Abstract
In this work, the carbon nanotubes (CNT)-supported nanosized, well-dispersed, CeZrO2 and Ni-CeZrO2 catalysts were obtained and tested for the first time in the reaction of methane dry reforming (DRM). The performance of the hybrid materials was compared with the performance of Ni/CNT catalyst. The mechanism of the DRM reaction and the occurrence of reverse water gas shift reaction (RWGS) and CO2 deoxidation were discussed in terms of catalysts composition. The contribution of RWGS and CO2 deoxidation in the DRM process, demonstrating an increased CO2 consumption when compared to CH4, and H2/CO < 1, varied depending on the catalyst composition, was also studied.
1. Introduction
The climate change is one of the most important issues the modern society struggles with. The main foundation of this transformation is the growing atmospheric pollution caused by the greenhouse gases (GHGs), i.e., water vapor (H2O), methane (CH4), carbon dioxide (CO2), fluorochlorocarbons and nitrous oxides (NOx). The highest contribution to the increasing CO2 and CH4 emissions has the agricultural industry, coal- and natural gas-based industry, and transportation. It is estimated, that increasing demand for energy (still mainly produced from fossil fuels) has 80–85% contribution to global warming [1]. Several methods for GHGs conversion have been developed in order to reduce their negative impact on the environment. One of them is the dry reforming of methane (DRM) (Equation (1)), which allows conversion of two GHGs (CH4 and CO2) to a syngas (H2 and CO), being a building block for various organic syntheses. Moreover, the DRM reaction can be also considered as a possible route for natural gas valorisation. The DRM reaction is accompanied by such side reactions as the Boudouard reaction (Equation (2)), the methane cracking (Equation (3)) and the reverse water–gas shift (Equation (4)) [2].
CH4 + CO2 = 2H2 +2CO ΔH = 247 kJ/mol
2CO = CO2 + C ΔH = 172.4 kJ/mol
CH4 = C + 2H2 ΔH = 74.9 kJ/mol
CO2 + H2 ↔︎ H2O + CO ΔH = 41.2 kJ/mol
The DRM is a more endothermic process compared to other popular reactions allowing CH4 conversion to syngas, i.e., the partial oxidation (POM) and steam reforming (SRM) [3]. Besides, compared to POM and SRM, the DRM is advantageous because it produces the syngas with the H2/CO ratio close to 1, which is proper for the production of liquid hydrocarbons via the Fischer–Tropsch synthesis of the dimethyl ether (DME) synthesis.
The DRM reaction requires temperatures as high as 800–1000 °C and is usually carried out over transition and noble metal-based catalysts; however, the most popular choice of the researchers in terms of catalyst active site is nickel, which is cheap and accessible. Other transition metals used for DRM, i.e., Fe and Co, are more prone to coking and thus the blockage of metallic active sites [4]. In general, the DRM reaction is at high risk of carbon deposition because it is thermodynamically favoured in the temperature window for DRM. According to Cao et al. [5], the decomposition of CH4 to C and H2 (Equation (3)) occurs from ca. 550 °C, whereas gasification of carbon deposit with CO2 takes place above 700 °C. Therefore, carbon formation during DRM is the most detrimental for the catalyst from 550 to 700 °C. Moreover, the process of carbon deposition is more likely to occur on larger Ni crystallites [6]. Another problem occurring during DRM reaction is the sintering of the active phase resulting from the high temperature of the reaction. The thermodynamics of DRM process hinder its practical industrial application; therefore, the main focus of researchers dealing with that reaction is currently on improving the thermal stability of Ni-based catalysts and their resistance to coke formation. It can be achieved e.g., by creating the bimetallic structures, proper selection of the support or promoter, or changing the preparation method [7]. One of the key strategies is to design the catalysts containing the active metal site attached to the high-surface-area support (usually an oxide), remembering that the essential role in this system plays the metal-support interface. The strong metal-support interaction effect (SMSI) is the reason for improved performance of the catalyst and has been studied for over 60 years now [8]. The electron transfer between support and metal or metal oxide leads to profound changes is the chemisorption and catalytic properties of the catalyst as well as the morphology of the metal or metal oxide particles [9]. As was found by Kim et al. [10], improved catalytic activity in CO oxidation of the nanostructure of Pt-NiO1−x supported on the bimetallic Pt3Ni(111) originates from the SMSI effect that provides thermodynamically efficient reaction pathways. In the case of studied catalyst, the formation of interfacial Pt-NiO1-x allowed lowering the heat of reaction on the surface. Therefore, it is important to provide such parameters of the catalyst synthesis, so that the interaction between metal site and the support was strong enough for good electron transfer between both phases, or the formation of the intermediate phases [11]. The Ni-based catalyst used for DRM are usually supported e.g., on Al2O3 [2,12,13], MgO [14], SiO2 [15], CeO2 [16,17], ZrO2 [18], CeO2-ZrO2 [18,19,20], zeolites [21] and others, including carbonaceous materials [22,23].
The CNTs have been used as catalysts and supports many heterogeneous reactions, e.g., water gas shift (WGS) [24] or preferential CO oxidation (PROX) [25,26] or already mentioned DRM [22,23]. The chemical inertness of CNTs, their thermal resistance, developed surface area, mesoporous structure, uniform pore size distribution and high heat transfer, make them a good catalysts support [27]. Ma et al. [22] studied the performance of Ni nanoparticles dispersed selectively inside (I) or outside (O) carbon nanotubes. The CH4 conversion in DRM over I–Ni/CNTs and r O–Ni/CNTs at 750 °C was 70 and 59%, respectively. The former was also more stable. Figueira et al. [23] studied the activity of Ce + Sr (or Ni) nanoparticles deposited inside the CNTs and Co (or Ni) nanoparticles anchored to the external CNT walls. According to their research, both the CeSr/MWCNT/Co and Ni/MWCNT/Ni catalyst showed high CO2 and CH4 conversions at 700 °C.
DRM reaction over Ni/CNT and Ni/SiO2 was studied by Donphai et al. [28]. Both catalysts showed similar performance in terms of methane conversion, but Ni/CNT showed much better stability owing to carbon deposition along the CNT tips instead of the Ni sites. Khavarian et al. [29] reported that Co/CNT catalyst showed higher CH4 conversions in DRM than Co/MgO, which was also attributed to lower rates of carbon deposition rates, and thus less catalyst deactivation, in the case of the former.
The cerium oxide (CeO2) is a very attractive component of the support or promoter in catalytic systems used in DRM [30]. It is well-known for its high oxygen storage arising from easy transformation between 3+ and 4+ oxidation state. The high surface ceria was found very promising candidate catalyst for DRM owing to very high resistance towards carbon deposition and good thermal stability compared to low surface area CeO2 [31]. The addition of zirconium oxide (ZrO2) promotes bulk reduction of CeO2-ZrO2-mixed oxide (denoted CeZrO2). The redox ageing often leads to improved TPR behaviour at moderate temperatures, which implies high thermal stability and oxygen storage capacity in the CeZrO2. The catalysts supported on CeZrO2 are more resistant to thermal ageing than those containing only CeO2. Moreover, the presence of Zr4+ shifts the temperature of Ce4+ reduction into lower values. Nevertheless, during the reduction of CeZrO2, only cerium is reduced from 4+ to 3+, while zirconium ions stay on +4 oxidation state [32]. It has been found that the thermodynamic properties of ceria-zirconia solid solutions differ dramatically from those of pure ceria [33]. Charisiou et al. [34] reported that alumina modified with CeO2 (and La2O3) was more active and stable during biogas dry reforming than alumina alone. The increased activity in DRM of NiMoMgAl hydrotalcite-derived catalyst was noticed when it was promoted with CeZrO2 [35]. The CeO2 and CeZrO2-promoted or supported catalysts were tested in DRM by others [36,37,38,39]. Wolfbeisser et al. [18] reported that the synthesis method had a strong impact on the performance of Ni/CeZrO2 catalyst in DRM, whereas the composition of the catalyst had been of minor importance. Moreover, there was no significant difference between the activity of Ni/CeZrO2 and Ni/ZrO2 catalysts; however, the former was more resistant to the deposition of filamentous carbon species, which reduced the risk of reactor blocking. The positive effect of CeO2 on catalyst resistance towards carbon deposition in DRM was also noticed by Mesrar et al. [40], who studied the activity of Ni/perlite catalysts. According to their research, the best performance revealed the CeO2/Ni/perlite containing 20 wt.% of ceria. The addition of CeO2 to Ni-supported catalysts was found beneficial for the nickel dispersion, which improved the catalytic performance and reduced the coke formation on the catalyst surface [20,41,42,43]. The Ni-CeO2 interaction was studied by Zhou et al. [44] using X-ray photoelectron spectroscopy (XPS) and scanning tunnelling microscopy (STM). It was found that redox properties of ceria and temperatures of Ni deposition over ceria, greatly influence the size, dispersion and electronic properties of Ni nanoparticles. However, Ni deposited on cerium oxides can agglomerate at high temperatures, resulting in decrease of catalyst activity [45]. At higher temperatures, owing to SMSI, new active sites can be formed on the Ni-ceria surface. Moreover, the charge transfer from Ni to CeO2 allows Ni oxidation to Ni2+ with simultaneous reduction of Ce4+ to Ce3+.
In this work we deal with CNT-supported Ni, CeZrO2 and Ni-CeZrO2, and their potential application in DRM, whereas the two latter catalysts are for the first time examined in terms of their performance in this reaction. The addition of CeZrO2 phase to the already known Ni/CNT catalyst aimed at reducing Ni deactivation caused by carbon deposition occurring during DRM. The dependence of DRM mechanism and the occurrence of RWGS and CO2 deoxidation is discussed in respect of catalysts composition.
2. Results and Discussion
2.1. Characterization
The X-ray diffraction (XRD) patterns for functionalized CNTs (denoted CNT_ox), CeZrO2/CNT (denoted CZ/CNT), NiCeZrO2/CNT (denoted NiCZ/CNT) and Ni/CNT catalysts are presented in Figure 1. The diffraction peak at ca. 26° visible for all samples is assigned to CNTs. Both the CZ/CNT and NiCZ/CNT diffractograms show reflexions at ca. 29.5, 33.9, 48.5 and 57.3° corresponding to (111), (200), (311) and (222) planes of CeZrO2, respectively.
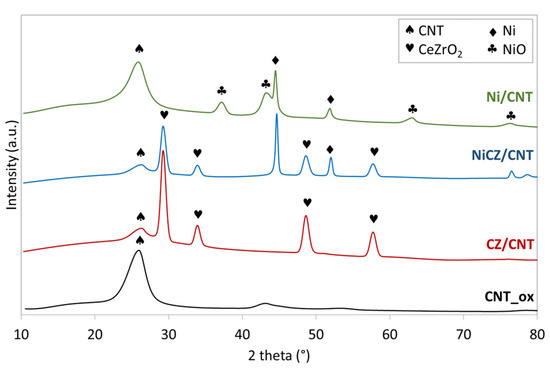
Figure 1.
XRD patterns of CNT_ox, CZ/CNT, NiCZ/CNT and Ni/CNT.
For the Ni/CNT and NiCZ/CNT samples, the diffraction peaks located at ca. 44.6 and 51.9° are assigned to (111) and (200) planes of Ni, respectively. The NiO phase occurs only in Ni/CZ sample and it is detected by the reflexions at 37.5, 43.5 and 63.0° corresponding to (101), (012) and (110) planes, respectively [46]. Hence, thermal decomposition of Ni(OH)2 on CNT support, precipitated from Ni(NO3)2 using NaOH, can be followed with partial (in the case of Ni/CNT) and total (for NiCZ/CNT) reduction of NiO to Ni. The presence of both NiO and Ni on the CNT walls in Ni/CNT catalyst after its annealing was reported e.g., in [47,48]. In the case of NiCZ/CNT catalyst annealing leads to Ni (the NiO phase is not detected by the XRD), and in this case, the total reduction of NiO to Ni can be caused by the strong contact between nickel phase and CeZrO2. Since the annealing of NiCZ/CNT was carried out at 500 °C, some oxygen could desorb from CeZrO2 crystal lattice, producing oxygen vacancies. The CeZrO2 is well-known for high oxygen mobility and redox properties that could facilitate the transformation of Ni(OH)2 to Ni.
The morphologies of CNT-supported Ni, CeZrO2 and Ni-CeZrO2 are presented in Figure 2, Figure 3 and Figure 4. The Ni and CeZrO2 crystals display grey or white on the bright and dark field TEM pictures, respectively. The distribution of the active phase in the CZ/CNT catalyst is good. It was detected by the energy dispersive spectroscopy (EDS) that Ce and Zr occur in the same area indicating the formation of CeZrO2. It can be seen in Figure 2c and d that the spherical particles of CeZrO2 are well dispersed over CNT surface. It was determined that the size of CeZrO2 crystallites varies from 1.21 to 4.22 nm, with 2.22 nm being the mean size. The histogram showing the size distribution of CeZrO2 particle is presented in Figure 2b.
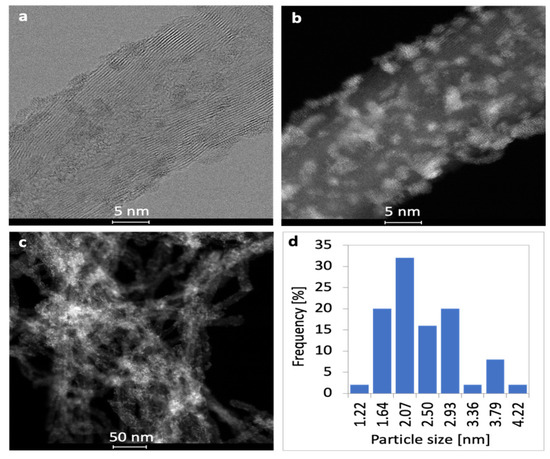
Figure 2.
Bright field (a) and dark field (b,c) TEM pictures of CZ/CNT with CeZrO2 particle size distribution (d).
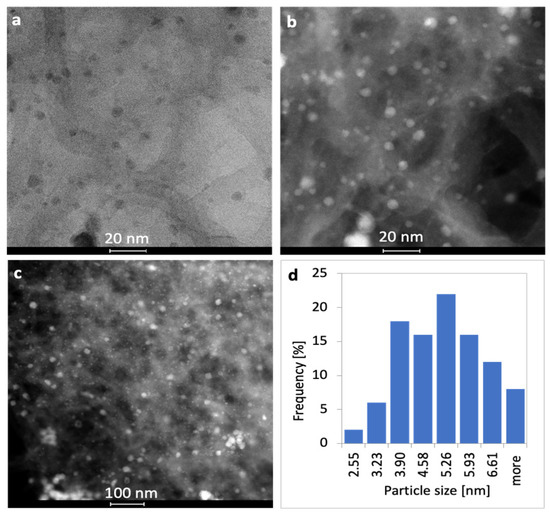
Figure 3.
Bright field (a) and dark field (b,c) TEM pictures of Ni/CNT with Ni particle size distribution (d).
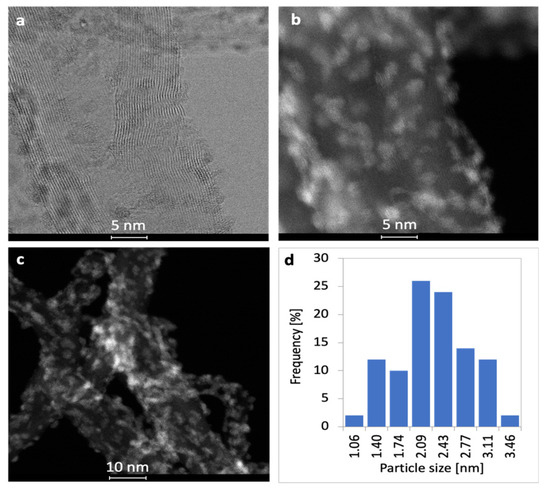
Figure 4.
Bright field (a) and dark field (b,c) TEM pictures of NiCZ/CNT with Ni-CeZrO2 particle size distribution (d).
The dispersion of nickel phase on CNTs in Ni/CNT catalyst (Figure 3) is also good; however, the presence of two nickel fractions can be observed, i.e.,: (i) small-size, spherical, non-agglomerated crystallites of the mean diameter close to 5 nm, and (ii) larger agglomerates whose size exceeds 15 nm.
Combination of both active phases, i.e., Ni and CeZrO2 in NiCZ/CNT catalyst, also results in their good dispersion over the CNT support (Figure 4). It was detected by elemental mapping and selected area electron diffraction that Ni occurs in proximity to the Ce and Zr (Figure 5). This indicates strong contact between Ni and CeZrO2. It was determined, that the size of Ni-CeZrO2 particles varies from 1.06 to 3.46 nm, with a mean diameter of 2.11 nm (Figure 4b).
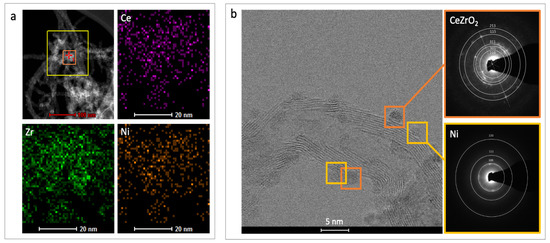
Figure 5.
The Ce, Zr and Ni mapping for NiCZ/CNT (a) and selected area electron diffraction for Ni and CeZrO2 nanocrystals supported over CNTs (b).
The N2 adsorption/desorption isotherms for CNT-supported CeZrO2, Ni and Ni-CeZrO2 are shown in Figure 6, and they are similar to type IV isotherm according to IUPAC classification. The occurrence of hysteresis loop visible for all samples prove the existence of mesopores. The textural properties of all samples, i.e., the specific surface area (SSA), the total pore volume (Vt), the volumes of micro-, meso- and macropores, and the average pore size (d), are presented in Table 1. The CeZrO2-containing catalysts (CZ/CNT and NiCZ/CNT) show significantly lower specific surface areas when compared to Ni/CNT, which can arise from higher loading of the active phase (CeZrO2 or Ni-CeZrO2) in those samples.
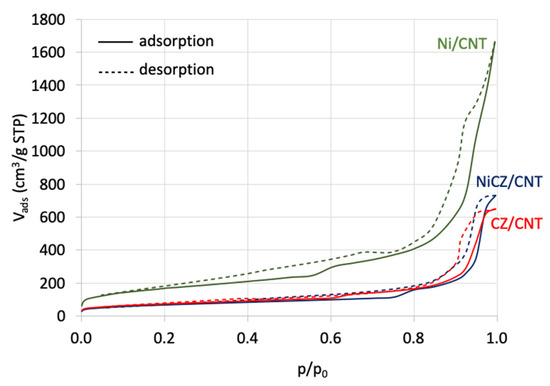
Figure 6.
The N2 adsorption/desorption isotherms for CZ/CNT, NiCZ/CNT and Ni/CNT catalysts.

Table 1.
Textural properties of CZ/CNT, NiCZ/CNT and Ni/CNT catalysts (SBET—BET surface area, Vt— total pore volume, Vmicro— volume of micropores, Vmacro— volume of macropores, d— mean pore size).
2.2. Catalytic Tests of DRM
The performance of obtained catalysts in the DRM reaction was tested in the temperature-programmed (TP) conditions to determine the temperature range for the reaction (Figure 7), and in isothermal mode (Figure 8).
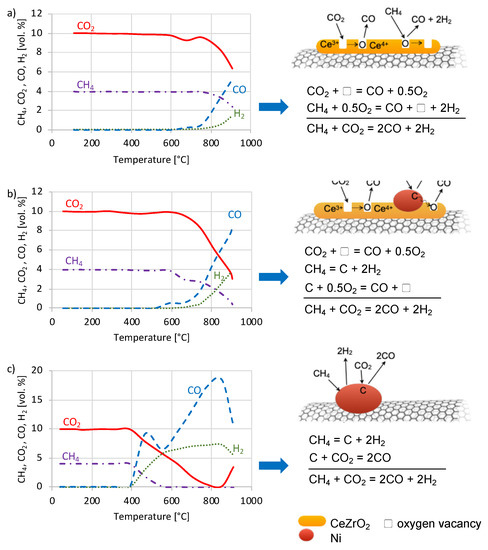
Figure 7.
The evolution of CH4, CO2, H2 and CO during tests of dry reforming of methane (DRM) carried out in temperature-programmed (TP) conditions over CZ/CNT (a), NiCZ/CNT (b) and Ni/CNT (c) and corresponding mechanisms of DRM reaction.
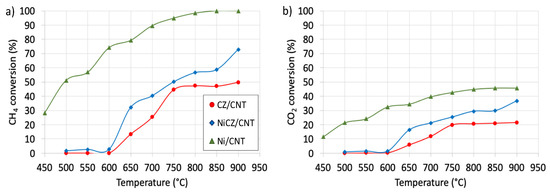
Figure 8.
CH4 (a) and CO2 (b) conversion during DRM on CZ/CNT, NiCZ/CNT and Ni/CNT catalysts.
From the TP tests (Figure 7) it is observed that Ni presence in the sample significantly decreases the temperature of DRM occurrence. The CO + H2 formation starts at 750 °C for CZ/CNT, 630 °C for NiCZ/CNT and 400 °C for Ni/CNT. Besides, all catalysts differ from each other regarding the reaction mechanism (shown on the right side of each TP test). For the CeZrO2-containing samples, i.e., CZ/CNT and NiCZ/CNT, some decrease in CO2 concentration due to its adsorption on the reduced and basic ceria surface is observed at temperatures below the temperature of DRM occurrence. The CO2 adsorption on CeZrO2 is followed with its decomposition to CO and ceria-zirconia re-oxidation. In the case of CZ/CNT, methane undergoes partial oxidation to CO and H2 using the lattice oxygen from CeZrO2. That elementary reaction leads to the formation of oxygen vacancies (□) that are next filled with oxygen from CO2 decomposition. In the case of NiCZ/CNT catalyst, methane adsorbs on the reduced Ni sites and undergoes dehydrogenation. Produced in that way carbon species are oxidized with oxygen from CO2 decomposition taking place over Ni0 or the oxygen vacancies in CeZrO2. While in the case of Ni/CNT catalyst, both the CH4 and CO2 activation occurs over reduced Ni active sites. Dehydrogenation of CH4 over Ni sites is followed by the oxidation of carbon species with CO2. It is known that if the rate of methane dehydrogenation is higher than the rate of carbon suppression with CO2, the Ni sites either will be covered with coke or will act as active sites for the formation of structural carbon species (e.g., CNTs, carbon nanofibers, etc.), whereas the type of carbon deposit formed over the Ni is temperature-dependent.
The CH4 and CO2 conversions during DRM after 2 h on-stream at each temperature over CZ/CNT, NiCZ/CNT and Ni/CNT are presented in Figure 8. The performance of Ni/CNT is significantly better than that of CZ/CNT and NiCZ/CNT catalysts. It shows 28 and 10% conversion of CH4 and CO2, respectively at a temperature as low as 450 °C, while the CZ/CNT and NiCZ/CNT catalysts are active in DRM above 600 °C. However, some minor activity can be observed in the 500–600 °C range for the latter. According to TEM observations, Ni and CeZrO2 remained in good contact; thus, one would expect such a catalyst to show at least similar activity to the Ni/CNT. The significantly lower performance of NiCZ/CNT compared to Ni/CNT may arise from the partial coverage of Ni crystals with CeZrO2; hence, not sufficient exposure of the Ni active sites for CH4 dehydrogenation. The most frequently used model for DRM is the Langmuir-Hinshelwood one, in which both the CH4 and CO2 are adsorbed on the surface. The most widely applied rate-determining step is CH4 decomposition and the surface reaction of two adsorbed species [49]. Reduced concentration of Ni sites accessible for CH4 adsorption in NiCZ/CNT compared to Ni/CNT will affect the DRM reaction. The Ni/CNT characterizes with very high SSA (about 2.3 times higher than for NiCZ/CNT) and the access of reagents to well-dispersed Ni nanocrystals is uninterrupted, making it very active in DRM.
At each temperature, the CO2 conversion was found to be higher than that arising from the stoichiometry of DRM reaction. It can be explained by CO2 consumption in the reverse water gas shift (RWGS) reaction (Equation (4)), that is known to occur during DRM [50], and in CO2 deoxidation on the reduced-state active sites (*) (Equation (5)) that can be either oxygen vacancies in the CeZrO2 lattice or Ni0. It must be noted here, that CO2 deoxidation is an elementary reaction both in the DRM and in the RWGS reactions; however, it can also take place beyond the DRM and RWGS catalytic cycles. From Table 2 it can be seen that the “non-DRM” conversion of CO2 (i.e., coming from RWGS or CO2 deoxidation) is the highest for the NiCZ/CNT sample which contains both types of active sites for CO2 deoxidation. Moreover, it is known that the presence of Zr in ceria lattice increases the rate of formation of oxygen vacancies. It results in higher oxygen capacity, and in consequence—in more important CO2 deoxidation. The contribution of CO2 in DRM, RWGS and deoxidation reactions varies depending on the active site supported on CNTs (Figure 9). The “non-DRM” consumption of CO2 over CZ/CNT is limited to CO2 deoxidation, whereas over Ni/CNT it is associated to RWGS only. The combination of Ce3+ (and oxygen vacancies) with Ni0 sites in NiCZ/CNT catalyst results in increased CO2 consumption owing to the occurrence of RWGS and CO2 deoxidation, whereas the contribution of the latter is bigger, especially at lower temperatures because of the thermodynamic of RWGS reaction that favours higher temperatures.

Table 2.
The “non-DRM” conversion of CO2 over CZ/CNT, NiCZ/CNT and Ni/CNT catalysts.
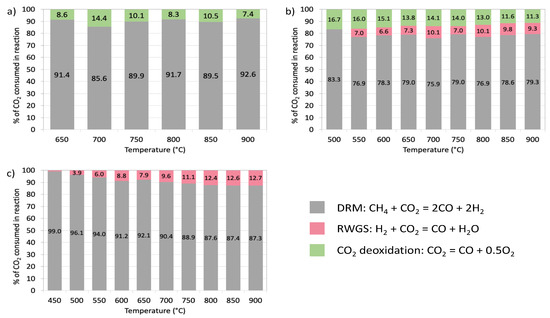
Figure 9.
Contribution of CO2 in DRM, reverse water gas shift reaction (RWGS) and CO2 deoxidation reactions during DRM over CZ/CNT (a), NiCZ/CNT (b) and Ni/CNT (c).
The occurrence of RWGS during DRM usually manifests by the H2/CO < 1, and such a phenomenon is observed in Figure 10. In addition, the decrease of H2/CO ratio can be caused by the CO2 deoxidation, that produces CO, thus decreases the overall contribution of H2 in the product gas. The reverse situation, i.e., the increase of H2/CO > 1, would speak either for the CH4 decomposition (Equation (3)) that generates more H2, or the Boudouard reaction (Equation (2)) that consumes CO. Both reactions can occur during DRM and result in carbon deposition. However, in the presented cases the H2/CO < 1.
CO2 + * ↔︎ CO + *O
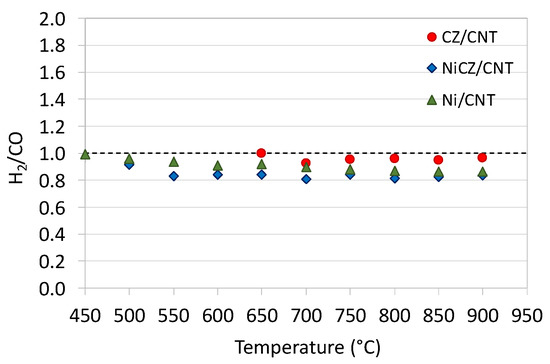
Figure 10.
The H2/CO ratio during DRM over CZ/CNT, NiCZ/CNT and Ni/CNT catalysts.
The spent CZ/CNT_s, NiCZ/CNT_s and Ni/CNT_s catalysts were subjected to XRD and TEM analyses. Some minor differences in catalysts composition and their morphology before and after catalytic tests, determined by XRD (Figure 11 and Table 3) and TEM (Figure 12), were noticed to occur. For example, it was calculated from Scherrer equation, that the mean size of CeZrO2 in [220] direction in CZ/CNT catalyst decreased after catalytic tests (Table 3) but TEM (Figure 12a) shows the presence of bigger CeZrO2 agglomerates of up to 17 nm dimension. In the case of NiCZ/CNT_s and Ni/CNT_s the increase of Ni and CeZrO2 crystallites size was noticed, compared to fresh catalysts. Nevertheless, the active phases deposited on CNTs maintained the nanosized character after exposition to DRM mixture at elevated temperatures. Moreover, the XRD of spent Ni/CNT_s proved that NiO that was initially observed in fresh Ni/CNT sample was totally reduced to Ni during DRM reaction, whereas in the case of NiCZ/CNT_s some oxidation of Ni to NiO can be observed. This supports the hypothesis that during DRM, the excess CO2 can dissociate on reduced active sites, especially on Ce3+ and oxidizes them to Ce4+ (hence, filling the oxygen vacancies in CeZrO2 lattice). In addition, high oxygen mobility in CeZrO2 lattice and strong Ni-CeZrO2 interaction allow the oxidation of Ni0 to NiO.
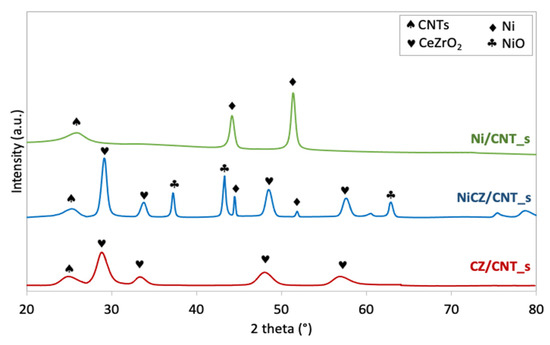
Figure 11.
XRD patterns of spent catalysts: CZ/CNT_s, NiCZ/CNT_s and Ni/CNT_s.

Table 3.
The mean crystallite size (D) for Ni[111] and CeZrO2[220] in fresh and spent catalysts.
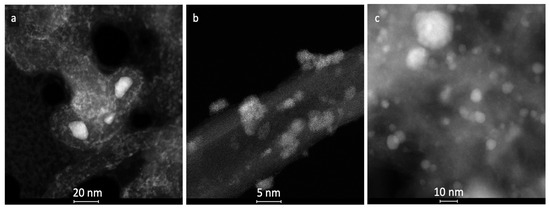
Figure 12.
TEM pictures of spent catalysts: CZ/CNT_s (a), NiCZ/CNT_s (b) and Ni/CNT_s (c).
3. Materials and Methods
3.1. Synthesis of the CNT-Supported Metal Catalysts
Three catalysts were synthesized using multiwall carbon nanotubes (Sigma Aldrich, the outer diameter of 6–13 nm; inner diameter of 2–6 nm; length of 2.5–20 μm, purity > 99%) as support. Prior to catalysts syntheses, the CNTs were oxidized (functionalized) in concentrated HNO3 at 110 °C for 24 h. After functionalisation the CNTs were washed with distilled water until pH = 7 and dried at 120 °C/24 h.
All the syntheses of the CNT-supported catalysts were carried out according to the same sequence of steps. In each synthesis, the suspension of 0.1 g of oxidized CNTs in 40 mL of ethanol and 50 mL of distilled was used. The CNT suspension was placed in the three-necked round-bottomed flask connected with the condenser. In order to provide the inert atmosphere during synthesis, the Ar (10 mL/min) flew through the suspension. Each of the metal salts—Ni(NO3)2·6H2O, Ce(NO3)3·6H2O and N2O7Zr·xH2O (both purchased in Sigma Aldrich)—was dissolved in 10 mL water + acetone mixture (v/v 1:1) and instilled (as required; Table 4) into the CNT suspension. The mixture was then sonicated for 1 h. Afterwards, the aqueous solution of NaOH (1M) was instilled into the mixture until pH = 10. The mixture was stirred at room temperature overnight, and then, at 70 °C for 4 h. After cooling down to the room temperature, the mixture was filtered off under reduced pressure and washed with large amounts of distilled water until the pH was neutral. The obtained material was dried at 120 °C for 24 h and calcined at 500 °C for 2 h under flowing argon (20 mL/min). In order to protect the CNT support from being oxidized with NOx produced during thermal decomposition of nitrates, the calcination was carried out in few steps: 1— heating to 150 °C (5 °C/min) followed by 30 min isotherm, 2—heating up to 350°C (5 °C/min) followed by 1 h isotherm, and finally, 3—heating to 500 °C (2 °C/min). After the 2 h isotherm at 500 °C, the sample was cooled down to room temperature in flowing Ar. As a result, three catalysts were obtained: Ni/CNT, CeZrO2/CNT (denoted CZ/CNT) and Ni-CeZrO2/CNT (denoted NiCZ/CNT). The cerium and zirconium salts were used in amounts allowing the formation of a cerium-zirconium mixed oxide of the following composition: Ce0.68Zr0.32O2. The composition of prepared catalysts is given in Table 4.

Table 4.
The composition of prepared catalysts.
3.2. Catalyst Characterization
The catalysts were characterized using typical analytical methods. The X-ray diffraction (XRD) was performed for 2ϴ from 10 to 80° with 0.03° steps using the MiniFlex diffractometer (Rigaku, Japan, Tokyo) with copper anticathode (λ = 1.54 Å). The specific surface area (SSA) of the catalyst samples was determined by the N2 adsorption/desorption at 77 K using the Autosorb 3.01 (Quantachrome Instruments, Boynton Beach, FL, USA). For determining the surface area of catalyst samples, the Brunauer-Emmett-Teller (BET) method was used. The pore volume was calculated using the Barrett-Joyner-Halenda (BJH) method. Before the analyses, the samples were degassed at 150 °C for 12 h. The morphology of the obtained catalysts was determined by the transmission electron microscopy using the S/TEM Titan 80–300 microscope (FEI Company, Hillsboro, Oregon, UK) equipped with EDAX EDS (energy dispersive X-ray spectroscopy) detector operating at accelerating voltage of 300 kV.
3.3. Catalytic Tests
The performance of CNT-supported catalysts in DRM reaction was determined by tests carried out in the temperature-programmed (TP) and isothermal conditions. All experiments were performed in a U-shape fixed bed reactor placed in an oven equipped with the temperature regulator. Tests were conducted in the atmospheric pressure using the reaction mixture composed of 4 vol.% CH4, 10 vol.% CO2 and balanced with Ar. The gas hourly space velocity (GHSV) was 15,000 h−1 for each test. The tests in TP conditions were carried out at temperature increasing linearly from 25 to 900 °C with a heating rate of 5 °C/min. The tests in isothermal conditions were conducted at temperatures ranging from 500 to 900 °C; however, the initial temperature was 900 °C and it was decreased by 50° after 2 h on-steam. The same sample was used for each temperature.
4. Conclusions
Three CNT-supported catalysts with well dispersed, nanosized CeZrO2, Ni-CeZrO2 and Ni active phases were obtained and tested in DRM reaction carried out in the excess of CO2 (CO2/CH4 = 2.5).
The DRM over obtained catalysts occurred via different mechanisms; however, the common elementary reaction was CO2 deoxidation that provided oxygen to the catalyst. The presence of oxygen vacancies in partially reduced and basic ceria (in CeZrO2/CNT and Ni-CeZrO2/CNT samples) facilitated CO2 deoxidation, protecting the catalyst from carbon deposition. Oxygen provided to the catalyst via CO2 deoxidation was used in the reaction of partial oxidation of CH4 adsorbed on CeZrO2 or oxidation of carbon species produced on Ni sites as a result of CH4 dehydrogenation.
The best performance in DRM showed the Ni/CNT catalyst, while the CeZrO2-containing samples exhibited much lower activity. A significant difference in the performance of Ni/CNT and Ni-CeZrO2/CNT might have been caused by the partial coverage of Ni nanocrystals with CeZrO2 in the latter; thus, the reduction in the concentration of Ni sites exposed to reagents. Catalytic tests revealed that the conversion of CO2 was higher than arising from the stoichiometry of DRM reaction, which was due to the occurrence of RWGS reaction over Ni-containing catalysts and CO2 deoxidation on CeZrO2-containing catalysts. The combination of two types of active sites in Ni-CeZrO2/CNT, i.e., oxygen vacancies and Ni0, resulted in increased CO2 consumption since both the RWGS and CO2 deoxidation took place.
CNTs as a support material allow to develop significantly the surface area of deposited active phases. The main drawback of DRM process is catalyst deactivation caused by carbon deposition, which is a result of insufficient suppression of carbon species formed during methane dehydrogenation. Since Ce3+ is an active site for CO2 activation followed by the oxidation of carbon species covering the surface of the catalyst during the DRM reaction, the combination of CeZrO2 and Ni phases over CNT support is a promising solution for developing an active catalyst resistant to carbon deposition. That could be achieved by modifying the synthesis method of Ni-CeZrO2/CNT, e.g., by the sequential deposition of both active phases.
Author Contributions
Conceptualization, A.Ł.; Investigation, A.Ł., P.J., M.S. and K.M.; Supervision, A.Ł.; Writing—original draft, A.Ł. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financed by the Ministry of Science and Higher Education from the statutory activity subsidy for the Faculty of Chemistry of Wrocław University of Science and Technology.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tuckett, R. Greenhouse Gases. In Encyclopedia of Analytical Sciences, 3rd ed.; Worsfold, P., Poole, C., Townshend, A., Miró, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 1, pp. 362–372. [Google Scholar] [CrossRef]
- Castro Luna, A.E.; Iriarte, M.E. Carbon dioxide reforming of methane over a metal modified Ni–Al2O3 catalyst. Appl. Catal. A Gen. 2008, 343, 10–15. [Google Scholar] [CrossRef]
- Aramouni, N.A.K.; Touma, J.G.; Tarboush, B.A.; Zeaiter, J.; Ahmad, M.N. Catalyst design for dry reforming of methane: Analysis review. Renew. Sustain. Energy Rev. 2018, 82, 2570–2585. [Google Scholar] [CrossRef]
- Hei, M.J.; Chen, H.B.; Yi, J.; Lin, Y.J.; Lin, Y.Z.; Wei, G.; Liao, D.W. CO2-reforming of methane on transition metal surfaces. Surf. Sci. 1998, 417, 82–96. [Google Scholar] [CrossRef]
- Cao, P.; Adegbite, S.; Wu, T. Thermodynamic equilibrium analysis of CO2 reforming of methane: Elimination of carbon deposition and adjustment of H2/CO ratio. Energy Procedia 2017, 105, 1864–1869. [Google Scholar] [CrossRef]
- Luisetto, I.; Tuti, S.; Battocchio, C.; Lo Mastro, S.; Sodo, A. Ni/CeO2–Al2O3 catalysts for the dry reforming of methane: The effect of CeAlO3 content and nickel crystallite size on catalytic activity and coke resistance. Appl. Catal. A Gen. 2015, 500, 12–22. [Google Scholar] [CrossRef]
- Parvary, M.; Jazayeri, S.; Taeb, A.; Petit, C.; Kiennemann, A. Promotion of active nickel catalysts in methane dry reforming reaction by aluminum addition. Catal. Commun. 2001, 2, 357–362. [Google Scholar] [CrossRef]
- Schwab, G.M.; Koller, K. Combined Action of Metal and Semiconductor Catalysts. J. Am. Chem. Soc. 1968, 90, 3078–3080. [Google Scholar] [CrossRef]
- Tauster, S.J.; Fung, S.C.; Baker, R.T.K.; Horsley, J.A. Strong Interactions in Supported-Metal Catalysts. Science 1981, 211, 1121–1125. [Google Scholar] [CrossRef]
- Kim, J.; Park, W.H.; Doh, W.H.; Lee, S.W.; Noh, M.C.; Gallet, J.J.; Bournel, F.; Kondoh, H.; Mase, K.; Jung, Y.; et al. Adsorbate-driven reactive interfacial Pt-NiO1−x nanostructure formation on the Pt3Ni(111) alloy surface. Sci. Adv. 2018, 4. [Google Scholar] [CrossRef]
- Pan, C.J.; Tsai, M.C.; Su, W.N.; Rick, J.; Akalework, N.G.; Agegnehu, A.K.; Cheng, S.Y.; Hwang, B.J. Tuning/exploiting Strong Metal-Support Interaction (SMSI) in Heterogeneous Catalysis. J. Taiwan Inst. Chem. Eng. 2017, 74, 154–186. [Google Scholar] [CrossRef]
- Huang, Q.; Fang, X.; Cheng, Q.; Li, Q.; Xu, X.; Xu, L.; Liu, W.; Gao, Z.; Zhou, W.; Wang, X. Synthesis of a Highly Active and Stable Nickel-Embedded Alumina Catalyst for Methane Dry Reforming: On the Confinement Effects of Alumina Shells for Nickel Nanoparticles. ChemCatChem 2017, 9, 3563–3571. [Google Scholar] [CrossRef]
- Shin, S.A.; Noh, Y.S.; Hong, G.H.; Park, J.I.; Song, H.T.; Lee, K.Y.; Moon, D.J. Dry reforming of methane over Ni/ZrO2-Al2O3 catalysts: Effect of preparation methods. J. Taiwan Inst. Chem. Eng. 2018, 90, 25–32. [Google Scholar] [CrossRef]
- Singha, R.K.; Shukla, A.; Sandupatla, A.; Deo, G.; Bal, R. Synthesis and catalytic activity of a Pd doped Ni–MgO catalyst for dry reforming of methane. J. Mater. Chem. A 2017, 5, 15688–15699. [Google Scholar] [CrossRef]
- Kroll, V.C.H.; Swaan, H.M.; Mirodatos, C. Methane Reforming Reaction with Carbon Dioxide over Ni/SiO2 Catalyst: I. Deactivation Studies. J. Catal. 1996, 161, 409–422. [Google Scholar] [CrossRef]
- Ay, H.; Üner, D. Dry reforming of methane over CeO2 supported Ni, Co and Ni–Co catalysts. Appl. Catal. B Environ. 2015, 179, 128–138. [Google Scholar] [CrossRef]
- Odedairo, T.; Chen, J.; Zhu, Z. Metal–support interface of a novel Ni–CeO2 catalyst for dry reforming of methane. Catal. Commun. 2013, 31, 25–31. [Google Scholar] [CrossRef]
- Wolfbeisser, A.; Sophiphun, O.; Bernardi, J.; Wittayakun, J.; Föttinger, K.; Rupprechter, G. Methane dry reforming over ceria-zirconia supported Ni catalysts. Catal. Today 2016, 277, 234–245. [Google Scholar] [CrossRef]
- Vasiliades, M.A.; Djinović, P.; Pintar, A.; Kovač, J.; Efstathiou, A.M. The effect of CeO2–ZrO2 structural differences on the origin and reactivity of carbon formed during methane dry reforming over NiCo/CeO2–ZrO2 catalysts studied by transient techniques. Catal. Sci. Technol. 2017, 7, 5422–5434. [Google Scholar] [CrossRef]
- Nguyen, T.G.H.; Tran, D.L.; Sakamoto, M.; Uchida, T.; Sasaki, K.; To, T.D.; Doan, D.C.T.; Dang, M.C.; Shiratori, Y. Ni-loaded (Ce,Zr)O2-δ-dispersed paper-structured catalyst for dry reforming of methane. Int. J. Hydrog. Energy 2018, 43, 4951–4960. [Google Scholar] [CrossRef]
- Luengnaruemitchai, A.; Kaengsilalai, A. Activity of different zeolite-supported Ni catalysts for methane reforming with carbon dioxide. Chem. Eng. J. 2008, 144, 96–102. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, D.; Wu, M.; Zhao, T.; Yoneyama, Y.; Tsubaki, N. Effect of catalytic site position: Nickel nanocatalyst selectively loaded inside or outside carbon nanotubes for methane dry reforming. Fuel 2013, 108, 430–438. [Google Scholar] [CrossRef]
- Figueira, C.E.; Moreira, P.F.; Giudici, R.; Alves, R.M.B.; Schmal, M. Nanoparticles of Ce, Sr, Co in and out the multi-walled carbon nanotubes applied for dry reforming of methane. Appl. Catal. A Gen. 2018, 550, 297–307. [Google Scholar] [CrossRef]
- Łamacz, A.; Matus, K.; Liszka, B.; Silvestre-Albero, J.; Lafjah, M.; Dintzer, T.; Janowska, I. The impact of synthesis method of CNT supported CeZrO2 and Ni-CeZrO2 on catalytic activity in WGS reaction. Catal. Today 2018, 301, 172–182. [Google Scholar] [CrossRef]
- Jardim, E.O.; Goncalves, M.; Rico-Francés, S.; Sepúlveda-Escribano, A.; Silvestre-Albero, J. Superior performance of multi-wall carbon nanotubes as support of Pt-based catalysts for the preferential CO oxidation: Effect of ceria addition. Appl. Cat. B Environ. 2012, 113, 72–78. [Google Scholar] [CrossRef]
- Tanaka, K.; Shou, M.; Zhang, H.; Yuan, Y.; Hagiwara, T.; Fukuoka, A.; Nakamura, J.; Lu, D. An Extremely Active Pt/Carbon Nano-Tube Catalyst for Selective Oxidation of CO in H2 at Room Temperature. Catal. Lett. 2008, 126, 89–95. [Google Scholar] [CrossRef]
- Ajayan, P.M. Nanotubes from carbon. Chem. Rev. 1999, 99, 1787–1800. [Google Scholar] [CrossRef]
- Donphai, W.; Faungnawakij, K.; Chareonpanich, M.; Limtrakul, J. Effect of Ni-CNTs/mesocellular silica composite catalysts on carbon dioxide reforming of methane. Appl. Catal. A Gen. 2014, 475, 16–26. [Google Scholar] [CrossRef]
- Khavarian, M.; Chai, S.P.; Mohamed, A.R. The effects of process parameters on carbon dioxide reforming of methane over Co–Mo–MgO/MWCNTs nanocomposite catalysts. Fuel 2015, 158, 129–138. [Google Scholar] [CrossRef]
- Chein, R.Y.; Fung, W.Y. Syngas production via dry reforming of methane over CeO2 modified Ni/Al2O3 catalysts. Int. J. Hydrog. Energy 2019, 44, 14303–14315. [Google Scholar] [CrossRef]
- Laosiripojana, N.; Assabumrungrat, S. Catalytic Dry Reforming of Methane Over High Surface Area Ceria. Appl. Catal. B Environ. 2005, 60, 107–116. [Google Scholar] [CrossRef]
- Diaz, E.; de Rivas, B.; Lopez-Fonseca, R.; Ordóñez, S. Characterization of ceria–zirconia mixed oxides as catalysts for the combustion of volatile organic compounds using inverse gas chromatography. J. Chromatogr. A 2006, 116, 230–239. [Google Scholar] [CrossRef]
- Shah, P.R.; Kim, T.; Zhou, G.; Fornasiero, P.; Gorte, R.J. Evidence for Entropy Effects in the Reduction of Ceria−Zirconia Solutions. Chem. Mater. 2006, 18, 5363–5369. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Siakavelas, G.; Papageridis, K.N.; Baklavaridis, A.; Tzounis, L.; Avraam, D.G.; Goula, M.A. Syngas production via the biogas dry reforming reaction over nickel supported on modified with CeO2 and/or La2O3 alumina catalysts. J. Nat. Gas Sci. Eng. 2016, 31, 164–183. [Google Scholar] [CrossRef]
- Li, C.; Tan, P.J.; Li, X.D.; Du, Y.L.; Gao, Z.H.; Huang, W. Effect of the addition of Ce and Zr on the structure and performances of Ni-Mo/CeZr-MgAl(O) catalysts for CH4-CO2 reforming. Fuel Process. Technol. 2015, 140, 39–45. [Google Scholar] [CrossRef]
- Liu, Z.; Lustemberg, P.; Gutiérrez, R.A.; Carey, J.J.; Palomino, R.M.; Vorokhta, M.; Grinter, D.C.; Ramírez, P.J.; Matolín, V.; Nolan, M.; et al. In Situ Investigation of Methane Dry Reforming on Metal/Ceria(111) Surfaces: Metal-Support Interactions and C−H Bond Activation at Low Temperature. Angew. Chem. Int. Ed. 2017, 56, 13041–13046. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, F.; Rui, N.; Li, X.; Lin, L.; Betancourt, L.E.; Su, D.; Xu, W.; Cen, J.; Attenkofer, K.; et al. Highly Active Ceria-Supported Ru Catalyst for the Dry Reforming of Methane: In Situ Identification of Ruδ+-Ce3+ Interactions for Enhanced Conversion. ACS Catal. 2019, 9, 3349–3359. [Google Scholar] [CrossRef]
- Smirnova, M.Y.; Bobin, A.S.; Pavlova, S.N.; Ishchenko, A.V.; Selivanova, A.V.; Kaichev, V.V.; Cherepanova, S.V.; Krieger, T.A.; Arapova, M.V.; Roger, A.C.; et al. Methane dry reforming over Ni catalysts supported on Ce–Zr oxides prepared by a route involving supercritical fluids. Open Chem. 2017, 15, 412–425. [Google Scholar] [CrossRef]
- Simonov, M.N.; Rogov, V.A.; Smirnova, M.Y.; Sadykov, V.A. Pulse Microcalorimetry Study of Methane Dry Reforming Reaction on Ni/Ceria-Zirconia Catalyst. Catalysts 2017, 7, 268. [Google Scholar] [CrossRef]
- Mesrar, F.; Kacimi, M.; Liotta, L.F.; Puleo, F.; Ziyad, M. Syngas production from dry reforming of methane over ni/perlite catalysts: Effect of zirconia and ceria impregnation. Int. J. Hydrog. Energy 2018, 43, 17142–17155. [Google Scholar] [CrossRef]
- Chen, W.; Zhao, G.; Xue, Q.; Chen, L.; Lu, Y. High carbon-resistance Ni/CeAlO3-Al2O3 catalyst for CH4/CO2 reforming. Appl. Catal. B Eniviron. 2013, 136, 260–268. [Google Scholar] [CrossRef]
- Taufiq-Yap, Y.H.; Sudarno, U.R.; Zainal, Z. CeO2-SiO2 supported nickel catalysts for dry reforming of methane toward syngas production. Appl. Catal. A Gen. 2013, 468, 359–369. [Google Scholar] [CrossRef]
- Sepehri, S.; Rezaei, M. Ce promoting effect on the activity and coke formation of Ni catalysts supported on mesoporous nanocrystalline γ-Al2O3 in autothermal reforming of methane. Int. J. Hydrog. Energy 2017, 42, 11130–11138. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, J. Interactions of Ni Nanoparticles with Reducible CeO2(111) Thin Films. J. Phys. Chem. C 2012, 116, 9544–9549. [Google Scholar] [CrossRef]
- Zhou, Y.; Perket, J.M.; Crooks, A.B.; Zhou, J.J. Effect of Ceria Support on the Structure of Ni Nanoparticles. Phys. Chem. Lett. 2010, 1, 1447–1453. [Google Scholar] [CrossRef]
- Elizabeth, I.; Nair, A.K.; Singh, B.P.; Gopukumar, S. Multifunctional Ni-NiO-CNT Composite as High Performing Free Standing Anode for Li Ion Batteries and Advanced Electro Catalyst for Oxygen Evolution Reaction. Electrochim. Acta 2017, 230, 98–105. [Google Scholar] [CrossRef]
- Gong, M.; Zhou, W.; Tsai, M.C.; Zhou, J.; Guan, M.; Lin, M.C.; Zhang, B.; Hu, Y.; Wang, D.Y.; Yang, J.; et al. Nanoscale nickel oxide/nickel heterostructures for active hydrogen evolution electrocatalysis. Nat. Commun. 2014, 5, 4695. [Google Scholar] [CrossRef]
- Wang, J.; Teschner, D.; Yao, Y.; Huang, X.; Willinger, M.; Shao, L.; Schlögl, R. Fabrication of nanoscale NiO/Ni heterostructures as electrocatalysts for efficient methanol oxidation. J. Mater. Chem. 2017, 5, 9946–9951. [Google Scholar] [CrossRef]
- Zhang, J.; Hui, W.; Ajay, K.D. Kinetic studies of carbon dioxide reforming of methane over Ni−Co/Al−Mg−O bimetallic catalyst. Ind. Eng. Chem. Res. 2008, 48, 677–684. [Google Scholar] [CrossRef]
- Wei, J.; Iglesia, E. Mechanism and Site Requirements for Activation and Chemical Conversion of Methane on Supported Pt Clusters and Turnover Rate Comparisons among Noble Metals. J. Phys. Chem. B 2004, 108, 4094–4103. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).