Synthesis of Valeric Acid by Selective Electrocatalytic Hydrogenation of Biomass-Derived Levulinic Acid
Abstract
1. Introduction
2. Results
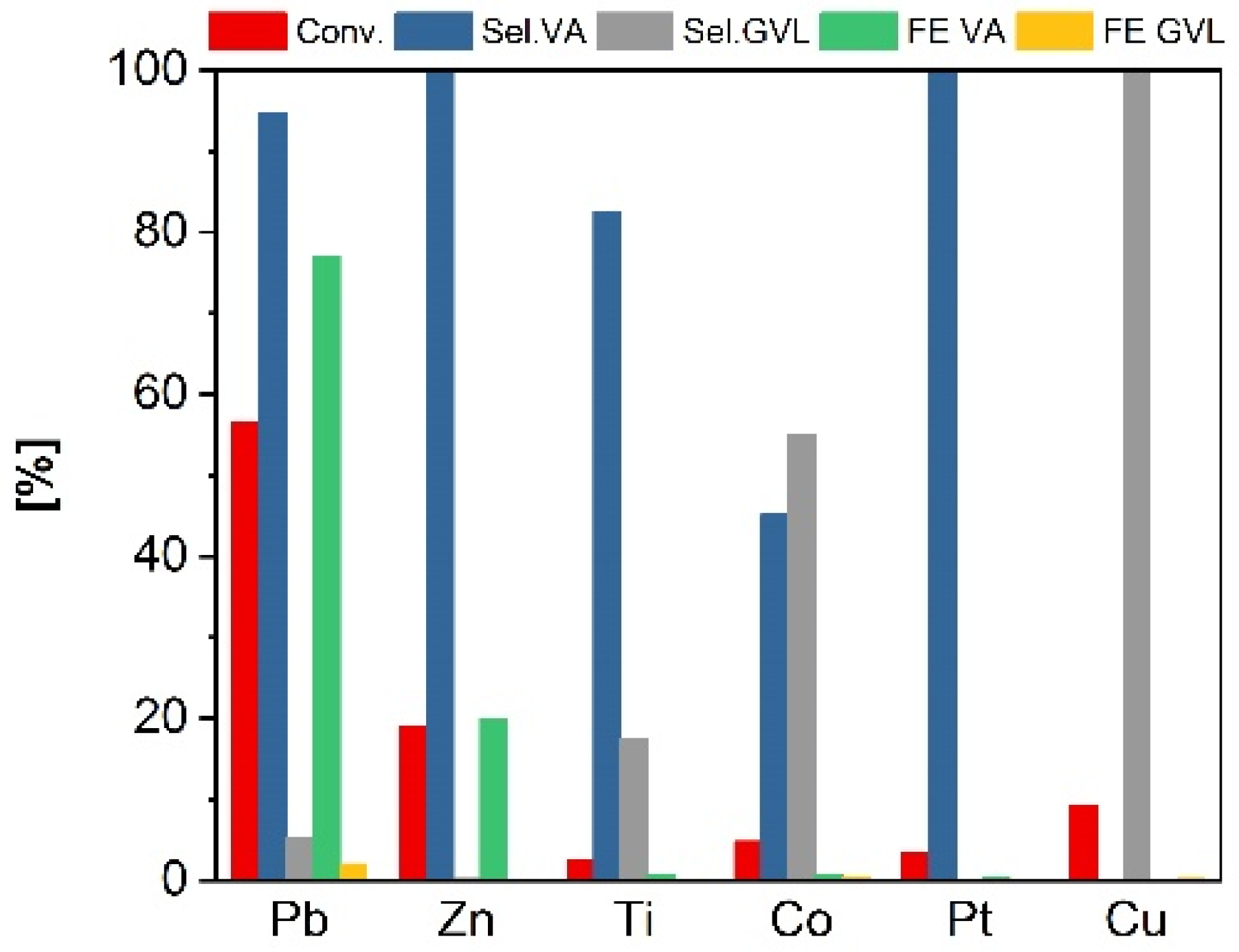
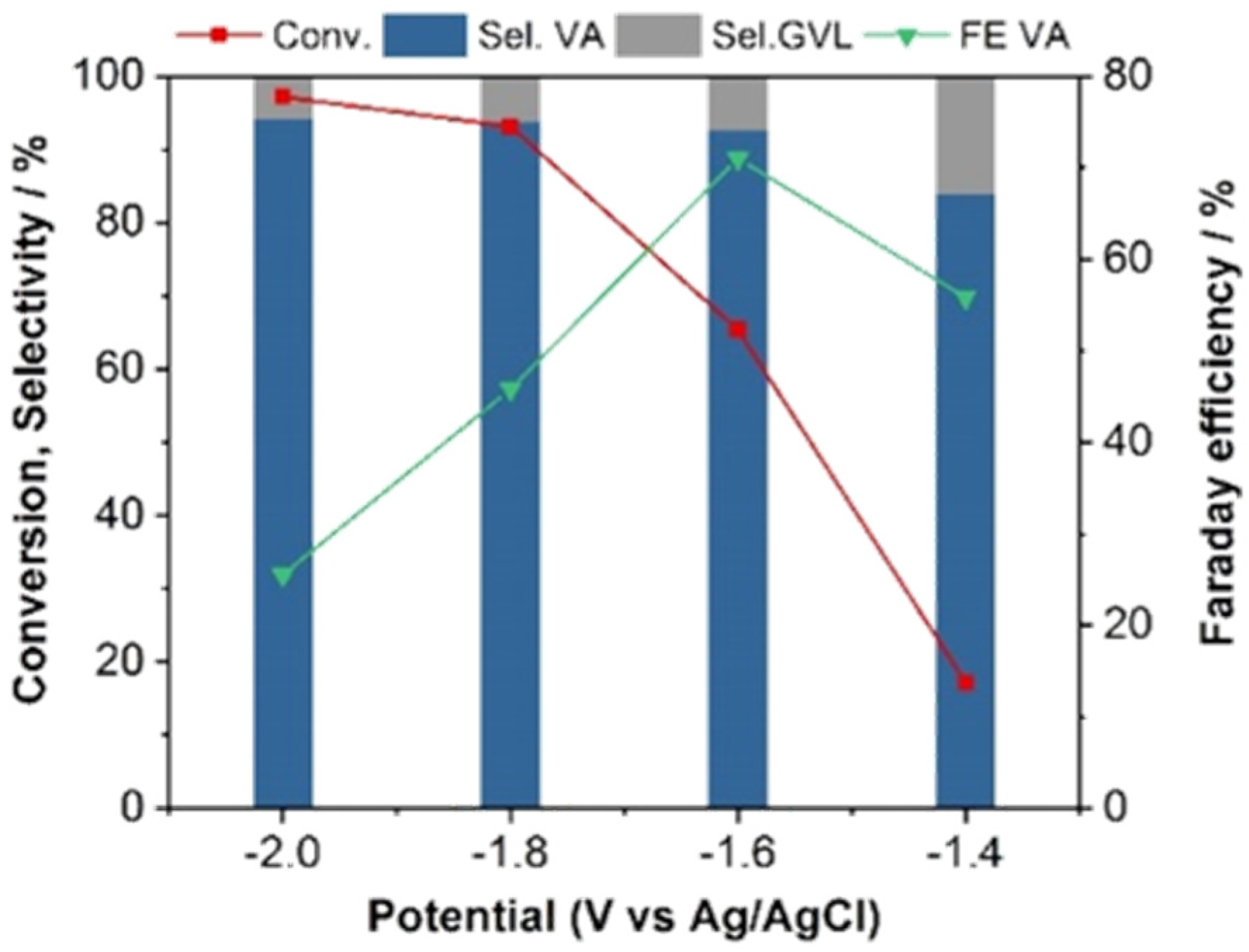
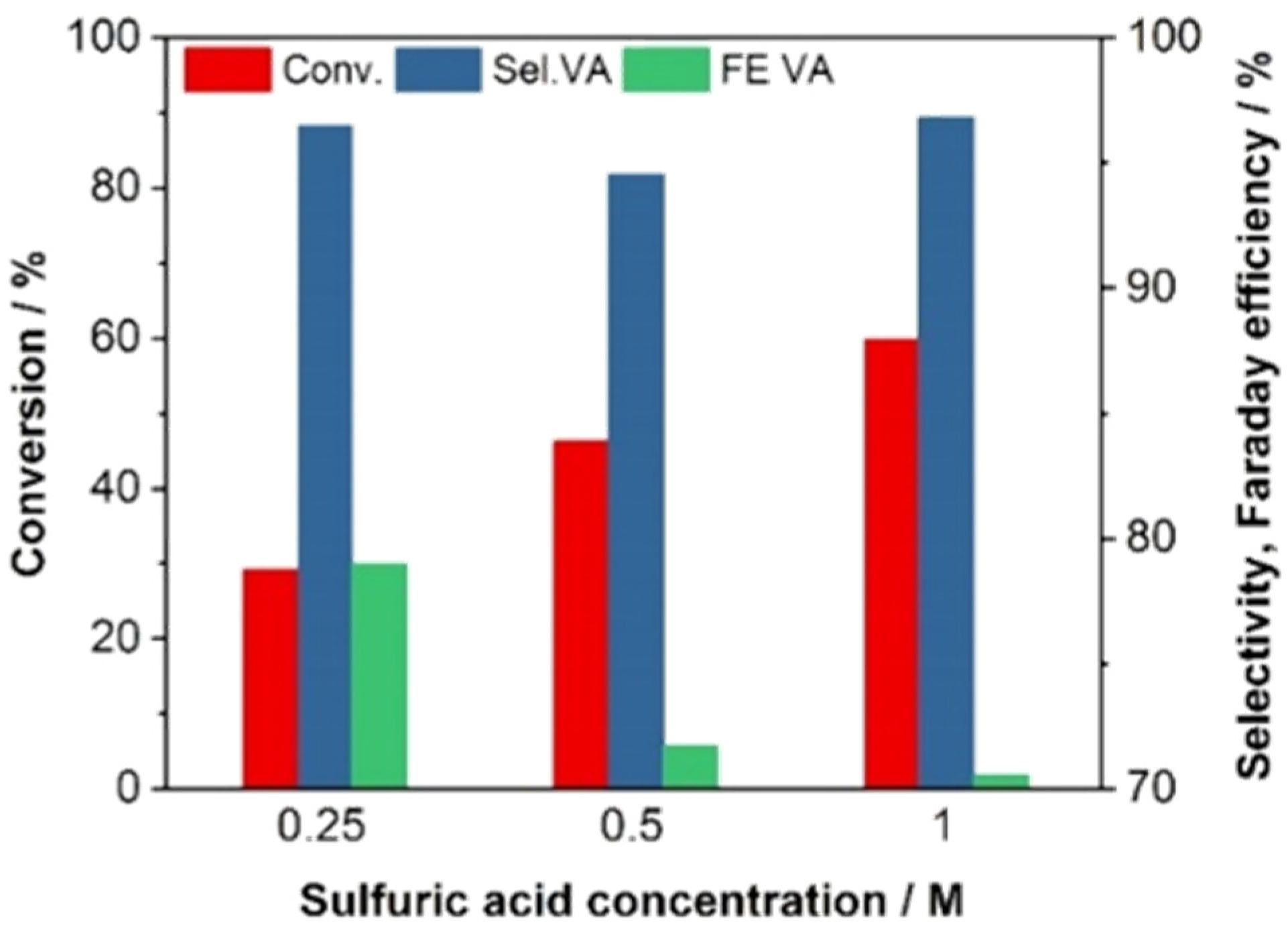
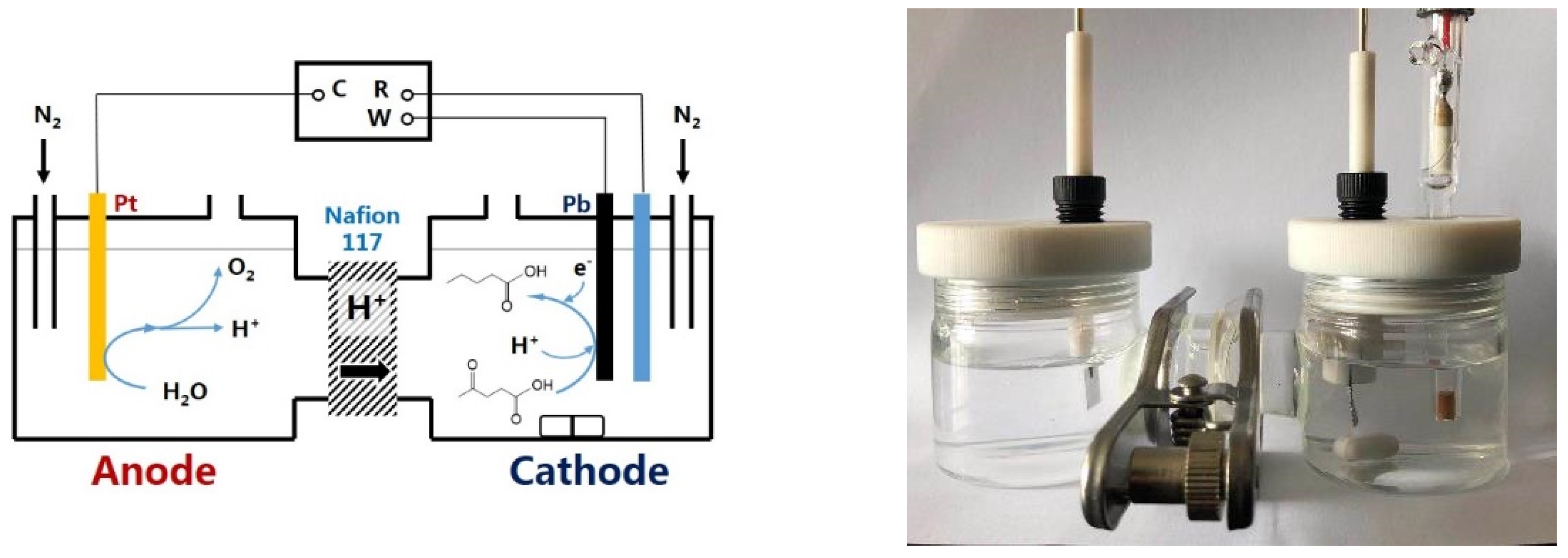
2.1. ECH of LA in H-Type Cell
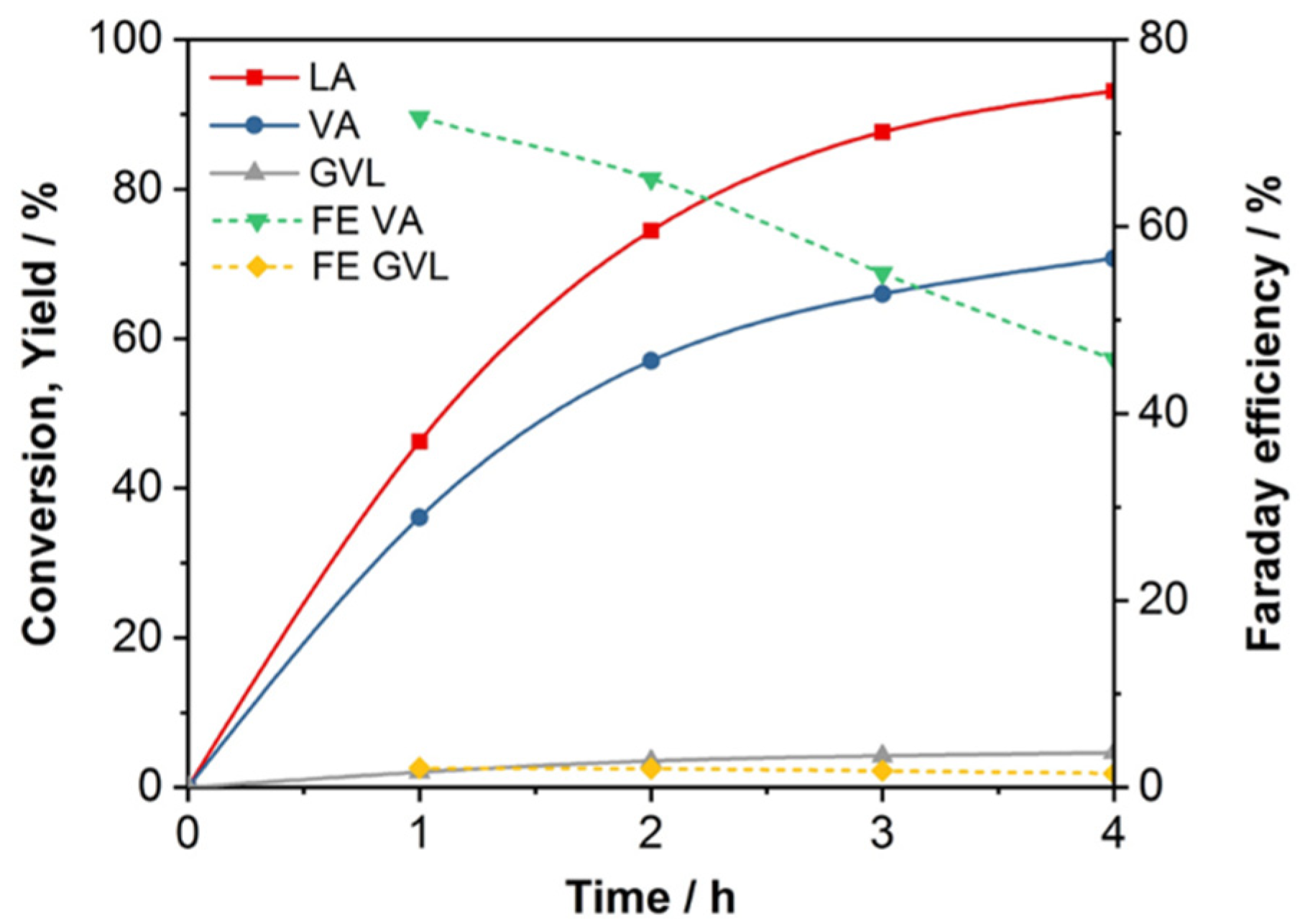
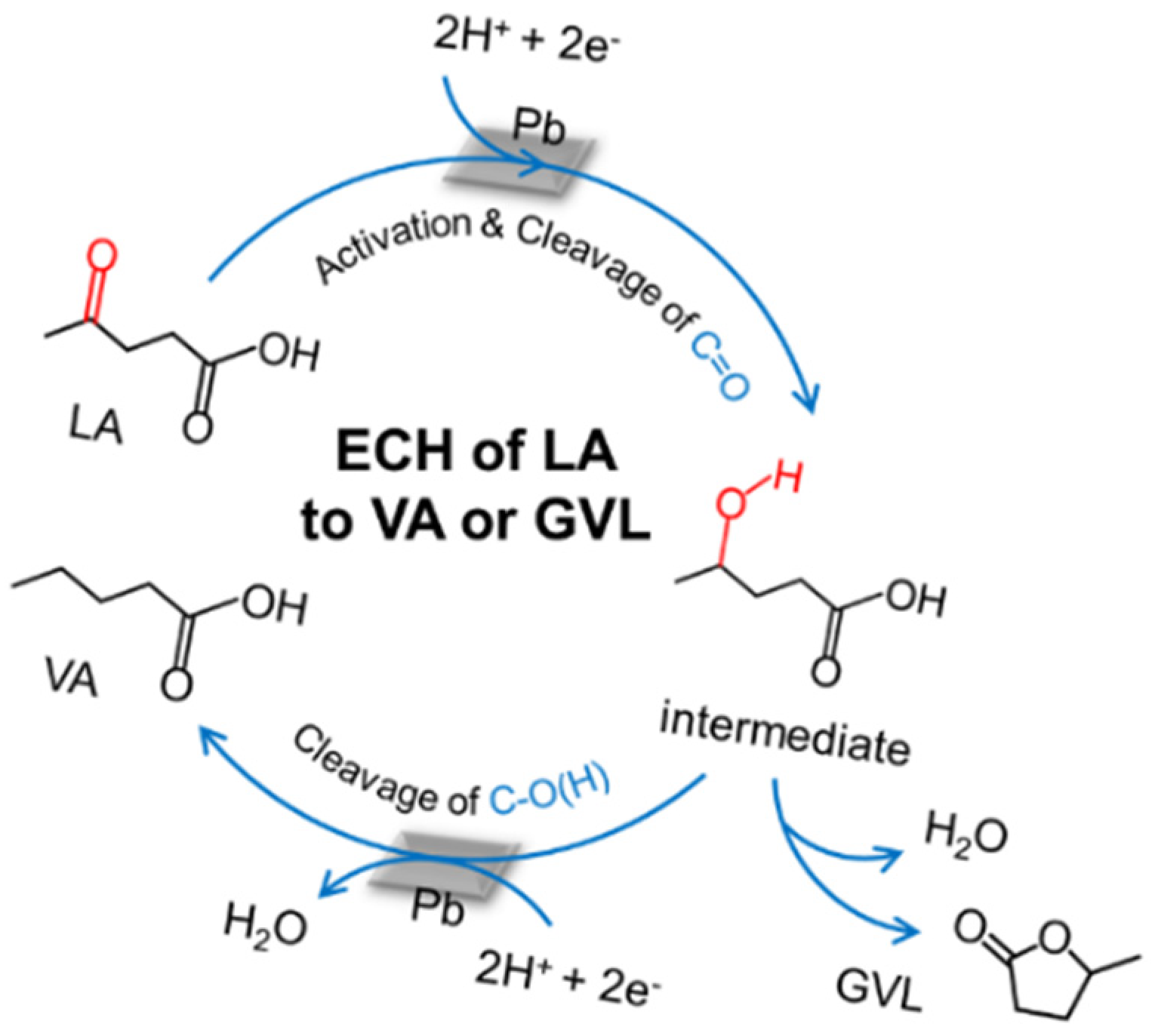
2.2. Reaction Process and Mechanism
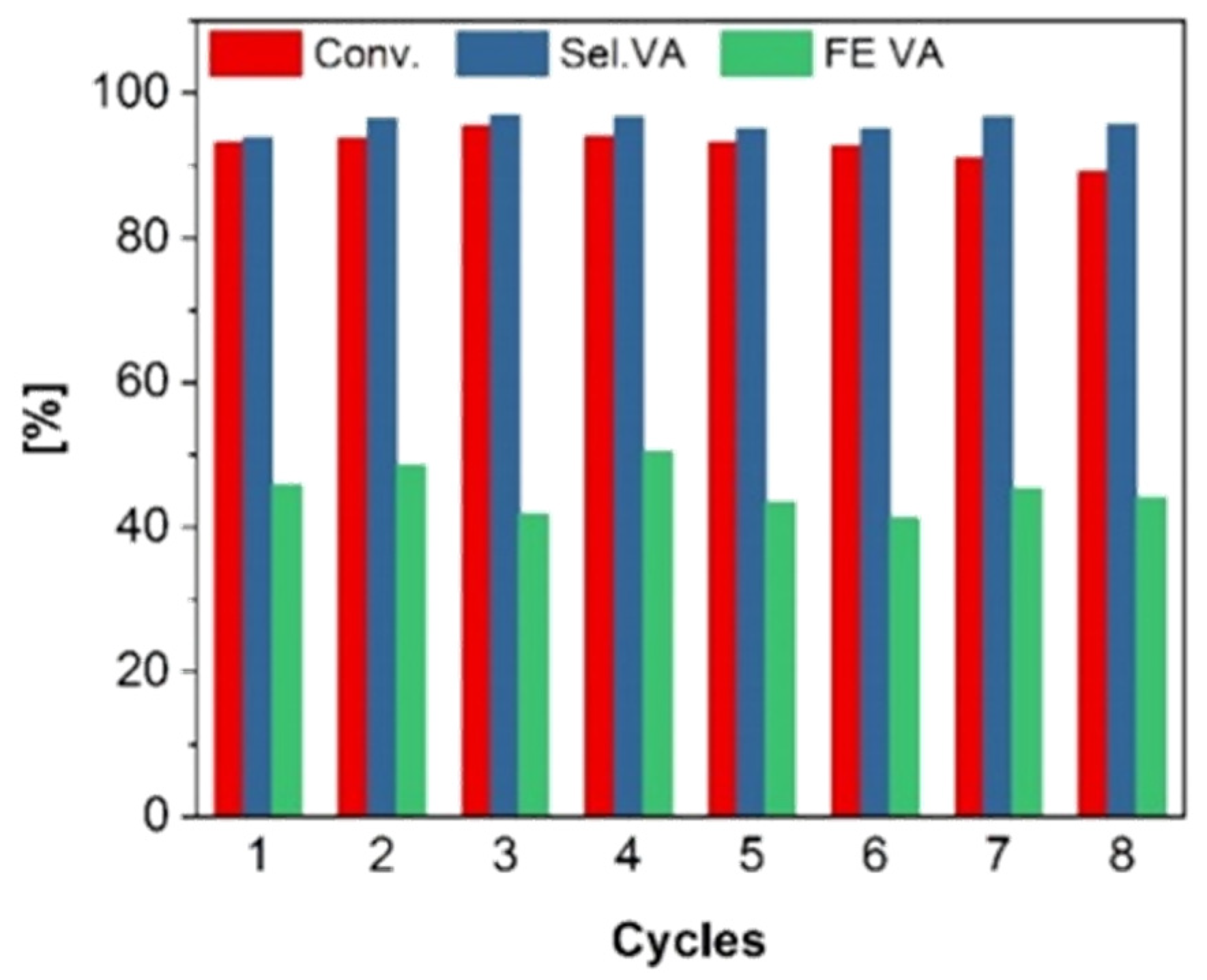
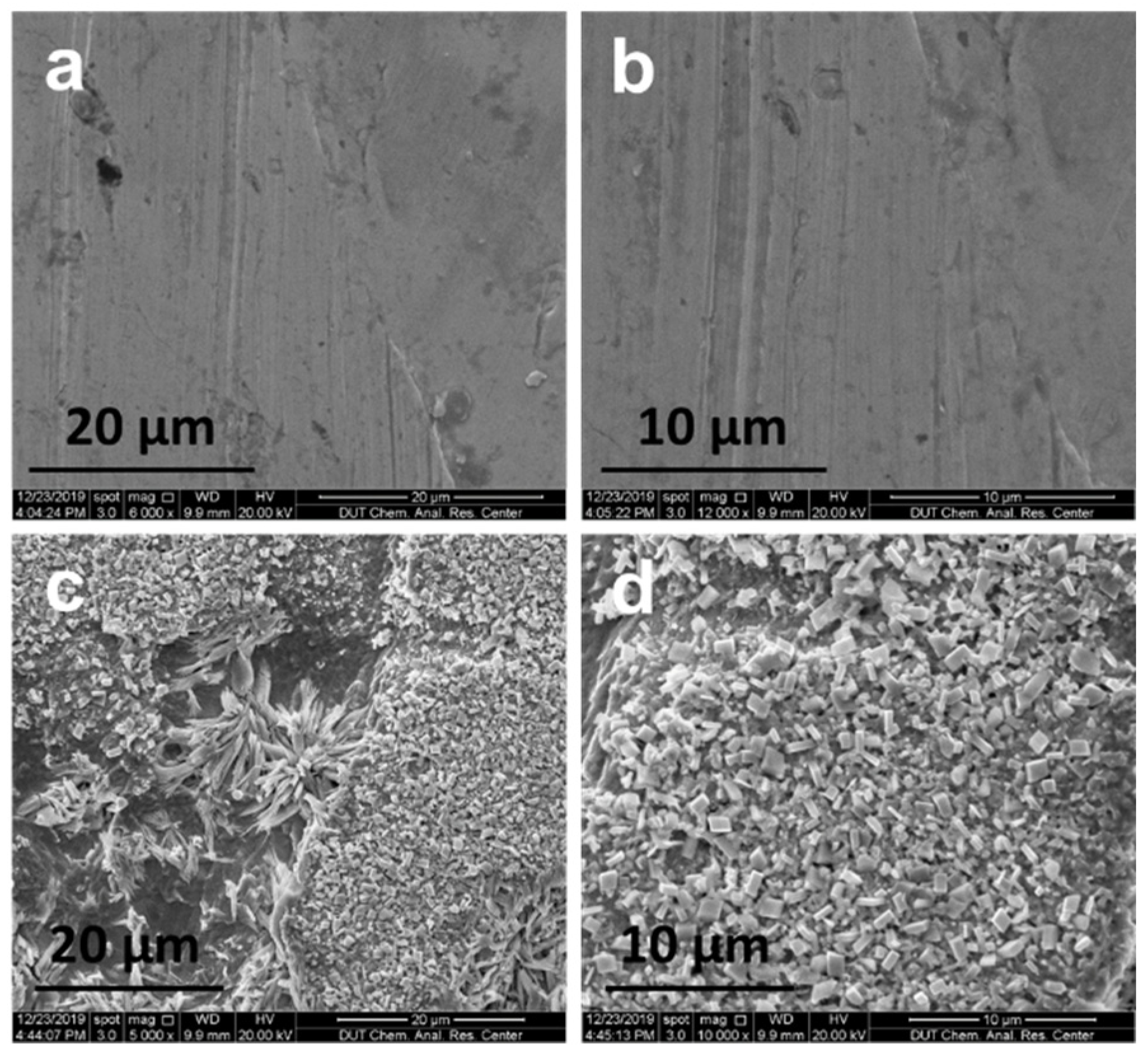
2.3. Stability Testing
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Electrochemical Measurements
3.3. Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Luterbacher, J.S.; Rand, J.M.; Alonso, D.M.; Han, J.; Youngquist, J.T.; Maravelias, C.T.; Pfleger, B.F.; Dumesic, J.A. Nonenzymatic Sugar Production from Biomass Using Biomass-Derived γ-Valerolactone. Science 2014, 343, 277. [Google Scholar] [CrossRef]
- Wang, G.-H.; Hilgert, J.; Richter, F.H.; Wang, F.; Bongard, H.J.; Spliethoff, B.; Weidenthaler, C.; Schüth, F. Platinum–cobalt bimetallic nanoparticles in hollow carbon nanospheres for hydrogenolysis of 5-hydroxymethylfurfural. Nat. Mater. 2014, 13, 293–300. [Google Scholar] [CrossRef]
- Lynd, L.R.; Wyman, C.E.; Gerngross, T.U. Biocommodity Engineering. Biotechnol. Progr. 1999, 15, 777–793. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Iborra, S.; Velty, A. Chemical Routes for the Transformation of Biomass into Chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Ruiz, J.C.; Dumesic, J.A. Catalytic routes for the conversion of biomass into liquid hydrocarbon transportation fuels. Energy Environ. Sci. 2011, 4, 83–99. [Google Scholar] [CrossRef]
- Sun, P.; Gao, G.; Zhao, Z.; Xia, C.; Li, F. Stabilization of Cobalt Catalysts by Embedment for Efficient Production of Valeric Biofuel. ACS Catal. 2014, 4, 4136–4142. [Google Scholar] [CrossRef]
- Luo, W.; Deka, U.; Beale, A.M.; van Eck, E.R.H.; Bruijnincx, P.C.A.; Weckhuysen, B.M. Ruthenium-catalyzed hydrogenation of levulinic acid: Influence of the support and solvent on catalyst selectivity and stability. J. Catal. 2013, 301, 175–186. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Kaburagi, W.; Osada, Y.; Fujitani, T.; Yamashita, H. Catalytic transfer hydrogenation of biomass-derived levulinic acid and it esters to γ-valerolactone over ZrO2 catalyst supported on SBA-15 silica. Catal. Today 2017, 281, 418–428. [Google Scholar] [CrossRef]
- Lomate, S.; Sultana, A.; Fujitani, T. Effect of SiO2 support properties on the performance of Cu–SiO 2 catalysts for the hydrogenation of levulinic acid to gamma valerolactone using formic acid as a hydrogen source. Catal. Sci. Technol. 2017, 7, 3073–3083. [Google Scholar] [CrossRef]
- Lomate, S.; Sultana, A.; Fujitani, T. Vapor Phase Catalytic Transfer Hydrogenation (CTH) of Levulinic Acid to γ-Valerolactone Over Copper Supported Catalysts Using Formic Acid as Hydrogen Source. Catal. Lett. 2018, 148, 348–358. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Kango, H.; Yamashita, H. Catalytic Transfer Hydrogenation of Biomass-Derived Levulinic Acid and Its Esters to γ- Valerolactone over Sulfonic Acid-Functionalized UiO-66. ACS Sustain. Chem. Eng. 2017, 5, 1141–1152. [Google Scholar] [CrossRef]
- Wijaya, Y.P.; Grossmann-Neuhaeusler, T.; Dhewangga Putra, R.D.; Smith, K.J.; Kim, C.S.; Gyenge, E.L. Electrocatalytic Hydrogenation of Guaiacol in Diverse Electrolytes Using a Stirred Slurry Reactor. ChemSusChem 2020, 13, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhou, P.; Zhang, Z.; Yang, C.; Zhang, B.; Deng, K.; Bottle, S.; Zhu, H. Selective Oxidation of 5-Hydroxymethylfurfural to 2,5-Furandicarboxylic Acid Using O2 and a Photocatalyst of Co-thioporphyrazine Bonded to g-C3N4. J. Am. Chem. Soc. 2017, 139, 14775–14782. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Garedew, M.; Lam, C.H.; Jackson, J.E.; Miller, D.J.; Saffron, C.M. Mild electrocatalytic hydrogenation and hydrodeoxygenation of bio-oil derived phenolic compounds using ruthenium supported on activated carbon cloth. Green Chem. 2012, 14, 2540–2549. [Google Scholar] [CrossRef]
- Li, Z.; Kelkar, S.; Raycraft, L.; Garedew, M.; Jackson, J.E.; Miller, D.J.; Saffron, C.M. A mild approach for bio-oil stabilization and upgrading: Electrocatalytic hydrogenation using ruthenium supported on activated carbon cloth. Green Chem. 2014, 16, 844–852. [Google Scholar] [CrossRef]
- Song, Y.; Gutiérrez, O.Y.; Herranz, J.; Lercher, J.A. Aqueous phase electrocatalysis and thermal catalysis for the hydrogenation of phenol at mild conditions. Appl. Catal. B 2016, 182, 236–246. [Google Scholar] [CrossRef]
- Chadderdon, X.H.; Chadderdon, D.J.; Pfennig, T.; Shanks, B.H.; Li, W. Paired electrocatalytic hydrogenation and oxidation of 5-(hydroxymethyl)furfural for efficient production of biomass-derived monomers. Green Chem. 2019, 21, 6210–6219. [Google Scholar] [CrossRef]
- Zhang, P.; Sheng, X.; Chen, X.; Fang, Z.; Jiang, J.; Wang, M.; Li, F.; Fan, L.; Ren, Y.; Zhang, B.; et al. Paired Electrocatalytic Oxygenation and Hydrogenation of Organic Substrates with Water as the Oxygen and Hydrogen Source. Angew. Chem. Int. Ed. 2019, 58, 9155–9159. [Google Scholar] [CrossRef]
- Zhang, Y.R.; Wang, B.X.; Qin, L.; Li, Q.; Fan, Y.M. A non-noble bimetallic alloy in the highly selective electrochemical synthesis of the biofuel 2,5-dimethylfuran from 5-hydroxymethylfurfural. Green Chem. 2019, 21, 1108–1113. [Google Scholar] [CrossRef]
- Zhang, X.; Han, M.; Liu, G.; Wang, G.; Zhang, Y.; Zhang, H.; Zhao, H. Simultaneously high-rate furfural hydrogenation and oxidation upgrading on nanostructured transition metal phosphides through electrocatalytic conversion at ambient conditions. Appl. Catal. B 2019, 244, 899–908. [Google Scholar] [CrossRef]
- Nilges, P.; Dos Santos, T.R.; Harnisch, F.; Schröder, U. Electrochemistry for biofuel generation: Electrochemical conversion of levulinic acid to octane. Energy Environ. Sci. 2012, 5, 5231–5235. [Google Scholar] [CrossRef]
- Dos Santos, T.R.; Nilges, P.; Sauter, W.; Harnisch, F.; Schröder, U. Electrochemistry for the generation of renewable chemicals: Electrochemical conversion of levulinic acid. RSC Adv. 2015, 5, 26634–26643. [Google Scholar] [CrossRef]
- Xin, L.; Zhang, Z.; Qi, J.; Chadderdon, D.J.; Qiu, Y.; Warsko, K.M.; Li, W. Electricity Storage in Biofuels: Selective Electrocatalytic Reduction of Levulinic Acid to Valeric Acid or γ-Valerolactone. ChemSusChem 2013, 6, 674–686. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Xin, L.; Chadderdon, D.J.; Qi, J.; Liang, C.; Li, W. Integrated electrocatalytic processing of levulinic acid and formic acid to produce biofuel intermediate valeric acid. Green Chem. 2014, 16, 1305–1315. [Google Scholar] [CrossRef]
- Wu, H.; Song, J.; Xie, C.; Hu, Y.; Zhang, P.; Yang, G.; Han, B. Surface engineering in PbS via partial oxidation: Towards an advanced electrocatalyst for reduction of levulinic acid to γ-valerolactone. Chem. Sci. 2019, 10, 1754–1759. [Google Scholar] [CrossRef]
- Sanyal, U.; Lopez-Ruiz, J.; Padmaperuma, A.B.; Holladay, J.; Gutiérrez, O.Y. Electrocatalytic Hydrogenation of Oxygenated Compounds in Aqueous Phase. Org. Process Res. Dev. 2018, 22, 1590–1598. [Google Scholar] [CrossRef]
- Zhao, B.; Chen, M.; Guo, Q.; Fu, Y. Electrocatalytic hydrogenation of furfural to furfuryl alcohol using platinum supported on activated carbon fibers. Electrochim. Acta 2014, 135, 139–146. [Google Scholar] [CrossRef]
- Jung, S.; Karaiskakis, A.N.; Biddinger, E.J. Enhanced activity for electrochemical hydrogenation and hydrogenolysis of furfural to biofuel using electrodeposited Cu catalysts. Catal. Today 2019, 323, 26–34. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, F.; Michel, F.C., Jr.; Co, A.C. Efficient Electrochemical Hydrogenation of 5-Hydroxymethylfurfural to 2,5-Bis(hydroxymethyl)furan on Ag-Displaced Nanotextured Cu Catalysts. ChemElectroChem 2019, 6, 4739–4749. [Google Scholar] [CrossRef]
- Serrano-Ruiz, J.C.; Wang, D.; Dumesic, J.A. Catalytic upgrading of levulinic acid to 5-nonanone. Green Chem. 2010, 12, 574–577. [Google Scholar] [CrossRef]
- Jung, S.; Biddinger, E.J. Electrocatalytic Hydrogenation and Hydrogenolysis of Furfural and the Impact of Homogeneous Side Reactions of Furanic Compounds in Acidic Electrolytes. ACS Sustain. Chem. Eng. 2016, 4, 6500–6508. [Google Scholar] [CrossRef]
- Bisselink, R.J.M.; Crockatt, M.; Zijlstra, M.; Bakker, I.J.; Goetheer, E.; Slaghek, T.M.; van Es, D.S. Identification of More Benign Cathode Materials for the Electrochemical Reduction of Levulinic Acid to Valeric Acid. ChemElectroChem 2019, 6, 3285–3290. [Google Scholar] [CrossRef]
- Holzhäuser, F.J.; Artz, J.; Palkovits, S.; Kreyenschulte, D.; Büchs, J.; Palkovits, R. Electrocatalytic upgrading of itaconic acid to methylsuccinic acid using fermentation broth as a substrate solution. Green Chem. 2017, 19, 2390–2397. [Google Scholar] [CrossRef]
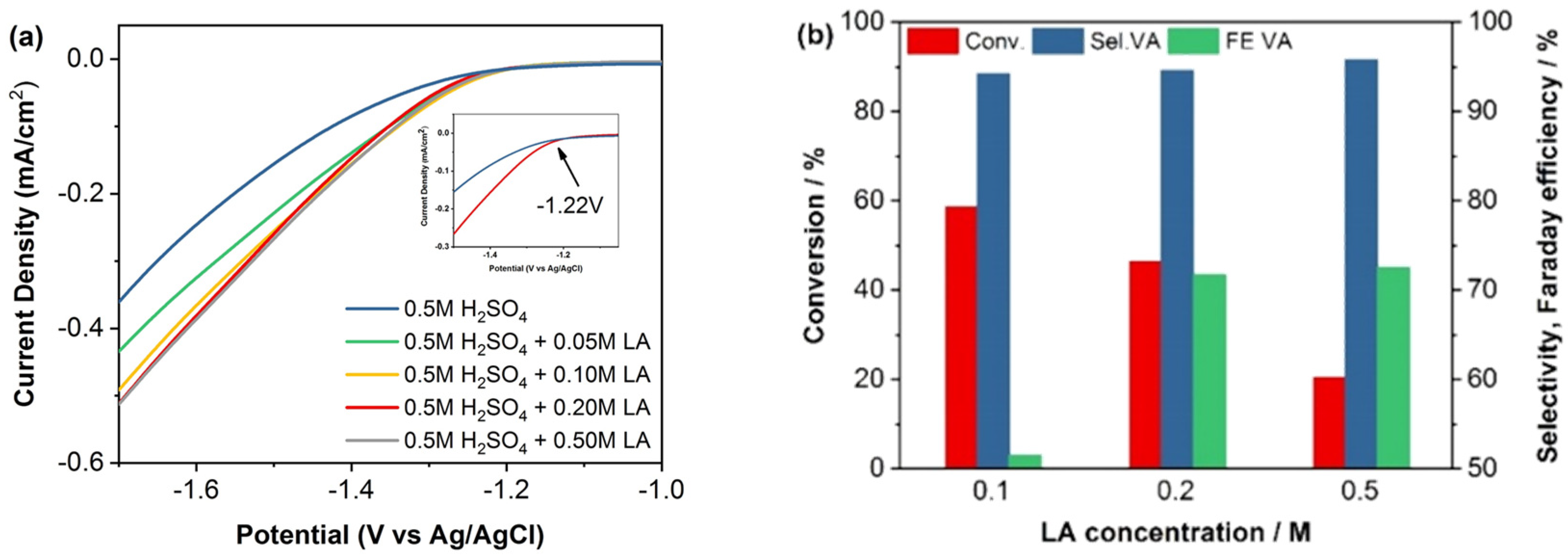
| Temperature [°C] | Conversion [%] | Selectivity VA [%] | Faradaic Efficiency VA [%] | Electrocatalytic Hydrogenation Rate [mmol L−1 min−1] |
|---|---|---|---|---|
| 25 | 46 | 94 | 72 | 14 |
| 50 | 56 | 97 | 72 | 18 |
| 65 | 66 | 96 | 74 | 21 |
| 80 | 68 | 91 | 59 | 23 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, Y.; Chen, X.; Qi, J.; Wang, P.; Liang, C. Synthesis of Valeric Acid by Selective Electrocatalytic Hydrogenation of Biomass-Derived Levulinic Acid. Catalysts 2020, 10, 692. https://doi.org/10.3390/catal10060692
Du Y, Chen X, Qi J, Wang P, Liang C. Synthesis of Valeric Acid by Selective Electrocatalytic Hydrogenation of Biomass-Derived Levulinic Acid. Catalysts. 2020; 10(6):692. https://doi.org/10.3390/catal10060692
Chicago/Turabian StyleDu, Yan, Xiao Chen, Ji Qi, Pan Wang, and Changhai Liang. 2020. "Synthesis of Valeric Acid by Selective Electrocatalytic Hydrogenation of Biomass-Derived Levulinic Acid" Catalysts 10, no. 6: 692. https://doi.org/10.3390/catal10060692
APA StyleDu, Y., Chen, X., Qi, J., Wang, P., & Liang, C. (2020). Synthesis of Valeric Acid by Selective Electrocatalytic Hydrogenation of Biomass-Derived Levulinic Acid. Catalysts, 10(6), 692. https://doi.org/10.3390/catal10060692







