - |
| LSCFsyn-SC | Synthesized | Spin-Coating | 2–5 | 2.7 ± 0.2 | - |
| LSCFcom-SP | Commercial | Screen-Printing | 11 | 11 ± 1 | - |
| Ag/LSCFcom-SC | Synthesized | Spin-Coating | 2–5 | 2.7 ± 0.2 | 50 |
| Ag/LSCFsyn-SC | Synthesized | Spin-Coating | 2–5 | 2.7 ± 0.2 | 50 |
| Ag/LSCFcom-SP | Commercial | Screen-Printing | 11 | 11 ± 1 | 50 |
| Catalytic Coatings | Propene Conversion at 300 °C/% | Specific Catalytic Rate at 300 °C /µmol C3H6 s−1.g−1 of Ag | OCV/mV |
|---|---|---|---|
| LSCFsyn-SC | 4 | - | +160 |
| LSCFcom-SC | 4.1 | - | +206 |
| LSCFcom-SP | 4.8 | - | +212 |
| Ag/LSCFsyn-SC | 14 | 95 | +303 |
| Ag/LSCFcom-SC | 17 | 115 | +220 |
| Ag/LSCFcom-SP | 33 | 225 | +251 |
| Sputtered Ag film [3] | 16 | 0.27 | +250 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Truong, T.G.; Rotonnelli, B.; Rieu, M.; Viricelle, J.-P.; Kalaitzidou, I.; Marinha, D.; Burel, L.; Caravaca, A.; Vernoux, P.; Kaper, H. Catalytic and Electrochemical Properties of Ag Infiltrated Perovskite Coatings for Propene Deep Oxidation. Catalysts 2020, 10, 729. https://doi.org/10.3390/catal10070729
Truong TG, Rotonnelli B, Rieu M, Viricelle J-P, Kalaitzidou I, Marinha D, Burel L, Caravaca A, Vernoux P, Kaper H. Catalytic and Electrochemical Properties of Ag Infiltrated Perovskite Coatings for Propene Deep Oxidation. Catalysts. 2020; 10(7):729. https://doi.org/10.3390/catal10070729
Chicago/Turabian StyleTruong, Thai Giang, Benjamin Rotonnelli, Mathilde Rieu, Jean-Paul Viricelle, Ioanna Kalaitzidou, Daniel Marinha, Laurence Burel, Angel Caravaca, Philippe Vernoux, and Helena Kaper. 2020. "Catalytic and Electrochemical Properties of Ag Infiltrated Perovskite Coatings for Propene Deep Oxidation" Catalysts 10, no. 7: 729. https://doi.org/10.3390/catal10070729
APA StyleTruong, T. G., Rotonnelli, B., Rieu, M., Viricelle, J.-P., Kalaitzidou, I., Marinha, D., Burel, L., Caravaca, A., Vernoux, P., & Kaper, H. (2020). Catalytic and Electrochemical Properties of Ag Infiltrated Perovskite Coatings for Propene Deep Oxidation. Catalysts, 10(7), 729. https://doi.org/10.3390/catal10070729