Abstract
The major challenge in the production of xylene from benzene alkylation with methanol is to avoid the side reaction of methanol with olefins, and this leads to the low utilization efficiency of methanol and the generation of byproduct ethylbenzene. Hierarchical porous Ti-ZSM-5 with appropriate acidity was achieved by substituting part of Al by Ti in the synthesis process, which exhibited the high utilization efficiency of methanol and high suppression of the ethylbenzene formation by the efficient suppression of methanol to olefins.
1. Introduction
Xylene is an essential intermediate to produce fine chemicals [1]. In recent years, alkylation of benzene has received substantial attention due to its possible role in the production of xylene from fossil fuels [2,3]. Zeolite Socony Mobil-5 (ZSM-5) zeolite with excellent catalytic activity and selectivity is often considered as a good catalyst in industries [4]. However, the conversion of benzene and the selectivity of xylene are both low by using conventional ZSM-5 due to its intrinsic micropores [5,6]. Moreover, the remained methanol would convert into alkanes and alkenes by side reactions, which led to the ethylbenzene formation and the catalyst deactivation [7]. Unfortunately, it remains difficult to separate or remove ethylbenzene from C8 aromatic [8]. Recently, some studies indicated that HZSM-5 (hierarchical porous zeolite) could reveal excellent catalytic performance in the reaction of benzene alkylation with the introduction of the mesopores into zeolite framework [5,9]. However, the formation of ethylbenzene was not successfully inhibited even over HZSM-5 [6,10]. It is found that ethylene is produced from the competitive reaction of methanol to olefins for aromatics alkylation. Thus, the key to inhibit the ethylbenzene formation is to inhibit the reaction of methanol to olefins. So far, there has not been many studies that have shown a significant improvement in inhibiting the competitive reaction of methanol to olefins in benzene alkylation.
Modifying the acidity of ZSM-5 could change the distribution of products in many alkylation reactions, in which decreasing the Brönsted acid could improve the utilization of methanol [11]. However, using an impregnation method with metal nitrates to modify the acidity would result in the decrease in the pore diameter [12]. Moreover, modification of the acidity by the direct adjustment of the Si/Al ratio was not effective mainly due to the presence of hexadecyltrimethoxysilane (HTS) inhibiting the movement of Al in the framework for the healing the defective sites [13]. We noted that Ti could substitute Si at tetrahedral framework sites to form TS-1 with MFI topology and Al could incorporate with Ti simultaneously into the TS-1 framework in order to form the catalysts with bifunction [14,15]. Specifically, Ti could not form Brönsted acid sites [15]. These prompted us that using Ti to replace a part of Al might be able to synthesize the hierarchical porous Ti-ZSM-5 with relatively low acidity and avoid the change of the channel at the same time.
In this work, we synthesized hierarchical porous Ti-ZSM-5 by dry gel conversion method. For comparison, Ti-ZSM-5 with different Ti content were prepared using the same procedure and tested in the reaction of benzene alkylation with methanol. The effect of Ti substitution on the catalytic performance of the catalyst was investigated. It was observed that hierarchical porous Ti-ZSM-5 catalysts could improve the utilization efficiency of methanol and suppress the ethylbenzene formation. The corresponding mechanism was also discussed in detail.
2. Results and Discussion
2.1. Catalyst Characterization
Through XRD characterization (Figure 1), it can be seen that all of the samples with a different substitution amount of Ti exhibit the characteristic diffraction peaks at 2θ of 8.0°, 8.9°, 23.2°, 24.0° and 24.5°, which corresponds to the standard MFI crystalline structure [16]. Moreover, the relative crystallinities of all Ti-ZSM-5 samples were nearly identical, indicating that incorporating Ti into ZSM-5 had no effect on the crystallinity of the synthesized samples. SEM images also proved that the hierarchical porous structure of the samples remained unchanged with the substitution of Ti (Figure S1).
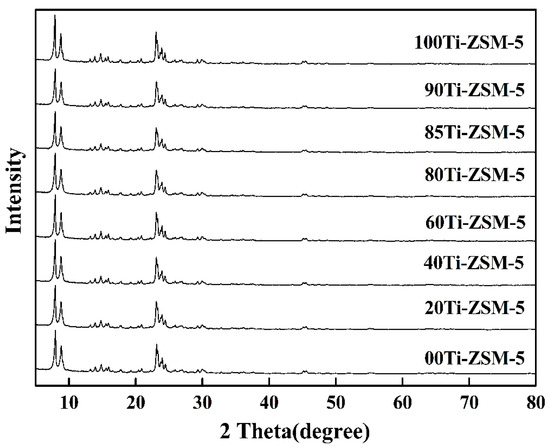
Figure 1.
X-Ray diffractograms of hierarchical porous Ti-ZSM-5.
Figure 2 shows the UV-Vis analyses of Ti-ZSM-5 samples. The presence of an absorption peak at 205–220 nm and the absence of a peak at 330 nm revealed that Ti-ZSM-5 mainly contained the framework Ti, and was free of non-desired extra framework TiO2 phases [17]. The increase in absorption peak at 205–220 nm indicated that more Ti was present in the framework [18].
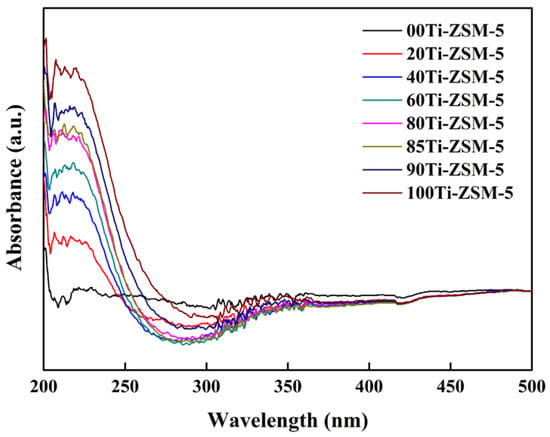
Figure 2.
Diffuse reflectance UV-Vis absorbance spectra of hierarchical porous Ti-ZSM-5.
Table 1 shows the element molar ratio in gel and surface of Ti-ZSM-5 was also summarized. It can be observed that the amount of Ti in Ti-ZSM-5 increased with an increase in the substitution amount of Al by Ti (consistent results by ICP-AES in Table S1). It is noteworthy that the Ti content in the outer shell of the crystals was slightly smaller than that in the gel and the Al content showed the opposite trend. This might be ascribed to the fact that Al limited the presence of Ti in the zeolite and tended to be present on the rim of the crystal [14,19]. Although 100Ti-ZSM-5 was absent of Al, the relatively smaller Ti content in the surface might result from the loss of a small amount of Ti in the process of synthesis. SEM-EDS mapping also proved that Ti was well distributed on the surface of zeolite (Figure S2).

Table 1.
Element molar ratio in gel and surface of hierarchical porous Ti-ZSM-5.
The Brunauer–Emmett–Teller (BET) surface area and pore size of the synthesized catalysts were calculated by N2 adsorption isotherms and desorption isotherms (Table 2). The results indicated that substituting part of Al by Ti had no major influence in the BET surface area (~450.0 m2/g) and pore size (~4 nm) and all the synthesized samples were with mesopores.

Table 2.
Textural properties of hierarchical porous Ti-ZSM-5 catalysts.
NH3-TPD was performed to study the effect of the substitution of Ti on the acidity of the catalyst. As shown in Figure 3, there are two peaks of NH3 desorption which correspond to stronger (situated above 300 °C) and weaker acidic sites (located below 200 °C), respectively. With the increase in Ti content, the peak area between 400 and 500 °C decreased, indicating the reduction in the number of stronger acidic sites of the samples. Replacing Si with Al in zeolite framework can create a defect (acidic site) [20]. With more Al substituted by Ti, the number of acidic sites was reduced due to the reason that titanium could not form the substitutional defect [21]. According to the results above, we could conclude that the substitution of Al by Ti was an effective way to control the acidity of the samples without the adverse effect on the diffusion of the catalyst.
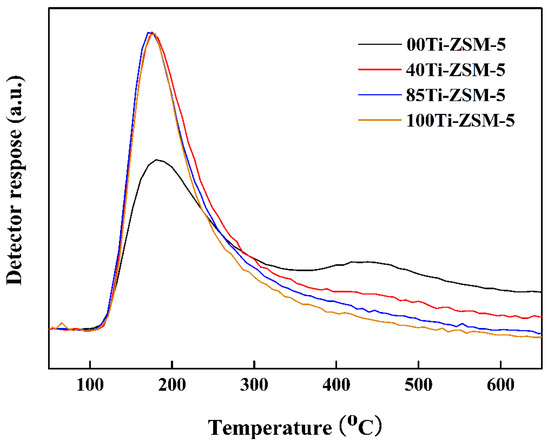
Figure 3.
NH3-TPD patterns of hierarchical porous Ti-ZSM-5.
2.2. Benzene Alkylation with Methanol
Table 3 shows the catalytic activity of hierarchical porous Ti-ZSM-5 in the alkylation of benzene with methanol. It is found that the selectivity to ethylbenzene reduced from 3.88 to 0.13% and the conversion of benzene and the selectivity to xylene rose from 48.94 to 57.25% and 33.02 to 37.87%, respectively, after substituting more Ti from 0 to 85%, suggesting that the Ti substitution on the framework of HZSM-5 could suppress the ethylbenzene formation and improve the production of xylene. However, increasing the substitution content of Ti to 90% caused the benzene conversion to drop. Therefore, 85% of Ti was considered as the best substitution content for the synthesized Ti-ZSM-5 catalyst and was selected as a representative in later analysis.

Table 3.
Catalytic activity of hierarchical porous Ti-ZSM-5 samples in benzene alkylation with methanol.
In order to investigate the suppression mechanism of hierarchical porous Ti-ZSM-5 on ethylbenzene formation, we carefully compared the products’ distribution of hierarchical porous ZSM-5 (00Ti-ZSM-5) and hierarchical porous Ti-ZSM-5 (85Ti-ZSM-5) (Table 4). As shown in Table 4, the products using hierarchical porous 85Ti-ZSM-5 catalyst contain more alkyl aromatics and less light alkenes and alkanes. According to literatures, ethylene converted from methanol by the reaction of methanol to olefins is the major reactant for the ethylbenzene formation via the alkylation of benzene and ethylene [6,7]. Moreover, ethylene could further alkylate with methanol to produce heavier olefins which further leading to the coke formation (large molecules or aromatics) via polymerization [7,22,23]. Differential thermal analysis (TG-DTA) was conducted to evaluate the content of coke on the catalyst. As seen in Figure 4b, it was clear that the coke content on 85Ti-ZSM-5 (1.86%) was slightly smaller than that of 00Ti-ZSM-5 (2.40%), indicating that the total content of olefins on synthesized Ti-ZSM-5 reduced as there was not more olefins transformed into coke. Therefore, we could conclude that the suppression of methanol to olefins was successful by the substitution of Al by Ti in the synthesis process. Furthermore, the ethylbenzene formation was also suppressed due to the success of suppressing the reaction of methanol to olefins and methanol might be utilized more efficiently (Table 5).

Table 4.
Products distribution of hierarchical 00Ti-ZSM-5 and hierarchical 85Ti-ZSM-5 in benzene alkylation with methanol.
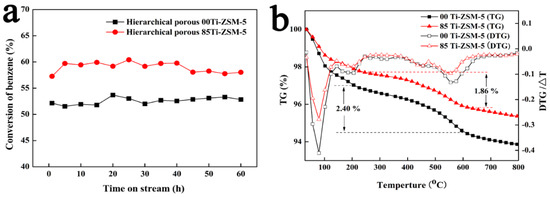
Figure 4.
Stability of hierarchical porous Ti-ZSM-5 samples (a) and TG-DTA profiles of the catalysts after successive reaction (b).

Table 5.
Utilization efficiency of methanol of hierarchical porous Ti-ZSM-5 catalysts.
The high activity of 85Ti-ZSM-5 (Table 3) clearly showed that the acidity of the sample was sufficient to catalyze the alkylation of aromatics but insufficient to catalyze the reaction of methanol to olefins, although Brönsted acid was the key to catalyze both the reaction of methanol to olefins and the alkylation of aromatics [7]. This result suggested that only a small number of acid sites were necessary for benzene alkylation with methanol and this was consistent with the result reported by Adebajo et al. [24]. The excess acidity on hierarchical porous Ti-ZSM-5 played a major role in catalyzing the competitive reaction of methanol to olefins during benzene alkylation with methanol. We also noted that increasing the substitution content of Ti to 90% caused a dramatic drop in the conversion of benzene, indicating that the acidity of 90Ti-ZSM-5 was no longer sufficient to catalyze the alkylation of benzene (Table 3). Moreover, an amount of methanol and dimethyl ether converted from methanol which was catalyzed by Lewis acid was found in the products, indicating that the acidity of 90Ti-ZSM-5 was also not sufficient enough to catalyze the reaction of methanol to olefins. Therefore, it is reasonable enough to conclude that the acidity of HZSM-5 is the important factor determining the catalytic activity on benzene alkylation with methanol. The substitution of Al by Ti in hierarchical porous Ti-ZSM-5 could sufficiently control the acidity of the catalyst and 85Ti-ZSM-5 was at the most appropriate acidity for our chosen reaction.
It is worth noting that although the benzene conversion of 85Ti-ZSM-5 remained between 58 and 60% during the first 40 h reaction on stream, the conversion of benzene showed a slight decrease in the next 20 h (Figure 4a). However, the benzene conversion of 00Ti-ZSM-5 showed the better stability in the 60 h reaction on stream. Comparing the content of coke on 85Ti-ZSM-5 and 00Ti-ZSM-5 (Figure 4b), the carbon deposition on 85Ti-ZSM-5 was still serious. Schmidt et al. [25] had reported that the coke distribution for HZSM-5 showed a homogeneous distribution of coke residuals over both inside and outside of the particle due to less diffusion restraints and the strong acid sites of deactivated material were not available for ammonia anymore due to the coverage of coke. The deactivation of 85Ti-ZSM-5 might be attributed to less strong acidic sites while 00Ti-ZSM-5 contained more strong acidic sites which could resist more coke. Therefore, reducing the amount of strong acid sites of HZSM-5 is favorable to suppress the side reaction of methanol to olefins but unfavorable to resist the carbon deposition, and it is important to investigate the method for suppressing coke formation on the HZSM-5 in future work.
3. Experimental
3.1. Catalyst Preparation
Solvent evaporation assisted dry-gel route was used to prepare required Ti-ZSM-5 catalysts in our experiments [6,13,26]. The reaction solution consisted of tetraethylorthosilicate (TEOS), aluminum isopropoxide (AIP), tetraethyl orthotitanate (TEOT), tetra-n-propylammonium hydroxide (TPAOH), hexadecyltrimethoxysilane (HTS) and ethanol (EtOH) with the molar ratio of 1:0.0056 (1−x):0.0056x:0.2:0.05:15, respectively. x represents the different substitution of Ti content (n(Ti)/n(Al + Ti)) which was equal to 0, 0.2, 0.4, 0.6, 0.8, 0.85, 0.9, and 1.0.
The solid gel was obtained through stirring of the reaction solution with the above composition. The gel was then air dried and synthesized at 180 °C for 72 h inside an autoclave. The autoclave was made of stainless steel with a PTFE liner equipped. The product was then dried after being filtered and calcined for 7 h. The dry temperature was 110 °C and the calcinating temperature was 550 °C (every 10 °C per min), respectively.
3.2. Catalyst Characterization
X-ray diffraction (XRD, SCINTAG X” TRA (Thermo Fisher, Waltham, MA, USA) with Cu Kα-radiation (1542 A), at 30 mA and 40 kV, scanning angle (2θ) = 5°–80°) was used to measure the crystallinity. ARL ADVANT’ X IntelliPower TM 4200 from Thermo Fisher (Waltham, MA, USA) was used to conduct X-ray fluorescence spectrum (XRF). Brunauer–Emmett–Teller (BET) data were measured by nitrogen physical adsorption–desorption obtained from a NOVA 1000 e surface area analyzer (Quantachrome, Shanghai, China) at 77 K. Diffuse reflectance UV-VIS spectra (DR UV-VIS) were measured by a UV-2550 spectrophotometer (Shimadzu, Tyoto, Japan) at ambient conditions. Differential thermal analysis (TG-DTA) was measured by Netzsch STA 449 C apparatus (Selb/Bayern, Germany). The temperature was heated from 30 to 800 °C (every 10 °C per min) in O2. Ammonia temperature-programmed desorption (NH3-TPD) was measured using QIC20 Characterization System (Hiden, Beijing, China). The evacuation of the sample was conducted for 1 h at 400 °C in He before cooling down to 50 °C. The adsorption of NH3 was conducted at 50 °C in 10 wt% NH3 and He co-flow. The desorption was conducted from 50 to 650 °C at 10 °C per min.
3.3. Catalytic Activity Test
The fixed-bed reactor with continuous-flow was used to carry out all tests at 1 atm. It was equipped with a stainless-steel tube with inner diameter of 8 mm. An amount of 0.5 g of catalyst was firstly mixed with 5.0 g inert quartz sand at the beginning of each test before loading into the reactor. The temperature of the catalyst was monitored through the thermocouple in the bed center. The sample was heated in situ to 400 °C with 10 °C per min and then maintained for 3 h in N2 with a space velocity of 2400 h−1. A 1:1 molar ratio of Benzene/Methanol mixture with WHSV = 2.0 h−1 was later fed into the fix-bed reactor with 40 mL/min of N2. Online analysis of the effluent was determined by the gas chromatography and a flame ionization detector. The chromatography from Fuli (model GC9790, Wenling, China) was equipped with a DB-1 capillary column (30 m × 0.25 mm × 1.00 μm). The heating belt was used to ensure the gas phase of the products by maintaining the effluent line at 200 °C. Conversion and selectivity of some targeted chemicals were defined in Supplementary Materials.
4. Conclusions
Hierarchical porous Ti-ZSM-5 catalysts exhibited excellent ability to suppress the side reaction of methanol to olefins and this effect could be ascribed to the substitution of Al by Ti which effectively decreased the acidity of the samples. The suppression of the reaction of methanol to olefins not only could help to improve the utilization efficiency of methanol, but also could suppress the ethylbenzene formation via decreasing the amount of ethylene. Using a simple modifying technique which substitutes a part of Al with Ti in the synthesis process could produce a highly active catalyst for benzene alkylation with methanol to produce xylene and improve the reaction’s commercial viability.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4344/10/6/693/s1, Table S1: Composition of Ti in hierarchical porous Ti-ZSM-5 catalysts, Figure S1: SEM images of hierarchical porous Ti-ZSM-5: a.20Ti-ZSM-5 b.40Ti-ZSM-5 c.60Ti-ZSM-5, Figure S2: SEM-EDS mapping (Si, Al and Ti) of 40Ti-ZSM-5 catalyst.
Author Contributions
Conceptualization, X.L. F.F. and C.L.; methodology, J.C.; formal analysis, N.Y.; investigation, H.H.; resources, J.C. and X.L.; writing—original draft preparation, N.Z. and J.C.; writing—review and editing, J.C. and N.Z.; supervision, J.C. and Z.L. and N.Y.; project administration, L.Y.; funding acquisition, F.F. and C.L.; All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Educational Commission of Zhejiang Province of China (Y201534876) and National Natural Science Foundation of China (21776258, 21978265) and the Technology Department of Zhejiang Province (LGG18B060004).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Alabi, W.; Atanda, L.; Jermy, R.; Al-Khattaf, S. Kinetics of toluene alkylation with methanol catalyzed by pure and hybridized HZSM-5 catalysts. Chem. Eng. J. 2012, 195, 276–288. [Google Scholar] [CrossRef]
- Dong, P.; Zhang, Y.; Li, Z.; Yong, H.; Li, G.; Ji, D. Enhancement of the utilization of methanol in the alkylation of benzene with methanol over 3-aminopropyltriethoxysilane modified HZSM-5. Catal. Commun. 2019, 123, 6–10. [Google Scholar] [CrossRef]
- Wang, X.; Xu, J.; Qi, G.; Li, B.; Wang, C.; Deng, F. Alkylation of benzene with methane over ZnZSM-5 zeolites studied with solid-state NMR spectroscopy. J. Phys. Chem. C 2013, 117, 4018–4023. [Google Scholar] [CrossRef]
- Inagaki, S.; Shinoda, S.; Kaneko, Y.; Takechi, K.; Komatsu, R.; Tsuboi, Y.; Yamazaki, H.; Kondo, J.N.; Kubota, Y. Facile fabrication of ZSM-5 zeolite catalyst with high durability to coke formation during catalytic cracking of paraffins. ACS Catal. 2013, 3, 74–78. [Google Scholar] [CrossRef]
- Christensen, C.H.; Johannsen, K.; Schmidt, I.; Christensen, C.H. Catalytic benzene alkylation over mesoporous zeolite single crystals: Improving activity and selectivity with a new family of porous materials. J. Am. Chem. Soc. 2003, 125, 13370–13371. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, Q.; Cen, J.; Li, X. Catalytic activity of Pt modified hierarchical ZSM-5 catalysts in benzene alkylation with methanol. Catal. Lett. 2014, 145, 715–722. [Google Scholar] [CrossRef]
- Anderson, J.R.; Mole, T.; Christov, V. Mechanism of some conversions over ZSM-5 catalyst. J. Catal. 1980, 61, 477–484. [Google Scholar] [CrossRef]
- Rui, J.; Lyu, J.; Hu, H.; Zhang, Q.; Wang, Q.; Li, X. Synthesized high-silica hierarchical porous ZSM-5 and optimization of its reaction conditions in benzene alkylation with methanol. Chin. Chem. Lett. 2019, 30, 757–761. [Google Scholar] [CrossRef]
- Christensen, C.H.; Johannsen, K.; Törnqvist, E.; Schmidt, I.; Topsøe, H.; Christensen, C.H. Mesoporous zeolite single crystal catalysts: Diffusion and catalysis in hierarchical zeolites. Catal. Today 2007, 128, 117–122. [Google Scholar] [CrossRef]
- Hu, H.; Lyu, J.; Rui, J.; Cen, J.; Zhang, Q.; Wang, Q.; Han, W.; Li, X. The effect of Si/Al ratio on the catalytic performance of hierarchical porous ZSM-5 for catalyzing benzene alkylation with methanol. Catal. Sci. Technol. 2016, 6, 2647–2652. [Google Scholar] [CrossRef]
- Zhao, S.F.; Yao, X.T.; Yan, B.H.; Li, L.; Liu, Y.M.; He, M.Y. Flexible regulation of C3=/C2= ratio in methanol-to-hydrocarbons by delicate control of acidity of ZSM-5 catalyst. Chin. Chem. Lett. 2017, 28, 1318–1323. [Google Scholar] [CrossRef]
- Li, J.; Xiang, H.; Liu, M.; Wang, Q.; Zhu, Z.; Hu, Z. The deactivation mechanism of two typical shape-selective HZSM-5 catalysts for alkylation of toluene with methanol. Catal. Sci. Technol. 2014, 4, 2639–2649. [Google Scholar] [CrossRef]
- Deng, W.; Xuan, H.; Zhang, C.; Gao, Y.; Zhu, X.; Zhu, K.; Huo, Q.; Zhou, Z. Promoting xylene production in benzenemethylation using hierarchically porous ZSM-5 derived from a modified dry-gel route. Chin. J. Chem. Eng. 2014, 22, 921–929. [Google Scholar] [CrossRef]
- Ovejero, G.; van Grieken, R.; Uguina, M.A.; Serrano, D.P.; Melero, J.A. Study on the Ti and Al coincorporation into the MFI zeolitic structure. J. Mater. Chem. 1998, 8, 2269–2276. [Google Scholar] [CrossRef]
- Shen, L.; Deng, X.; Liu, Y. Synthesis and catalytic oxidation performance of Al–TS-1. Chin. J. Catal. 2013, 34, 1232–1241. [Google Scholar] [CrossRef]
- Wu, E.L.; Lawton, S.L.; Olson, D.H.; Rohrman, A.C.; Kokotailo, G.T. ZSM-5-type materials. Factors affecting crystal symmetry. J. Phys. Chem. 1979, 83, 2777–2781. [Google Scholar] [CrossRef]
- Serrano, D.; Sanz, R.; Pizarro, P.; Moreno, I. Turning TS-1 zeolite into a highly active catalyst for olefin epoxidation with organic hydroperoxides. Chem. Commun. 2009, 11, 1407–1409. [Google Scholar] [CrossRef]
- Choi, K.-M.; Yokoi, T.; Tatsumi, T.; Kuroda, K. A novel route for preparation of Ti-containing mesoporous silica with high catalytic performance by using a molecular precursor tetrakis(tris-tert-butoxysiloxy)titanium. J. Mater. Chem. A 2013, 1, 2485–2494. [Google Scholar] [CrossRef]
- Danilina, N.; Krumeich, F.; Castelanelli, S.A.; van Bokhoven, J.A. Where are the active sites in zeolites? Origin of aluminum zoning in ZSM-5. J. Phys. Chem. C 2010, 114, 6640–6645. [Google Scholar] [CrossRef]
- Moors, S.L.C.; De Wispelaere, K.; Van der Mynsbrugge, J.; Waroquier, M.; Van Speybroeck, V. Molecular dynamics kinetic study on the zeolite-catalyzed benzene methylation in ZSM-5. ACS Catal. 2013, 3, 2556–2567. [Google Scholar] [CrossRef]
- Drago, R.S.; Dias, S.C.; McGilvray, J.M.; Mateus, A.L.M.L. Acidity and hydrophobicity of TS-1. J. Phys. Chem. B 1998, 102, 1508–1514. [Google Scholar] [CrossRef]
- Hu, H.L.; Zhang, Q.; Cen, J.; Li, X. High suppression of the formation of ethylbenzene in benzene alkylation with methanol over ZSM-5 catalyst modified by platinum. Catal. Commun. 2014, 57, 129–133. [Google Scholar] [CrossRef]
- Zhao, Y.; Tan, W.; Wu, H.; Zhang, A.; Liu, M.; Li, G.; Wang, X.; Song, C.; Guo, X. Effect of Pt on stability of nano-scale ZSM-5 catalyst for toluene alkylation with methanol into p-xylene. Catal. Today 2011, 160, 179–183. [Google Scholar] [CrossRef]
- Adebajo, M.O.; Howe, R.F.; Long, M.A. Methylation of benzene with methanol over zeolite catalysts in a low pressure flow reactor. Catal. Today 2000, 63, 471–478. [Google Scholar] [CrossRef]
- Schmidt, F.; Hoffmann, C.; Giordanino, F.; Bordiga, S.; Simon, P.; Carrillo-Cabrera, W.; Kaskel, S. Coke location in microporous and hierarchical ZSM-5 and the impact on the MTH reaction. J. Catal. 2013, 307, 238–245. [Google Scholar] [CrossRef]
- Zhu, K.; Sun, J.; Liu, J.; Wang, L.; Wan, H.; Hu, J.; Wang, Y.; Peden, C.H.F.; Nie, Z. Solvent evaporation assisted preparation of oriented nanocrystalline mesoporous MFI zeolites. ACS Catal. 2011, 1, 682–690. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).