Advances in Recombinant Lipases: Production, Engineering, Immobilization and Application in the Pharmaceutical Industry
Abstract
1. Introduction
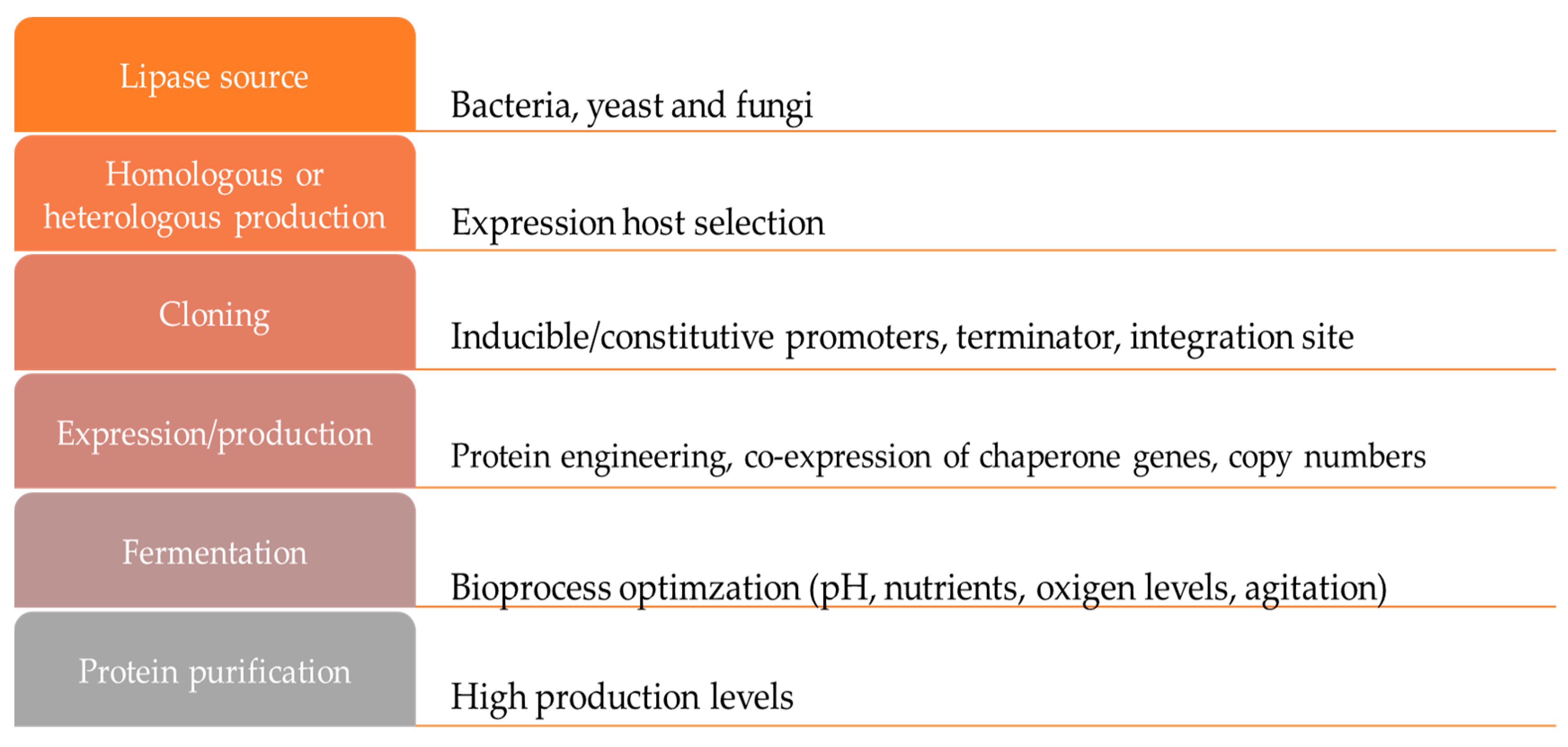
2. Production of Lipases
2.1. Microbial Sources of Lipases
2.2. Production of Recombinant Lipases
2.2.1. Prokaryotic Expression Systems
2.2.2. Eukaryotic Expression Systems
Alternative Eukaryotic Hosts
3. Lipase Characteristics and Engineering
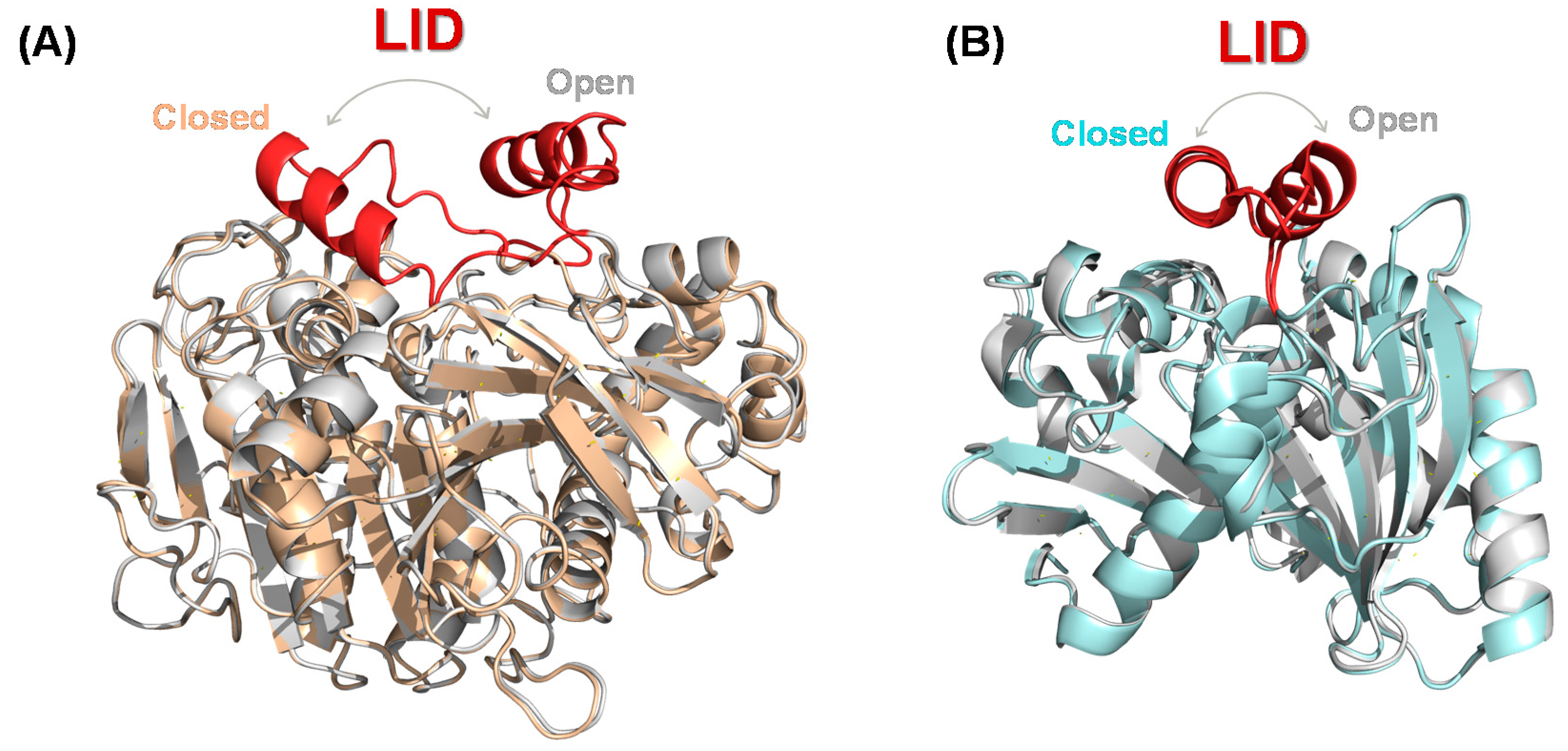
3.1. Structural Characteristics of Lipases
3.2. Lipase Engineering
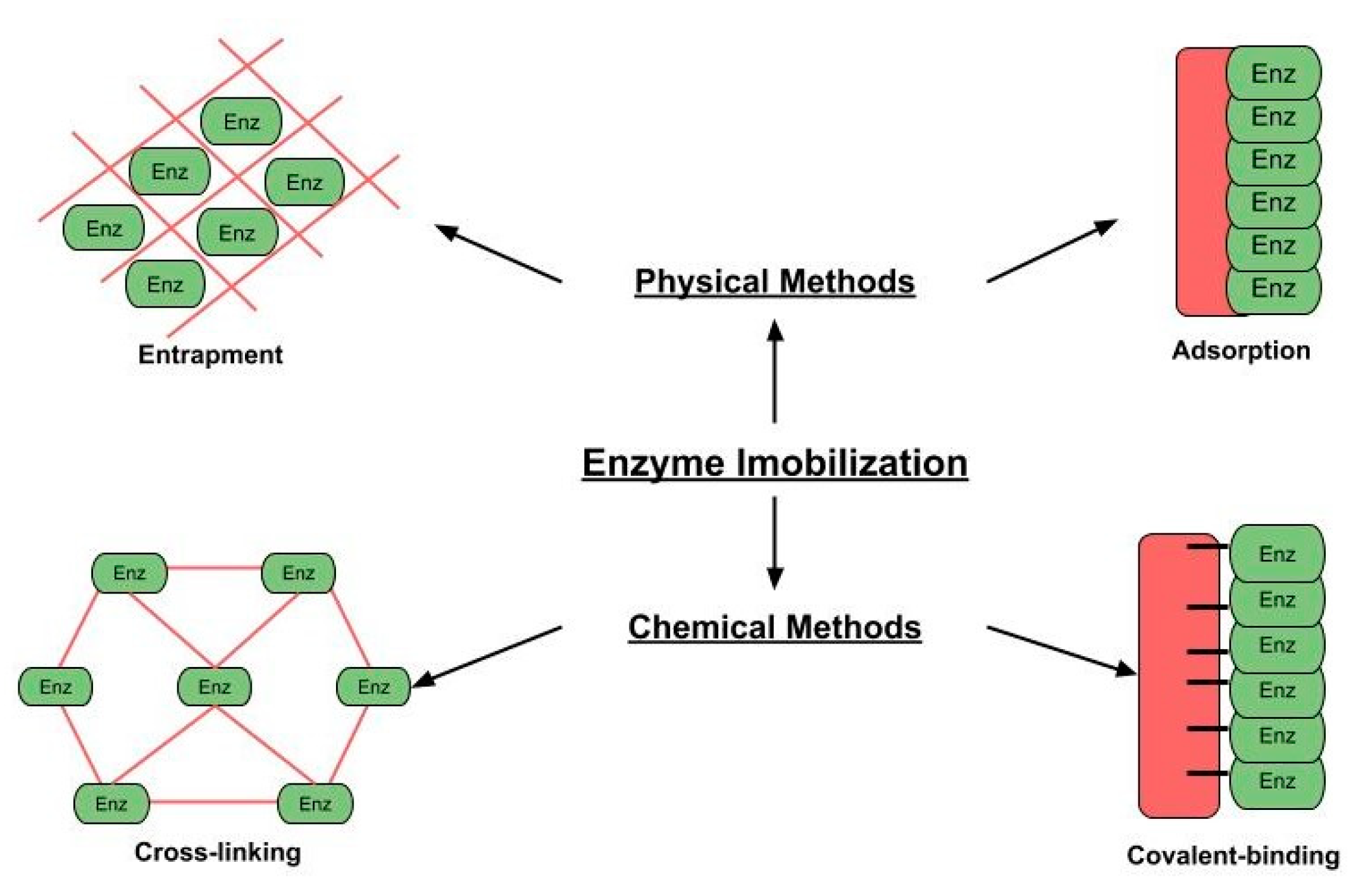
4. Relevant Immobilization Methods for Recombinant Lipases
5. Application of Recombinant Lipases
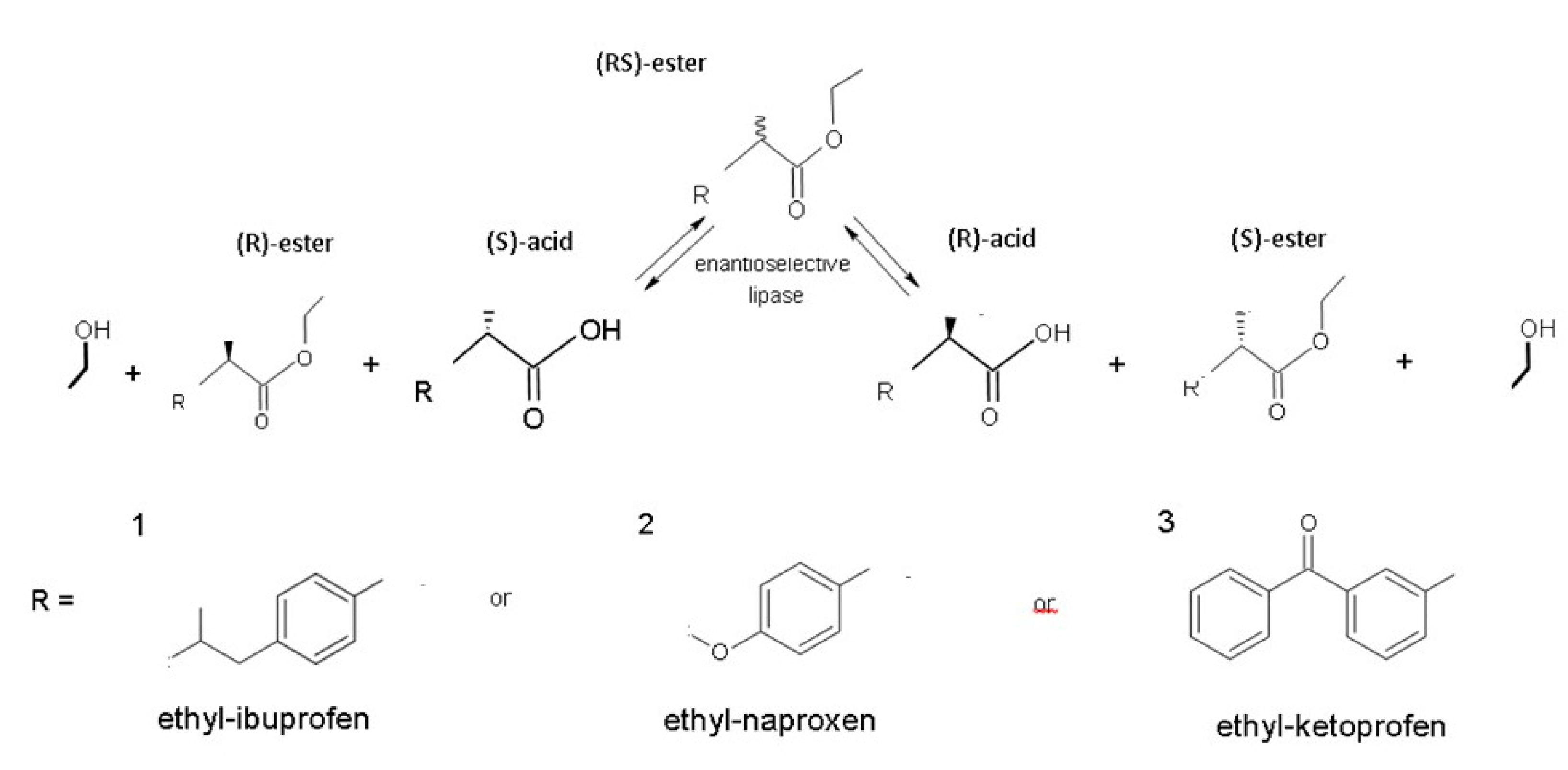
Pharmaceutical Applications of Recombinant Lipases
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gerits, L.R.; Pareyt, B.; Decamps, K.; Delcour, J.A. Lipases and their functionality in the production of wheat-based food systems. Compr. Rev. Food Sci. Food Saf. 2014, 13, 978–989. [Google Scholar] [CrossRef]
- Rani, S.; Jagtap, S. Acceleration of Swiss cheese ripening by microbial lipase without affecting its quality characteristics. J. Food Sci. Technol. 2019, 56, 497–506. [Google Scholar] [CrossRef]
- Antonini, F.; Crippa, S.; Falconi, M.; Macarri, G.; Pezzilli, R. Pancreatic enzyme replacement therapy after gastric resection: An update. Dig. Liver Dis. 2018, 50, 1–5. [Google Scholar] [CrossRef]
- Amini, Z.; Ong, H.C.; Harrison, M.D.; Kusumo, F.; Mazaheri, H.; Ilham, Z. Biodiesel production by lipase-catalyzed transesterification of Ocimum basilicum L. (sweet basil) seed oil. Energy Convers. Manag. 2017, 132, 82–90. [Google Scholar] [CrossRef]
- Carvalho, P.D.O.; Contesini, F.J.; Ikegaki, M. Enzymatic resolution of (R, S)-ibuprofen and (R, S)-ketoprofen by microbial lipases from native and commercial sources. Braz. J. Microbiol. 2006, 37, 329–337. [Google Scholar] [CrossRef]
- Da Silva, V.C.F.; Contesini, F.J.; de Oliveira Carvalho, P. Enantioselective behavior of lipases from Aspergillus niger immobilized in different supports. J. Ind. Microbiol. Biotechnol. 2009, 36, 949–954. [Google Scholar] [CrossRef]
- Svendsen, A. Lipase protein engineering. Biochim. Biophys. Acta (BBA)/Protein Struct. Mol. Enzym. 2000, 1543, 223–238. [Google Scholar] [CrossRef]
- Naik, S.; Basu, A.; Saikia, R.; Madan, B.; Paul, P.; Chaterjee, R.; Brask, J.; Svendsen, A. Lipases for use in industrial biocatalysis: Specificity of selected structural groups of lipases. J. Mol. Catal. B Enzym. 2010, 65, 18–23. [Google Scholar] [CrossRef]
- Saxena, R. Microbial lipases: Potential biocatalysts for the future industry. Curr. Sci. 1999, 77, 101–115. [Google Scholar]
- Haki, G.; Rakshit, S. Developments in industrially important thermostable enzymes: A review. Bioresour. Technol. 2003, 89, 17–34. [Google Scholar] [CrossRef]
- Rueda-López, S.; Martínez-Montaño, E.; Viana, M.T. Biochemical characterization and comparison of pancreatic lipases from the Pacific bluefin tuna, Thunnus orientalis; totoaba, Totoaba macdonaldi; and striped bass, Morone saxatilis. J. World Aquac. Soc. 2017, 48, 156–165. [Google Scholar] [CrossRef]
- Rivera, I.; Gutiérrez-Ortega, A.; Sandoval, G. Functional Expression of Plant Lipases: The Case of CpLip1 from Carica papaya. In Lipases and Phospholipases; Humana Press: New York, NY, USA, 2018; pp. 169–178. [Google Scholar]
- Li, M.; Yang, L.-R.; Xu, G.; Wu, J.-P. Cloning and characterization of a novel lipase from Stenotrophomonas maltophilia GS11: The first member of a new bacterial lipase family XVI. J. Biotechnol. 2016, 228, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, T.; Finotelli, P.V.; Bonomo, R.C.; Franco, M.; Amaral, P.F. Evaluating aqueous two-phase systems for Yarrowia lipolytica extracellular lipase purification. Process Biochem. 2017, 53, 259–266. [Google Scholar] [CrossRef]
- Rehman, S.; Wang, P.; Bhatti, H.N.; Bilal, M.; Asgher, M. Improved catalytic properties of Penicillium notatum lipase immobilized in nanoscale silicone polymeric films. Int. J. Biol. Macromol. 2017, 97, 279–286. [Google Scholar] [CrossRef]
- Lanka, S.; Latha, J.N.L. A short review on various screening methods to isolate potential lipase producers: Lipases-the present and future enzymes of biotech industry. Int. J. Biol. Chem. 2015, 9, 207–219. [Google Scholar] [CrossRef]
- Almeida, J.M.; Martini, V.P.; Iulek, J.; Alnoch, R.C.; Moure, V.R.; Müller-Santos, M.; Souza, E.M.; Mitchell, D.A.; Krieger, N. Biochemical characterization and application of a new lipase and its cognate foldase obtained from a metagenomic library derived from fat-contaminated soil. Int. J. Biol. Macromol. 2019, 137, 442–454. [Google Scholar] [CrossRef]
- Dartois, V.; Baulard, A.; Schanck, K.; Colson, C. Cloning, nucleotide sequence and expression in Escherichia coli of a lipase gene from Bacillus subtilis 168. Biochim. Biophys. Acta (BBA)-Gene Struct. Expr. 1992, 1131, 253–260. [Google Scholar] [CrossRef]
- Van Tassel, L.; Moilanen, A.; Ruddock, L.W. Efficient production of wild-type lipase B from Candida antarctica in the cytoplasm of Escherichia coli. Protein Expr. Purif. 2020, 165, 105498. [Google Scholar] [CrossRef]
- Minning, S.; Serrano, A.; Ferrer, P.; Solá, C.; Schmid, R.D.; Valero, F. Optimization of the high-level production of Rhizopus oryzae lipase in Pichia pastoris. J. Biotechnol. 2001, 86, 59–70. [Google Scholar] [CrossRef]
- Huang, J.; Zhao, Q.; Chen, L.; Zhang, C.; Bu, W.; Zhang, X.; Zhang, K.; Yang, Z. Improved production of recombinant Rhizomucor miehei lipase by coexpressing protein folding chaperones in Pichia pastoris, which triggered ER stress. Bioengineered 2020, 11, 375–385. [Google Scholar] [CrossRef]
- Kaieda, M.; Nagayoshi, M.; Hama, S.; Kondo, A.; Fukuda, H. Enantioselective transesterification using immobilized Aspergillus oryzae overexpressing lipase. Appl. Microbiol. Biotechnol. 2004, 65, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, D.B. Improvement of enzyme production in Aspergillus. Antonie Van Leeuwenhoek 1987, 53, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Cherry, J.R.; Fidantsef, A.L. Directed evolution of industrial enzymes: An update. Curr. Opin. Biotechnol. 2003, 14, 438–443. [Google Scholar] [CrossRef]
- Bischof, R.H.; Ramoni, J.; Seiboth, B. Cellulases and beyond: The first 70 years of the enzyme producer Trichoderma Reesei. Microb. Cell Fact. 2016, 15, 106. [Google Scholar] [CrossRef]
- Harwood, C.R.; Cranenburgh, R. Bacillus protein secretion: An unfolding story. Trends Microbiol. 2008, 16, 73–79. [Google Scholar] [CrossRef]
- Zhu, S.-Y.; Xu, Y.; Yu, X.-W. Improved Homologous Expression of the Acidic Lipase from Aspergillus niger. J. Microbiol. Biotechnol. 2020, 30, 196–205. [Google Scholar]
- Ma, R.J.; Wang, Y.H.; Liu, L.; Bai, L.L.; Ban, R. Production enhancement of the extracellular lipase LipA in Bacillus subtilis: Effects of expression system and Sec pathway components. Protein Expr. Purif. 2018, 142, 81–87. [Google Scholar] [CrossRef]
- Rantasalo, A.; Vitikainen, M.; Paasikallio, T.; Jäntti, J.; Landowski, C.P.; Mojzita, D. Novel genetic tools that enable highly pure protein production in Trichoderma reesei. Sci. Rep. 2019, 9, 5032. [Google Scholar] [CrossRef]
- Helisto, P.; Korpela, T. Effects of detergents on activity of microbial lipases as measured by the paranitrophenyl alkanoate esters method. Enzym. Microb. Technol. 1998, 23, 113–117. [Google Scholar] [CrossRef]
- Kulkarni, N.; Gadre, R.V. Production and properties of alkaline, thermophilic lipase from Pseudomonas fluorenscens NS2W. J. Ind. Microbiol. Biotechnol. 2002, 28, 344–348. [Google Scholar] [CrossRef]
- Verger, R. ‘Interfacial activation’of lipases: Facts and artifacts. Trends Biotechnol. 1997, 15, 32–38. [Google Scholar] [CrossRef]
- Khan, F.I.; Lan, D.; Durrani, R.; Huan, W.; Zhao, Z.; Wang, Y. The lid domain in lipases: Structural and functional determinant of enzymatic properties. Front. Bioeng. Biotechnol. 2017, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Bodh, U.; Gupta, R. Fungal lipases: A review. J. Biotech Res. 2017, 8, 58–77. [Google Scholar]
- Contesini, F.J.; da Silva, V.C.F.; Maciel, R.F.; de Lima, R.J.; Barros, F.F.C.; de Oliveira Carvalho, P. Response surface analysis for the production of an enantioselective lipase from Aspergillus niger by solid-state fermentation. J. Microbiol. 2009, 47, 563–571. [Google Scholar] [CrossRef]
- Skjold-Jørgensen, J.; Vind, J.; Svendsen, A.; Bjerrum, M.J. Understanding the activation mechanism of Thermomyces lanuginosus lipase using rational design and tryptophan-induced fluorescence quenching. Eur. J. Lipid Sci. Technol. 2016, 118, 1644–1660. [Google Scholar] [CrossRef]
- Li, G.; Fang, X.; Su, F.; Chen, Y.; Xu, L.; Yan, Y. Enhancing the thermostability of Rhizomucor miehei lipase with a limited screening library by rational-design point mutations and disulfide bonds. Appl. Environ. Microbiol. 2018, 84. [Google Scholar] [CrossRef]
- Markel, U.; Zhu, L.; Frauenkron-Machedjou, V.J.; Zhao, J.; Bocola, M.; Davari, M.D.; Jaeger, K.-E.; Schwaneberg, U. Are directed evolution approaches efficient in exploring nature’s potential to stabilize a lipase in organic cosolvents? Catalysts 2017, 7, 142. [Google Scholar] [CrossRef]
- Cen, Y.; Singh, W.; Arkin, M.; Moody, T.S.; Huang, M.; Zhou, J.; Wu, Q.; Reetz, M.T. Artificial cysteine-lipases with high activity and altered catalytic mechanism created by laboratory evolution. Nat. Commun. 2019, 10, 3198. [Google Scholar] [CrossRef]
- Sarmah, N.; Revathi, D.; Sheelu, G.; Yamuna Rani, K.; Sridhar, S.; Mehtab, V.; Sumana, C. Recent advances on sources and industrial applications of lipases. Biotechnol. Prog. 2018, 34, 5–28. [Google Scholar] [CrossRef]
- Samoylova, Y.V.; Sorokina, K.; Piligaev, A.; Parmon, V. Application of Bacterial Thermostable Lipolytic Enzymes in the Modern Biotechnological Processes: A Review. Catal. Ind. 2019, 11, 168–178. [Google Scholar] [CrossRef]
- Borrelli, G.M.; Trono, D. Recombinant lipases and phospholipases and their use as biocatalysts for industrial applications. Int. J. Mol. Sci. 2015, 16, 20774–20840. [Google Scholar] [CrossRef] [PubMed]
- Ghasemian, A.; Moradpour, Z. Production of recombinant microbial thermostable lipases. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 133–150. [Google Scholar]
- Atalah, J.; Cáceres-Moreno, P.; Espina, G.; Blamey, J.M. Thermophiles and the applications of their enzymes as new biocatalysts. Bioresour. Technol. 2019, 280, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Memarpoor-Yazdi, M.; Karbalaei-Heidari, H.R.; Khajeh, K. Production of the renewable extremophile lipase: Valuable biocatalyst with potential usage in food industry. Food Bioprod. Process. 2017, 102, 153–166. [Google Scholar] [CrossRef]
- Samoylova, Y.V.; Sorokina, K.N.; Romanenko, M.V.; Parmon, V.N. Cloning, expression and characterization of the esterase estUT1 from Ureibacillus thermosphaericus which belongs to a new lipase family XVIII. Extremophiles 2018, 22, 271–285. [Google Scholar] [CrossRef]
- Chen, K.-C.; Zheng, M.-M.; Pan, J.; Li, C.-X.; Xu, J.-H. Protein engineering and homologous expression of Serratia marcescens lipase for efficient synthesis of a pharmaceutically relevant chiral epoxyester. Appl. Biochem. Biotechnol. 2017, 183, 543–554. [Google Scholar] [CrossRef]
- Wang, X.; Yu, X.; Xu, Y. Homologous expression, purification and characterization of a novel high-alkaline and thermal stable lipase from Burkholderia cepacia ATCC 25416. Enzym. Microb. Technol. 2009, 45, 94–102. [Google Scholar] [CrossRef]
- Yang, J.; Guo, D.; Yan, Y. Cloning, expression and characterization of a novel thermal stable and short-chain alcohol tolerant lipase from Burkholderia cepacia strain G63. J. Mol. Catal. B Enzym. 2007, 45, 91–96. [Google Scholar] [CrossRef]
- Yang, H.; Li, J.; Du, G.; Liu, L. Microbial production and molecular engineering of industrial enzymes: Challenges and strategies. In Biotechnology of Microbial Enzymes; Elsevier: Amsterdam, The Netherlands, 2017; pp. 151–165. [Google Scholar]
- Tang, W.; Lan, D.; Zhao, Z.; Li, S.; Li, X.; Wang, Y. A thermostable monoacylglycerol lipase from marine Geobacillus sp. 12AMOR1: Biochemical characterization and mutagenesis study. Int. J. Mol. Sci. 2019, 20, 780. [Google Scholar] [CrossRef]
- Almeida, J.M.; Alnoch, R.C.; Souza, E.M.; Mitchell, D.A.; Krieger, N. Metagenomics: Is it a powerful tool to obtain lipases for application in biocatalysis? Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2020, 1868, 140320. [Google Scholar] [CrossRef]
- Cristakopoulos, P.; Tzia, C.; Kekos, D.; Macris, B.J. Production and characterization of extracellular lipase from Calvatia gigantean. Appl. Microbiol. Biotechnol. 1992, 38, 194–197. [Google Scholar]
- Salleh, A.B.; Musani, R.; Basri, M.; Ampon, K.; Yunus, W.M.Z.; Razac, C.N.A. Extra- and intra-cellular lipases from a thermophilic Rhizopus oryzae and factors affecting their production. Can. J. Microbiol. 1993, 39, 978–981. [Google Scholar] [CrossRef]
- Pokorny, D.; Friedrich, J.; Cimerman, A. Effect of nutritional factors on lipase biosynthesis by Aspergillus Niger. Biotechnol. Lett. 1994, 16, 363–366. [Google Scholar] [CrossRef]
- Papaparaskevas, D.; Christakopoulos, P.; Kekos, D.; Macris, B.J. Optimizing production of extracellular lipase from Rhodotorula glutinis. Biotechnol. Lett. 1992, 14, 397–402. [Google Scholar] [CrossRef]
- Shimada, Y.; Sugihara, A.; Nagao, T.; Tominaga, Y. Induction of Geotrichum candidum lipase by long-chain fatty acids. J. Ferment. Bioeng. 1992, 74, 77–80. [Google Scholar] [CrossRef]
- Rapp, P. Production, regulation, and some properties of lipase activity from Fusarium oxysporum f. sp. Vasinfectum. Enzym. Microb. Technol. 1995, 17, 832–838. [Google Scholar] [CrossRef]
- Brunel, L.; Neugnot, V.; Landucci, L.; Boze, H.; Moulin, G.; Bigey, F.; Dubreucq, E. High-level expression of Candida parapsilosislipase/acyltransferase in Pichia pastoris. J. Biotechnol. 2004, 111, 41–50. [Google Scholar] [CrossRef]
- Jahic, M.; Wallberg, F.; Bollok, M.; Garcia, P.; Enfors, S.O. Temperature limited fed-batch technique for control of proteolysis in Pichia pastoris bioreactor cultures Microb. Cell Fact. 2003, 2, 6. [Google Scholar] [CrossRef][Green Version]
- Yamaguchi, S.; Takeuchi, K.; Mase, T.; Matsuura, A. Efficient Expression of Mono- and Diacylglycerol Lipase Gene from Penicillium camembertii U-150 in Aspergillus oryzae under the Control of Its Own Promoter. Biosci. Biotechnol. Biochem. 1997, 61, 800–805. [Google Scholar] [CrossRef]
- Khuarana, J.; Pratibha, C.S.; Kaur, J. Studies on recombinant lipase production by E. coli: Effect of media and bacterial expression system optimization. Int. J. Mol. Biol. 2017, 2, 17–23. [Google Scholar]
- Batumalaie, K.; Khalili, E.; Mahat, N.A.; Huyop, F.Z.; Wahab, R.A. A statistical approach for optimizing the protocol for overexpressing lipase KV1 in Escherichia coli: Purification and characterization. Biotechnol. Biotechnol. Equip. 2018, 32, 69–87. [Google Scholar] [CrossRef]
- Nooh, H.M.; Masomian, M.; Salleh, A.B.; Mohamad, R.; Ali, M.S.M.; Rahman, R.N.Z.R.A. Production of Thermostable T1 Lipase Using Agroindustrial Waste Medium Formulation. Catalysts 2018, 8, 485. [Google Scholar] [CrossRef]
- Robert, J.M.; Betancur, M.O.; Machado, A.C.O.; Arruda, A.; Reis, V.C.B.; Almeida, R.V.; Torres, F.A.G.; Alegre, P.F.; Valero, F.; Freire, D.M.G. Increase of Candida antarctica lipase B production under PGK promoter in Pichia pastoris: Effect of multicopies. Braz. J. Microbiol. 2019, 50, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Ellis, R.J.; Hemmingsen, S.M. Molecular chaperones: Proteins essential for the biogenesis of some macromolecular structures. Trends Biochem. Sci. 1989, 14, 339–342. [Google Scholar] [CrossRef]
- Yoshida, K.; Konishi, K.; Magana-Mora, A.; Rougny, A.; Yasutake, Y.; Muramatsu, S.; Murata, S.; Kumagai, T.; Aburatani, S.; Sakasegawa, S.-I. Production of recombinant extracellular cholesterol esterase using consistently active promoters in Burkholderia stabilis. Biosci. Biotechnol. Biochem. 2019, 83, 1974–1984. [Google Scholar] [CrossRef]
- Sahoo, R.K.; Kumar, M.; Sukla, L.B.; Subudhi, E. Bioprospecting hot spring metagenome: Lipase for the production of biodiesel. Environ. Sci. Pollut. Res. 2017, 24, 3802–3809. [Google Scholar] [CrossRef]
- Kaur, R.; Kumar, R.; Verma, S.; Kumar, A.; Rajesh, C.; Sharma, P.K. Structural and functional insights about unique extremophilic bacterial lipolytic enzyme from metagenome source. Int. J. Biol. Macromol. 2020, 152, 593–604. [Google Scholar] [CrossRef]
- Kavitha, M. Cold active lipases–An update. Front. Life Sci. 2016, 9, 226–238. [Google Scholar] [CrossRef]
- Khan, M.T.; Kaushik, A.C.; ul ain Rana, Q.; Malik, S.I.; Khan, A.S.; Wei, D.-Q.; Sajjad, W.; Ahmad, S.; Ali, S.; Irfan, M. Characterization and synthetic biology of lipase from Bacillus amyloliquefaciens strain. Arch. Microbiol. 2020, 202, 1497–1506. [Google Scholar] [CrossRef]
- Salwoom, L.; Salleh, A.B.; Convey, P.; Mohamad Ali, M.S. New recombinant cold-adapted and organic solvent tolerant lipase from psychrophilic Pseudomonas sp. LSK25, isolated from Signy Island Antarctica. Int. J. Mol. Sci. 2019, 20, 1264. [Google Scholar] [CrossRef]
- Alnoch, R.C.; Stefanello, A.A.; Martini, V.P.; Richter, J.L.; Mateo, C.; de Souza, E.M.; Mitchell, D.A.; Muller-Santos, M.; Krieger, N. Co-expression, purification and characterization of the lipase and foldase of Burkholderia contaminans LTEB11. Int. J. Biol. Macromol. 2018, 116, 1222–1231. [Google Scholar] [CrossRef]
- Putra, L.; Natadiputri, G.H.; Meryandini, A.; Suwanto, A. Isolation, Cloning and Co-Expression of Lipase and Foldase Genes of Burkholderia territorii GP3 from Mount Papandayan Soil. J. Microbiol. Biotechnol. 2019, 29, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Nagaroor, V.; Gummadi, S.N. Biochemical characterization of an esterase from Clostridium acetobutylicum with novel GYSMG pentapeptide motif at the catalytic domain. J. Ind. Microbiol. Biotechnol. 2020, 47, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Alfaro-Chávez, A.L.; Liu, J.-W.; Porter, J.L.; Goldman, A.; Ollis, D.L. Improving on nature’s shortcomings: Evolving a lipase for increased lipolytic activity, expression and thermostability. Protein Eng. Des. Sel. 2019, 32, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Ishak, S.N.H.; Masomian, M.; Kamarudin, N.H.A.; Ali, M.S.M.; Leow, T.C.; Rahman, R.N.Z.R.A. Changes of thermostability, organic solvent, and pH stability in Geobacillus zalihae HT1 and its mutant by calcium ion. Int. J. Mol. Sci. 2019, 20, 2561. [Google Scholar] [CrossRef]
- Li, P.-Y.; Zhang, Y.-Q.; Zhang, Y.; Jiang, W.-X.; Wang, Y.-J.; Zhang, Y.-S.; Sun, Z.-Z.; Li, C.-Y.; Zhang, Y.-Z.; Shi, M. Study on a Novel Cold-Active and Halotolerant Monoacylglycerol Lipase Widespread in Marine Bacteria Reveals a New Group of Bacterial Monoacylglycerol Lipases Containing Unusual C (A/S) HSMG Catalytic Motifs. Front. Microbiol. 2020, 11, 9. [Google Scholar] [CrossRef]
- Shao, H.; Hu, X.; Sun, L.; Zhou, W. Gene cloning, expression in E. coli, and in vitro refolding of a lipase from Proteus sp. NH 2-2 and its application for biodiesel production. Biotechnol. Lett. 2019, 41, 159–169. [Google Scholar] [CrossRef]
- Yaacob, N.; Kamarudin, N.H.A.; Leow, A.T.C.; Salleh, A.B.; Abd Rahman, R.N.Z.R.; Ali, M.S.M. Effects of Lid 1 Mutagenesis on Lid Displacement, Catalytic Performances and Thermostability of Cold-active Pseudomonas AMS8 Lipase in Toluene. Comput. Struct. Biotechnol. J. 2019, 17, 215–228. [Google Scholar] [CrossRef]
- Gonzalez, T.; M’Barek, H.N.; Gomaa, A.E.; Hajjaj, H.; Zhen, C.; Dehua, L. Molecular Cloning and Expression of Candida antarctica lipase B in Corynebacterium genus. Microbiol. Biotechnol. Lett. 2019, 47, 546–554. [Google Scholar]
- Robinson, P.J.; Pringle, M.A.; Woolhead, C.A.; Bulleid, N.J. Folding of a single domain protein entering the endoplasmic reticulum precedes disulfide formation. J. Biol. Chem. 2017, 292, 6978–6986. [Google Scholar] [CrossRef]
- Lan, D.; Qu, M.; Yang, B.; Wang, Y. Enhancing production of lipase MAS1 from marine Streptomyces sp. strain in Pichia pastoris by chaperones co-expression. Electron. J. Biotechnol. 2016, 22, 62–67. [Google Scholar] [CrossRef]
- Sha, C.; Yu, X.-W.; Lin, N.-X.; Zhang, M.; Xu, Y. Enhancement of lipase r27RCL production in Pichia pastoris by regulating gene dosage and co-expression with chaperone protein disulfide isomerase. Enzym. Microb. Technol. 2013, 53, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Samuel, P.; Vadhana, A.K.P.; Kamatchi, R.; Antony, A.; Meenakshisundaram, S. Effect of molecular chaperones on the expression of Candida antarctica lipase B in Pichia pastoris. Microbiol. Res. 2013, 168, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Zhou, Q.; Su, Z.; Xu, L.; Yan, Y. High-level extracellular production of Rhizopus oryzae lipase in Pichia pastoris via a strategy combining optimization of gene-copy number with co-expression of ERAD-related proteins. Protein Expr. Purif. 2018, 147, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Resina, D.; Cos, O.; Ferrer, P.; Valero, F. Developing high cell density fed-batch cultivation strategies for heterologous protein production in Pichia pastoris using the nitrogen source-regulated FLD1 Promoter. Biotechnol. Bioeng. 2005, 91, 760–767. [Google Scholar] [CrossRef]
- Wongwatanapaiboon, J.; Malilas, W.; Ruangchainikom, C.; Thummadetsak, G.; Chulalaksananukul, S.; Marty, A.; Chulalaksananukul, W. Overexpression of Fusarium solani lipase in Pichia pastoris and its application in lipid degradation. Biotechnol. Biotechnol. Equip. 2016, 30, 885–893. [Google Scholar] [CrossRef]
- Theron, C.W.; Vandermies, M.; Telek, S.; Steels, S.; Fickers, P. Comprehensive comparison of Yarrowia lipolytica and Pichia pastoris for production of Candida antarctica lipase B. Sci. Rep. 2020, 10, 1741. [Google Scholar] [CrossRef]
- Prathumpai, W.; Flitter, S.J.; McIntyre, M.; Nielsen, J. Lipase production by recombinant strains of Aspergillus niger expressing a lipase-encoding gene from Thermomyces lanuginosus. Appl. Microbiol. Biotechnol. 2004, 65, 714–719. [Google Scholar] [CrossRef]
- Qin, L.-N.; Cai, F.-R.; Dong, X.-R.; Huang, Z.-B.; Tao, Y.; Huang, J.-Z.; Dong, Z.-Y. Improved production of heterologous lipase in Trichoderma reesei by RNAi mediated gene silencing of an endogenic highly expressed gene. Bioresour. Technol. 2012, 109, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, Z.; Zhang, T.; Wang, Y.; Yang, B. High-level expression of Thermomyces dupontii thermophilic lipase in Pichia pastoris via combined strategies. 3 Biotech 2019, 9, 62. [Google Scholar] [CrossRef]
- Cregg, J.M.; Cereghino, J.L.; Shi, J.; Higgins, D.R. Recombinant protein expression in Pichia pastoris. Mol. Biotechnol. 2000, 16, 23–52. [Google Scholar] [CrossRef]
- Vogl, T.; Glieder, A. Regulation of Pichia pastoris promoters and its consequences for protein production. New Biotechnol. 2013, 30, 385–404. [Google Scholar] [CrossRef] [PubMed]
- Hartner, F.S.; Glieder, A. Regulation of methanol utilisation pathway genes in yeasts. Microb. Cell Fact. 2006, 5, 39. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yu, J.; Wang, A.; Zuo, C.; Li, H.; Chen, X.; Pei, X.; Zhang, P. Efficient synthesis of vitamin A palmitate in nonaqueous medium using self-assembled lipase TLL@ apatite hybrid nanoflowers by mimetic biomineralization. Green Chem. Lett. Rev. 2018, 11, 476–483. [Google Scholar] [CrossRef]
- Hohenblum, H.; Gasser, B.; Maurer, M.; Borth, N.; Mattanovich, D. Effects of gene dosage, promoters, and substrates on unfolded protein stress of recombinant Pichia pastoris. Biotechnol. Bioeng. 2004, 85, 367–375. [Google Scholar] [CrossRef]
- Craig, E.A.; Gambill, B.D.; Nelson, R.J. Heat shock proteins: Molecular chaperones of protein biogenesis. Microbiol. Mol. Biol. Rev. 1993, 57, 402–414. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, H.L.; Xue, C.; Xiong, X.H.; Yao, X.Q.; Li, X.Y.; Chen, H.P.; Liu, Z.M. Enhanced secretion of heterologous proteins in Pichia pastoris following overexpression of Saccharomyces cerevisiae chaperone proteins. Biotechnol. Prog. 2006, 22, 1090–1095. [Google Scholar] [CrossRef]
- Park, Y.-K.; Vandermies, M.; Soudier, P.; Telek, S.; Thomas, S.; Nicaud, J.-M.; Fickers, P. Efficient expression vectors and host strain for the production of recombinant proteins by Yarrowia lipolytica in process conditions. Microb. Cell Factories 2019, 18, 167. [Google Scholar] [CrossRef]
- Fleißner, A.; Dersch, P. Expression and export: Recombinant protein production systems for Aspergillus. Appl. Microbiol. Biotechnol. 2010, 87, 1255–1270. [Google Scholar] [CrossRef]
- Keränen, S.; Penttilä, M. Production of recombinant proteins in the filamentous fungus Trichoderma reesei. Curr. Opin. Biotechnol. 1995, 6, 534–537. [Google Scholar] [CrossRef]
- Schrag, J.D.; Cygler, M. Lipases and αβ hydrolase fold. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1997; Volume 284, pp. 85–107. [Google Scholar]
- Gupta, R.; Kumari, A.; Syal, P.; Singh, Y. Molecular and functional diversity of yeast and fungal lipases: Their role in biotechnology and cellular physiology. Prog. Lipid Res. 2015, 57, 40–54. [Google Scholar] [CrossRef]
- Eggert, T.; van Pouderoyen, G.; Dijkstra, B.W.; Jaeger, K.-E. Lipolytic enzymes LipA and LipB from Bacillus subtilis differ in regulation of gene expression, biochemical properties, and three-dimensional structure. FEBS Lett. 2001, 502, 89–92. [Google Scholar] [CrossRef]
- Brady, L.; Brzozowski, A.M.; Derewendat, Z.S.; Dodson, E.; Dodson, G.; Tolley, S.; Turkenburg, J.P.; Christiansent, L.; Huge-Jensent, B.; Norskovt, L.; et al. A serine protease triad forms the catalytic centre of a triacylglycerol lipase. Nature 1990, 343, 767–770. [Google Scholar] [CrossRef] [PubMed]
- Schrag, J.D.; Li, Y.; Wu, S.; Cygler, M. Ser-His-Glu triad forms the catalytic site of the lipase from Geotrichum candidum. Nature 1991, 351, 761–764. [Google Scholar] [CrossRef] [PubMed]
- Mala, J.G.S.; Takeuchi, S. Understanding Structural Features of Microbial Lipases—An Overview. Anal. Chem. Insights 2008, 3, ACI.S551. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, M.K.; Singh, R.; Singh, R.K.; Kim, I.-W.; Lee, J.-K. Computational approaches for rational design of proteins with novel functionalities. Comput. Struct. Biotechnol. J. 2012, 2, e201204002. [Google Scholar] [CrossRef]
- Garmroodi, M.; Mohammadi, M.; Ramazani, A.; Ashjari, M.; Mohammadi, J.; Sabour, B.; Yousefi, M. International Journal of Biological Macromolecules Covalent Binding of Hyper-Activated Rhizomucor miehei Lipase (RML) on Hetero-Functionalized Siliceous Supports. Int. J. Biol. Macromol. 2016, 86, 208–215. [Google Scholar] [CrossRef]
- Kim, H.S.; Le, Q.A.T.; Kim, Y.H. Development of thermostable lipase B from Candida antarctica (CalB) through in silico design employing B-factor and RosettaDesign. Enzym. Microb. Technol. 2010, 47, 1–5. [Google Scholar] [CrossRef]
- Bornscheuer, U.T. Enzymes in lipid modification: Past achievements and current trends. Eur. J. Lipid Sci. Technol. 2014, 116, 1322–1331. [Google Scholar] [CrossRef]
- Brundiek, H.B.; Evitt, A.S.; Kourist, R.; Bornscheuer, U.T. Creation of a lipase highly selective for trans fatty acids by protein engineering. Angew. Chem. Int. Ed. 2012, 51, 412–414. [Google Scholar] [CrossRef]
- Warwel, S.; Borgdorf, R. Substrate Selectivity of Lipases in the Esterification of Cistrans-Isomers and Positional Isomers of Conjugated Linoleic Acid (CLA). Biotechnol. Lett. 2000, 22, 1151–1155. [Google Scholar] [CrossRef]
- Childers, M.C.; Daggett, V. Insights from molecular dynamics simulations for computational protein design. Mol. Syst. Des. Eng. 2017, 2, 9–33. [Google Scholar] [CrossRef] [PubMed]
- Haque, N.; Prabhu, N.P. Lid dynamics of porcine pancreatic lipase in non-aqueous solvents. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2016, 1860, 2326–2334. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Sang, J.; Zhang, Y.; Sun, T.; Liu, H.; Yue, R.; Zhang, J.; Wang, H.; Dai, Y.; Lu, F. Rational design of a Yarrowia lipolytica derived lipase for improved thermostability. Int. J. Biol. Macromol. 2019, 137, 1190–1198. [Google Scholar] [CrossRef] [PubMed]
- Sayous, V.; Lubrano, P.; Li, Y.; Acevedo-Rocha, C.G. Unbiased libraries in protein directed evolution. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2020, 1868, 140321. [Google Scholar] [CrossRef]
- Tripathi, A.; Varadarajan, R. Residue specific contributions to stability and activity inferred from saturation mutagenesis and deep sequencing. Curr. Opin. Struct. Biol. 2014, 24, 63–71. [Google Scholar] [CrossRef]
- Guisan, J.M. Immobilization of enzymes as the 21st century begins. In Immobilization of Enzymes and Cells; Humana Press: Totowa, NJ, USA, 2006; pp. 1–13. [Google Scholar]
- Hernandez, K.; Fernandez-Lafuente, R. Control of protein immobilization: Coupling immobilization and site-directed mutagenesis to improve biocatalyst or biosensor performance. Enzym. Microb. Technol. 2011, 48, 107–122. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Sorgedrager, M.; Janssen, M.H. Use of cross-linked enzyme aggregates (CLEAs) for performing biotransformations. Chim. Oggi 2007, 25, 62. [Google Scholar]
- Saifuddin, N.; Raziah, A.; Junizah, A. Carbon nanotubes: A review on structure and their interaction with proteins. J. Chem. 2013, 2013, 676815. [Google Scholar] [CrossRef]
- Guillén, M.; Benaiges, M.D.; Valero, F. Immobilization and stability of a Rhizopus oryzae lipase expressed in Pichia pastoris: Comparison between native and recombinant variants. Biotechnol. Prog. 2011, 27, 1232–1241. [Google Scholar] [CrossRef]
- Song, C.; Sheng, L.; Zhang, X. Immobilization and characterization of a thermostable lipase. Mar. Biotechnol. 2013, 15, 659–667. [Google Scholar] [CrossRef]
- Branco, R.V.; Gutarra, M.L.; Guisan, J.M.; Freire, D.M.; Almeida, R.V.; Palomo, J.M. Improving the thermostability and optimal temperature of a lipase from the hyperthermophilic archaeon Pyrococcus furiosus by covalent immobilization. Biomed Res. Int. 2015, 2015, 250532. [Google Scholar] [CrossRef] [PubMed]
- Chronopoulou, L.; Kamel, G.; Sparago, C.; Bordi, F.; Lupi, S.; Diociaiuti, M.; Palocci, C. Structure–activity relationships of Candida rugosa lipase immobilized on polylactic acid nanoparticles. Soft Matter 2011, 7, 2653–2662. [Google Scholar] [CrossRef]
- Bavaro, T.; Ubiali, D.; Brocca, S.; Rocchietti, S.; Nieto, I.; Pregnolato, M.; Lotti, M.; Terreni, M. Recombinant lipase from Candida rugosa for regioselective hydrolysis of peracetylated nucleosides. A comparison with commercial non-recombinant lipases. Biocatal. Biotransform. 2010, 28, 108–116. [Google Scholar] [CrossRef]
- Yao, C.; Lin, W.; Yue, K.; Ling, X.; Jing, K.; Lu, Y.; Tang, S.; Fan, E. Biocatalytic synthesis of vitamin A palmitate using immobilized lipase produced by recombinant Pichia pastoris. Eng. Life Sci. 2017, 17, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Benaiges, M.D.; Alarcón, M.; Fuciños, P.; Ferrer, P.; Rua, M.; Valero, F. Recombinant Candida rugosa lipase 2 from Pichia pastoris: Immobilization and use as biocatalyst in a stereoselective reaction. Biotechnol. Prog. 2010, 26, 1252–1258. [Google Scholar] [CrossRef] [PubMed]
- Badillo-Zeferino, G.L.; Ruiz-López, I.I.; Oliart-Ros, R.; Sánchez-Otero, M.G. Improved expression and immobilization of Geobacillus thermoleovorans CCR11 thermostable recombinant lipase. Biotechnol. Appl. Biochem. 2017, 64, 62–69. [Google Scholar] [CrossRef]
- Rios, N.S.; Pinheiro, B.B.; Pinheiro, M.P.; Bezerra, R.M.; dos Santos, J.C.S.; Gonçalves, L.R.B. Biotechnological potential of lipases from Pseudomonas: Sources, properties and applications. Process Biochem. 2018, 75, 99–120. [Google Scholar] [CrossRef]
- Zdarta, J.; Meyer, A.S.; Jesionowski, T.; Pinelo, M. A general overview of support materials for enzyme immobilization: Characteristics, properties, practical utility. Catalysts 2018, 8, 92. [Google Scholar] [CrossRef]
- Ghiaci, M.; Aghaei, H.; Soleimanian, S.; Sedaghat, M. Enzyme immobilization: Part 1. Modified bentonite as a new and efficient support for immobilization of Candida rugosa lipase. Appl. Clay Sci. 2009, 43, 289–295. [Google Scholar] [CrossRef]
- Manoel, E.A.; Robert, J.M.; Pinto, M.C.; Machado, A.C.; Besteti, M.D.; Coelho, M.A.Z.; Simas, A.B.; Fernandez-Lafuente, R.; Pinto, J.C.; Freire, D.M. Evaluation of the performance of differently immobilized recombinant lipase B from Candida antarctica preparations for the synthesis of pharmacological derivatives in organic media. RSC Adv. 2016, 6, 4043–4052. [Google Scholar] [CrossRef]
- Manoel, E.A.; Ribeiro, M.F.; Dos Santos, J.C.; Coelho, M.A.Z.; Simas, A.B.; Fernandez-Lafuente, R.; Freire, D.M. Accurel MP 1000 as a support for the immobilization of lipase from Burkholderia cepacia: Application to the kinetic resolution of myo-inositol derivatives. Process Biochem. 2015, 50, 1557–1564. [Google Scholar] [CrossRef]
- Ortiz, C.; Ferreira, M.L.; Barbosa, O.; dos Santos, J.C.; Rodrigues, R.C.; Berenguer-Murcia, Á.; Briand, L.E.; Fernandez-Lafuente, R. Novozym 435: The “perfect” lipase immobilized biocatalyst? Catal. Sci. Technol. 2019, 9, 2380–2420. [Google Scholar] [CrossRef]
- George, R.; Sugunan, S. Kinetic and thermodynamic parameters of immobilized glucoamylase on different mesoporous silica for starch hydrolysis: A comparative study. J. Mol. Catal. B Enzym. 2014, 106, 81–89. [Google Scholar] [CrossRef]
- Grollmisch, A.; Kragl, U.; Großeheilmann, J. Enzyme immobilization in polymerized ionic liquids-based hydrogels for active and reusable biocatalysts. SynOpen 2018, 2, 0192–0199. [Google Scholar] [CrossRef]
- Reetz, M.T. Practical protocols for lipase immobilization via sol–gel techniques. In Immobilization of Enzymes and Cells; Humana Press: Totowa, NJ, USA, 2013; pp. 241–254. [Google Scholar]
- Kovalenko, G.; Beklemishev, A.; Perminova, L.; Chuenko, T.; Mamaev, A.; Ivanov, I.; Moseenkov, S.; Kuznetsov, V. Recombinant strain producing thermostable lipase from Thermomyces lanuginosus immobilized into nanocarbon-in-silica matrices and properties of the prepared biocatalysts. Appl. Biochem. Microbiol. 2013, 49, 296–305. [Google Scholar] [CrossRef]
- Lee, D.H.; Park, C.H.; Yeo, J.M.; Kim, S.W. Lipase immobilization on silica gel using a cross-linking method. J. Ind. Eng. Chem. 2006, 12, 777–782. [Google Scholar]
- Wang, W.; Zhou, W.; Li, J.; Hao, D.; Su, Z.; Ma, G. Comparison of covalent and physical immobilization of lipase in gigaporous polymeric microspheres. Bioprocess Biosyst. Eng. 2015, 38, 2107–2115. [Google Scholar] [CrossRef]
- Oláh, M.; Suba, S.; Boros, Z.; Kovács, P.; Gosselin, M.; Gaudreault, C.; Hornyánszky, G. Lipase B from Candida antarctica Immobilized on Epoxy-functionalized Hollow Silica Microspheres: Efficient Biocatalysts for Enantiomer Selective Acylation of Alcohols and Amines. Period. Polytech. Chem. Eng. 2018, 62, 519–532. [Google Scholar] [CrossRef]
- Diaz-Vidal, T.; Armenta-Perez, V.P.; Rosales-Rivera, L.C.; Mateos-Díaz, J.C.; Rodríguez, J.A. Cross-linked enzyme aggregates of recombinant Candida antarctica lipase B for the efficient synthesis of olvanil, a nonpungent capsaicin analogue. Biotechnol. Prog. 2019, 35, e2807. [Google Scholar] [CrossRef]
- Zlateski, V.; Fuhrer, R.; Koehler, F.M.; Wharry, S.; Zeltner, M.; Stark, W.J.; Moody, T.S.; Grass, R.N. Efficient magnetic recycling of covalently attached enzymes on carbon-coated metallic nanomagnets. Bioconjugate Chem. 2014, 25, 677–684. [Google Scholar] [CrossRef]
- El-Aziz, A.M.A.; Shaker, M.A.; Shaaban, M.I. Enhanced Biocatalytic Activity of Recombinant Lipase Immobilized on Gold Nanoparticles. Curr. Pharm. Biotechnol. 2019, 20, 497–505. [Google Scholar] [CrossRef]
- Dandavate, V.; Keharia, H.; Madamwar, D. Ethyl isovalerate synthesis using Candida rugosa lipase immobilized on silica nanoparticles prepared in nonionic reverse micelles. Process Biochem. 2009, 44, 349–352. [Google Scholar] [CrossRef]
- Yilmaz, E.; Can, K.; Sezgin, M.; Yilmaz, M. Immobilization of Candida rugosa lipase on glass beads for enantioselective hydrolysis of racemic Naproxen methyl ester. Bioresour. Technol. 2011, 102, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, R.R.C.; Lima, P.J.M.; Pinheiro, B.P.; Freire, T.M.; Dutra, L.U.; Fechine, P.B.A.; Gonçalves, L.R.B.; de Souza, M.C.M.; dos Santos, J.C.S.; Lafuente, R.F. Immobilization of Lipase A from Candida antarctica onto Chitosan-Coated Magnetic Nanoparticles. Int. J. Mol. Sci. 2019, 20, 4018. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Shi, J.; Deng, Q.; Zheng, M.; Wan, C.; Zheng, C.; Li, Y.; Huang, F. Preparation of carriers based on ZnO nanoparticles decorated on graphene oxide (GO) nanosheets for efficient immobilization of lipase from Candida rugosa. Molecules 2017, 22, 1205. [Google Scholar] [CrossRef] [PubMed]
- Cassimjee, K.E.; Kadow, M.; Wikmark, Y.; Humble, M.S.; Rothstein, M.; Rothstein, D.; Bäckvall, J.-E. A general protein purification and immobilization method on controlled porosity glass: Biocatalytic applications. Chem. Commun. 2014, 50, 9134–9137. [Google Scholar] [CrossRef] [PubMed]
- Yadav, D.K.; Yadav, N.; Yadav, S.; Haque, S.; Tuteja, N. An insight into fusion technology aiding efficient recombinant protein production for functional proteomics. Arch. Biochem. Biophys. 2016, 612, 57–77. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodi, S.; Pourhassan-Moghaddam, M.; Wood, D.W.; Majdi, H.; Zarghami, N. Current affinity approaches for purification of recombinant proteins. Cogent Biol. 2019, 5, 1665406. [Google Scholar] [CrossRef]
- Couto, R.S.; Sanromán, M. Effect of Two Wastes Fromgroundnut Processing on Laccase Production and Dye Decolouriza-Tion Ability. J. Food Eng. 2006, 73, 388–393. [Google Scholar] [CrossRef]
- Carvalho, A.C.L.D.M.; Fonseca, T.D.S.; Mattos, M.C.D.; Oliveira, M.D.C.F.D.; Lemos, T.L.G.D.; Molinari, F.; Romano, D.; Serra, I. Recent advances in lipase-mediated preparation of pharmaceuticals and their intermediates. Int. J. Mol. Sci. 2015, 16, 29682–29716. [Google Scholar] [CrossRef]
- Gandhi, N.N. Applications of lipase. J. Am. Oil Chem. Soc. 1997, 74, 621–634. [Google Scholar] [CrossRef]
- Abdelmoez, W.; Mustafa, A. Oleochemical industry future through biotechnology. J. Oleo Sci. 2014, 63, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Wang, X.; Shang, M.; Huang, J.; Guan, G.; Li, Y.; Shi, B. Isolation of a novel alkaline-stable lipase from a metagenomic library and its specific application for milkfat flavor production. Microb. Cell Factories 2014, 13, 1–9. [Google Scholar] [CrossRef]
- Huang, J.; Yang, Z.; Guan, F.; Zhang, S.; Cui, D.; Guan, G.; Li, Y. A novel mono-and diacylglycerol lipase highly expressed in Pichia pastoris and its application for food emulsifier preparation. Process Biochem. 2013, 48, 1899–1904. [Google Scholar] [CrossRef]
- Neta, N.D.A.S.; dos Santos, J.C.S.; de Oliveira Sancho, S.; Rodrigues, S.; Gonçalves, L.R.B.; Rodrigues, L.R.; Teixeira, J.A. Enzymatic synthesis of sugar esters and their potential as surface-active stabilizers of coconut milk emulsions. Food Hydrocoll. 2012, 27, 324–331. [Google Scholar] [CrossRef]
- Patel, R.N. Biocatalytic synthesis of intermediates for the synthesis of chiral drug substances. Curr. Opin. Biotechnol. 2001, 12, 587–604. [Google Scholar] [CrossRef]
- Kim, M.G.; Lee, E.G.; Chung, B.H. Improved enantioselectivity of Candida rugosa lipase towards ketoprofen ethyl ester by a simple two-step treatment. Process Biochem. 2000, 35, 977–982. [Google Scholar] [CrossRef]
- Sikora, A.; Siódmiak, T.; Marszałł, M.P. Kinetic resolution of profens by enantioselective esterification catalyzed by Candida antarctica and Candida rugosa lipases. Chirality 2014, 26, 663–669. [Google Scholar] [CrossRef]
- Ceynowa, J.; Rauchfleisz, M. High enantioselective resolution of racemic 2-arylpropionic acids in an enzyme membrane reactor. J. Mol. Catal. B Enzym. 2003, 23, 43–51. [Google Scholar] [CrossRef]
- Siódmiak, T.; Mangelings, D.; Vander Heyden, Y.; Ziegler-Borowska, M.; Marszałł, M.P. High enantioselective novozym 435-catalyzed esterification of (R, S)-flurbiprofen monitored with a chiral stationary phase. Appl. Biochem. Biotechnol. 2015, 175, 2769–2785. [Google Scholar] [CrossRef]
- Gerard, D.; Gueroult, M.; Casas-Godoy, L.; Condoret, J.-S.; Andre, I.; Marty, A.; Duquesne, S. Efficient resolution of profen ethyl ester racemates by engineered Yarrowia lipolytica Lip2p lipase. Tetrahedron Asymmetry 2017, 28, 433–441. [Google Scholar] [CrossRef][Green Version]
- Long, Z.-D.; Xu, J.-H.; Zhao, L.-L.; Pan, J.; Yang, S.; Hua, L. Overexpression of Serratia marcescens lipase in Escherichia coli for efficient bioresolution of racemic ketoprofen. J. Mol. Catal. B Enzym. 2007, 47, 105–110. [Google Scholar] [CrossRef]
- Lee, K.-W.; Bae, H.-A.; Lee, Y.-H. Molecular cloning and functional expression of esf gene encoding enantioselective lipase from Serratia marcescens ES-2 for kinetic resolution of optically active (S)-flurbiprofen. J. Microbiol. Biotechnol. 2007, 17, 74–80. [Google Scholar] [PubMed]
- Xu, N.; Zhu, J.; Zhu, Q.; Xing, Y.; Cai, M.; Jiang, T.; Zhou, M.; Zhang, Y. Identification and characterization of novel promoters for recombinant protein production in yeast Pichia pastoris. Yeast 2018, 35, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-J.; Zheng, R.-C.; Ma, H.-Y.; Zheng, Y.-G. Engineering of Thermomyces lanuginosus lipase Lip: Creation of novel biocatalyst for efficient biosynthesis of chiral intermediate of Pregabalin. Appl. Microbiol. Biotechnol. 2014, 98, 2473–2483. [Google Scholar] [CrossRef]
- Ding, X.; Tang, X.-L.; Zheng, R.-C.; Zheng, Y.-G. Efficient biocatalytic synthesis of chiral intermediate of Pregabalin using immobilized Talaromyces thermophilus lipase. BioMed Res. Int. 2018, 2018, 6192059. [Google Scholar] [CrossRef]
- Martinez, C.A.; Hu, S.; Dumond, Y.; Tao, J.; Kelleher, P.; Tully, L. Development of a chemoenzymatic manufacturing process for pregabalin. Org. Process Res. Dev. 2008, 12, 392–398. [Google Scholar] [CrossRef]
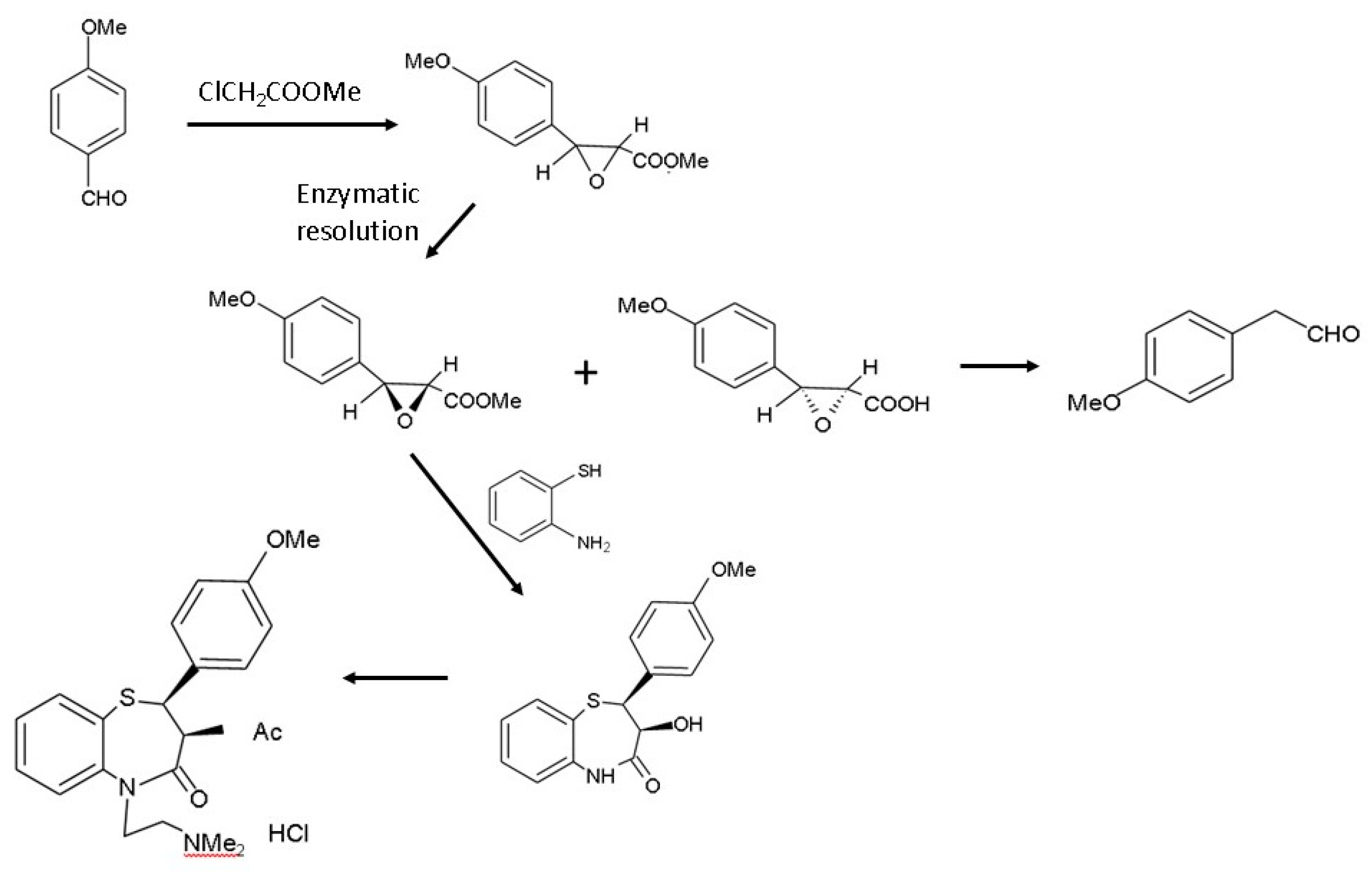
- Shibatani, T.; Omori, K.; Akatsuka, H.; Kawai, E.; Matsumae, H. Enzymatic resolution of diltiazem intermediate by Serratia marcescens lipase: Molecular mechanism of lipase secretion and its industrial application. J. Mol. Catal. B Enzym. 2000, 10, 141–149. [Google Scholar] [CrossRef]
- Yin, Y.-C.; Li, H.-Q.; Wu, X.-S. Refolding with Simultaneous Purification of Recombinant Serratia marcescens Lipase by One-Step Ultrasonication Process. Appl. Biochem. Biotechnol. 2020, 191, 1670–1683. [Google Scholar] [CrossRef]
- Dézsi, C.A.; Szentes, V. The Real Role of β-Blockers in Daily Cardiovascular Therapy. Am. J. Cardiovasc. Drugs 2017, 17, 361–373. [Google Scholar] [CrossRef]
- Kuyper, L.M.; Khan, N.A. Atenolol vs. nonatenolol β-blockers for the treatment of hypertension: A meta-analysis. Can. J. Cardiol. 2014, 30, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.A.; He, H.; Pham-Huy, C. Chiral drugs: An overview. Int. J. Biomed Sci. 2006, 2, 85–100. [Google Scholar] [PubMed]
- Dwivedee, B.P.; Ghosh, S.; Bhaumik, J.; Banoth, L.; Banerjee, U.C. Lipase-catalyzed green synthesis of enantiopure atenolol. RSC Adv. 2015, 5, 15850–15860. [Google Scholar] [CrossRef]
- María, P.D.; de Gonzalo, G.; Alcántara, A.R. Biocatalysis as Useful Tool in Asymmetric Synthesis: An Assessment of Recently Granted Patents. Catalysts 2019, 9, 802. [Google Scholar] [CrossRef]
- Xia, J.; Chen, D. Method for Preparing L-Valsartan by Separating DL-Valsartan Ester with Lipase. CN Patent 105420338A, 23 March 2016. [Google Scholar]
- Ji, Y.; Chen, B.; Quian, F.; He, Y.; Gao, X.; Hong, X. A Method for Preparing Levetiracetam. CN Patent 105063120B, 18 October 2015. [Google Scholar]
- Zheng, G.; Chen, Q. Method for Producing Ticagrelor Chiral Drug Intermediates by Using Candida Antarctica Lipase B. CN Patent 104164469A, 26 November 2014. [Google Scholar]
- Gaboardi, M.; Pallanza, G.; Baratella, M.; Castaldi, G.; Castaldi, M. Chemoenzymic Preparation of Sofosbuvir Involving a Lipase Catalyzed Regioselective Deacetylation. WO Patent 2017144423A1, 31 August 2017. [Google Scholar]
- Ku, L. Process for Synthesis of Posaconazole Intermediate 2-Methylpropionic Acid 4-(2,4-Difluorophenyl)-2-(2S)-hydroxymethyl-4-pentenyl Ester. CN Patent 105753693A, 13 July 2016. [Google Scholar]
- Wang, Q.; Wang, T.; Sun, Y.; Wang, B.; You, B.; Pu, J.; Li, X.; Jiang, Y. Preparation of Levocarnitine. CN Patent 106748843A, 31 May 2017. [Google Scholar]
- Ueda, T.; Abe, Y. Novel Method for Producing 1-(acyloxy)alkyl Carbamate Derivatives. WO Patent 2016208709A1, 29 December 2016. [Google Scholar]
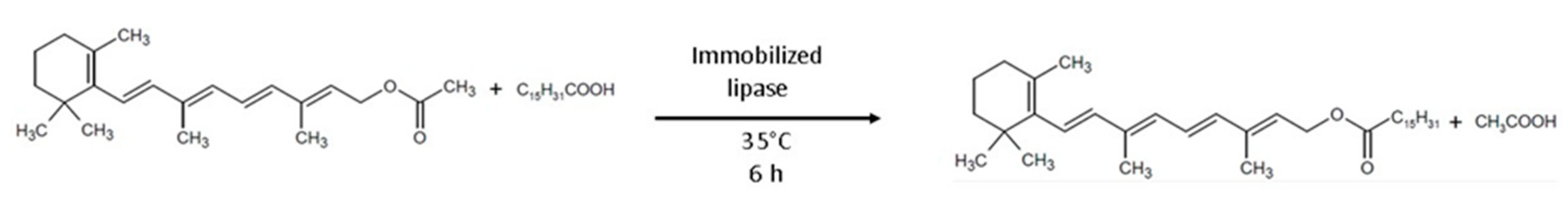

| Enzyme | Enzyme Source | Expression Vector (Expression Host) | Cloning Vector (Recipient Strain) | Expression Vector | Molecular Mass (kDa) | Optimal Temperature and pH | Thermal Stability | Additional Remarks | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Lipase KV1 | Acinetobacter haemolyticus | E. coli BL21 (DE3) | pGEM-T Easy (E. coli JM109) | pET-30a (+) | 39 | 40 °C, 8.0 | T1/2 −40 °C, 24 h | Optimization of production conditions using response surface methodology (RSM) | [63] |
| Lipase BaG7Lip | Bacillus amyloliquefaciens G7 | E. coli BL21-Star (DE3) | p15TV-L (E. coli BL21-Star (DE3)) | p15TV-L | 26 | 50 °C, 8.0 | Remaining activity of ~85% at 50 °C for 250 min | Construction of metagenomics libraries + prediction of the best producing conditions using a Boolean network analysis. Activity enhancement with acetone, glycerol and K+ ions. | [71] |
| Lipase LipBC (LipA) + foldase LifBC (LipB) | Burkholderia contaminans LTEB11 | E. coli BL21 (DE3) | - | pET28a(+) and pT7-7 for LipA and LipB, respectively | 36 (LipA), 37 (LipB) and 66 (complex LipA–LipB) | 25–45 °C, 6.5–10.0 | T1/2 −50 °C, 1.5 h | Co-expression of lipase and chaperone genes. Specific activity of 1426 U/mg (tributyrin); > 80% conversion of ethyl-oleate in n-hexane over five reaction cycles of 6 h at 45 °C. | [73] |
| Cholesterol esterase + foldase | Burkholderia stabilis FERMP-21014 | E. coli BL21 (DE3), E. coli Rosetta (DE3) and B. stabilis | E. coli DH5α and JM109 | pET26b(+), pET39b(+), pET40b(+), pBBR122 | - | - | - | Promoters screening by RNA-Seq + co-expression of lipase and foldase + heterologous and homologous expression. Recombinant activity was 243 fold higher than the WT without oleic acid; B. stabilis system was more efficient to produce esterase. | [67] |
| Lipase LipBT and foldase LifBT | Burkholderia territorii GP3 | E. coli DH5α, E. coli DH10β, E. coli BL21 (DE3) pLysS, E. coli Origami B, E. coli Shuffle B, and E. coli SHuffle K | pGEM-T Easy (E. coli DH5α), pET15b (E. coli DH10β) | pGEM-T Easy and pET15b | 30 | 80 °C, 11.0 | T1/2 −70 °C, 30 min, pH 8.0 | Metagenomics for screening and identification of lipolytic strains + evaluation of the best expression systems. Higher lipase activity in E. coli BL21 (DE3) pLysS (pET15b) (6.73 ± 0.24 U/mg); optimum substrate pNP-C10; activity enhancement in n-hexane, Triton X100, and Ca2+ and Mg2+ ions. | [74] |
| Lipase Ca-Est | Clostridium acetobutylicum (ATCC 824) | E. coli BL21 (DE3) | pMCSG7 (E. coli DH5α) | pMCSG7 | 29 | 60 °C, 7.0 | Remaining activity of ~70% at 30 °C for 300 min | Rational design + docking analysis + site-directed mutagensis. Activity enhancement with methanol; residues Ser89 and His224 are crucial for catalysis. | [75] |
| Lipase Lip3 | Drosophila melanogaster | E. coli BL21 (DE3) | - | pETMCSIII | 43 | - | T1/2 −37.3 °C (WT) and 52.9 °C (R7_47D) after 45 min | Directed evolution + error-prone PCR + construction of variant libraries. R7_59A mutant activity was higher than the WT towards tributyrin, glyceryl trioctanoate, coconut oil, glyceryl trioleate and pNP (pNP-C3, pNP-C8, pNP-C16, pNP-C18) substrates; 57_59A activity enhancement of 228 fold compared to the WT using pNP-C8 substrate. | [76] |
| Lipase HT1-5M | Geobacillus zalihae | E. coli BL21 (DE3)pLysS | pUC57 and pGEX-4T1 (E. coli TOP10) | pLysS | 44 | 70 °C, 9.0 | Stable at 30–60 °C for 30 min | Rational design + molecular dynamics (MD) + site-directed mutagenesis. Activity enhancement with Ca2+ ions; more stable in DMSO, n-hexane, and n-heptane with Ca2+ ions. | [77] |
| Lipase GnMgl | Glaciecola nitratireducens FR1064T | E. coli BL21 (DE3) | - | pET22b | 39 | 30 °C, 9.0 | T1/2 −35 °C, 30 min; lipase retained 30% activity at 0 °C | Site-directed mutagenesis. Higher activity towards monoacylglycerols C12:0 and C14:0, pNP-C6 and pNP-C8, and tributyrin; tolerance to 3.5 M NaCl; improved activity with detergents and organic solvents; mutation of residues Ser156, Asp290, or His318 fully disrupted the lipase activity. | [78] |
| Lipase LipPN1 | Proteus sp. NH 2-2 | E. coli BL21 (DE3) | - | pET-28a(+) | 31 | 40 °C, 9.0 | Remaining activity of 61.75% (40 °C), 58.30% (50 °C) and 19.63% (60 °C) after 30 min | Site-directed mutagenesis. Highest activity towards pNP-butyrate (pH 9.0, 40 °C); activity enhancement with acetone and ions Ca2+, Mn2+ and Mg2+; rLipPN1 and rLipPN1_C85S reached 1662 U/L and 1436 U/L, respectively; 91.5% of soybean oil was converted into biodiesel. | [79] |
| G55Y, T52Y and AMS8 recombinant lipases | Pseudomonas fluorescens AMS8 | E. coli BL21 (DE3) | - | pET32b | - | - | T1/2 −25 °C, 5 h + 37 °C, 5 h (AMS8); 25 °C, > 10 h + 37 °C, > 9 h (T52Y); 25 °C, 2.5 h + 37 °C, 1.5 h (G55Y) | Rational design + site-directed mutagenesis. G55Y and T52Y lipases had lower affinity by pNP-palmitate, laurate and caprylate substrates compared to WT AMS8 lipase; efficiency of G55Y lipase was higher than T52Y for pNPL and pNPP substrates. | [80] |
| Lipase LSK25 | Pseudomonas sp. LSK25 | E. coli BL21 (DE3) | pGEMT Easy (-) | pET32b(+) | 65 | 30 °C, 6.0 | Stable at 5–30 °C and at pH 6–8 | Activity enhancement with Ca2+ ions; activity boosted in toluene, xylene, n-hexane, n-heptane and n-hexadecane; higher activities towards long chain fatty acids contained in coconut oil and rice brain oil, and pNP-C12. | [72] |
| Lipase RK-lip479 | Uncultured microorganism isolated from hot springs | E. coli BL21 (DE3) | pUC19 (E. coli DH5α) | pETite C-His Kan | 42 | 65 °C, 8.0 | Remaining activity of 89, 92 and 60% after 6 h at 55, 65 and 75 °C, respectively | Construction of metagenomics libraries. Remaining activity of 50% after 72 h in 25% (v/v) methanol; maximum activity towards pNP-C12; activity enhancement with DMSO and DMF; yield of biodiesel production was 40–76%. | [68] |
| Lipase RPK01 | Uncultured microorganism isolated from hot springs | E. coli BL21 (DE3) | pGEM-T Easy (E. coli DH5α) | pET23(+) | 24 | 40 °C, 8.0 | Stable at 30 °C after 3h; remaining activity of ~50% at 50 and 60 °C up to 3 h | MD simulations. Preference for pNP-decanoate substrate, specific activity 6.2 ± 0.065 U/mg, activity improvement with Ca2+ ions, Tween 20 and Triton X-100; tolerance to 1% methanol and n-hexane. | [69] |
| Lipase CALB | Candida antarctica | Corynebacterium glutamicum MB001 | pEC-CALB and pEC-H36-CALB (E. coli DH5α) | pEC-CALB and pEC-H36-CALB | 33 | 40 °C, 9.0 | Stable at 30–50; lipase activity was reduced to 60% at ≥ 60 °C | Activity inhibited by 31% with 10 mM MgSO4. | [81] |
| Enzyme | Enzyme Source | Expression Vector (Expression Host) | Cloning Vector (Recipient Strain) | Expression Vector | Molecular Mass (kDa) | Optimal Temperature and pH | Thermal Stability | Additional Remarks | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Lipase MAS1 | Marine Streptomyces sp. strain W007 | K. phaffii X-33 | pPICZαA (E. coli DH5α) | pPICZαA | 30 | 25 °C, 8.0 | - | PDI co-expression gives 1.7 fold increase in lipase expression. The highest lipase production was achieved at pH 6.0 and at 24 °C with an activity of 440 U/mL. | [83] |
| r27RCL | Rhizopus chinensis | K. phaffii GS115 | pPIC9K | pPIC9K | 37 | 37 °C, 8.0 | - | PDI co-expression gives 1 fold increase. The highest lipase activity reached 355 U/mL with one copy of PDI and five copies of r27RCL gene. | [84] |
| CALB | Candida antarctica | K. phaffii GS115 | pPICZαB (E. coli TOP10F’) | pPICZαB | 33 | 40 °C, 7.0 | - | Combinatorial overexpression of Ydj1p-Ssa1p resulted in the highest fold increase, 2.5. Individual overexpression of Ydj1p, Ssa1p and Sec63p increased CALB expression level by 1.6, 1.4 and 1.4 fold, respectively. Co-expression of Ydjlp-Sec63p Kar2p-Ssalp, Kar2p-Sec63p, resulted in 1.5, 1.5 and 1.5 fold increase, respectively. Kar2p-Ydj1p combination resulted in decreased CALB secretion. | [85] |
| CALB | Candida antarctica | K. phaffii X-33 and M12 (leu2) | E. coli Stellar™ and XL10-Gold® | pBluescript II SK pPGKΔ3PRO_LIPB | 33 | 40 °C, 7.0 | Remaining activity of 15% at 70 °C after 20 min | Strain with three copies achieved 48.760 U/L enzyme yield, 2.3 fold higher than the one-copy strain. | [65] |
| ROL | Rhizopus oryzae | K. phaffii GS115 | pPICZα and pAO815 (E.coli Top10 cells) | pPICZα-ROL | 35 | 35 °C, 8.0 | - | Strain with five copies resulted in 8 fold increase in ROL expression. Co-expression of both genes Ubc1 and Hrd1 resulted in.54.2% higher ROL activity, 4750 U/mL. | [86] |
| ROL | Rhizopus oryzae | K. phaffii X-33 | pPICZFLDαROL | 35 | 30 °C, 7.25 | - | pFLD gave a 1.9 fold increase compared to pAOX1. The best ROL production strategy with the PFLD-based system is a fed-batch induction phase with a constant sorbitol excess. | [87] | |
| FSL | Fusarium solani | K. phaffii X-33 | pPICZαA and pGAPZαA (E. coli DH5α) | pPICZαA-FSL and pGAPZαA-FSL | 30 | 35 °C, 7.0 | Remaining activity of 96% at 20 °C and 81% at 25 °C after 30 min | Strain expressing pGAPZαA-FSL produced the highest specific lipase activity, 18.81 ± 1.98 U/mg, in 3 days of cultivation time. Lipase activity was enhanced by Mn2+, Ba2+, Li+, Ca2+, Ni2+, CHAPS and Triton X-100 but was inhibited by Hg2+, Ag+ and SDS. | [88] |
| CALB | Candida antarctica | K. phaffi GS115 and Y. lipolytica RIY368 | E. coli | K. phaffii (pIB4, promoter pAOX1, HIS4 marker) and Y. lipolytica (JMP4266, promoter pEYK1-A3B, URA3 marker) | 33 | 40 °C, 7.0 | - | The lipase activity after 72 h cultivation was for Y. lipolytica strain RIY368 5540 U/mg dcw and for K. phaffii strain RIY311 1066 U/mg dcw. For Y. lipolytica, the lipase activity increased during the growth phase, whereas for P. pastoris it increased both during growth and stationary phase. | [89] |
| Lipase with accession no. AF054513 | Thermomyces lanuginosus | A. niger NW297 | - | pBOEL960-24 | 30 | 30 °C, 7.5 | - | During chemostat cultivations, the maximal lipase production was observed during sporulation. The heterologous TAKA amylase promoter from Aspergillus oryzae was demonstrated to express high levels of lipase in A. niger. | [90] |
| Lipase lip | A. niger | T. reesei Tu6 strain | pBluescript II SK(+) and pMD18-T Simple (E. coli DH5α) | pSKpyr4 and pSK-lip | 30 | 45 °C, 7.5 | - | All cph1 gene silencing transformants expressed higher levels of lipase and less cbh 1 transcript than the reference strain, approximately lower than 2%. The RNAi mediated gene silencing of cbh 1 did not negatively affect the lipase transcript abundance. | [91] |
| TDL | Thermomyces dupontii | K. phaffii X33 | pPICZαA (E. coli Top 10) | PICZαA–tdl-opt | 30 and 38 (N-glycosylation) | 60 °C, 9.5 | - | Of 15 methanol-inducible promoters, the highest TDL activity was achieved with pFLD1 (27.076 U/mL), whereas of nine constitutive promoters, pGCW14 gave the highest activity (17.353 U/mL). | [92] |
| Substance | Reaction Analyzed | Application | Patent Number | Sponsor | Reference |
|---|---|---|---|---|---|
| Valsartan | Stereoselective hydrolysis | Enantioselective hydrolysis of racemic esters of valsartan | CN105420338A | Tiantai Yisheng Biochemical Technology Co. Ltd. | [181] |
| Levetiracetam | Stereoselective hydrolysis | Kinetic resolution of a racemic 2-haloester | CNA2009100263523A | Zhejiang Changming Pharmaceutical Co. Ltd. | [182] |
| Ticagrelor | Stereoselective hydrolysis | Kinetic resolution of an alcohol used for the drug preparation | CN104164469A | Beijing University of Chemical Technology | [183] |
| Sofosbuvir | Site-selective acyl-transfer | Mono-deacetylation of a precursor for sofosbuvir preparation | WO2017144423A1 | HC-Pharma AG | [184] |
| Posaconazole | Stereoselective acyl-transfer | Stereoselective monoacylation of prochiral intermediate with isopropanoic anhydride. | CN105753693A | Ningbo Xinkai Biotechnology Co. Ltd. | [185] |
| L-carnitine | Stereoselective acyl-transfer | Kinetic resolution of racemic hidroxynitrile | CN106748843A | Wuxi Fortune Pharmaceutical Co. Ltd. | [186] |
| Carbamate prodrug | Stereoselective hydrolysis | Kinetic resolution of an intermediate | WO2016208709A1 | Daiichi Sankyo Co. Ltd. | [187] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Contesini, F.J.; Davanço, M.G.; Borin, G.P.; Vanegas, K.G.; Cirino, J.P.G.; Melo, R.R.d.; Mortensen, U.H.; Hildén, K.; Campos, D.R.; Carvalho, P.d.O. Advances in Recombinant Lipases: Production, Engineering, Immobilization and Application in the Pharmaceutical Industry. Catalysts 2020, 10, 1032. https://doi.org/10.3390/catal10091032
Contesini FJ, Davanço MG, Borin GP, Vanegas KG, Cirino JPG, Melo RRd, Mortensen UH, Hildén K, Campos DR, Carvalho PdO. Advances in Recombinant Lipases: Production, Engineering, Immobilization and Application in the Pharmaceutical Industry. Catalysts. 2020; 10(9):1032. https://doi.org/10.3390/catal10091032
Chicago/Turabian StyleContesini, Fabiano Jares, Marcelo Gomes Davanço, Gustavo Pagotto Borin, Katherina Garcia Vanegas, João Pedro Gonçalves Cirino, Ricardo Rodrigues de Melo, Uffe Hasbro Mortensen, Kristiina Hildén, Daniel Rossi Campos, and Patricia de Oliveira Carvalho. 2020. "Advances in Recombinant Lipases: Production, Engineering, Immobilization and Application in the Pharmaceutical Industry" Catalysts 10, no. 9: 1032. https://doi.org/10.3390/catal10091032
APA StyleContesini, F. J., Davanço, M. G., Borin, G. P., Vanegas, K. G., Cirino, J. P. G., Melo, R. R. d., Mortensen, U. H., Hildén, K., Campos, D. R., & Carvalho, P. d. O. (2020). Advances in Recombinant Lipases: Production, Engineering, Immobilization and Application in the Pharmaceutical Industry. Catalysts, 10(9), 1032. https://doi.org/10.3390/catal10091032







