1. Introduction
Cheno- and ursodeoxycholic acid (CDCA and UDCA, respectively) are used for the treatment of cholestatic diseases by reducing the absorption of cholesterol and improving the digestion of fatty acids through stimulation of the liver functions [
1,
2,
3,
4]. CDCA can be produced via dehydroxylation of the readily available cholic acid (CA) at C
12, and subsequent epimerization at C
7 affords UDCA.
However, chemical synthesis routes for the conversion of CA to CDCA or UDCA employ toxic chemicals in stochiometric amounts, suffer from low yields and lead to the formation of large amounts of waste. Despite these disadvantages, the chemical transformation of CA to CDCA or UDCA is still currently used, making a replacement with a benign approach urgent [
5,
6,
7].
Alternatively, the dehydroxylation at C
12 can be performed in a two-step chemo-enzymatic reaction employing: (i) the selective oxidation of the 12-OH group to form 12-oxo-CDCA catalyzed by the enzyme 12α-hydroxysteroid dehydrogenase (12α-HSDH,
Scheme 1); (ii) the Wolff-Kishner reduction of 12-oxo-CDCA or 12-oxo-UDCA to form CDCA or UDCA, respectively.
12α-HSDHs specifically oxidize the C
12 hydroxyl group to a ketone, with a concomitant reduction of NAD
+ [
5]. To obtain an economical and sustainable process, a regeneration system for the nicotinamide cofactors is required. Previously, the enzyme NAD(P)H oxidase (NOX) was applied for the regeneration of NAD
+ in the enzymatic preparation of 12-oxo-CDCA [
6]. NOX uses O
2 for the oxidation of NAD(P)H and only generates water as a side product. However, NOX regeneration systems suffer from low activity, low stability and high costs. Moreover, the substrate loading is limited to 10 mM.
Further NAD(P)
+ regeneration systems, such as glutamate dehydrogenase,
iPrOH (alcohol) dehydrogenase and lactate dehydrogenase were recently reviewed for the enzymatic synthesis of mono- and diketo hydroxysteroids [
7]. These methods involve the reduction of an organic sacrificial substrate (α-ketoglutarate, acetone or pyruvate, respectively) to the corresponding alcohol/amine. Other solutions for the regeneration of NAD
+, employing molecular O
2 as final electron acceptor, were also identified. Profiting from the spontaneous auto-oxidation of FMN, a flavin reductase was employed to regenerate NAD
+ in the biocatalytic oxidation of CDCA to 7-oxo-LCA [
8]. Laccase/mediator systems have been similarly applied in the same reaction [
9,
10]. Specifically, laccases catalyze the oxidation of several low molecular weight compounds, which function as mediators by spontaneously oxidizing NADH without interfering with the enzymatic oxidation of CDCA [
9,
11,
12,
13].
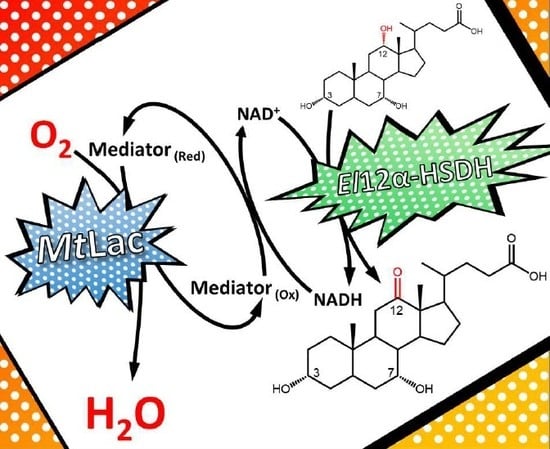
To develop a feasible, preparative-scale oxidation of CA to 12-oxo-CDCA relying on oxygen as oxidant, the laccase/mediator system was coupled with the
El12α-HSDH enzyme (
Scheme 1).
The influence of several factors on the enzymatic activity and stability of the system were tested (pH values, different mediator types and cosolvents, enzyme and mediator concentrations and substrate loading). Finally, a preparative scale reaction was performed under optimized conditions, employing a substrate loading of 120 mM CA (corresponding to 50 g/L) with as little as 150 μM of syringaldazine as mediator.
The production of the enzyme El12α-HSDH was improved, and the overall environmental impact was analyzed to ensure a functional process.
2. Results
2.1. El12α-HSDH Expression Trials
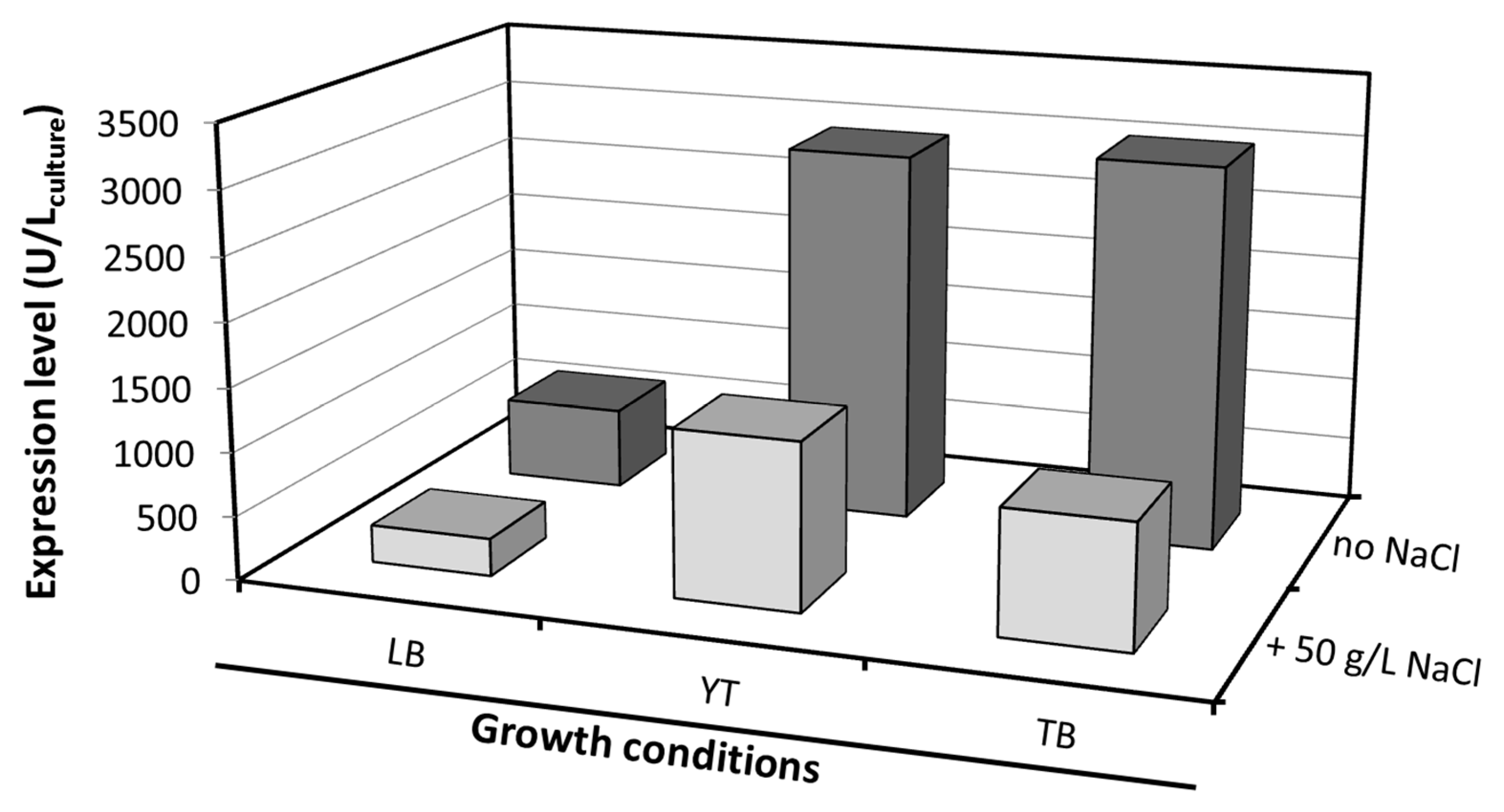
Expression trials were initially carried out to optimize the heterologous production of El12α-HSDH in E. coli. Several modifications of the commonly used Lysogeny Broth (LB) medium have been described with the aim of increasing cell yield, such as supplementing both fermentable and non-fermentable carbon sources and phosphate buffers. Based on this, different media compositions and the addition of NaCl at the moment of induction were investigated. The expression of El12α-HSDH enzyme was investigated by measuring its activity in the crude extract.
The enzymatic activity varied considerably depending on the type of media used. A high expression of
El12α-HSDH was measured in YT (2928 U/L
broth) and TB (3020 U/L
broth) medium (
Figure 1). The addition of NaCl to the medium subjects the cells to osmotic stress, and should have a positive effect on protein expression [
14]. In this case, a negative effect was observed, lowering the expression of
El12α-HSDH in all the three different media. Overall, a five-fold increase in volumetric expression was obtained, in comparison with the previously reported expression in LB medium (638 U/L
broth) [
6].
2.2. Laccase/Mediator System for the Regeneration of NAD+
To establish a suitable and efficient regeneration system for NAD
+, several laccases from different origins were tested for their ability to oxidize NADH with three different mediators (acetosyringone, syringaldehyde and syringaldazine,
Table S1). Laccase activity was assayed in the presence of 2 μM mediator. The spectrophotometric assay was carried out at two different pH values (5 and 8) by following the consumption of NADH at 340 nm (
Figure 2).
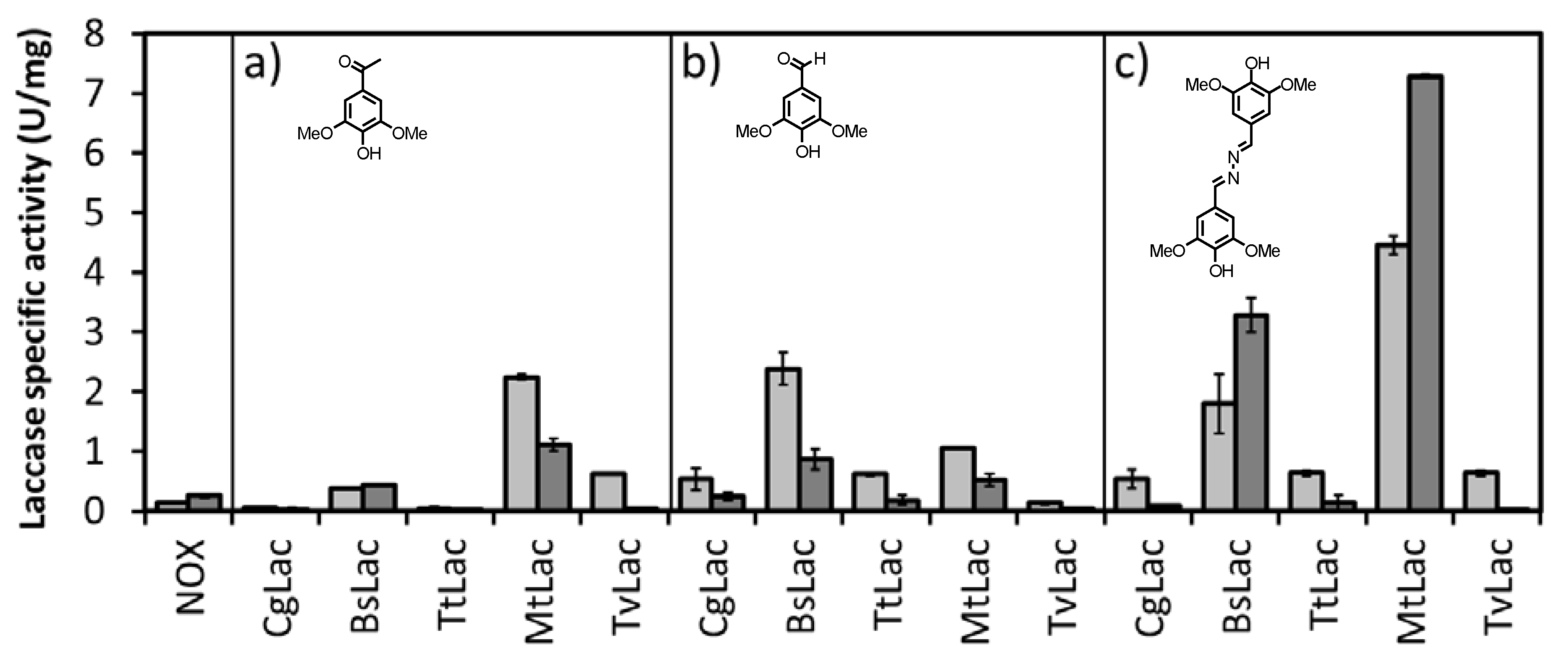
The laccase from
Myceliophthora thermophilia (
MtLac) showed the highest activity at pH 8.0 (
El12α-HSDH is active and stable at this pH) [
6], displaying 30-fold higher activity than the NOX system.
MtLac is a commercially available enzyme, with a moderate-low price. Its safety in use, high activity and stability have been reported for other applications [
15]. Therefore,
MtLac was chosen for the subsequent studies towards the development of the proposed biocatalytic cascade.
In the next step, a wider selection of mediators was investigated with
MtLac (
Table S2). In this case, we chose to directly evaluate the bio-production of the desired 12-oxo-CDCA through incubation of 10 mM CA with
El12α-HSDH, in the presence of 0.5 mM of NAD
+, laccase (0.34 mg/mL) and mediators (all at a concentration of 5 µM) at pH 8.0. High conversions of CA to 12-oxo-CDCA were obtained within 4 h with 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS) and syringaldazine as mediators, leading to 99% and 95% conversion, respectively. In contrast to previous studies, low reaction rates were observed with Meldola’s Blue [
9]. This difference can be attributed to the different type of laccases used and to the lower concentration of mediator in the bioconversion (
Table S2). To compare the different mediators, the specific activity of the different
MtLac/mediator systems was evaluated by a spectrophometric assay, following the oxidation of NADH at 340 nm (
Figure 3). A high activity was observed when syringaldazine or ABTS were used as mediator, confirming the results obtained for the conversion of CA. Notably, syringaldazine is slowly oxidized by molecular oxygen and shows absorbance at 340 nm for different redox states. This results in an apparent background activity when syringaldazine is used as mediator [
13]. Thus, ABTS and syringaldazine were selected for subsequent investigations.
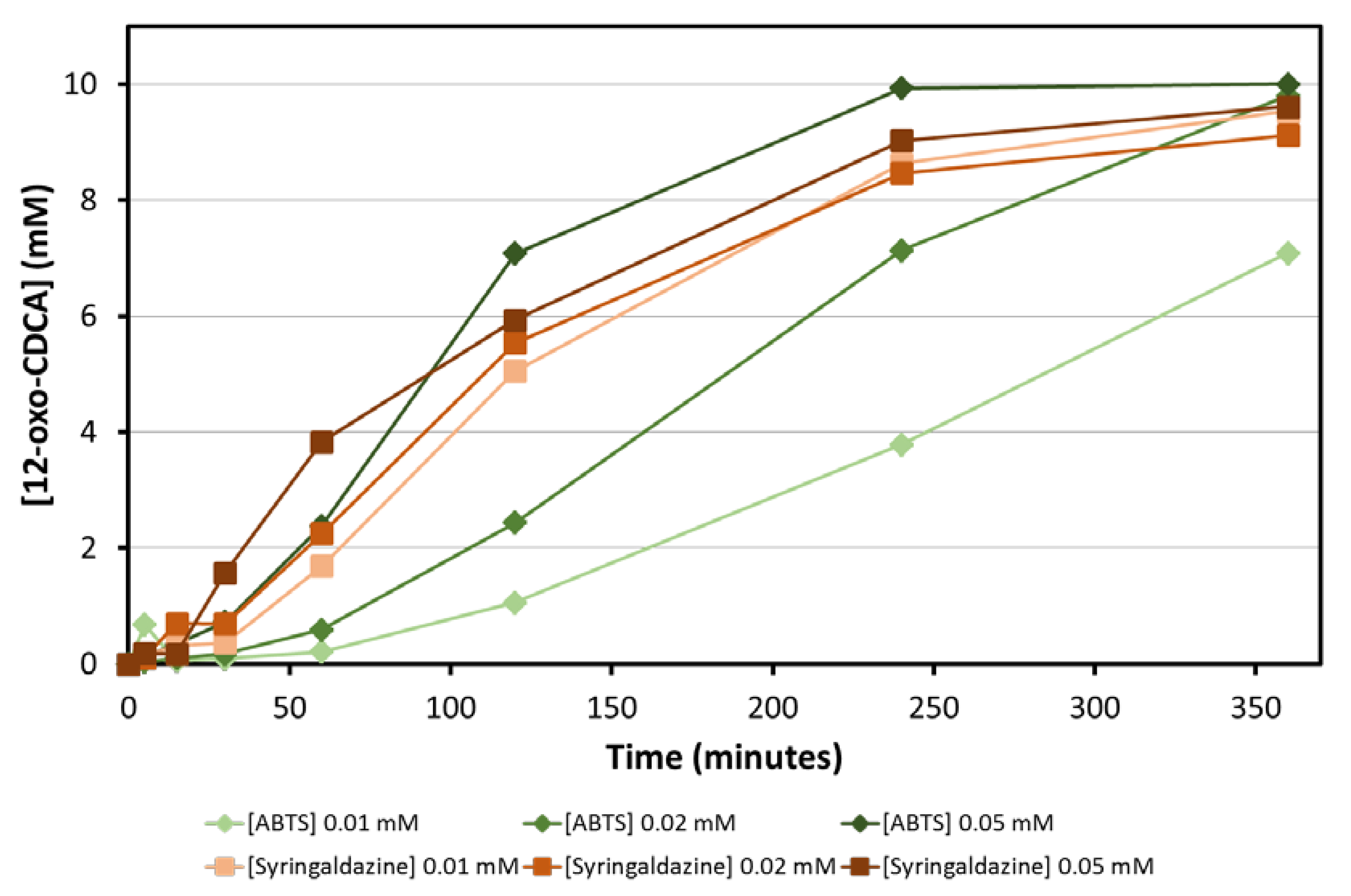
The amount of mediator is another parameter that needs to be optimized for the bioconversion: ideally, mediators should be used at the lowest concentration possible, in order to obtain a clean reaction and minimize the generation of waste. Bioconversions of CA with three different concentrations of ABTS and syringaldazine (0.01, 0.02 and 0.05 mM) were followed over time. Full conversion of 10 mM CA was obtained for all combinations within 6 h. While bioconversions with syringaldazine as mediator are faster and less dependent on the concentration of mediator used, bioconversions with ABTS are slower and the rate of the reaction depends on the concentration of mediator used (
Figure 4). This can be attributed to the higher K
M of fungal laccases, such as
MtLac for ABTS at basic pH values [
16].
The pH optimum of
MtLac depends on the respective substrate/mediator pair used and was previously reported by Berka et al. [
17]. When ABTS is used as substrate,
MtLac shows optimal activity in the acidic range (3–6), which is mismatched with the pH optimum of HSDHs (pH 7.0–9.0). In contrast,
MtLac shows maximal activity around pH 7.0–7.5 when syringaldazine is used as mediator. Consequently, a pH value of 7.2 was chosen for further studies. Notably, CA is comparably soluble at pH 7.2 and 8.0 (pKa = 4.6) [
18,
19].
2.3. Substrate Loading and Cosolvent
Preceding publications have highlighted the low solubility of hydroxysteroids in aqueous media as the main bottleneck for the biocatalytic preparation of 12-oxo-hydroxysteroids [
5,
20]. Having established the laccase/mediator system as a suitable way to regenerate NAD
+, our focus changed toward increasing the substrate loading. Both monophasic and biphasic cosolvent systems have been shown to increase the substrate loading of hydroxysteroids in aqueous environments. However, the addition of organic solvents to the reaction mixture can influence the enzymatic activities [
21].
Based on preliminary studies, MeOH and DMSO were selected as cosolvents and their effect on the activities of the enzymes involved in the biotransformation was investigated. Bioconversions with 20, 50, 100 and 250 mM of CA were set up in the presence of MeOH (10%, 20% and 40%) and DMSO (10%). Notably, 10% of DMSO was enough to fully solubilize 250 mM of CA, and 85.8 mM of 12-oxo-CDCA were obtained. For this reason, higher concentrations of DMSO were not investigated.
In contrast, >10% methanol is needed to increase the substrate concentration above 10 mM, resulting in a loss of activity and thus lower conversions (
Table 1). Previous studies showed no decrease of activity for
El12α-HSDH in the presence of 10% MeOH [
6], while
MtLac activity was reported to decrease over time when incubated for long periods with MeOH [
22].
According to the GlaxoSmithKline (GSK) solvent sustainability guide [
23,
24], DMSO shows moderate complications when used at low temperatures. The main disadvantage consists in its difficult removal from the product mixture due to a high boiling point. However, since it enables a high enzyme activity as well as a better solubilization of CA in water, it was chosen as cosolvent for the preparative scale production of 12-oxo-CDCA.
2.4. 20 g Scale Preparation of 12-oxo-CDCA
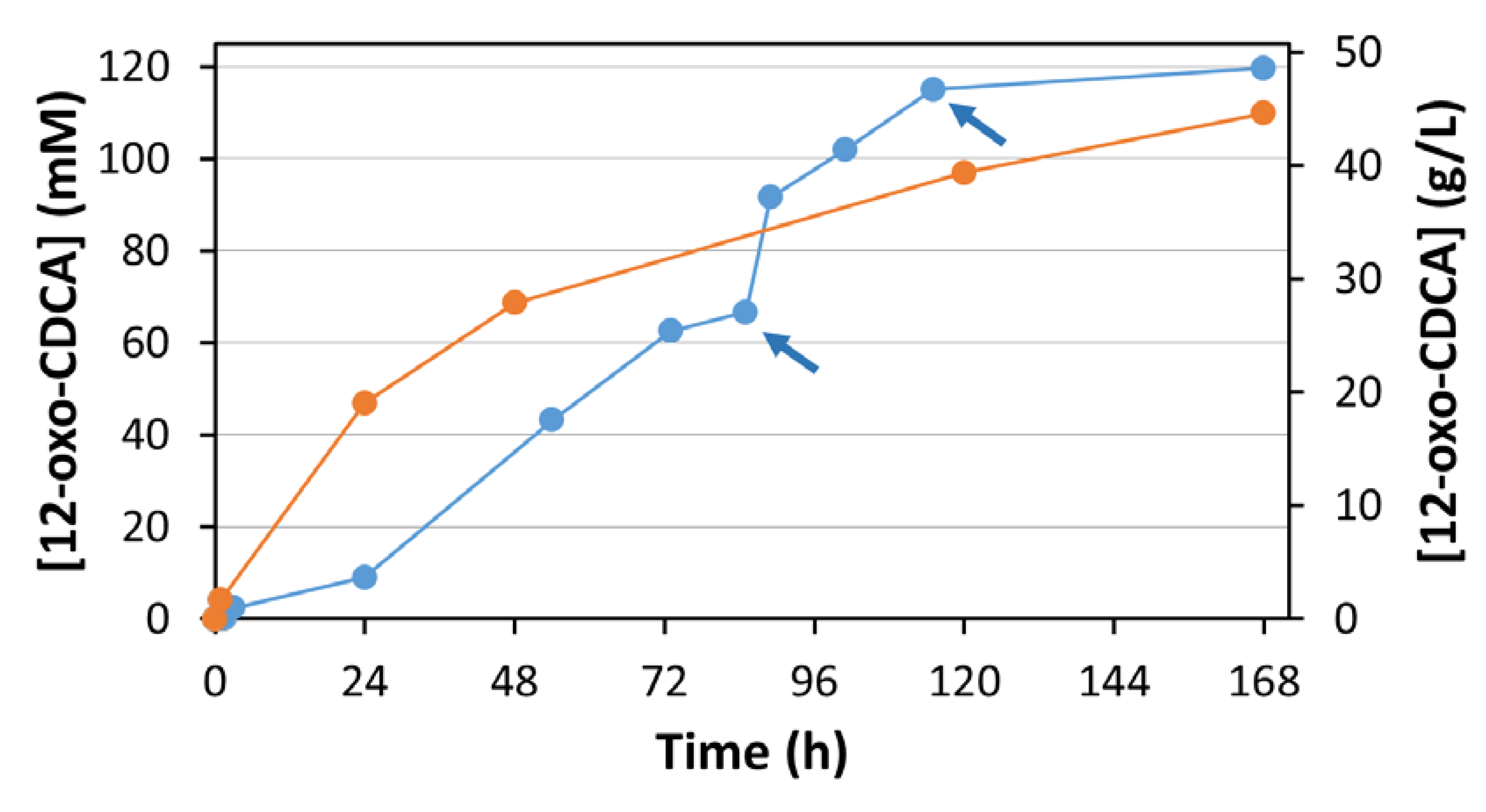
To demonstrate the synthetic potential of the proposed system, a preparative scale bioconversion was carried out. 20 g of CA were dissolved in 100 mM KPi buffer, pH 7.2 in the presence of 10% DMSO, resulting in a final concentration of 50 g/L for CA (corresponding to 120 mM).
The solution was initially heated in order to completely dissolve the CA. Once the solution reached homogeneity, the mixture was cooled (25 °C) and supplemented with 0.5 mM NAD+, 50 μM of syringaldazine, 0.013 mg/mL (0.8 U/mL) of El12α-HSDH and 0.34 mg/mL (1.8 U/mL) of MtLac.
The reaction proceeded for 7 days, and samples were analyzed by HPLC at different times (
Figure 5, blue line). The pH was checked every 24 h. When a small shift in pH was observed (0.1–0.2 pH points) the pH was adjusted with 1 M KOH.
After 85 h, a decrease of reaction rate was observed. This can be attributed to the degradation of the mediator, which was observed in previous experiments. The addition of 7 mg of syringaldazine (corresponding to a concentration of 50 μM) resulted in the restart of the reaction. A second addition of syringaldazine after 115 h similarly restarted the reaction, driving it finally to completion (98%). A total of 21 mg of syringaldazine (final concentration of 150 μM) were required to convert 20 g of CA.
The product (12-oxo-CDCA) was precipitated by acidification of the reaction mixture to pH 3 with 6 M HCl. It was subsequently extracted with diethyl ether (5 × 200 mL). Notably, this volume could be reduced by removing the enzymes from the reaction mixture by ultrafiltration. The organic phase was dried over MgSO4 and the ether was evaporated under reduced pressure. Finally, 18.4 g of product was obtained (92% isolated yield).
2.5. Cofactor Regeneration with a Classical Regeneration System
To compare the performance of the
MtLac/syringaldazine system with a classical regeneration system (employing a ketone as stoichiometric oxidant by ADHs), 14 commercial NAD
+-dependent enzymes from the EvoCatal (ADH Kit) were screened for their ability to oxidize
i-PrOH with concomitant reduction of NAD
+ to NADH (
Table S3) under the conditions employed in our experiments. Acetone and
i-PrOH are cheap sacrificial substrates that can ideally also be used as cosolvents.
The enzyme ADH420 (from unknown source) was selected for its ability to efficiently oxidize i-PrOH, showing a specific activity of 1.2 U/mg. Additionally, this enzyme does not accept CA as substrate, avoiding the formation of side-products.
In the next step, CA (2 g) was incubated with
El12a-HSDH and ADH420. 15% acetone (corresponding to a final concentration of 2 M) was simultaneously used as cosolvent and sacrificial substrate. The reaction mixture was sampled at regular intervals and analyzed by HPLC (
Figure 5, orange line). After 168 h of incubation, 12-oxo-CDCA was obtained in 92% isolated yield.
3. Discussion
To date, there is no cofactor recycling system that fully complies with the principles of Green Chemistry. Several enzymatic, chemical and electrochemical methods have been proposed in literature for the regeneration of NAD
+, but it is still not possible to clearly state which one outperforms the others [
25]. In the particular case of ADHs, biocatalytic reactions are driven by the addition of sacrificial substrates that generate waste compounds of no particular interest. In addition, due to the reversibility of the reactions involved, these sacrificial substrates have to be used in excess.
NOX has been identified as a possible candidate for the clean regeneration of NAD
+. This enzyme catalyzes the oxidation of NAD(P)H using molecular oxygen as sacrificial substrate and generates water as the sole side product. Since this reaction is irreversible (K
eq = 10
80), oxygen is not needed in excess. Nonetheless, the poor solubility of molecular oxygen in water is frequently limiting its application as a stoichiometric reagent. However, different technologies can be used to overcome this limitation: for example, segmented-flow reactors have been shown to maximize mass transfer between the gas and the water phase, allowing for quicker reactions than the corresponding batch mode. Other types of reactor design (e.g., bubble-column reactors, sparger reactors, etc.) have been employed in biocatalytic processes [
26,
27].
In the particular case of hydroxysteroid oxidation, a previous study employing
El12α-HSDH (for the C
12 oxidation of CA) and NOX (for the regeneration of NAD
+) showed that the rate limiting reaction is the oxidation of NADH (produced by
El12α-HSDH) by NOX. For this reason, we focused our investigation on the development of a laccase/mediator system for the regeneration of oxidized nicotinamide cofactors. In fact, this system catalyzes the same overall reaction as NOX, profiting additionally from the use of the highly active, stable and cheap enzyme
MtLac. The application of the laccase/mediator system has already been shown by Monti et al. for the preparation of 7-oxo-CDCA, another important intermediate for the production of UDCA. By analogy, we focused on the identification of a laccase/mediator couple that can be used at higher pH value (7.0–8.0) compatible with the activity of ADHs (pH range 7.0–9.0). Previous studies showed high laccase activity towards phenolic compounds at pH > 7.0 [
13,
28,
29]. Accordingly, a panel of phenolic mediators was selected and screened for their ability to oxidize NADH.
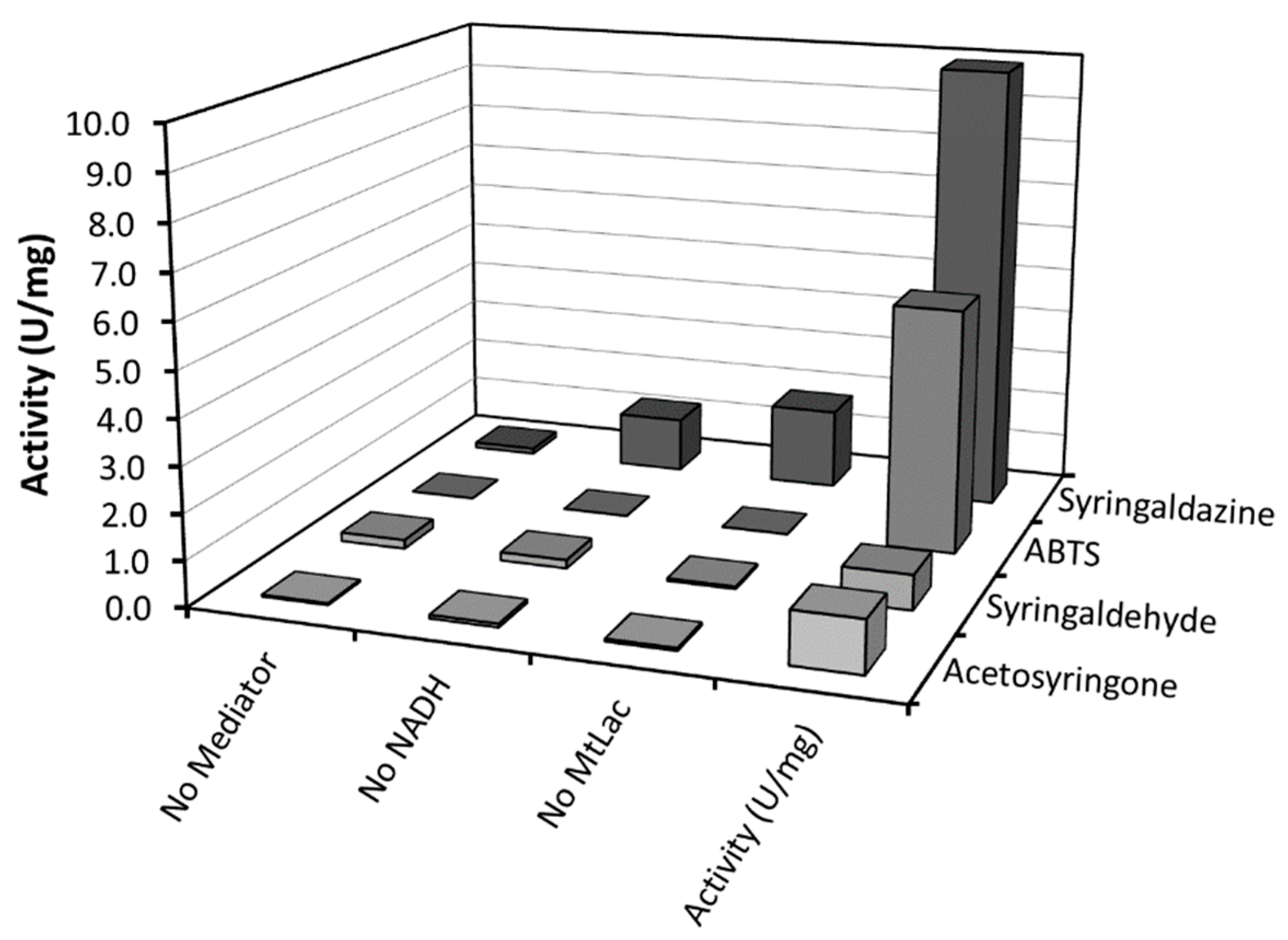
From the starting panel of selected laccases, the blue fungal laccase from Mycelyophthora thermophilia (MtLac) displayed high activity (7.2 U/mg) for the oxidation of NADH in combination with syringaldazine as mediator. Interestingly, its activity towards syringaldazine as substrate at pH 8.0 is even higher than at pH 5.0. In comparison to the NOX system (0.24 U/mg for NADH oxidation), the MtLac/syringaldazine couple showed a 30-fold higher activity. Furthermore, other mediators were tested for their ability to oxidize NADH. A detectable activity was observed with acetosyringone, syringaldehyde and ABTS. However, the maximal NADH oxidation rate was observed when syringaldazine was used as mediator. Several bioconversions with different amounts of mediator were set up in order to establish the minimal amount of mediator required for the reaction. Observing the time-course of the bioconversions, no particular differences were detected when 10, 20 and 50 µM of syringaldazine were employed in the biotransformation. On the other hand, when ABTS was used, a dependence of the reaction rate on the amount of mediator was observed. This particular behavior can be related to a higher KM of MtLac for ABTS at basic pH values. Full conversion of 10 mM of CA to the desired product (12-oxo-CDCA) was observed in all cases.
Having established MtLac/syringaldazine as a suitable system for the proposed biocatalytic process, the focus was shifted toward increasing the substrate loading to enable preparative and industrial scale applications.
DMSO and MeOH were evaluated as possible cosolvents for the reaction. At equal concentration, DMSO is a better cosolvent than MeOH; 10% (v/v) DMSO is enough to solubilize 120 mM of CA (in KPi buffer, pH 7.2). However, the use of this solvent can result in complications during downstream processing and in wastewater treatment. Notably, the activity of the enzymes involved in the biotransformation (El12α-HSDH and MtLac) are not compromised by the presence of this cosolvent.
Once the critical parameters for the biotransformation were identified and optimized, a preparative scale reaction starting from 20 g of CA was set up. A decrease in conversion rate was observed after 85 h of reaction. This effect is mainly due to slow degradation of syringaldazine under the reaction conditions. The addition of 7 mg of syringaldazine (50 µM) enabled the restart of the reaction, yielding > 90% conversion within 120 h. A further addition of mediator was necessary to obtain a final conversion of 98%. The reaction was stopped after 168 h by the addition of HCl, leading to the inactivation of the enzyme and precipitation of the product. 18 g of 12-oxo-CDCA were isolated. Notably, the extraction procedure was not optimized, and a better extraction efficiency may well be obtained if the enzymes were to be removed before the acidification and extraction process. Nonetheless, the product was recovered with high yield and purity.
To compare this O2 dependent enzymatic system with a classical regeneration system, El12α-HSDH was combined with ADH420 from the EvoCatal screening kit. This enzyme was identified for its ability to oxidize and reduce i-PrOH and acetone, respectively. It does not accept hydroxysteroids as substrate. A preparative scale reaction starting from 2 g of CA was performed. Interestingly, conversion values similar to the laccase mediator system were obtained: in both cases, >90% conversion was achieved after 7 days of reaction. The initial rate appears to be faster than that of the laccase/mediator system. This effect can be related to gas-liquid mass transfer limitations of oxygen, which is required in the El12α-HSDH/MtLac system.
Despite the fact that both processes perform equally, several observations can be made (
Table 2): (i) From the evaluation of green metrics (
Table S4), the laccase mediator system is preferable, due to the absence of the sacrificial substrate (acetone) required by the classical system: this is apparent from a lower E-value (2.9 vs. 3.1) and a higher atom efficiency; (ii) the main difference between the two processes is the cosolvent employed in the biotransformation: DMSO (for the laccase/mediator NAD
+ regeneration system) and acetone (for the classical regeneration system). Notably, acetone also functions as final electron acceptor and is converted to
i-PrOH during the reaction. Based on the GSK solvent sustainability guide, there are no particular health hazard related to the use of these solvents. Because of its low boiling point, acetone is easier to recycle than DMSO. In addition, it does not form an azeotrope with water, enabling a straightforward separation; (iii) the specific productivity of the ADH420/acetone regeneration system appears to be higher than the
MtLac/syringaldazine system, while the other process metrics are similar for the two setups. However, for the
MtLac/mediator system, difficulties could be encountered with larger reaction volumes, due to oxygen solubility and mass-transfer limitations.
The results obtained in this study were compared with the 7α-HSDH/laccase/mediator system developed by Ferrandi et al. (for the oxidation of the 7α-OH group of cholic acid) and with the 12α-HSDH/acetone/ADH system developed by Fossati et al. (for the oxidation of the 12α-OH group of cholic acid).
The here presented system (MtLac/syringaldazine) appears to be less productive than the Meldola’s Blue/Trametes pubescens laccase system (specific productivity at full conversion for the regeneration systems are 0.4 vs. 7.4 mmol kU−1 h−1, respectively). However, a sparger was used to increase the O2 concentration in the preparative reaction performed by Ferrandi et al., while in our setup the reaction was performed in a simple round bottom flask in a shaker. The oxygen availability can certainly influence the rate of the laccase reaction and therefore the efficiency of the regeneration system. On the other hand, the ADH420/acetone system shows similar process metrics to the system developed by Fossati et al. Particularly, the specific productivity values indicate that the use of the ADH420 for the regeneration of the NAD+ is advantageous.
In conclusion, the MtLac/syringaldazine and ADH420/acetone systems are suitable for the regeneration of the NAD+ in the preparation of 12-oxo-hydroxysteroids. In contrast to the NOX regeneration system, higher concentrations (120 vs. 10 mM) of CA can be successfully converted into 12-oxo-CDCA.
In addition, a productivity value of 6.4 g L−1d−1 was obtained for the MtLac/syringaldazine system: under these conditions, it will require a 500 L reaction run for 1 week to produce the amount of CDCA necessary to treat the 60 CTX Dutch patients for 1 year (roughly 16 kg). In our opinion, this is a feasible volume that is suitable for this biocatalytic process.
4. Materials and Methods
4.1. El12α-HSDH Expression Trials
The NAD
+-dependent HSDH from
Eggerthella lenta (
El12α-HSDH; GenBank: WP_114518444.1) was expressed as reported previously [
6]. Expression trials of
El12α-HSDH were performed in Erlenmeyer flasks with 500 mL of different media. LB media: 10 g/L Bacto-tryptone, 5 g/L Yeast extract, 10 g/L NaCl; TB media: 20 g/L Bacto-tryptone, 24 g/L Yeast extract, 0.231 g/L KH
2PO
4, 1.254 g/L K
2HPO
4, 4 mL/L Glycerol; YT media: 16 g/L Bacto-tryptone, 10 g/L Yeast extract, 5 g/L NaCl. The presence of NaCl as co-inducer was evaluated by adding 100 mL of NaCl solution (of a 250 g/L stock solution) at the moment of the induction (final concentration of 50 g/L).
E. coli cells were grown at 37 °C in a shaker incubator (200 rpm). When an OD600nm value of 1.0 was reached, the expression of the gene El12α-HSDH was induced by the addition of 0.250 mM IPTG and the temperature lowered to 25 °C. After 24 h of incubation under these conditions, cells were separated from the broth by centrifugation. The crude extract was obtained by resuspension of the cells in lysis buffer, sonication and centrifugation at 50,000× g (20 min, 4 °C).
Enzyme expression was evaluated measuring the El12α-HSDH activity in the crude extract: 10 mL of the cultures were centrifuged at 7000 rpm for 10 min. The supernatant was discarded and the pellet was resuspended in 2 mL of potassium phosphate buffer (100 mM, pH 8.0). Samples were then sonicated and subsequently centrifuged (7000 rpm, 10 min) to discard cell debris. Activity assay was carried out via spectroscopy, measuring the formation of NADH at the wavelength of 340 nm for 10 min. 25 μL of crude extract obtained by cells expressing the El12α-HSDH enzyme grew in the different media were added to 200 μL of a stock solution consisting of 10 mM CA, 0.5 mM of NAD+ and 50 mM potassium phosphate buffer (pH 8.0). Assays were carried out in triplicates.
4.2. Purification of MtLac
Laccase from
Myceliophthora thermophila (
MtLac) was purchased (see Laccases screening). The original sample consists in a centrifuged and concentrated broth sample of laccase with a brown color. Enzyme purification was performed by using a NGC apparatus (BioRad, Hercules, U.S.). The sample (17 mL, was diluted ten-times in 10 mM Tris-HCl, pH 7.2 and loaded onto a 70 mL Q-Sepharose column (GE Healtcare, Chicago, Illinois, U.S.) pre-equilibrated with the same buffer (accordingly to the procedure reported by Berka et al [
17]).
MtLac was eluted with 20% of the same buffer containing 2 M NaCl (purification chromatogram is reported in
Figure S1).
MtLac has an intense blue color which corresponds to an absorbance peak at 600 nm, typical of multicopper oxidases. The blue fractions were collected, pooled together and concentrated by Amicon apparatus equipped with a membrane with a cut-off of 30 kDa.
4.3. Laccase Screening
Laccases from Casuarina glauca (CgLac), Bacillus subtilis (BsLac) and Trametes trogii (TtLac) were kindly donated by Vlada B. Urlacher (Heinrich-Heine-Universität, Düsseldorf, Germany). Laccase from Myceliophthora thermophila (MtLac) was purchased from Novozymes (trade name Flavorstar). Laccase from Trametes versicolor (TvLac) was purchased from Sigma-Aldrich. NAD(P)H oxidase (PRO-NOX001) was purchased from Prozomix (Haltwhistle, UK). Enzyme concentrations were spectrophotometrically determined with Bradford assay, measuring the absorbance at 595 nm in a plate reader, using a calibration curve with BSA from 2 μg/mL to 50 μg/mL.
Laccase screening was carried out with 2 µM of mediators (syringaldazine, syringaldehyde and acetosyringone) in presence of 0.5 mM NADH and 100 mM potassium phosphate buffer (KPi), pH 8.0 or 100 mM sodium acetate buffer (NaAcO), pH 5.0. Reactions (200 µL) were started by the addition of 20 µL of 100-fold diluted enzyme stock solutions (final concentration in the assay: 5.9 µg/mL
CgLac, 3.3 µg/mL
BsLac, 5.5 µg/mL
TtLac, 11.5 µg/mL
MtLac, 90.9 µg/mL
TvLac). Activity was spectrophotometrically assayed following the oxidation of NADH at 340 nm (ε
340nm = 6.2 mM
−1 cm
−1) (
Figure 2 and
Figure 3). Assays were performed in 200 µL volume in a 96-well plate reader at 25 °C. Assays were carried out in triplicate.
4.4. Mediator Screening
All mediators used in this study are commercially available. Syringaldazine, acetosyringone, syringaldehyde, thionine, tartrazine, resazurin and Meldola’s blue were purchased from Sigma-Aldrich (Germany). ABTS (2,2’-Azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt) was purchased from Fluka (Germany).
Biocatalytic reactions (2 mL) were carried out in 15 mL tube in presence of different mediators. They were set up incubating 10 mM CA, 0.5 mM NAD
+, 0.34 mg/mL
MtLac, 0.013 mg/mL
El12α-HSDH and 5 µM of different mediators. Reactions were carried out at 25 °C for 24 h. Samples were analyzed by HPLC chromatography (
Table S2).
4.5. Enzymatic Activity Assays
The El12α-HSDH activity was determined spectrophotometrically following the production of NADH (εNADH at 340 nm = 6.2 mM−1 cm−1) for ten minutes. Assay conditions: 1 mM CA, 1 mM NAD+, 50 mM KPi buffer pH 8.0, 25 °C. One unit of enzymatic activity was defined as the amount of enzyme that reduces 1 µmol of NAD+ per minute under the assay conditions. Specific activity of El12α-HSDH is 59.2 U mg−1.
The MtLac activity was determined spectrophotometrically following the oxidation of ABTS (εABTS at 420 nm = 36.0 mM−1 cm−1) for one minute. Assay conditions: 0.5 mM ABTS, 50 mM sodium acetate buffer pH 5.0, 25 °C. One unit of enzymatic activity was defined as the amount of enzyme that oxidizes 1 µmol of ABTS per minute under the assay conditions. Specific activity of MtLac is 5.4 U mg−1.
The ADH420 activity was determined spectrophotometrically following the production of NADH (εNADH at 340 nm = 6.2 mM−1 cm−1) for ten minutes. Assay conditions: 15% (v/V) isopropanol, 0.5 mM NAD+, 50 mM KPi buffer pH 8.0, 25 °C. One unit of enzymatic activity was defined as the amount of enzyme that reduces 1 µmol of NAD+ per minute under the assay conditions. Specific activity of ADH420 is 1.2 U mg−1.
4.6. Optimization Studies
All bioconversions were carried out employing 0.34 mg/mL (1.8 U/mL) MtLac and 0.013 mg/mL (0.8 U/mL) El12α-HSDH. Analytical scale conversions were carried out in a 15 mL tube filled with 2 mL of reaction mixture and they were incubated at 25 °C on a rotator shaker (45 rpm) (IntelliMixer, Neolab Migge GmbH, Heidelberg, DE).
Effect of the mediator concentration: reaction mixtures containing different amounts of syringaldazine or ABTS (10, 20 and 50 µM), 10 mM CA, 0.5 mM NAD
+ and 100 mM KPi buffer at pH 8.0 were prepared. The reaction was started by the addition of the enzymes and the reaction mixture was sampled at given times (
Figure 4).
Effect of the cosolvent and substrate loading: reaction mixtures containing different amount of CA (from 10 to 250 mM), 50 µM of syringaldazine, 0.5 mM NAD
+ and 100 mM KPi buffer at pH 7.2 and different amount of MeOH (10–40% v/v) or DMSO (10%) were prepared (see
Table 1). After the addition of the enzymes, the reaction mixture was sampled after 48 h.
4.7. Preparation of 12-oxo-CDCA (MtLac + Mediator Regeneration System)
In a 2 L round-bottom flask 20 g (49.2 mmol) of CA, 136 mg (0.205 mmol) of NAD
+ and 7 mg (0.019 mmol) of syringaldazine were dissolved in 100 mM of KPi buffer, pH 7.2 with 10% DMSO (final volume 410 mL). The reaction was initiated by adding 5.33 mg (314 U) of
El12α-HSDH and 139 mg (750 U) of
MtLac (Flavostar, Novozyme). The reactions were gently stirred at 25 °C in an orbital shaker. Conversion was followed with HPLC. After 85 and 115 h, 7 mg (0.019 mmol) of syringaldazine was added to the reaction mixture. When the reaction was complete (168 h) the solution was acidified with HCl to pH 3.0 and extracted five times with diethyl ether (200 mL for each extraction). Solvent was dried with MgSO
4 and evaporated giving the final product (white powder). 12-oxo-CDCA: Isolated yield: 18.4 g (92%);
13C NMR (100 MHz, DMSO-
d6): δ 214.0 (C12=O), 174.8 (COOH), 70.0 (C3α-OH), 66.0 (C7α-OH), 56.3, 53.4, 46.1, 40.9, 37.4, 36.6, 35.5, 35.1, 35.1, 34.6, 31.1, 30.4, 30.3, 27.1, 23.3, 22.0, 18.5, 11.2. In accordance with literature data [
30].
4.8. Preparation of 12-oxo-CDCA (ADH420 + Acetone)
In a 50 mL falcon tube, 2 g (4.92 mmol) of CA and 13.6 mg (0.021 mmol) of NAD
+ were dissolved in 100 mM of KPi buffer, pH 7.2 with 15% acetone (83 mM) (final volume 41 mL). The reaction was initiated by addition of 0.53 mg (31.4 U) of
El12α-HSDH and 2.05 mg (2.5 U) of ADH 420 (EvoCatal ADH screening kit). The reaction mixture was incubated at 25 °C on a rotatory shaker. Conversion was checked with HPLC. When the reaction was complete (168 h) the solution was acidified with HCl to pH 3.0 and extracted three times with diethyl ether (20 mL for each extraction). Solvent was dried with MgSO
4 and evaporated giving the final product (white powder). 12-oxo-CDCA: Isolated yield: 1.83 g (92%);
13C NMR (100 MHz, DMSO-
d6): δ 214.0 (C12=O), 174.8 (COOH), 70.0 (C3α-OH), 66.0 (C7α-OH), 56.3, 53.4, 46.1, 40.9, 37.4, 36.6, 35.5, 35.1, 35.1, 34.6, 31.1, 30.4, 30.3, 27.1, 23.3, 22.0, 18.5, 11.2. In accordance with literature data [
30].
4.9. HPLC Analyses
The bioconversions were analyzed by HPLC: 50 μL of sample were diluted with water/CH3CN/TFA (70/30/0.1) solution to reach a final concentration of hydroxysteroid of 2 mM and centrifuged at 14,000× g for 2 min. 10 μL of the obtained samples was analyzed by HPLC.
Reversed-phase, isocratic gradient HPLC analyses were performed on a Shimadzu (Kyoto, Japan) apparatus equipped with a LC20AT pump and an ELSD-LTII detector and fitted with a XTerra RP C18 column (Waters, Milford, Massachusetts, USA) (length/internal diameter 150/4.6 mm, pore size 3.5 μm) under the following conditions: eluent H2O/CH3CN/TFA (70:30:0.1), flow 1.0 mL min−1, temperature 35 °C.