Abstract
In the present study, four types of sulfonation method, including thermal treatment with concentrated sulfuric acid (H2SO4), thermal decomposition of ammonium sulphate (NHSO4), thermal treatment with chlorosulfonic in chloroform (HSO3Cl), and in situ polymerization of poly(sodium4-styrenesulfonate) (PSS), were employed to convert incomplete carbonized glucose (ICG) to sulfonated heterogeneous catalysts for the fatty acid methyl ester (FAME) production. The characteristics of synthesized catalysts were further examined using Raman spectroscopy, Fourier transformation infrared (FT-IR), ammonia temperature programmed desorption (NH3-TPD), Brunauer–Emmett–Teller (BET), thermogravimetric analysis (TGA), scanning electron microscopy (SEM), and energy dispersive X-ray (EDX). According to experiments, the sulfonic acid density was varied in a range from 4.408 to 14.643 mmol g−1 over various sulfonation methods. The catalytic activity of synthesized catalysts over different sulfonation methods was determined by performing the conversion of palm fatty acid distillate (PFAD) to ester synthesis in a batch-system reactor. The findings reveal that using PSS-ICG resulted in the highest FAME yield of 96.3% followed by HSO3Cl-ICG of 94.8%, NHSO4-ICG of 84.2%; and H2SO4-ICG of 77.2%. According to results, the ICG sulfonated by PSS method with the highest acid density (14.643 mmol g−1) gave the highest catalytic activity over PFAD conversion to biodiesel. According to experiment results, acid density played a crucial role over FAME yield percentage. Besides acid density, it is also worth mentioning that various sulfonation methods including different mechanisms, chemicals and sulfonating agents played crucial roles in the FAME yield percentage.
1. Introduction
It is not deniable that conventional fossil fuels are being swiftly depleted which has raised a global concern. Moreover, the consumption of the current fuels has negatively affected the global climate due to the emission of the hazardous particulates such as carbon monoxide, sulphur dioxide, etc., which lead to the greenhouse gases (GHG) emissions [1,2,3,4]. The harmful consequences of GHG emission on the wellbeing of individuals has expanded public awareness to employ renewable resources of energy.
Biodiesel, as a promising alternative for diesel fuels, has substantially attracted the awareness of scientists. Moreover, biodiesel is considered as an environmentally friendly source of energy that is able to lessen the illness possibility by up to 90%. Biodiesel, fatty acid methyl ester (FAME), is generally derived from esterification of the free fatty acids (FFAs) or transesterification of triglycerides (TGs) over a suitable catalyst [5,6,7].
There are several important factors that highly influence the general expense of ester production: (i) accessibility of the feedstock, (ii) FFA content, (iii) synthesis route, and (iv) type of catalyst. The refined edible vegetable oils such as sunflower, rapeseed, cottonseed, soybean, palm oil, and canola have all been employed as raw feedstocks [8,9,10]. Nevertheless, the consumption of these edible oils always threatens the food crop sources, resulting in high costs of fuel production. To minimize the expense of analysis, there are demands to switch over non-eatable feedstocks. Castor oil [11], rubber seed [11], jojoba oil [12], Jatropha curcas [13], Cynara cardunculus oil [13], waste oils (trap grease, frying oil) [14] and microalgae [15] are some examples of non-edible oil used for biodiesel production. Between all the nonedible oil sources, palm fatty acid distillate (PFAD), as a by-product from the palm oil milling process, has drawn current attention due to its low-grade oil and its availability in Malaysia (which is recognized as the second major palm oil producer in the world). However, because of the high content of FFA in PFAD (85%–95%) [16], a highly stable acid catalyst is required rather than a base catalyst in order to avoid soap formation.
In contrast with homogeneous catalysts, heterogeneous acid catalysts are preferable through the process of ester generation due to the remarkable advantages that they possess such as the easy and inexpensive separation process, higher recyclability, highly intensive to FFA and aquatic content in raw materials and being environmentally friendly. Conversely, homogeneous acid catalysts are capable of concurrently catalyzing together esterification-transesterification reactions due to the presence of hydrophobic and strong acid sites. Recently, sulfonated carbon-based solid catalysts [17,18,19,20] have been significantly studied regarding their catalysis activities through biodiesel production due to possessing unique characteristics like a hydrophobic surface area, excellent thermal and mechanical stability, and low cost [21]. In a specific study, D-glucose was initially carbonized to obtain rigid graphite and then post-sulfonation treatment took place using concentrated sulfuric acid (H2SO4) [18]. The synthesized starch derived solid acid catalyst successfully converted PFAD to biodiesel using conventional batch system. The utmost FAME yield and FFA transformation gained 90.4% and 94.6%, respectively, in presence of 2 wt.% of catalyst at 75 °C applying 10:1 methanol:PFAD molar ratio within 3 h. In another study, a carbohydrate-derived mesoporous ZnO-TiO2 hollow sphere [22] and a mesoporous NiO core–shell solid sphere [23] were initially produced over incomplete carbonized glucose (ICG) as a template agent via the one-pot hydrothermal technique. The as-prepared composites were later immobilized by thermal treatment with chlorosulfonic acid in chloroform and thermal decomposition of ammonium sulphate, respectively, to add -SO3H functional species to the surface of the catalysts as well as the mesopore walls. The SO3H-ZnO-TiO2-ICG and SO3H-NiO-ICG catalysts showed promising FAME yield of 96.1% and 95.6%, respectively, by using an autoclave reactor.
The selection of sulfonation route depends on several considerations. One of the most crucial factors is the expected characteristics of the desired products. Certain methods are incredibly adaptable, whilst others generate a small variety of products. Every single procedure makes somewhat unique products. For instance, the air/SO3 method has the potential to sulfonate a broad range of feedstocks and synthesize exceptional property products. The second important element to choose a sulfonation process is the capacity of the expected fabrication. Some batch procedures suit the fabrication of minor capacities of material and some are capable of large-scale continuous processes to produce tons per hour of product. Some processes such as chlorosulfuric acid can be operated as either continuous or batch processes. Chemical expense, initial equipment cost and required safety systems may possibly have a notable effect on picking a sulfonation procedure. The apparatus cost is practically the opposite of the reactants cost. Here, the air/SO3 route is the top in cost, whilst the uncomplicated batch sulfamic method is the lowest. The last aspect to consider in the choosing of sulfonation procedures is the cost of waste discarding. The chlorosulfuric acid route generates significant by-product streams of either sulfuric acid or hydrochloric acid where the vital apparatus is expensive, and the dumping costs would be ridiculous.
In the current research, the principal aim was to fabricate various sort of carbohydrate-derived solid acid catalyst using different sulfonation methods to maximize the FAME yield over esterification of PFAD. In this regard, ICG was initially fabricated over pyrolysis of D-glucose and further sulfonated over various sulfonating agents and conditions. The synthesized catalysts were characterized via state-of-the-art analytical methods. Moreover, the produced catalysts were later employed at a fixed esterification conditions to examine their catalytic activity over biodiesel production yield. According to our knowledge, no detailed study has been conducted on the characterization and comparison of different sulfonation methods to convert ICG to sulfonated catalysts for ester production using high FFA feedstock.
2. Results and Discussion
2.1. Characterization of Catalysts
2.1.1. Fourier Transformation Infrared (FT-IR) Analysis
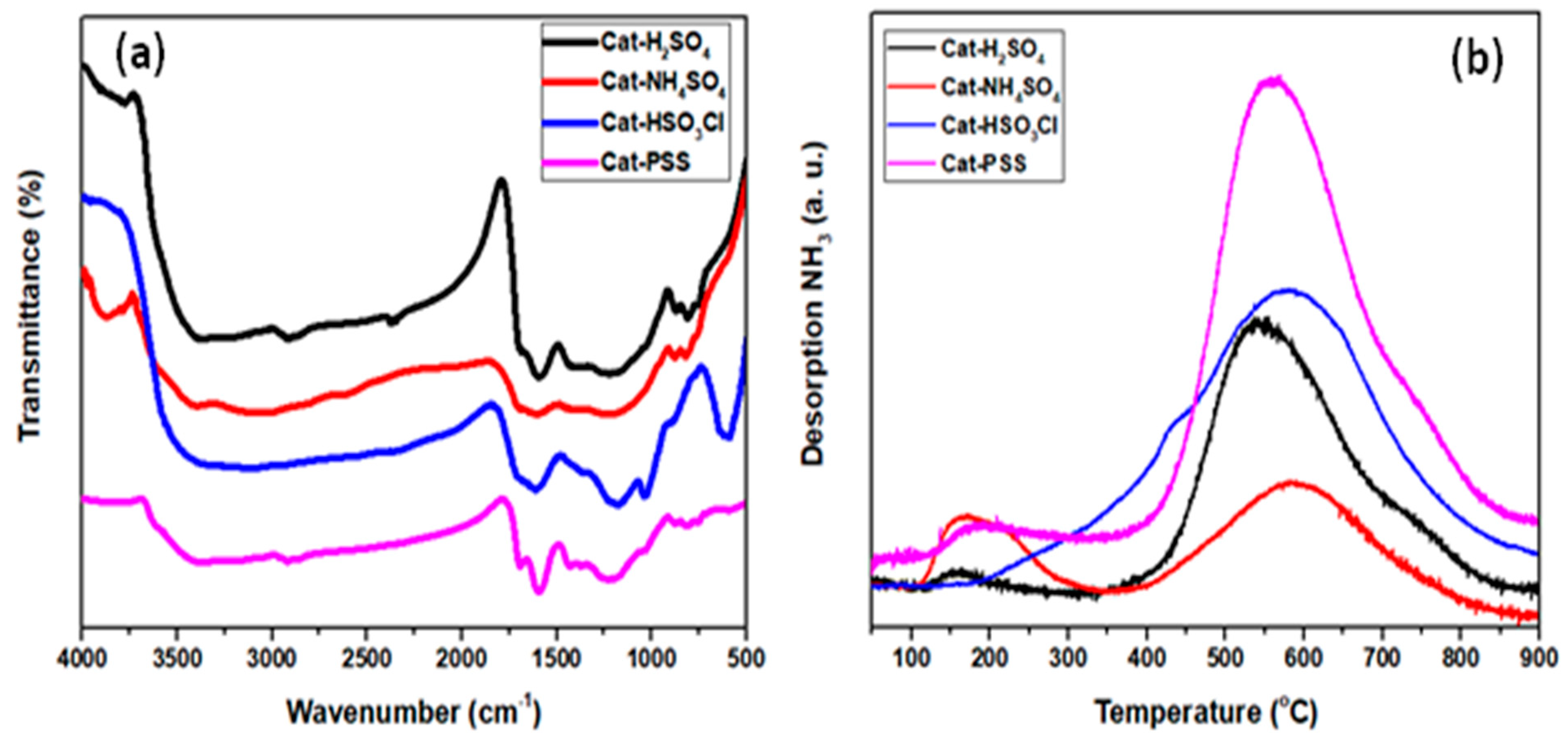
The FT-IR spectroscopy was applied for analyzing the nature of functional species linked to the active sites. From the spectrum shown in Figure 1a, all sulfonated catalysts had clear and strong vibration at around 1597.31 and 1686.02 cm−1, which corresponded to the C=C aromatic ring vibration mode and C=O stretching mode of the carboxyl spices in turn [16]. The presence of sulfonic groups was confirmed by clear peak at 1036.70 and 1227.21 cm−1, which were attributed to symmetric and asymmetric O=S=O stretching vibration [17]. From the spectra, the synthesized HSO3Cl-ICG (C) and PSS-ICG (D) catalysts had stronger vibration at peak 1036.70 cm-1 as compared to those H2SO4-ICG (A) and NHSO4-ICG (B). Moreover, all four catalysts showed a peak at 750.10 cm−1 which proved attachment of S−O group to the active sites.
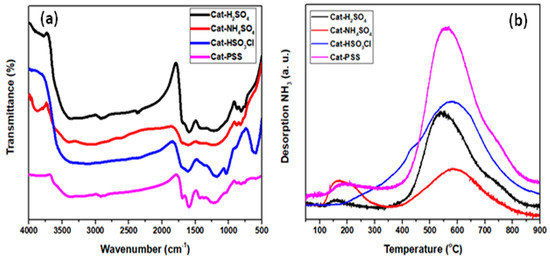
Figure 1.
(a) Fourier transformation infrared (FT-IR) spectrum; and (b) ammonia temperature programmed desorption (NH3-TPD) profiles of sulfonated incomplete carbonized glucose (ICG) catalysts; ICG sulfonated by thermal treatment with concentrated sulfuric acid; ICG sulfonated by thermal treatment with ammonium sulphate; ICG sulfonated by thermal treatment with chlorosulfonic acid in chloroform; ICG sulfonated by in situ polymerization of poly(sodium4-styrene sulfonate).
2.1.2. Ammonia Temperature Programmed Desorption (NH3-TPD) and Brunauer–Emmett–Teller (BET) Analysis
The acid density of the sulfonated ICG can highly manipulate the catalytic performance through esterification. According to the previous literature, an excess of acid density increased the catalytic performance and subsequently enhanced the conversion rate of biodiesel production [24]. The NH3-TPD was used to determine the intensity and dispersal of the acid sites. TPD profiles (Figure 1b) revealed that all the catalysts except HSO3Cl-ICG (C) possessed two unique desorption peaks from 150, 350, and 400 to 850 °C, which were attributed to weak and strong brönsted acids on the surface in turn. However, it was observed that HSO3Cl-ICG (C) had only one peak, which was a broad peak as of 400 to 850 °C and maximized at 600 οC. The presence of these peaks ensured that the synthesized catalysts in this research were chemically and thermally steady up to 300 °C prior to the decomposition of –SO3H species. According to the results indicated in Table 1, the sulfonic acid density was varied between 4.408 and 14.643 mmol g−1 over various sulfonation methods. The specific surface areas (SBET) of the synthesized sulfonated catalysts were analyzed from the N2 adsorption/desorption isotherm. The synthesized sulfonated catalysts possessed specific surface areas within the range of 4.27–8.70 m2g−1, as summarized in Table 1. As expected, the specific surface area of the synthesized catalysts was very low and had minor effect on catalysis process, hence much more attention was paid to studying the differentiation of the sulfonic methods and their effects on the catalytic activity.

Table 1.
Elemental composition, total acid density, surface area and fatty acid methyl ester (FAME) yield using different type of sulfonation methods.
2.1.3. Raman Spectroscopy Analysis
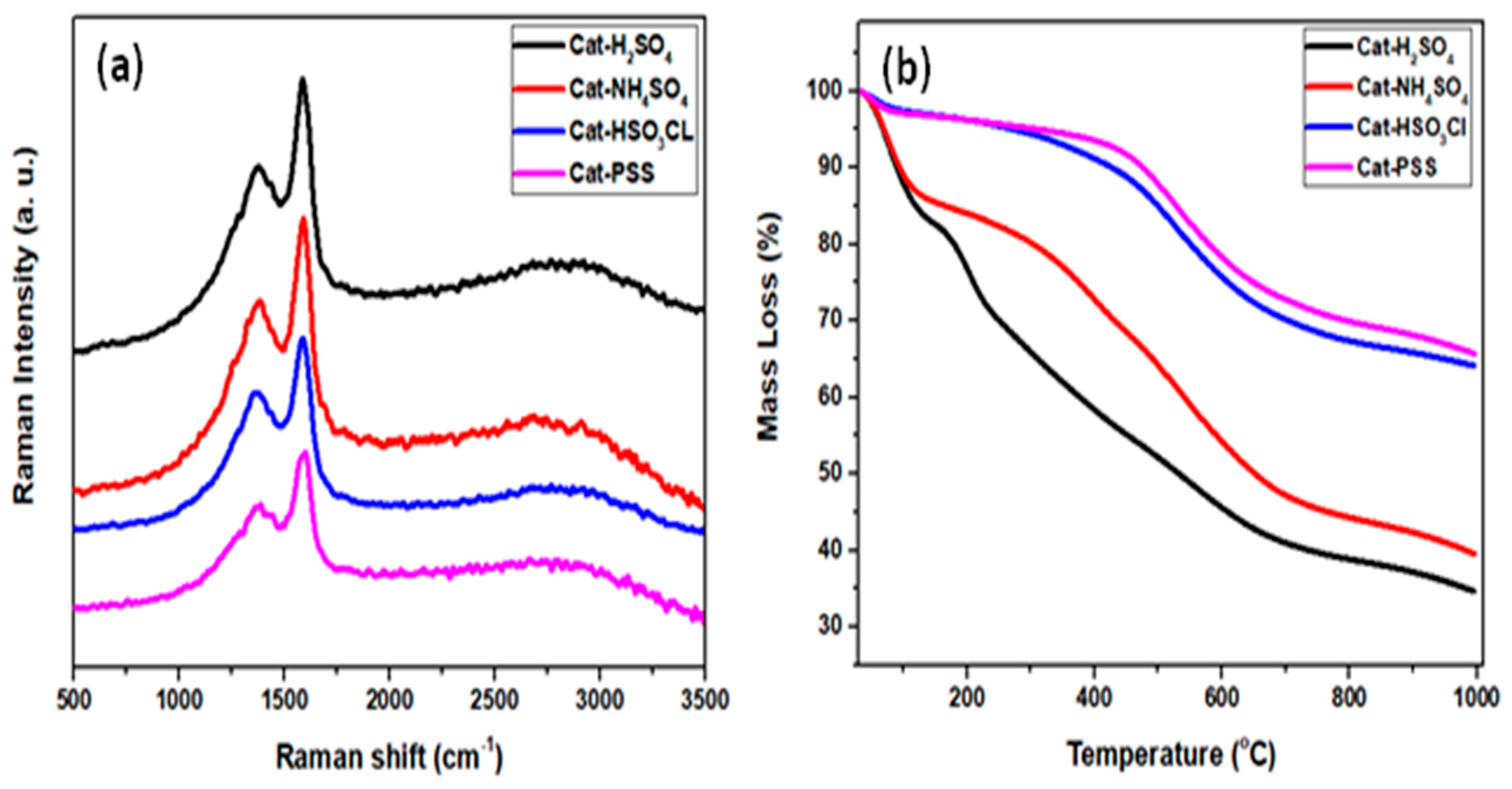
Figure 2a depicts Raman spectra for all sulfonated ICG catalysts. The spectra showed two distinct bands occurring at wavelength 1580 and 1370 cm−1 that are referred to as G and D-bands, respectively [25,26]. The presence of these two signals proved that polycyclic aromatic carbon sheets were successfully formed. In detail, the D-band with A1g D breathing mode corresponded to the deficiencies in the carbon sheets or presence of amorphous carbon which confirmed that the carbonization process had already taken place [27]. The G band was assigned to carbon atoms with a single crystal that vibrated in the opposite direction, which confirmed the formation of graphitic structures. The degree of structural order changes of graphite and defect was determined using the intensity ratio of amorphous carbon I(D)/I(G) [28]. The intensity ratio for all the synthesized catalysts—H2SO4-ICG (A); NHSO4-ICG (B); HSO3Cl-ICG (C); and PSS-ICG (D)—were calculated to be 0.714, 0.711, 0.704, and 0.615, respectively. It disclosed that the sulfonation treatment through inserting −SO3 species on the imperfect carbonized sp2 caused the imperfections of the carbon structure [26].
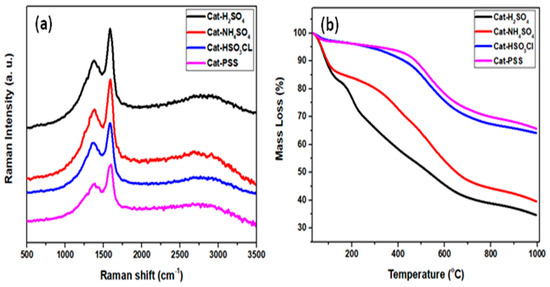
Figure 2.
(a) Raman spectra and (b) thermogravimetric analysis (TGA) profiles of sulfonated ICG catalysts; ICG sulfonated by thermal treatment with concentrated sulfuric acid; ICG sulfonated by thermal treatment with ammonium sulphate; ICG sulfonated by thermal treatment with chlorosulfonic acid in chloroform; ICG sulfonated by in-situ polymerization of poly (sodium4-styrene sulfonate).
2.1.4. Thermogravimetric Analysis (TGA)
The thermal stability of each prepared catalyst was examined using the TGA technique (Figure 2b). The thermal disintegration of the synthesized catalysts demonstrated that slight and gradual weight losses of about 5%–10% in the temperature up to 150 °C could be assigned to dihydroxylation. This was followed by substantial weight losses between 150 and 360 °C that could be assigned to decarboxylation. The third strong desorption was distinguished in the array of 360–600 °C that could be corresponded to the de-sulfonation. This implied that the attached sulfonic group could remain highly stable at 360 °C [29,30,31].
From the analysis, it was notified that the ICG sulfonated by thermal treatment with chlorosulfonic acid in chloroform (C) and ICG sulfonated by in situ polymerization of poly(sodium4-styrene sulfonate) (D) had quite a similar pattern of TGA as compared to ICG sulfonated by thermal treatment with concentrated sulfuric acid (A) and ICG sulfonated by thermal treatment with ammonium sulphate (A), which were slightly different. This may be because of the different type of sulfonation method which affected the stability of the acid site through the attachment of the –SO3H group to the carbon sheets.
2.1.5. Scanning Election Microscopy (SEM) and Energy Dispersive X-ray (EDX) Analysis
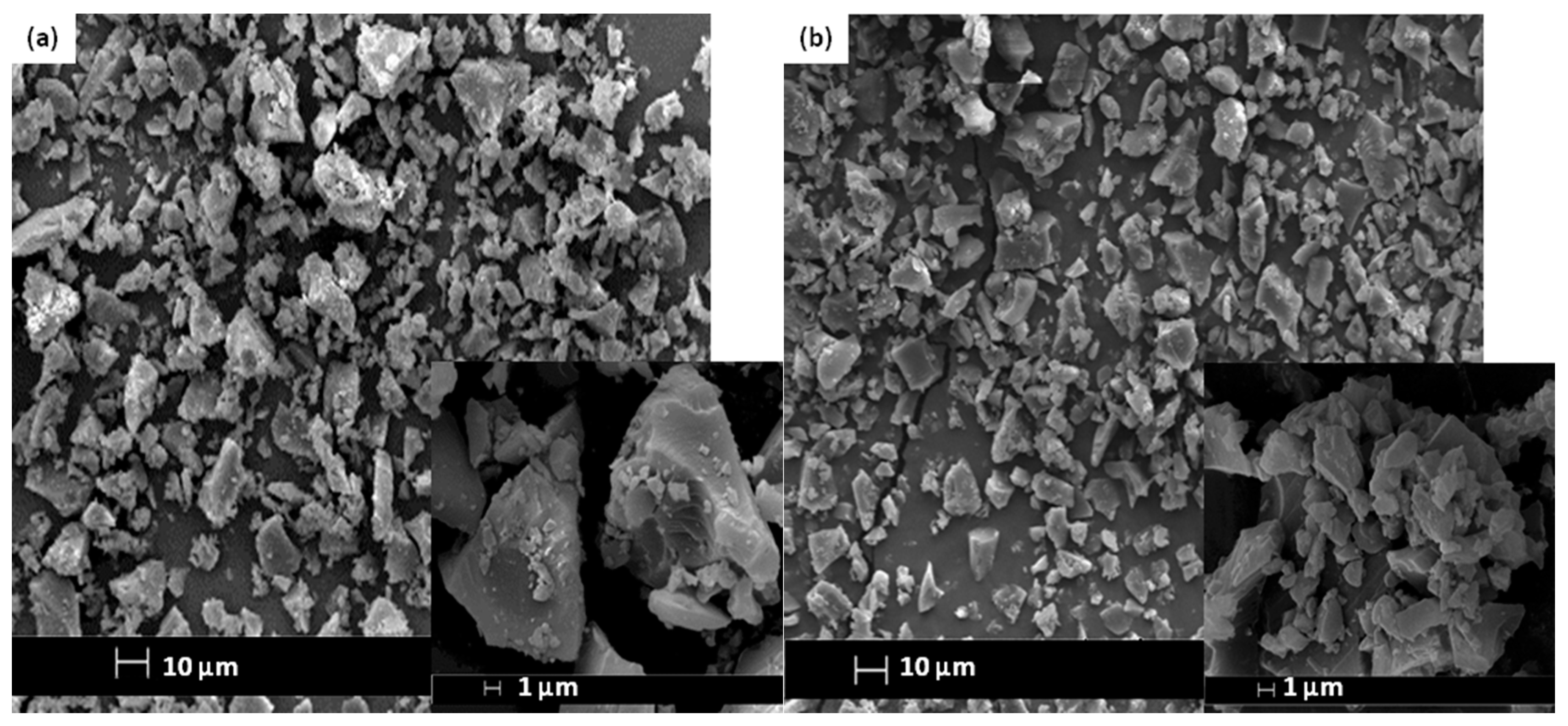
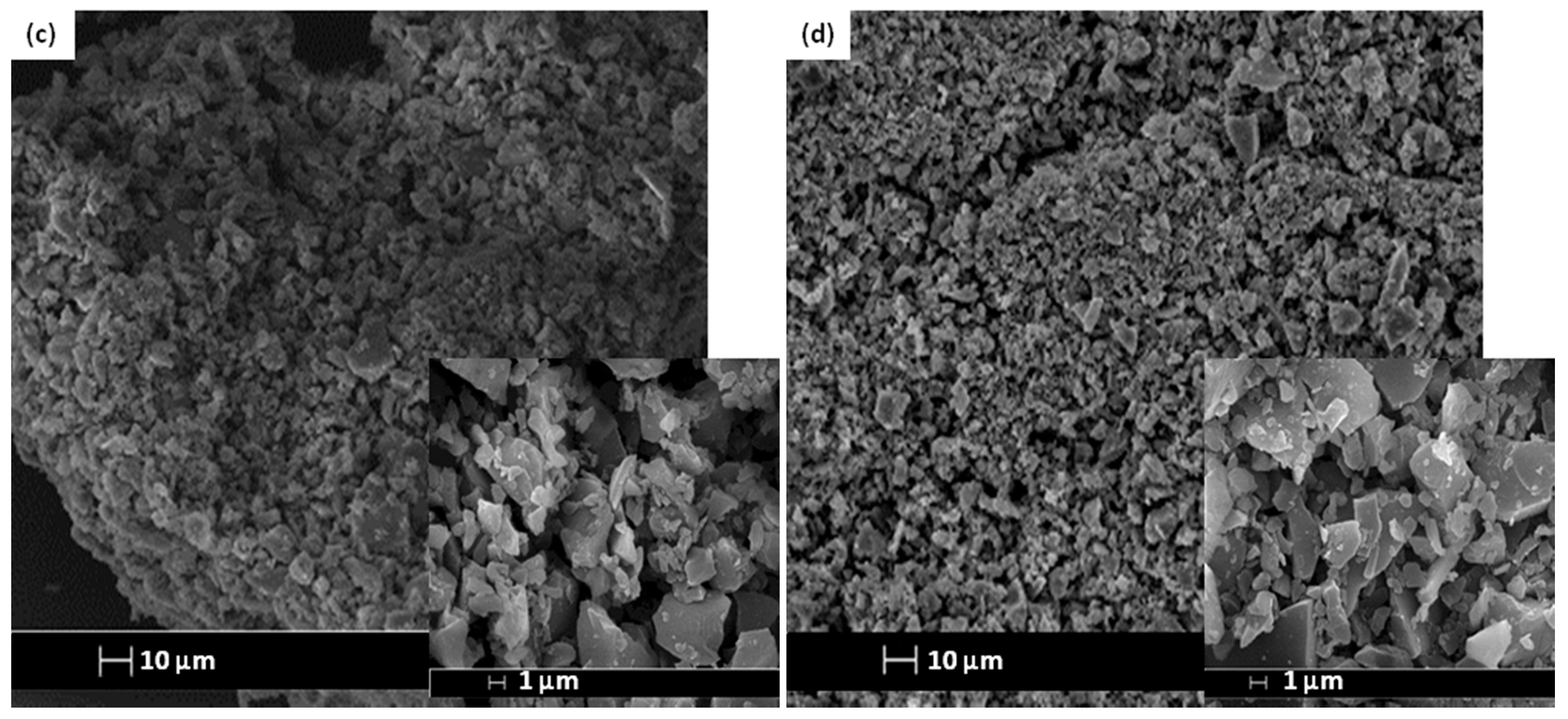
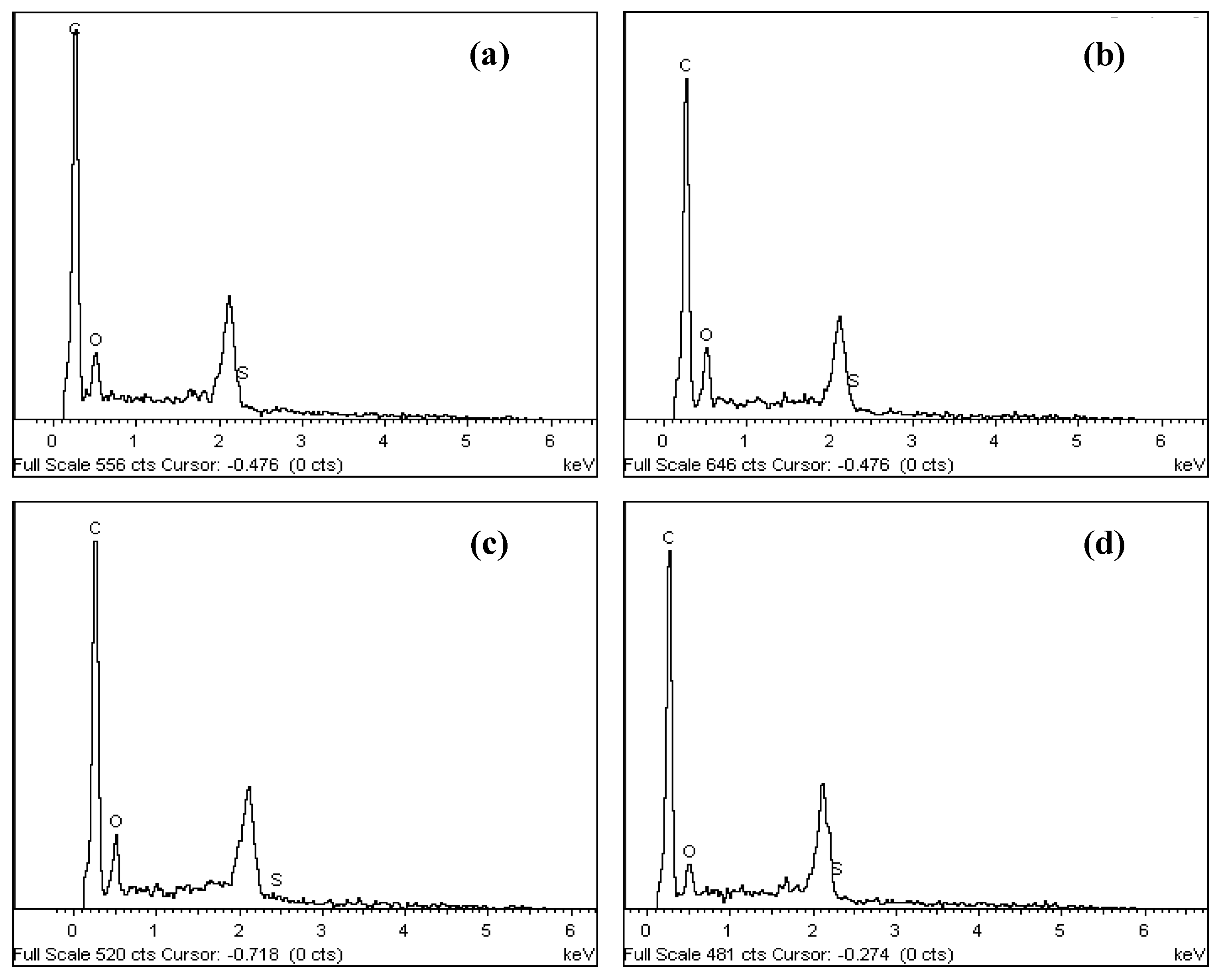
The SEM images and elemental arrangement for all the sulfonated products are shown in Figure 3; Figure 4, respectively. As expected, the carbon sheets composed predominantly of polycyclic aromatic species coupling with enormous quantities of SO3H groups, which were similar to images depicted by other researchers [28]. As outlined in Table 1 from the EDX data, PSS-ICG possessed the highest binding content of S element (7.87%) and O (10.85%), followed by HSO3Cl-ICG with content of S element (6.26%) and O (9.82%); NHSO4-ICG; S element (4.51%), O element (36.16%), H2SO4-ICG with S element (1.91 %), O element (33.83%).
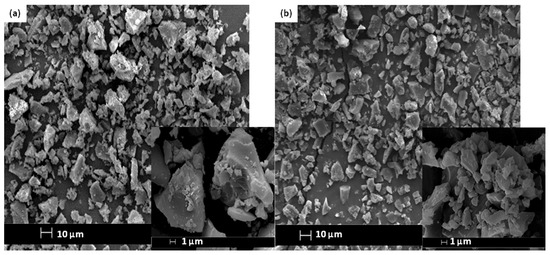
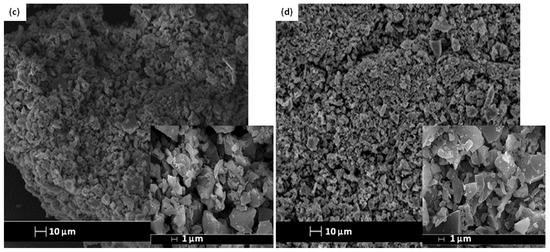
Figure 3.
Scanning electron microscopy (SEM) images of ICG sulfonated by thermal treatment with concentrated sulfuric acid (a); ICG sulfonated by thermal treatment with ammonium sulphate (b); ICG sulfonated by thermal treatment with chlorosulfonic acid in chloroform (c); ICG sulfonated by in situ polymerization of poly(sodium4-styrene sulfonate) (d) at 500× and 5000× magnification
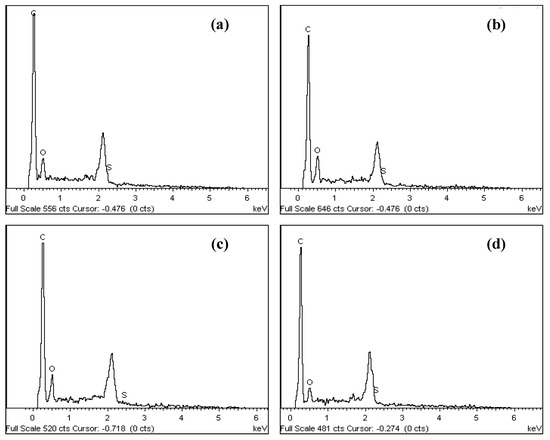
Figure 4.
Spectrum of elemental composition for ICG sulfonated by thermal treatment with concentrated sulfuric acid (a); ICG sulfonated by thermal treatment with ammonium sulphate (b); ICG sulfonated by thermal treatment with chlorosulfonic acid in chloroform (c); ICG sulfonated by in situ polymerization of poly(sodium4-styrene sulfonate) (d) using energy dispersive X-ray (EDX).
2.2. Comparisons of the Catalytic Activities using Different Sulfonation Methods
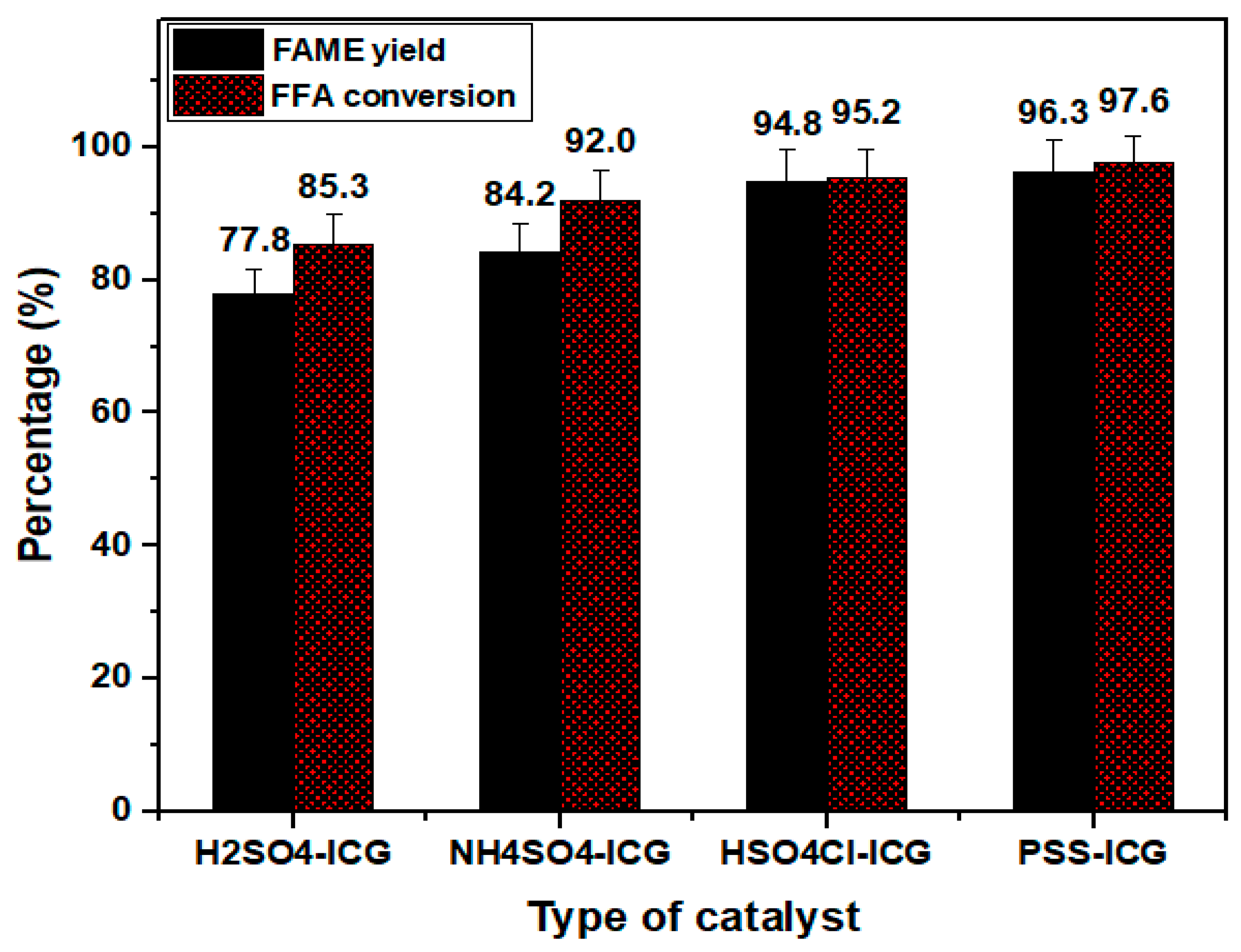
The catalytic performance of sulfonated catalysts was examined through the esterification of PFAD. The FFA conversions for each catalyst were assessed via titration route and later the values were calculated using Equation (2). The esterification reaction conditions were used as follows; operating temperature of 80 °C, operating time of 4 h, methanol to oil molar ratio of 10:1, and catalyst amount of 2.5%. According to the obtained results shown in Figure 5, the presence of PSS-ICG (D) gave the highest FFA conversion of 97.6% followed by HSO3Cl-ICG (C) of 95.2%, NHSO4-ICG (B) of 89.8%; and H2SO4-ICG (A) of 85.3%. It can be concluded that catalysts with a superior total acidity yielded higher FFA conversion in turn.
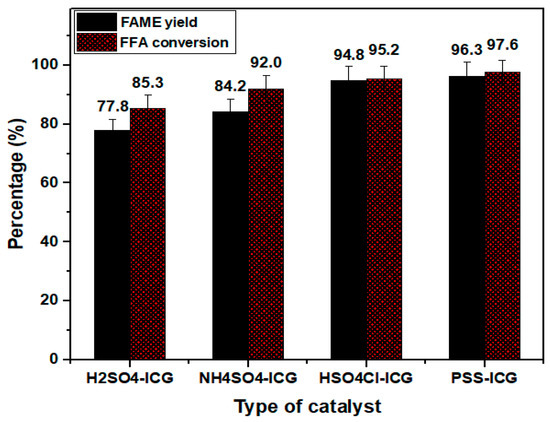
Figure 5.
FFA conversion and FAME yield of synthesized catalysts ICG sulfonated by thermal treatment with concentrated sulfuric acid; ICG sulfonated by thermal treatment with ammonium sulphate; ICG sulfonated by thermal treatment with chlorosulfonic acid in chloroform; ICG sulfonated by in situ polymerization of poly(sodium4-styrene sulfonate). (Esterification conditions: operating temperature of 80 °C, catalysts amount of 2.5 wt.%, methanol to oil ratio of 10:1, operating time of 4 h)
Subsequently, the percentage of total FAME yield produced was determined via Equation (1) and illustrated in Figure 5. From the calculation, ICG sulfonated by in situ polymerization of poly (sodium4-styrene sulfonate) (D) offered the highest FAME yield of 96.3% followed by ICG sulfonated via thermal treatment with chlorosulfonic acid in chloroform (C) of 94.8%, ICG sulfonated by thermal treatment with ammonium sulphate (B) of 84.2%, and ICG sulfonated by thermal treatment with concentrated sulfuric acid (A) of 77.8%. These results were in accordance with the FFA conversion rate, whereas esterification of PFAD over ICG sulfonated by in situ polymerization of poly(sodium4-styrene sulfonate) also offered the highest FFA conversion.
The summary of FAME yield and total acidity over the four unique sulfonation methods is shown in Table 1. From all the results obtained, it was clearly notified that acid density played a crucial role in the FFA conversion rate and FAME yield percentage. FAME yield increased with the increase in total acid density. It was observed that PSS-ICG (D) gave the highest FAME yield among other prepared catalysts with acidity of 14.463 mmol g-1. This may result from the significant forms caused by the benzenesulfonic acid species suppressed in poly(sodium4-styrenesulfonate). The SO3H sulfonic group became stronger in the existence of the three electronegative oxygen atoms and also increased the properties of an electron-withdrawing group. It is also worth mentioning that using sulfonated ICG as a catalyst gave higher FAME yield (96.3%) as compared to sulfonated multi-walled carbon nanotubes and SO3H-ICS catalyst with the ester yield of 93% [32] and 90.4% [18], respectively.
On the other hand, not only different type of sulfonation methods may influence the FAME yield percentage, but also different mechanisms and chemicals involved sulfonating agents and conditions may influence sulfonation process and subsequently FAME yield percentage. Sulfonation via poly (sodium-4-styrenesulfonate) method at lower temperature may influence the polymerization process and bonding of SO3H species to the active sites. This was supported by Kanokwan et al. [33] study, whereas with rising the sulfonation temperature, the overall acidity decreased. It may result in increasing of oxygen to carbon ratio because of the fact that oxidation, dehydrogenation, or condensation may take place. As indicated in Table 1, the oxygen to carbon ratios of PSS-ICG (D) and HSO3Cl-ICG (C) were much lower as compared to those of NHSO4-ICG (B) and H2SO4-ICG (A) where both of them (B and A) were treated at a higher sulfonation temperature. A higher oxygen:carbon ratio may increase the number of weak acid groups on the ICG, resulting in a lower total acid density [34]. Although post-sulfonation treatment of ICG sulfonated by thermal treatment with concentrated sulfuric acid (A) was conducted at extremely elevated temperatures (150 °C), it possessed the lowest acidity of 4.408 mmol g-1. However, for the ICG by thermal treatment with ammonium sulphate (B), the low point of acidity of 5.299 mmol g-1 might correspond to the fact that post-sulfonation over a closed system may reduce the effect of oxidation, dehydrogenation, or condensation.
3. Materials and Methods
3.1. Materials
The initial material, D-glucose, was purchased from R&M Chemicals. All the required acidic agents were supplied accordingly; concentrated sulfuric acid [H2SO4; J.T Baker, ~98%], ammonium sulphate [(NH4)2SO4; HmBG Chemicals, 99.5%], chlorosulfonic acid [HSO3Cl; R&M Chemicals], chloroform [CHCl3; R&M Chemicals], ammonium per sulphate, [(NH4)2S2O8; R&M Chemicals, 98%], and poly(sodium4-styrene sulfonate) [Sigma Aldrich]. Ethanol and methanol as reagents were supplied by Merck. The PFAD was delivered by Jomalina R&D. Sime. Darby. Sdn. Bhd, Malaysia. The standard methyl esters for gas chromatography (GC) analysis were supplied by Fluka, USA.
3.2. Methodology of the Catalyst Synthesis
3.2.1. Synthesis of Incomplete Carbonized Glucose (ICG)
To produce ICS, the pyrolysis procedure was adopted using a furnace tube, where 10 g of D-glucose was filled in the calcination boat and put into the furnace under the following conditions: temperature of 400 °C for 12 h with nitrogen (N2) gas flow rate at 1 mL/min to provide the inert atmosphere. After the calcination, the calcined sample was cooled down to room temperature and later was processed via milling at 1000 rpm for 60 min to get the fine black powder.
3.2.2. Sulfonation by Thermal Treatment with Concentrated Sulfuric Acid
Conventionally, acid treatment is thermally carried out using concentrated sulfuric acid (H2SO4) in order to produce carbon solid acid catalysts to synthesize biodiesel [7,20]. In this regard, 2.0 g of ICG was interspersed together with 50 mL of H2SO4 and then sonicated for half an hour. The blend was later stirred and refluxed for 12 h at 150 °C, over N2 flow at 100 mL/min to give an inert condition. The calcined sample was cooled down to room temperature then filtered and washed with double distilled water (DW) until the pH of the residual water became neutral and dried out at 120 °C, overnight. The final produced catalyst sample was labeled as H2SO4-ICG.
3.2.3. Sulfonation by Thermal Decomposition of Ammonium Sulphate
This sulfonation method was implemented and amended as proposed by Siew et al. [32], where 0.4 g of ICG was interspersed with 30 mL ammonium sulphate (NH4)2SO4 and sonicated for 10 min. Then, the mixture was flowed into an autoclave and sealed at 200 °C for half an hour under autogenous pressure. As it cooled down, the mixture was then filtered using a vacuum pump and washed ruinously using DW to eliminate dissipation of (NH4)2SO4. The mixture was later dried in the oven at 120 °C, overnight. The final sample was labeled as NHSO4-ICG.
3.2.4. Sulfonation by Thermal Treatment with Chlorosulfonic Acid in Chloroform
This method was referred from Pravin et al. [35], where 2.0 g of ICG was dissolved into 50 mL of chloroform for 60 min in a sonicator, resulted in a black distributed ICG suspension. Then, 5 mL of chlorosulfonic acid was cautiously poured into the mixture, stirred and refluxed at 70 °C for 4 h. As the mixture cooled down, it was then filtered and washed with a mixture of DW/Ethanol to eliminate organic moieties till the pH of the filtrate turn into neutral. The sample was then dried at 120 °C for 12 h in the oven. The final product was labeled as HSO3Cl-ICG.
3.2.5. Sulfonation by in situ Polymerization of Poly(Sodium4-styrene Sulfonate)
This sulfonation system was adopted as proposed by Shuit et al. [32], where 0.4 g of ICG was stirred in a mixture of 0.8 g of poly(sodium 4-styrene sulfonate) and 100 mL deionized (DI) water in a round bottom flask at room temperature for 10 h. Then, ammonium persulphate (NH4)2S2O8 was poured into the blend, stirred and warmed up to 65 °C for 48 h, using a hot plate stirrer. As the mixture cooled down, 100 mL of DI was poured into the cooled blend, sonicated for 60 min, then filtered and washed frequently by DW. Next, the filtrate was added to 500 mL of 4 M H2SO4 and stirred at lab temperature for 24 h. After this, the blend was filtered using a vacuum pump and washed again with DW until the pH of the filtrate turn into neutral. The sample was finally dried out at 120 °C in the oven for 12 h. The final product was labeled as PSS-ICG.
3.3. Characterization Methods
The morphology of the synthesized catalysts was examined via Raman spectroscopy (WITec; Alpha 300R). Fourier transformation Infrared (FT-IR; Perkin Elmer - model GX) was employed to determine type of functional spices connected to the active site of the catalyst. In addition, ammonia temperature programmed desorption (NH3-TPD; Thermo Finnigan, model TPDRO. 1100. series) was utilized to investigate the acid density of the synthesized catalysts. The specific surface area (SBET) of the synthesized sulfonated catalyst were analyzed using the Thermo Finnigan Sorptomatic 1990 series apparatus. Thermogravimetric analysis (TGA) was applied to determine the mass losses of the functional groups from room temperature to 1000 °C in an air flow of 200 mL min–1 at a rate of 10 °C min–1 using Mettler Toledo, 990. The morphology of the synthesized catalysts was studied by scanning electron microscopy (SEM; JEOL, JSM-6400) fitted with the energy dispersive X-ray (EDX) spectroscopy analysis system.
3.4. Catalytic Activity of the Catalysts
The catalytic performance of each prepared sulfonated catalyst was examined through the esterification of PFAD. The esterification reaction was performed with a reflux system where the round-bottom flask was connected to a condenser to re-condense the vaporized methanol to minimize the loss amount of methanol. An important point that needs to be taken into consideration is that high loss of evaporated methanol can significantly affect the FAME yield production during esterification reaction [7]. The esterification was done at a reaction temperature of 80 °C, methanol to PFAD molar ratio of 10:1, with catalyst concentration of 2.5 wt.%, in the presence of 5 g of PFAD feedstock for 4 h, as per our previous research [7]. During the esterification reaction, the blend was stirred at 600 rpm to have homogenized dispersed of catalyst in the mixture along the operating time. By the completion of the reaction, the catalyst was removed from the mixture by centrifuging at 5000 rpm for 20 min. After phase separation overnight, the methanol was withdrawn and recovered from the blend. The final FAME was collected for more evaluation.
3.5. FAME Analysis and FFA Conversion
The characteristics of the synthesized ester were examined utilizing a GC outfitted with a flame ionization detector (FID; HP, 6880). In addition, to separate the FAME compound, an extremely polar vessel column (BPX 70, SGE Company) was employed. N-hexane was introduced as the solvent for the weakening of the samples while helium gas as the carrier gas. In this process, 500 ppm of each standard methyl myristate, methyl palmitate, methyl oleate, methyl linoleate, and methyl stearate were used as the reference standard. Methyl heptadecanoate was mixed with the prepared ester to be used as an internal standard. Later, 1 µL of the sample solution was inserted into injector port. The oven temperature was initially adjusted at 100 °C and risen to 230 °C with the heating rate of 10 °C/min. The indicator temperature was put at 270 °C. The product yield was further calculated using Equation (1) as depicted below [36]:
where C is the calculation of ester yield, ∑A is the amount of maximum zone for the FA summits, Ameh is peak zone of methyl heptadecanoate, Cmeh is the internal standard strength, Vmeh is the volume utilized of internal standard, and Wt is the FAME’s mass.
The FFA conversion rate (%) from PFAD feedstock to FAME was determined using titration method. Equation (2) shows the calculation for acid value determination [37]:
where AVf and AVp are the acid values of the feedstock and the product, respectively.
4. Conclusions
In the present investigation, incomplete carbon glucose (ICG) was initially fabricated using D-glucose as carbon resources. Various sulfonation methods were introduced in order to convert the ICG to sulfonated heterogeneous catalysts for FAME generation. The esterification of PFAD was conducted over four different synthesized sulfonated catalysts upon using the batch technique. The present study showed that acidity played a crucial role in catalytic activity, where FAME yield improved with the rise in acidity. In addition, different types of sulfonation method along with different mechanisms and chemicals involved sulfonating agents and conditions influence the FAME yield percentage. The in situ polymerization of poly(sodium 4-styrene sulfonate) method resulted in the highest FFA conversion and FAME yield of 97.6% and 96.3%, respectively, as compared to other studied methods.
Author Contributions
U.R. and T.S.Y.C. conceived this work, designed the experimental work and wrote the final draft of the manuscript. N.H.D. performed the experiments, analyzed the data, performed the characterization of the catalyst and prepared the initial draft of the manuscript. I.A.N. provided the scientific guidance for successful completion of the project and S.S. helped to review the final draft of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge their gratitude to King Saud University (Riyadh, Saudi Arabia) for the funding of this research through Researchers Supporting Project number (RSP-2019/80).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chongkhong, S.; Tongurai, C.; Chetpattananondh, P.; Bunyakan, C. Biodiesel production by esterification of palm fatty acid distillate. Biomass. Bioenergy 2007, 31, 563–568. [Google Scholar] [CrossRef]
- Al-Haj, L.; Al-Hinai, M.A.; Hellier, P.; Rashid, U. Optimization of oil extraction from waste “Date pits” for biodiesel production. Energ. Conver. Manag. 2016, 117, 264–272. [Google Scholar]
- Rehan, M.; Gardy, J.; Demirbas, A.; Rashid, U.; Budzianowski, W.M.; Pant, D.; Nizami, A.S. Waste to biodiesel: A preliminary assessment for Saudi Arabia. Bioresour. Technol. 2018, 250, 17–25. [Google Scholar] [CrossRef]
- Cho, H.J.; Kim, J.-K.; Ahmed, F.; Yeo, Y.-K. Life-cycle greenhouse gas emissions and energy balances of a biodiesel production from palm fatty acid distillate (PFAD). Appl. Energy 2013, 111, 479–488. [Google Scholar] [CrossRef]
- Soltani, S.; Rashid, U.; Yunus, R.; Taufiq-Yap, Y.H.; Al-Resayes, S.I. Post-functionalization of polymeric mesoporous C@Zn core–shell spheres used for methyl ester production. Renew. Energy 2016, 99, 1235–1243. [Google Scholar] [CrossRef]
- Roschat, W.; Siritanon, T.; Yoosuk, B.; Sudyoadsuk, T.; Promarak, V. Rubber seed oil as potential non-edible feedstock for biodiesel production using heterogeneous catalyst in Thailand. Renew. Energy 2017, 101, 937–944. [Google Scholar] [CrossRef]
- Lokman, I.M.; Rashid, U.; Taufiq-Yap, Y.H.; Yunus, R. Methyl ester production from palm fatty acid distillate using sulfonated glucose-derived acid catalyst. Renew. Energy 2015, 81, 347–354. [Google Scholar] [CrossRef]
- Issariyakul, T.; Dalai, A.K. Biodiesel from vegetable oils. Renew. Sustain. Energy Rev. 2014, 31, 446–471. [Google Scholar] [CrossRef]
- Ashraful, A.M.; Masjuki, H.H.; Kalam, M.A.; Rizwanul Fattah, I.M.; Imtenan, S.; Shahir, S.A.; Mobarak, H.M. Production and comparison of fuel properties, engine performance, and emission characteristics of biodiesel from various non-edible vegetable oils: A review. Energy. Convers. Manag. 2014, 80, 202–228. [Google Scholar] [CrossRef]
- Dawodu, F.A.; Ayodele, O.; Xin, J.; Zhang, S.; Yan, D. Effective conversion of non-edible oil with high free fatty acid into biodiesel by sulphonated carbon catalyst. Appl. Energy 2014, 114, 819–826. [Google Scholar] [CrossRef]
- Baskar, G.; Soumiya, S. Production of biodiesel from castor oil using iron (II) doped zinc oxide nanocatalyst. Renew. Energy 2016, 98, 101–107. [Google Scholar] [CrossRef]
- Al-Hamamre, Z.; Al-Salaymeh, A. Physical properties of (jojoba oil + biodiesel), (jojoba oil + diesel) and (biodiesel + diesel) blends. Fuel 2014, 123, 175–188. [Google Scholar] [CrossRef]
- Martínez, G.; Sánchez, N.; Encinar, J.M.; González, J.F. Fuel properties of biodiesel from vegetable oils and oil mixtures. Influence of methyl esters distribution. Biomass. Bioenergy 2014, 63, 22–32. [Google Scholar] [CrossRef]
- Lou, W.-Y.; Zong, M.-H.; Duan, Z.-Q. Efficient production of biodiesel from high free fatty acid-containing waste oils using various carbohydrate-derived solid acid catalysts. Bioresour. Technol. 2008, 99, 8752–8758. [Google Scholar] [CrossRef]
- Sani, Y.M.; Daud, W.M.A.W.; Abdul Aziz, A.R. Solid acid-catalyzed biodiesel production from microalgal oil—The dual advantage. J. Environ. Chem. Eng. 2013, 1, 113–121. [Google Scholar] [CrossRef]
- Hidayat, A.; Wijaya, K.; Nurdiawati, A.; Kurniawan, W.; Hinode, H.; Yoshikawa, K.; Budiman, A. Esterification of palm fatty acid distillate with high amount of free fatty acids using coconut shell char based catalyst. Energy. Procedia 2015, 75, 969–974. [Google Scholar] [CrossRef]
- Nakajima, K.; Hara, M. Amorphous carbon with SO3H groups as a solid brønsted acid catalyst. ACS Catal. 2012, 2, 1296–1304. [Google Scholar] [CrossRef]
- Lokman, I.M.; Rashid, U.; Taufiq-Yap, Y.H. Meso- and macroporous sulfonated starch solid acid catalyst for esterification of palm fatty acid distillate. Arab. J. Chem. 2016, 9, 179–189. [Google Scholar] [CrossRef]
- Chouhan, A.S.; Sarma, A.K. Modern heterogeneous catalysts for biodiesel production: A comprehensive review. Renew. Sustain. Energy Rev. 2011, 15, 4378–4399. [Google Scholar] [CrossRef]
- Toda, M.; Takagaki, A.; Okamura, M.; Kondo, J.N.; Hayashi, S.; Domen, K.; Hara, M. Biodiesel made with sugar catalyst. Nature 2005, 438, 178. [Google Scholar] [CrossRef]
- Roldán, L.; Pires, E.; Fraile, J.M.; García-Bordejé, E. Impact of sulfonated hydrothermal carbon texture and surface chemistry on its catalytic performance in esterification reaction. Catal. Today 2015, 249, 153–160. [Google Scholar] [CrossRef]
- Soltani, S.; Khanian, N.; Rashid, U.; Choong, T.S.Y. Core-shell ZnO-TiO2 hollow spheres synthesized by in-situ hydrothermal method for ester production application. Renew. Energy 2019, 151, 1076–1081. [Google Scholar] [CrossRef]
- Soltani, S.; Khanian, N.; Rashid, U.; Yaw Choong, T.S. Synthesis and characterization of sulfonated mesoporous NiO–ICG core–shell solid sphere catalyst with superior capability for methyl ester production. RSC Adv. 2019, 9, 31306–31315. [Google Scholar] [CrossRef]
- Shu, Q.; Nawaz, Z.; Gao, J.; Liao, Y.; Zhang, Q.; Wang, D.; Wang, J. Synthesis of biodiesel from a model waste oil feedstock using a carbon-based solid acid catalyst: reaction and separation. Bioresour. Technol. 2010, 101, 5374–5384. [Google Scholar] [CrossRef]
- Suganuma, S.; Nakajima, K.; Kitano, M.; Kato, H.; Tamura, A.; Kondo, H.; Yanagawa, S.; Hayashi, S.; Hara, M. SO3H-bearing mesoporous carbon with highly selective catalysis. Microporous. Mesoporous. Mater. 2011, 143, 443–450. [Google Scholar] [CrossRef]
- Santos, E.M.; de Carvalho Teixeira, A.P.; da Silva, F.G.; Cibaka, T.E.; Araújo, M.H.; Oliveira, W.X.C.; Medeiros, F.; Brasil, A.N.; de Oliveira, L.S.; Lago, R.M. New heterogeneous catalyst for the esterification of fatty acid produced by surface aromatization/sulfonation of oilseed cake. Fuel 2015, 150, 408–414. [Google Scholar] [CrossRef]
- Zong, M.-H.; Duan, Z.-Q.; Lou, W.-Y.; Smith, T.J.; Wu, H. Preparation of a sugar catalyst and its use for highly efficient production of biodiesel. Green. Chem. 2007, 9, 434. [Google Scholar] [CrossRef]
- Konwar, L.J.; Boro, J.; Deka, D. Review on latest developments in biodiesel production using carbon-based catalysts. Renew. Sustain. Energy. Rev. 2014, 29, 546–564. [Google Scholar] [CrossRef]
- Zhang, L.; Yan, J.; Zhou, M.; Yang, Y.; Liu, Y.-N. Fabrication and photocatalytic properties of spheres-in-spheres ZnO/ZnAl2O4 composite hollow microspheres. Appl. Surf. Sci. 2013, 268, 237–245. [Google Scholar] [CrossRef]
- Li, S.; Shen, Y.; Xiao, M.; Liu, D.; Fa, L.; Wu, K. Intercalation of 2,4-dihydroxybenzophenone-5-sulfonate anion into Zn/Al layered double hydroxides for UV absorption properties. J. Ind. Eng. Chem. 2014, 20, 1280–1284. [Google Scholar] [CrossRef]
- Bruno, J.E.; Dooley, K.M. Double-bond isomerization of hexadecenes with solid acid catalysts. Appl. Catal. A Gen. 2015, 497, 176–183. [Google Scholar] [CrossRef]
- Shuit, S.H.; Tan, S.H. Feasibility study of various sulphonation methods for transforming carbon nanotubes into catalysts for the esterification of palm fatty acid distillate. Energy Convers. Manag. 2014, 88, 1283–1289. [Google Scholar] [CrossRef]
- Ngaosuwan, K.; Goodwin, J.G.; Prasertdham, P. A green sulfonated carbon-based catalyst derived from coffee residue for esterification. Renew. Energy 2016, 86, 262–269. [Google Scholar] [CrossRef]
- Fraile, J.M.; García-Bordejé, E.; Pires, E.; Roldán, L. New insights into the strength and accessibility of acid sites of sulfonated hydrothermal carbon. Carbon 2014, 77, 1157–1167. [Google Scholar] [CrossRef]
- Alhassan, F.H.; Yunus, R.; Rashid, U.; Sirat, K.; Islam, A.; Lee, H.V.; Taufiq-Yap, Y.H. Production of biodiesel from mixed waste vegetable oils using Ferric hydrogen sulphate as an effective reusable heterogeneous solid acid catalyst. Appl. Catal. A Gen. 2013, 456, 182–187. [Google Scholar] [CrossRef]
- Soltani, S.; Rashid, U.; Nehdi, I.A.; Al-Resayes, S.I. Esterification of palm fatty acid distillate using a sulfonated mesoporous CuO-ZnO mixed metal oxide catalyst. Chem. Eng. Technol. 2017, 40, 1931–1939. [Google Scholar] [CrossRef]
- Soltani, S.; Rashid, U.; Al-Resayes, S.I.; Nehdi, I.A. Sulfonated mesoporous ZnO catalyst for methyl esters production. J. Clean. Prod. 2017, 144, 482–491. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).