Water Depollution and Photo-Detoxification by Means of TiO2: Fluoroquinolone Antibiotics as a Case Study
Abstract
:1. Introduction
- (1)
- (2)
- they are very useful antibacterial agents and form the last class of antibiotics of large-scale use, particularly in highly developed countries. In the 1980s, molecules of this class were synthesized in Europe and the USA for human use, and about ten years later, as veterinary medicines. They show both a broad activity spectrum against Gram-positive and Gram-negative bacteria, excellent oral absorption, and are also used as a prophylactic in husbandry [48];
- (3)
- (4)
- (5)
- the broad knowledge gathered around their photochemical reactivity, photoproducts, and photoreaction mechanism allows a clear separation of the catalyst effect from baseline photoreactivity.
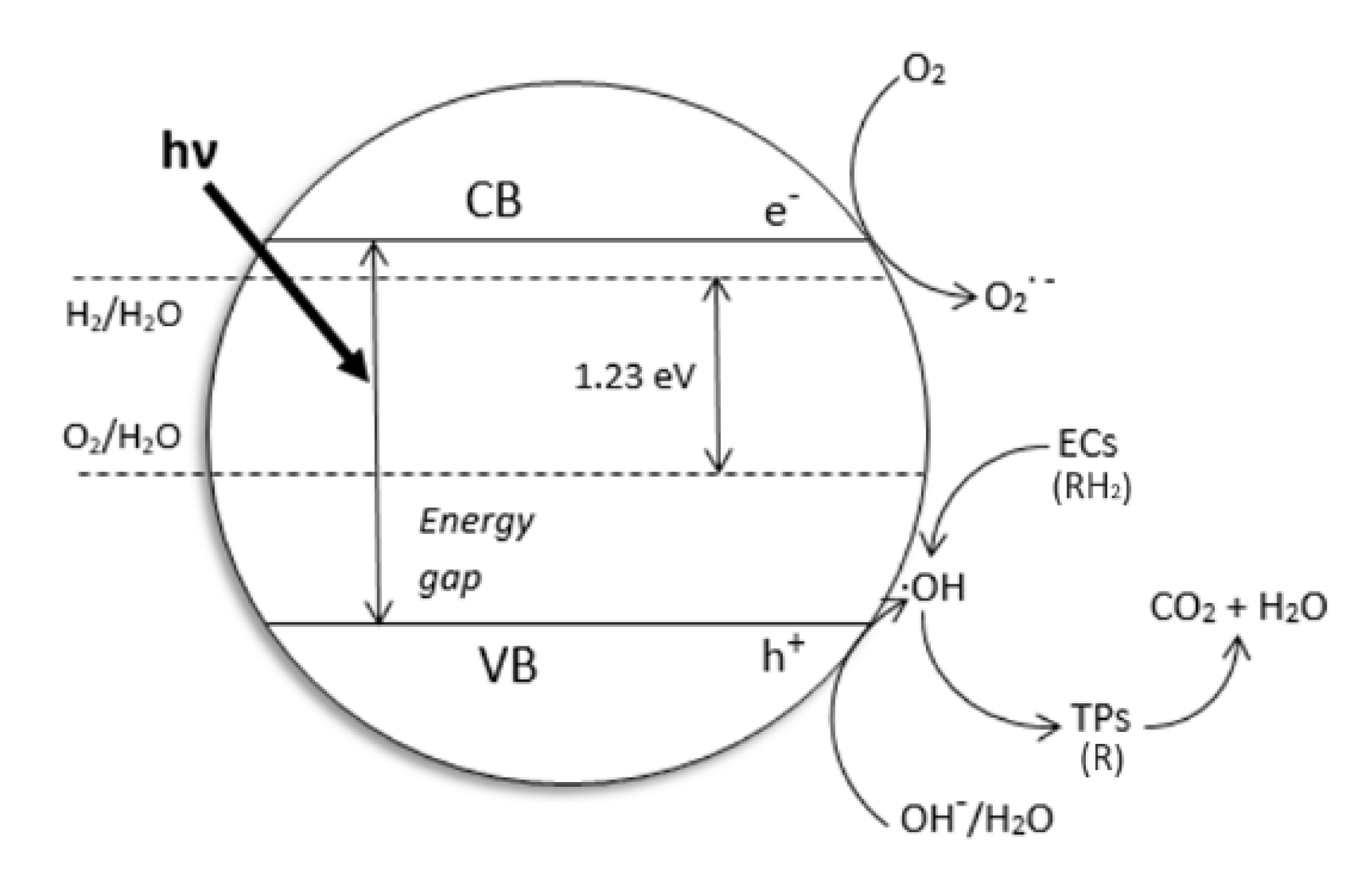
2. Why We Used TiO2 and Drugs as the Probe
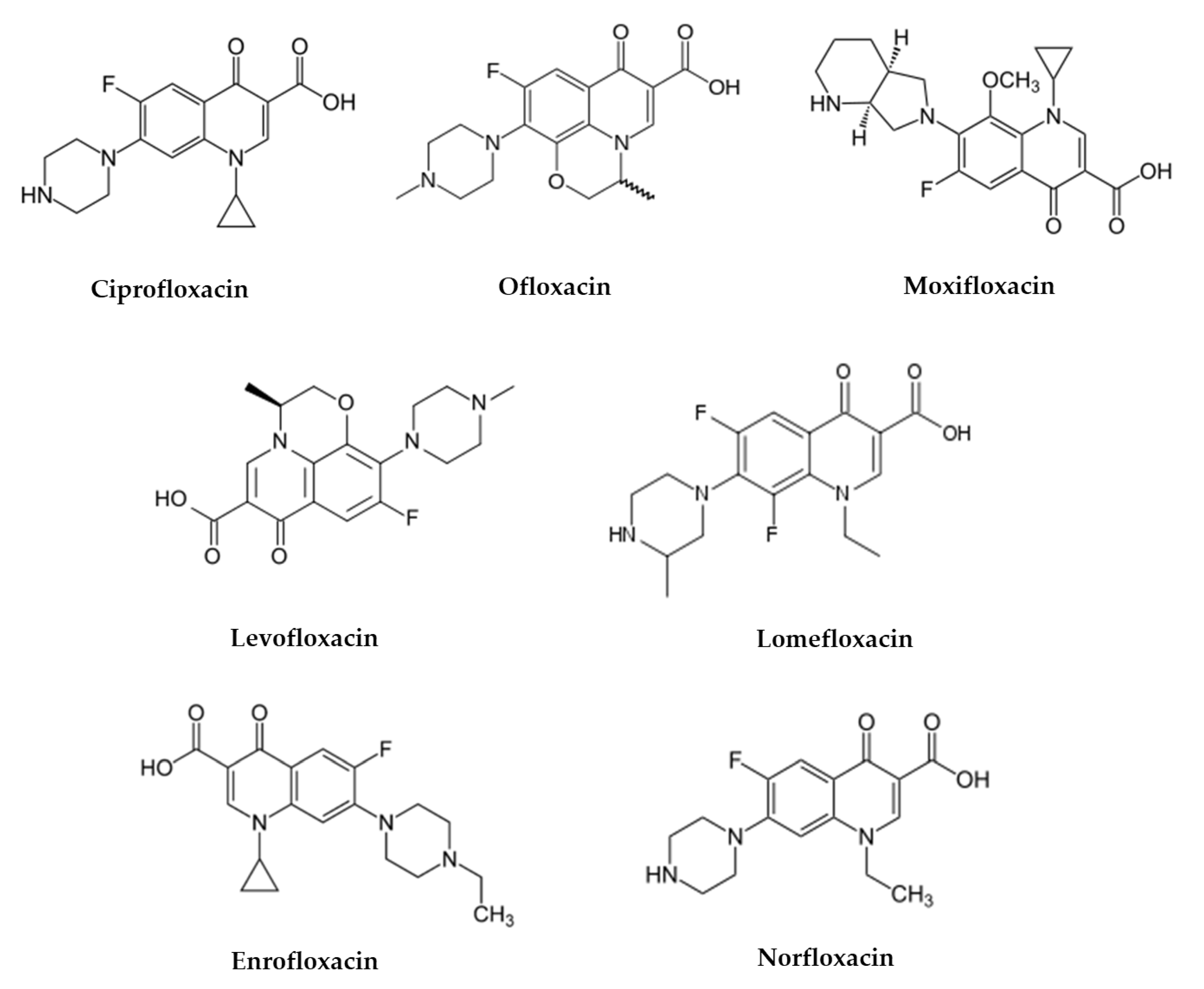
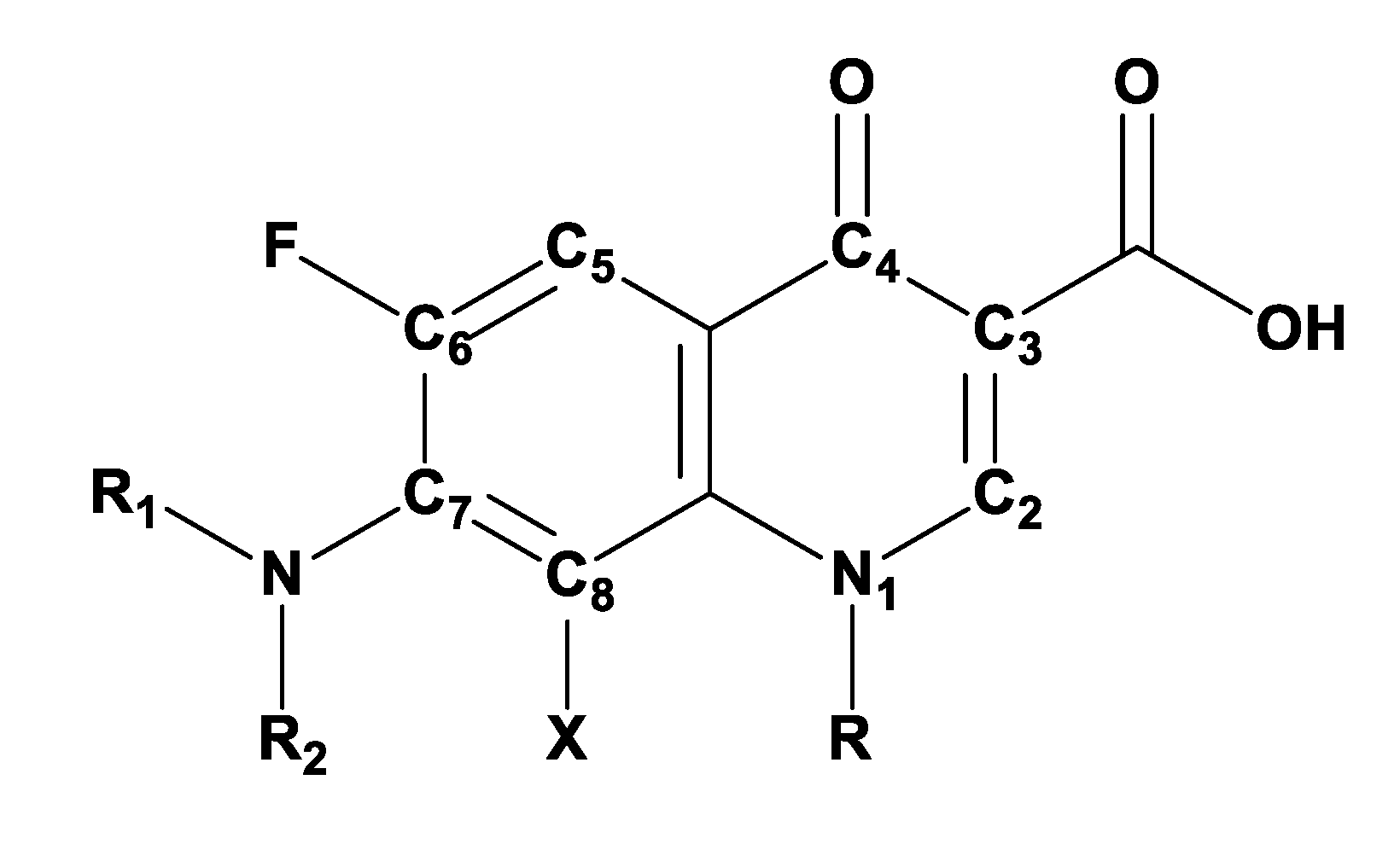
3. Fluoroquinolone Antibiotics
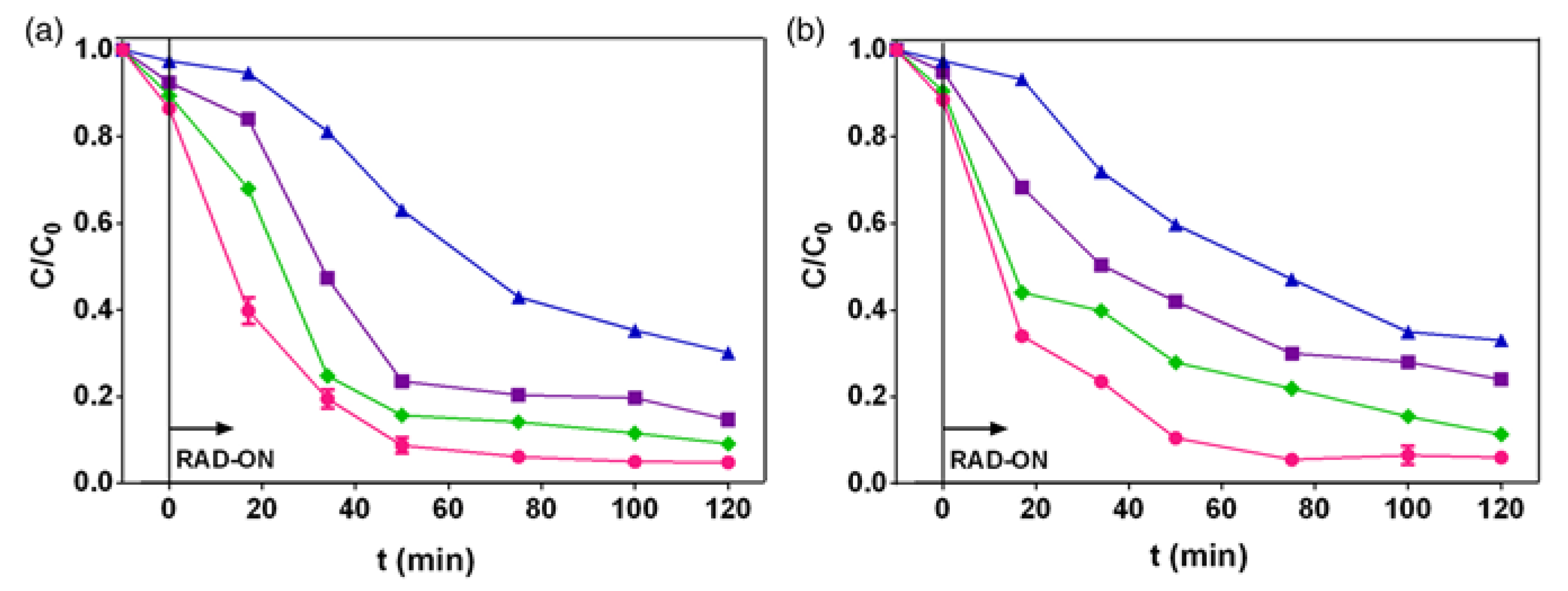
3.1. Fluoroquinolone Degradation Kinetics and Phototransformation Products
3.2. Antibacterial and Ecotoxicological Tests
4. Conclusions on Benefits and Drawbacks
Author Contributions
Funding
Conflicts of Interest
References
- Available online: https://www.britannica.com/science/solar-constant (accessed on 22 April 2020).
- Dionysiou, D.D.; Li Puma, G.; Jinhua Ye, J.; Jenny Schneider, J.; Bahnemann, D. Photocatalysis: Applications; RSC Publishing: Cambridge, UK, 2016. [Google Scholar]
- Hernández-Ramírez, A.; Medina-Ramírez, I. Photocatalytic Semiconductors. Synthesis, Characterization, and Environmental Applications; Springer: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]
- Pichat, P. A brief survey of the practicality of using photocatalysis to purify the ambient air (indoors or outdoors) or air effluents. Appl. Catal. B Environ. 2019, 245, 770–776. [Google Scholar] [CrossRef]
- Bahnemann, D.W.; Cunningham, J.; Fox, M.A.; Pelizzetti, E.; Pichat, P.; Serpone, N. Photocatalytic Treatment of Waters. In Aquatic and Surface Photochemistry, 1st ed.; Helz, R.G., Ed.; CRC Press: Boca Raton, FL, USA, 1994; pp. 261–316. [Google Scholar] [CrossRef]
- Guillard, C.; Amalric, L.; D’Oliveira, J.C.; Delprat, H.; Hoang-Van, C.; Pichat, P. Heterogeneous photocatalysis: Use in water treatment and involvement in atmospheric chemistry. In Aquatic and Surface Photochemistry, 1st ed.; Helz, R.G., Ed.; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar] [CrossRef]
- Serpone, N.; Horikoshi, S. Can the photocatalyst TiO2 be incorporated into a wastewater treatment method? Background and prospects. Catal. Today 2020, 340, 334–346. [Google Scholar] [CrossRef]
- Fagan, R.; McCormack, D.E.; Dionysiou, D.D.; Pillai, S.C. A review of solar and visible light active TiO2 photocatalysis for treating bacteria, cyanotoxins and contaminants of emerging concern. Mater. Sci. Semicond. Process 2016, 42, 2–14. [Google Scholar] [CrossRef] [Green Version]
- Kanakaraju, D.; Glass, B.D.; Oelgemöller, M. Titanium dioxide photocatalysis for pharmaceutical wastewater treatment. Environ. Chem. Lett. 2014, 12, 27–47. [Google Scholar] [CrossRef]
- Etacheri, V.; Di Valentin, C.; Schneider, J.; Bahnemann, D.; Pillai, S.C. Visible-light activation of TiO2 photocatalysts: Advances in theory and experiments. J. Photochem. Photobiol. C 2015, 25, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Kanakaraju, D.; Glass, B.D.; Oelgemöller, M. Advanced oxidation process-mediated removal of pharmaceuticals from water: A review. J. Environ. Manag. 2018, 219, 189–207. [Google Scholar] [CrossRef] [PubMed]
- Albini, A.; Chen, C.; Sturini, M. Use the drugs and respect the environment. Curr. Opin. Green Sustain. Chem. 2017, 6, A1–A4. [Google Scholar] [CrossRef]
- Nick Serpone, N.; Artemev, Y.M.; Ryabchuk, V.K.; Emeline, A.V.; Horikoshi, S. Light-driven advanced oxidation processes in the disposal of emerging pharmaceutical contaminants in aqueous media: A brief review. Curr. Opin. Green Sustain. Chem. 2017, 6, 18–33. [Google Scholar] [CrossRef]
- Mahmoud, W.M.M.; Rastogi, T.; Kümmerer, K. Application of titanium dioxide nanoparticles as a photocatalyst for the removal of micropollutants such as pharmaceuticals from water. Curr. Opin. Green Sustain. Chem. 2017, 6, 1–10. [Google Scholar] [CrossRef]
- Calvete, M.J.F.; Piccirillo, G.; Vinagreiro, C.S.; Pereira, M.M. Hybrid materials for heterogeneous photocatalytic degradation of antibiotics. Coordin. Chem. Rev. 2019, 395, 63–85. [Google Scholar] [CrossRef]
- Babić, S.; Ćurković, L.; Ljubas, D.; Čizmić, M. TiO2 assisted photocatalytic degradation of macrolide antibiotics. Curr. Opin. Green Sustain. Chem. 2017, 6, 34–41. [Google Scholar] [CrossRef]
- Sauvé, S.; Desrosiers, M. A review of what is an emerging contaminant. Chem. Central J. 2014, 8, 15–21. [Google Scholar] [CrossRef] [Green Version]
- Sousa, J.C.G.; Ribeiro, A.R.; Barbosa, M.O.; Fernando, M.; Pereira, R.; Silva, A.M.T. A review on environmental monitoring of water organic pollutants identified by EU guidelines. J Hazard. Mater. 2018, 344, 146–162. [Google Scholar] [CrossRef] [PubMed]
- European Union Strategic Approach to Pharmaceuticals in the Environment. Communication from the Commission to the European Parliament, the Council and the European Economic and Social Committee. Available online: https://ec.europa.eu/transparency/regdoc/rep/1/2019/EN/COM-2019-128-F1-EN-MAIN-PART-1.PDF (accessed on 22 October 2019).
- Yang, Y.; Ok, Y.S.; Kim, K.H.; Kwon, E.E.; Tsang, Y.F. Occurrences and removal of pharmaceuticals and personal care products (PPCPs) in drinking water and water/sewage treatment plants: A review. Sci. Total Environ. 2017, 596–597, 303–320. [Google Scholar] [CrossRef] [PubMed]
- Castiglioni, S.; Davoli, E.; Riva, F.; Palmiotto, M.; Camporini, P.; Manenti, A.; Zuccato, E. Data on occurrence and fate of emerging contaminants in a urbanized area. Data Brief 2018, 17, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Riva, F.; Castiglioni, S.; Fattore, E.; Manenti, A.; Davoli, E.; Zuccato, E. Monitoring in the drinking water of Milan and assessment of the human risk. Int. J. Environ. Health 2018, 221, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Ezzariai, A.; Hafidi, M.; Khadra, A.; Aemig, Q.; El Fels, L.; Barret, M.; Merlina, G.; Patureau, D.; Pinelli, E. Human and veterinary antibiotics during composting of sludge or manure: Global perspectives on persistence, degradation, and resistance genes. J. Hazard. Mater. 2018, 359, 465–481. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Ge, F.; Zhang, L.; Hou, X.; Cao, Y.; Gong, L.; Chen, M.; Wang, R.; Bao, E. Occurrence of 13 veterinary drugs in animal manure-amended soils in Eastern China. Chemosphere 2016, 144, 2377–2383. [Google Scholar] [CrossRef]
- Quaik, S.; Embrandiri, A.; Ravindran, B.; Hossain, K.; Al Dhabi, N.A.; Arasu, M.V.; Ignacimuthu, S.; Ismail, N. Veterinary antibiotics in animal manure and manure laden soil: Scenario and challenges in Asian countries. J. King Saud Univ. Sci. 2020, 32, 1300–1305. [Google Scholar] [CrossRef]
- Golet, E.M.; Strehler, A.; Alder, A.C.; Giger, W. Determination of fluoroquinolone antibacterial agents in sewage sludge and sludge-treated soil using accelerated solvent extraction followed by solid-phase extraction. Anal. Chem. 2002, 74, 5455–5462. [Google Scholar] [CrossRef]
- Hu, X.; Luo, Y.; Zhou, Q. Simultaneous analysis of selected typical antibiotics in manure by microwave-assisted extraction and LC–MS. Chromatographia 2010, 71, 217–223. [Google Scholar] [CrossRef]
- Adeel, M.; Song, X.; Wang, Y.; Francis, D.; Yang, Y. Environmental impact of estrogens on human, animal and plant life: A critical review. Environ. Int. 2017, 99, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Galbiati, V.; Papale, A.; Kummer, E.; Corsini, E. In vitro models to evaluate drug-induced hypersensitivity: Potential test based on activation of dendritic cells. Front. Pharmacol. 2016, 7, 204–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theuretzbacher, U. Global antibacterial resistance: The never-ending story. J. Glob. Antimicrob. Resist. 2013, 1, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Menz, J.; Baginska, E.; Arrhenius, Å.; Haiß, A.; Backhaus, T.; Kümmerer, K. Antimicrobial activity of pharmaceutical cocktails in sewage treatment plant effluent—An experimental and predictive approach to mixture risk assessment. Environ. Pollut. 2017, 231, 1507–1517. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K. Resistance in the environment. J. Antimicrob. Chemother. 2004, 54, 311–320. [Google Scholar] [CrossRef] [Green Version]
- Lindberg, R.H.; Björklund, K.; Rendhal, P.; Johansson, M.I.; Tysklind, M.; Andersson, B.A.V. Environmental risk assessment of antibiotics in the Swedish environment with emphasis on sewage treatment plants. Water Res. 2007, 41, 613–619. [Google Scholar] [CrossRef]
- Rodriguez-Mozaz, S.; Chamorro, S.; Marti, E.; Huerta, B.; Gros, M.; Sànchez-Melsió, A.; Borrego, C.M.; Barceló, D.; Balcázar, J.L. Occurrence of antibiotics and antibiotic resistance genes in hospital and urban wastewaters and their impact on the receiving river. Water Res. 2015, 69, 234–242. [Google Scholar] [CrossRef]
- Santos, L.H.M.L.M.; Gros, M.; Rodriguez-Mozaz, S.; Delerue-Matos, C.; Pena, A.; Barceló, D.; Montenegro, M.C.B.S.M. Contribution of hospital effluents to the load of pharmaceuticals in urban wastewaters: Identification of ecologically relevant pharmaceuticals. Sci. Total Environ. 2013, 461–462, 302–316. [Google Scholar] [CrossRef]
- Verlicchi, P.; Galletti, A.; Petrovic, M.; Barceló, D. Hospital effluents as a source of emerging pollutants: An overview of micropollutants and sustainable treatment options. J. Hydrol. 2010, 389, 416–428. [Google Scholar] [CrossRef]
- Mantovi, P.; Baldoni, G.; Toderi, G. Reuse of liquid, dewatered, and composted sewage sludge on agricultural land: Effects of long-term application on soil and crop. Water Res. 2005, 39, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Malato, S.; Fernández-Ibáñez, P.; Maldonado, M.I.; Blanco, J.; Gernjak, W. Decontamination and disinfection of water by solar photocatalysis: Recent overview and trends. Catal. Today 2009, 147, 1–59. [Google Scholar] [CrossRef]
- Di Li, D.; Shi, W. Recent developments in visible-light photocatalytic degradation of antibiotics. Chin. J. Catal. 2016, 37, 792–799. [Google Scholar] [CrossRef]
- Choi, H.; Al-Abed, S.R.; Dionysiou, D.D.; Stathatos, E.; Lianos, P. Chapter 8 TiO2-based advanced oxidation nanotechnologies for water purification and reuse. Sustain. Sci. Eng. 2010, 2, 229–254. [Google Scholar] [CrossRef]
- Wang, J.; Zhuan, R. Degradation of antibiotics by advanced oxidation processes: An overview. Sci. Total Environ. 2020, 701, 135023–135038. [Google Scholar] [CrossRef] [PubMed]
- Esposito, B.R.; Capobianco, M.L.; Martelli, A.; Navacchia, M.L.; Pretali, L.; Saracino, M.; Zanelli, A.; Emmi, S.S. Advanced water remediation from ofloxacin by ionizing radiation. Radiat. Phys. Chem. 2017, 141, 118–124. [Google Scholar] [CrossRef]
- Durán, A.; Monteagudo, J.M.; San Martín, I. Operation costs of the solar photo-catalytic degradation of pharmaceuticals in water: A mini-review. Chemosphere 2018, 211, 482–488. [Google Scholar] [CrossRef]
- Sturini, M.; Speltini, A.; Maraschi, F.; Profumo, A.; Pretali, L.; Irastorza, E.A.; Fasani, E.; Albini, A. Photolytic and photocatalytic degradation of fluoroquinolones in untreated river water under natural sunlight. Appl. Catal. B Environ. 2012, 32, 119–120. [Google Scholar] [CrossRef]
- Sturini, M.; Speltini, A.; Maraschi, F.; Pretali, L.; Ferri, E.N.; Profumo, A. Sunlight-induced degradation of fluoroquinolones in wastewater effluent: Photoproducts identification and toxicity. Chemosphere 2015, 134, 313–318. [Google Scholar] [CrossRef]
- Sturini, M.; Speltini, A.; Maraschi, F.; Pretali, L.; Profumo, A.; Fasani, E.; Albini, A.; Migliavacca, R.; Nucleo, E. Photodegradation of fluoroquinolones in surface water and antimicrobial activity of the photoproducts. Water. Res. 2012, 46, 5575–5582. [Google Scholar] [CrossRef]
- Albini, A.; Monti, S. Photophysics and photochemistry of fluoroquinolones. Chem. Soc. Rev. 2003, 32, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Thu, D.M.; Pham, T.D.M.; Ziora, Z.M.; Blaskovich, M.A.T. Quinolone antibiotics. Med. Chem. Commun. 2019, 10, 1719–1739. [Google Scholar] [CrossRef]
- Hurum, D.C.; Agrios, A.G.; Gray, K.A.; Rajh, T.; Thurnauer, M.C. Explaining the Enhanced Photocatalytic Activity of Degussa P25 Mixed-Phase TiO2 Using EPR. J. Phys. Chem. B 2003, 107, 4545–4549. [Google Scholar] [CrossRef]
- Ohtani, B.; Prieto-Mahaney, O.O.; Li, D.; Abe, R. What is Degussa (Evonik) P25? Crystalline composition analysis, reconstruction from isolated pure particles and photocatalytic activitytes. J. Photochem. Photobiol. A Chem. 2010, 216, 179–182. [Google Scholar] [CrossRef] [Green Version]
- Alonso-Tellez, A.; Masson, R.; Robert, D.; Keller, N.; Keller, V. Comparison of Hombikat UV100 and P25 TiO2 performance in gas-phase photocatalytic oxidation reaction. Chemistry 2012, 250, 58–65. [Google Scholar] [CrossRef]
- Reddy, P.A.K.; Reddy, P.V.L.; Kwon, E.; Kim, K.-H.; Akter, T.; Kalagara, S. Recent advances in photocatalytic treatment of pollutants in aqueous media. Environ. Int. 2016, 91, 94–103. [Google Scholar] [CrossRef]
- Sturini, M.; Maraschi, F.; Cantalupi, A.; Pretali, L.; Nicolis, S.; Dondi, D.; Profumo, A.; Caratto, V.; Sanguineti, E.; Ferretti, M.; et al. TiO2 and N-TiO2 Sepiolite and Zeolite Composites for Photocatalytic Removal of Ofloxacin from Polluted Water. Materials 2020, 13, 537. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Bolton, J.R. Determination of the Quantum Yield for the photochemical generation of hydroxyl radicals in TiO2 suspensions. J. Phys. Chem. 1996, 100, 4127–4134. [Google Scholar] [CrossRef]
- Serpone, N.; Salinaro, A. Terminology, relative photonic efficiencies and quantum yields in heterogeneous photocatalysis. Part I: Suggested protocol. Pure Appl. Chem. 1999, 71, 303–320. [Google Scholar] [CrossRef]
- Salinaro, A.; Emeline, A.V.; Zhao, J.; Hidaka, H.; Ryabchuk, V.K.; Serpone, N. Terminology, relative photonic efficiencies and quantum yields in heterogeneous photocatalysis. Part II: Experimental determination of quantum yields. Pure Appl. Chem. 1999, 71, 321–335. [Google Scholar] [CrossRef]
- Kisch, H. On the problem of comparing rates or apparent quantum yields in heterogeneous photocatalysis. Angew. Chem. Int. Ed. 2010, 49, 9588–9589. [Google Scholar] [CrossRef] [PubMed]
- Barbero, N.; Vione, D. Why Dyes should not be used to test the photocatalytic activity of semiconductor oxides. Environ. Sci. Technol. 2016, 50, 2130–2131. [Google Scholar] [CrossRef] [PubMed]
- Venancio, W.A.L.; Rodrigues-Silva, C.; Maniero, M.G.; Guimarães Lima, J.R. Photocatalytic removal of fluoroquinolones and their antimicrobial activity from water matrices at trace levels: A comparison of commercial TiO2 catalysts. Water Sci. Technol. 2018, 78, 1668–1678. [Google Scholar] [CrossRef]
- Villegas-Guzman, P.; Oppenheimer-Barrot, S.; Silva-Agredo, J.; Torres-Palma, R.A. Comparative Evaluation of Photo-Chemical AOPs for Ciprofoxacin degradation: Elimination in natural waters and analysis of pH effect, primary degradation by-roducts, and the relationship with the antibiotic activity. Water Air Soil Pollut. 2017, 228, 209–224. [Google Scholar] [CrossRef]
- Silva, A.R.; Martins, P.M.; Teixeira, S.; Carabineiro, S.A.C.; Kuehn, K.; Cuniberti, G.; Alves, M.M.; Lanceros-Mendezag, S.; Pereira, L. Ciprofloxacin wastewater treated by UVA photocatalysis: Contribution of irradiated TiO2 and ZnO nanoparticles on the final toxicity as assessed by Vibrio fischeri. RSC Adv. 2016, 6, 95494–95503. [Google Scholar] [CrossRef]
- Li, W.; Guo, C.; Su, B.; Xu, J. Photodegradation of four fluoroquinolone compounds by titanium dioxide under simulated solar light irradiation. J. Chem. Technol. Biotechnol. 2012, 87, 643–650. [Google Scholar] [CrossRef]
- Paul, T.; Dodd, M.C.; Strathmann, T.J. Photolytic and photocatalytic decomposition of aqueous ciprofloxacin: Transformation products and residual antibacterial activity. Water Res. 2010, 44, 3121–3132. [Google Scholar] [CrossRef]
- Calza, P.; Medana, C.; Carbone, F.; Giancotti, V.; Baiocchi, C. Characterization of intermediate compounds formed upon photoinduced degradation of quinolones by high performance liquid chromatography/high-resolution multiple-stage mass spectrometry. Rapid Commun. Mass Spectrom. 2008, 22, 1533–1552. [Google Scholar] [CrossRef]
- Biancullo, F.; Moreira, N.F.F.; Ribeiro, A.R.; Manaia, C.M.; Faria, J.L.; Nunes, O.C.; Castro-Silva, S.M.; Silva, A.M.T. Heterogeneous photocatalysis using UVA-LEDs for the removal of antibiotics and antibiotic resistant bacteria from urban wastewater treatment plant effluents. Chem. Eng. J. 2019, 367, 304–313. [Google Scholar] [CrossRef]
- Vasquez, M.I.; Hapeshi, E.; Fatta-Kassinos, D.; Kümmerer, K. Biodegradation potential of ofloxacin and its resulting transformation products during photolytic and photocatalytic treatment. Environ. Sci. Pollut. Res. 2013, 20, 1302–1309. [Google Scholar] [CrossRef]
- Hapeshi, E.; Fotiou, I.; Fatta-Kassinos, D. Sonophotocatalytic treatment of ofloxacin in secondary treated effluent and elucidation of its transformation products. Chem. Eng. J. 2013, 224, 96–105. [Google Scholar] [CrossRef]
- Michael, I.; Hapeshi, E.; Michael, C.; Fatta-Kassinos, D. Solar Fenton and solar TiO2 catalytic treatment of ofloxacin in secondary treated effluents: Evaluation of operational and kinetic parameters. Water Res. 2010, 44, 5450–5462. [Google Scholar] [CrossRef]
- Andreozzi, R.; Campanella, L.; Fraysse, B.; . Garric, J.; Gonnella, A.; Lo Giudice, R.; Marotta, R.; Pinto, G.; Pollio, A. Effects of advanced oxidation processes (AOPs) on the toxicity of a mixture of pharmaceuticals. Water Sci. Technol. 2004, 50, 23–28. [Google Scholar] [CrossRef]
- Sushil Kumar Kansal, S.K.; Kundu, P.; Sood, S.; Lamba, R.; Umar, A.; Mehta, S.K. Photocatalytic degradation of the antibiotic levofloxacin using highly crystalline TiO2 nanoparticles. New J. Chem. 2014, 38, 3220–3226. [Google Scholar] [CrossRef]
- Van Doorslaer, X.; Haylamicheal, I.D.; Dewulf, J.; Van Langenhove, H.; Janssen, C.R.; Demeestere, K. Heterogeneous photocatalysis of moxifloxacin in water: Chemical transformation and ecotoxicity. Chemosphere 2015, 119, S75–S80. [Google Scholar] [CrossRef]
- Vione, D.; Scozzaro, A. Photochemistry of surface fresh waters in the framework of climate change. Environ. Sci. Technol. 2019, 53, 7945–7963. [Google Scholar] [CrossRef]
- Jimenez-Villarin, J.; Serra-Clusellas, A.; Martínez, C.; Conesa, A.; Garcia-Montaño, J.; Moyano, E. Liquid chromatography coupled to tandem and high resolution mass spectrometry for the characterisation of ofloxacin transformation products after titanium dioxide photocatalysis. J. Chromatogr. A 2016, 1443, 201–210. [Google Scholar] [CrossRef]
- Paul, T.; Miller, P.L.; Strathmann, T.J. Visible-light-mediated TiO2 photocatalysis of fluoroquinolone antibacterial agents. Environ. Sci. Technol. 2007, 41, 4720–4727. [Google Scholar] [CrossRef]
- Van Doorslaer, X.; Heynderickx, P.M.; Demeestere, K.; Debevere, K.; Van Langenhove, H.; Dewulf, J. UV-A and UV-C induced photolytic and photocatalytic degradation of aqueous ciprofloxacin and moxifloxacin: Reaction kinetics and role of adsorption. Appl. Catal. B Environ. 2011, 101, 540–547. [Google Scholar] [CrossRef]
- Sturini, M.; Speltini, A.; Maraschi, F.; Vinci, G.; Profumo, A.; Pretali, L.; Albini, A.; Malavasi, L. g-C3N4-promoted degradation of ofloxacin antibiotic in natural waters under simulated sunlight. Environ. Sci. Pollut. Res. 2017, 24, 4153–4161. [Google Scholar] [CrossRef]
- Ola, O.; Maroto-Valer, M.M. Review of material design and reactor engineering on TiO2 photocatalysis for CO2 reduction. J. Photochem. Photobiol. C Photochem. Rev. 2015, 24, 16–42. [Google Scholar] [CrossRef] [Green Version]
- Ziental, D.; Czarczynska-Goslinska, B.; Mlynarczyk, D.T.; Glowacka-Sobotta, A.; Stanisz, B.; Goslinski, T.; Sobotta, L. Titanium dioxide nanoparticles: Prospects and applications in medicine. Nanomaterials 2020, 10, 387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoseinzadeh, E.; Makhdoumi, P.; Taha, P.; Hossini, H.; Stelling, J.; Kamal, M.A.; Ashraf, G.M. A review on nano-antimicrobials: Metal nanoparticles, methods and mechanisms. Curr. Drug Metab. 2017, 18, 120–128. [Google Scholar] [CrossRef]
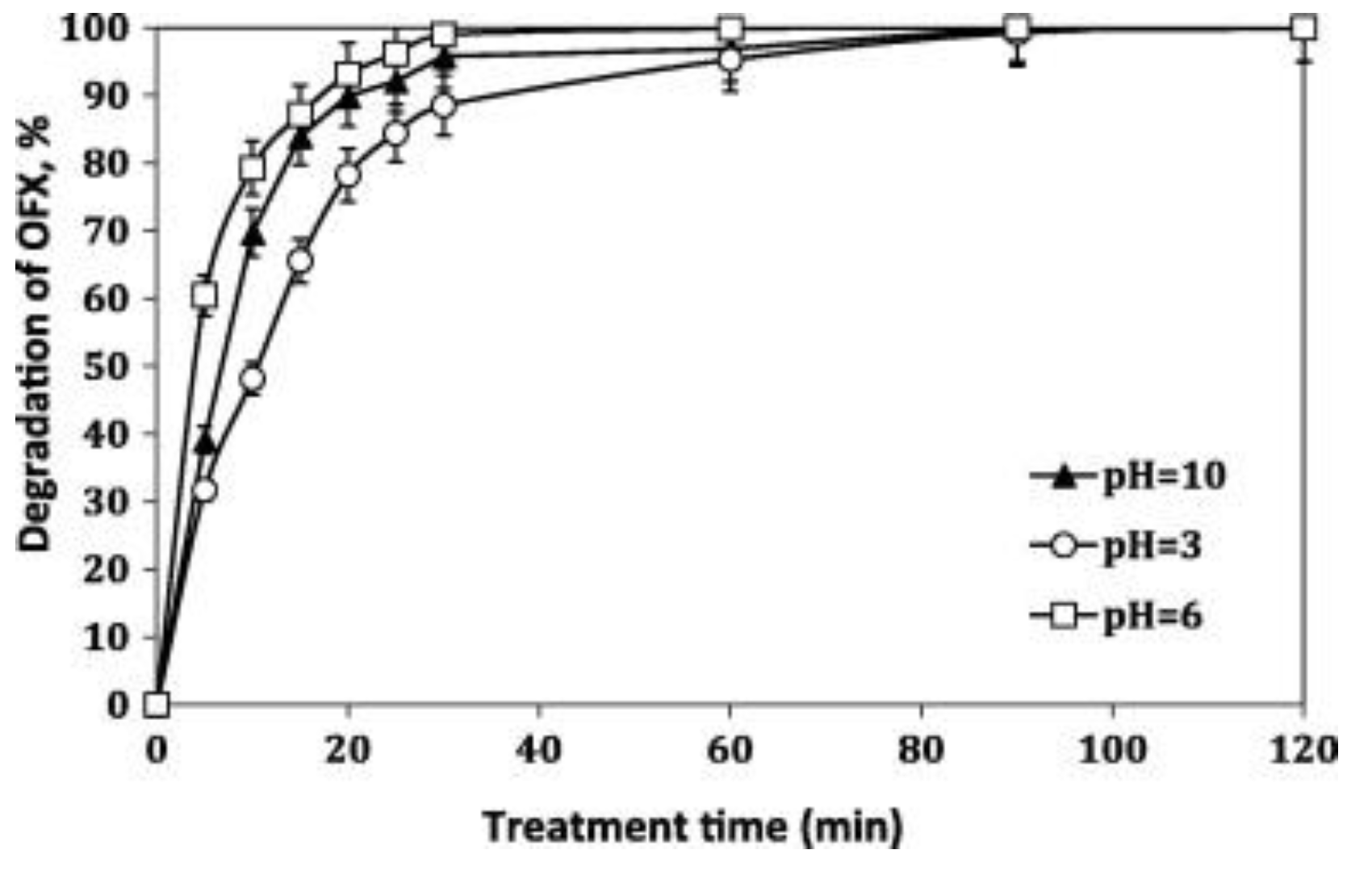
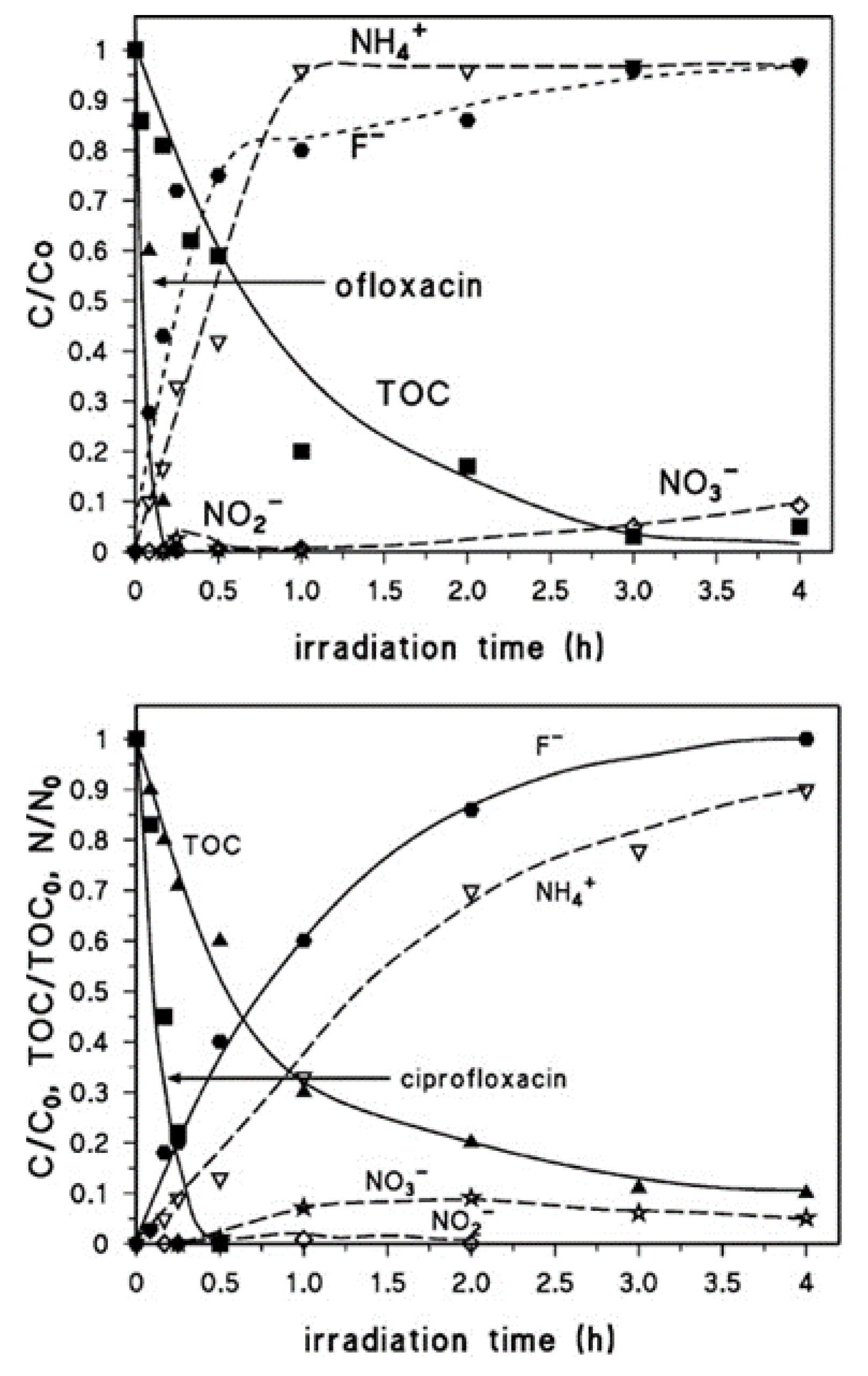

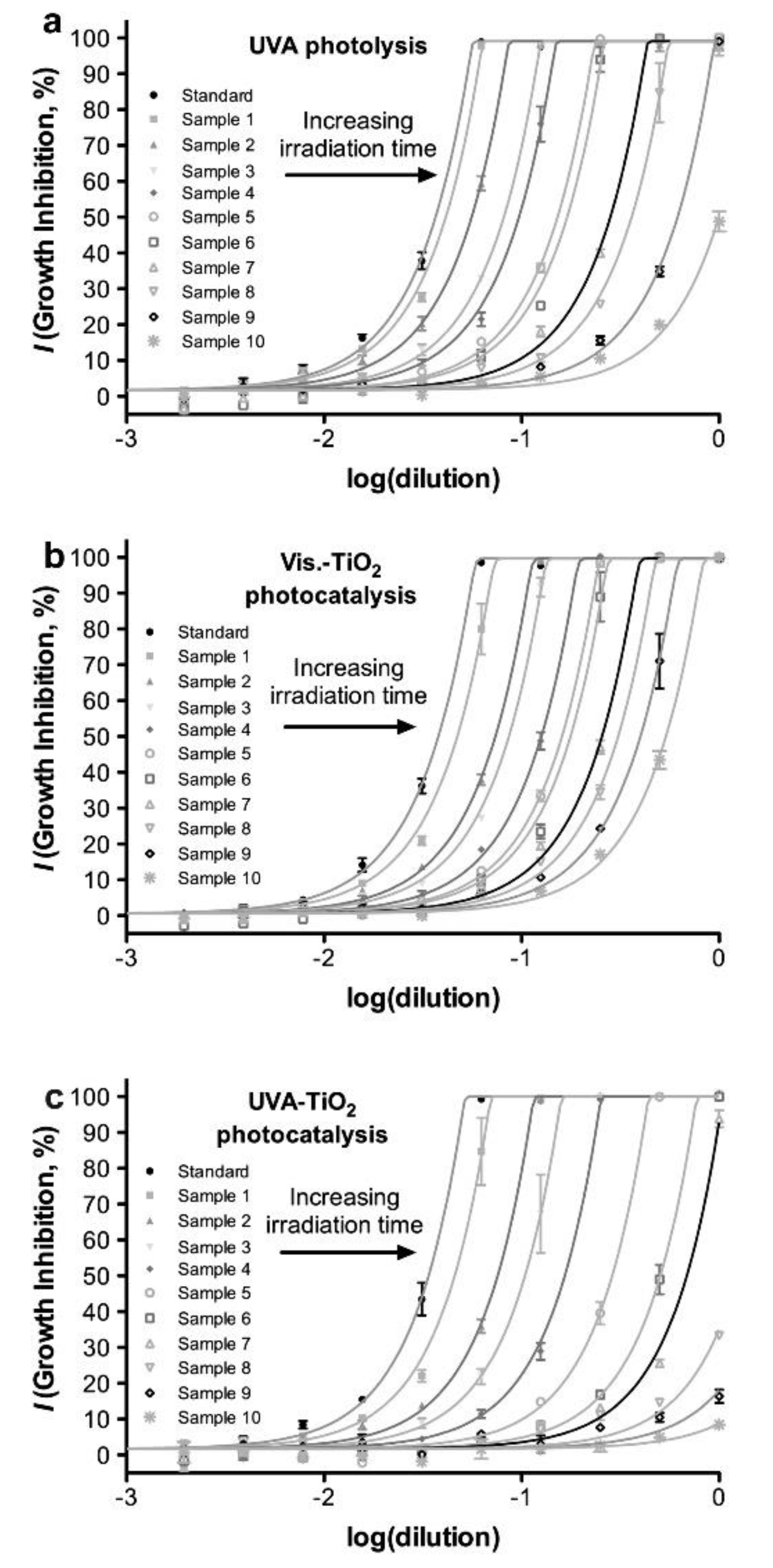

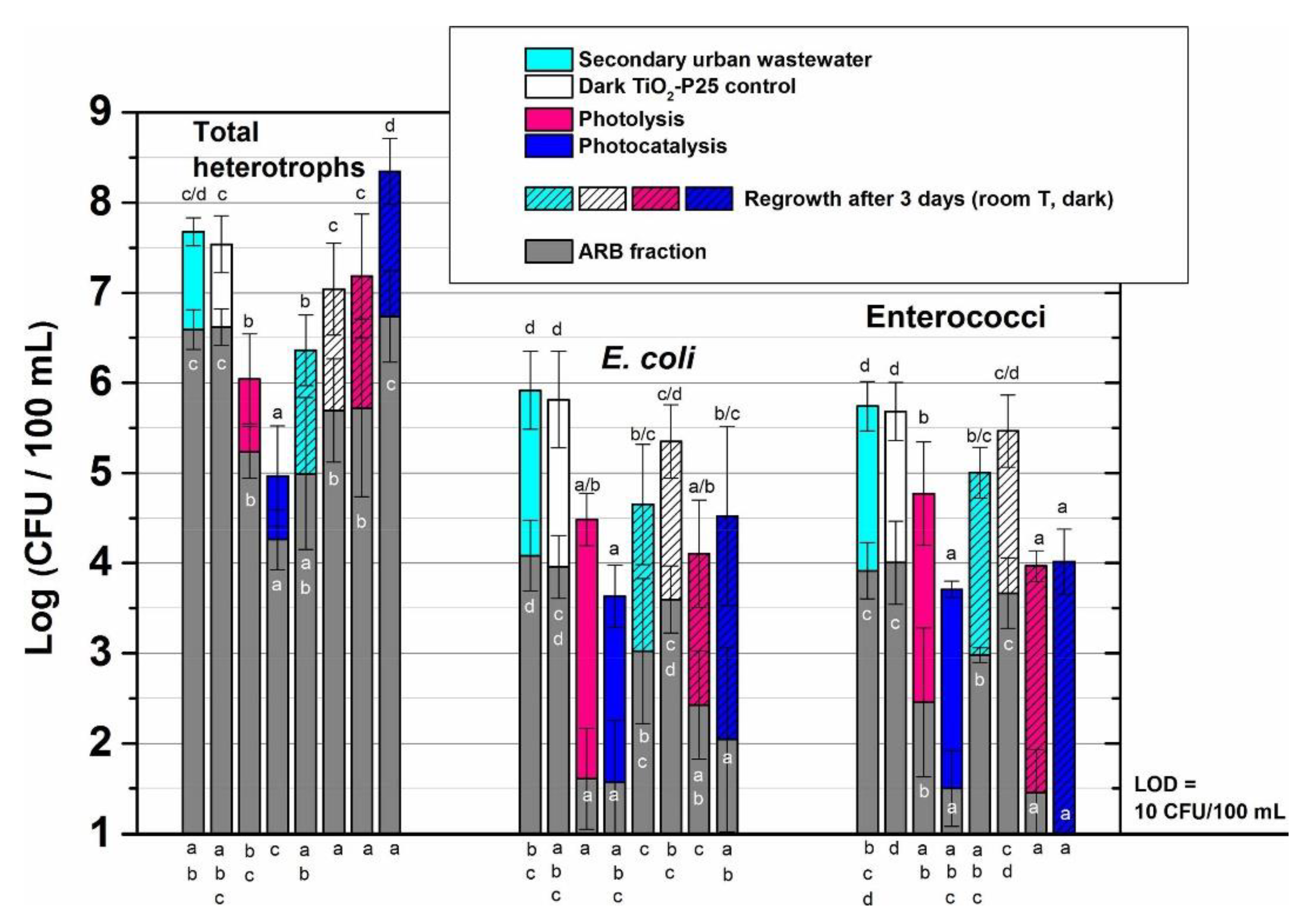
| FQ | Catalyst | Irradiation Source | Matrix | Model organism | Primary TPs/pathway | Abatement % | Ecotoxic effects | Ref. |
|---|---|---|---|---|---|---|---|---|
| [OFL] 100 µg L−1 mixed with other antibiotics | Evonik P25 (1 g L−1) | 4 UV high-intensity LEDs 9 W, 15–515 W m−2 | Secondary treated effluent from urban WWTP pH = 7.2 | E. coli, Enterococcaceae, heterotrophs | - | [OFL] < LOD in 15 min both actual and spiked samples with a variable antibiotic concentration Spiked: kdeg 0.290 min−1 Spiked (MeOH): kdeg0.270 min−1 | Bacterial activity inhibition: ≈ 2 log-units Bacteria regrowth after the treatment: similar (heterotrophs and E. coli) and lower (Enterococcaceae) than in the untreated sample | [65] |
| [CIP], [OFL], [LOM] 100 μg L−1 | Evonik P25 (0.1 g L−1); PC500 (0.05 g L−1) | UV-A lamp, 8W | Ultrapure water; tap water pH = 7.0; commercial bottle water pH = 7.6; simulated water USEPA pH = 7.0 | E. coli, B. subtilis | Primary TPs from attack on piperazine ring | 95% FQs degraded in 120 min and after 60 min with saturated oxygen P25: kdeg 0.038–0.045 min−1 PC500: kdeg 0.035–0.050 min−1 Matrix effect: Ultrapure < simulated < bottle < tap water PC500 more efficient than P25 | Bacterial activity reduced by 120 min PC500: 92% for E. coli, 78% for B. Subtilis P25: 95% for E. coli, 84% for B. subtilis P25 more efficient than PC500 | [59] |
| [CIP] 2–17 mg L−1 | Evonik P25 (0.05 g L−1) | 2 × 15 W lamps | Distilled water, mineral natural water pH = 6.4 | E. coli, S. aureus | 3 TPs, piperazine ring cleavage | 100% CIP degraded in 350 min (ultrapure water) 90% CIP degraded in 500 min (mineral natural water) Matrix effect: ultrapure < mineral natural water | Bacterial activity differently reduced Distilled water: 85% for S. aureus, 50% for E. coli Mineral natural water: 80% for S. aureus, 45% for E. coli Different effects of TPs on bacteria strains | [60] |
| [CIP] 300 μg L−1 | Evonik P25 (1 g L−1) | 6 UV-A lamps 1.6–1.7 mW cm−2 | Ultrapure water | V. fisheri | 7 TPs Degradation primarily on piperazine ring, defluorination | 100% CIP degraded in less than 6 min | Slight toxicity after 15 min irradiation; higher toxicity (70%) after 45 min irradiation. Not excluded the contribution of irradiated TiO2 nanoparticles | [61] |
| [MOX] 50 mg L−1 | Degussa P25 (1 g L−1) | UV-A pen ray 4 mW cm−2 | Phosphate buffer 10 mM pH = 7.0 | P. subcapitata | 13 TPs Core quinolone structure retained, no defluorination, transformation at R1 and R7. Presence of secondary TPs after MOX degradation | 100% MOX degraded after 90 min | EC50 0.78 mg L−1 I% from 72% to 14% after 150 min treatment MOX contributes to growth inhibition more than its TPs | [71] |
| [LEV] 25 g L−1 | TiO2 synthesized by a sol-gel method (1 g L−1) | 7 × 18 W UV-A lamps 0.5 mW cm−2 | Distilled water | E. coli | - | 90% LEV degraded in 120 min with home-made catalyst 78% and 80% LEV degraded in 120 min with P25 and PC50 Reusable catalyst | 80% bacterial inhibition after 25 min irradiation 100% bacterial inhibition after 60 min irradiation | [70] |
| [OFL] 10 mg L−1 | Aeroxide P25 (1 g L−1) | UV-A lamp 9W 3.16 W m−2 | Secondary treated effluent from urban WWTP pH = 8 | D. magna | Major changes in N-piperazine ring Core quinolone structure retained, several competing pathways 20 TPs | US-UV-A-TiO2: 100% OFL degraded, kdeg 0.105 min−1 UV-A-TiO2: 85% OFL degraded, kdeg 0.073 min−1 slightly increasing in the presence of 0.14 mM H2O2 | TPs exhibit a long term toxicity (I% = 55, 60 min irradiation, 48 h exposure) Removal of toxic TPs after 240 min treatment | [67] |
| [OFL] 20 mg L−1 | Aeroxide P25 (not specified) | UV-A medium pressure Hg lamp, 150 W | Mineral medium pH = 7.4 | Closed bottle tests (OECD 301, 1992) | Opening of the piperazine ring, demethylation, decarboxylation | - | Primary TPs non-readily biodegradable; TPs persistence attributed to the fluorine | [66] |
| [OFL],[NOR],[CIP], [ENR] 10 mg L−1 | Degussa P25 (0.5 g L−1) | Xenon lamp 800 W Simulated solar light irradiation | Deionized water pH = 6 | B. subtilus | - | FQs 100% degraded in 90 min irradiation and in presence of 2.4 mM H2O2 kdeg 0.022–0.027 min−1 | Bacterial activity completely removed in 180 min irradiation FQs contribute to the antibacterial activity TPs do not exhibit a long term toxicity | [62] |
| [CIP] 33 mg L−1 | Hombikat UV 100 (0.5 g L−1) | Xenon lamp 450 W UV-A, visible light 5.28 × 10−2–2.67 × 10−2 W cm−2 | Deionized water pH = 6 | E. coli | Piperazine ring cleavage Core quinolone structure retained | CIP 100% degraded in 30 min under UV-A/TiO2 CIP 90% degraded in 60 min under Vis/TiO2 | Total CIP inactivation under laboratory conditions TPs retain negligible antibacterial activity | [63] |
| [OFL] 10 mg L−1 | Degussa P25 (3 g L−1) | Xenon lamp 1 kW 272 W m−2 Simulated solar light irradiation | Secondary treated effluent from urban WWTP pH = 8 | D. magna | Piperazine ring, FQ moiety | kdeg 0.009 min −1, kdeg 0.016 min −1 (5.5 mM H2O2) | OFL treated effluents relatively non-toxic (highest immobilization at 48 h of exposure) | [68] |
| [CIP], [OFL] 15 mg L−1 | Degussa P25 (0.2 g L−1) | Xenon lamp 1500 W Simulated solar light irradiation | Deionized water | V. fisheri | CIP: piperazine moiety and quinolone moiety OFL: piperazine moiety and methyl groups, quinolone core unmodified | 100% CIP and OFL degraded in 30 min | CIP and its TPs do not exhibit acute toxicity OFL TPs exhibit toxicity (I % = 55, 10 min irradiation, 15 min incubation) | [64] |
| [OFL] 560 µg L−1 mixed with other drugs | Degussa P25 (0.3 g L−1) | 300W Simulated solar light irradiation | Moderately hard synthetic medium (MHW-EPA) pH = 7.6 | S. leopoliensis, B. calyciflorus | - | 80% mixture degraded both suspended and immobilized TiO2 in 48 h irradiation | 9% bacterial inactivation with suspended TiO2, no inactivation with supported TiO2 potentially toxic TPs | [69] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pretali, L.; Maraschi, F.; Cantalupi, A.; Albini, A.; Sturini, M. Water Depollution and Photo-Detoxification by Means of TiO2: Fluoroquinolone Antibiotics as a Case Study. Catalysts 2020, 10, 628. https://doi.org/10.3390/catal10060628
Pretali L, Maraschi F, Cantalupi A, Albini A, Sturini M. Water Depollution and Photo-Detoxification by Means of TiO2: Fluoroquinolone Antibiotics as a Case Study. Catalysts. 2020; 10(6):628. https://doi.org/10.3390/catal10060628
Chicago/Turabian StylePretali, Luca, Federica Maraschi, Alice Cantalupi, Angelo Albini, and Michela Sturini. 2020. "Water Depollution and Photo-Detoxification by Means of TiO2: Fluoroquinolone Antibiotics as a Case Study" Catalysts 10, no. 6: 628. https://doi.org/10.3390/catal10060628
APA StylePretali, L., Maraschi, F., Cantalupi, A., Albini, A., & Sturini, M. (2020). Water Depollution and Photo-Detoxification by Means of TiO2: Fluoroquinolone Antibiotics as a Case Study. Catalysts, 10(6), 628. https://doi.org/10.3390/catal10060628






