Synthesis of Titanium Dioxide via Surfactant-Assisted Microwave Method for Photocatalytic and Dye-Sensitized Solar Cells Applications
Abstract
1. Introduction
2. Results and Discussion
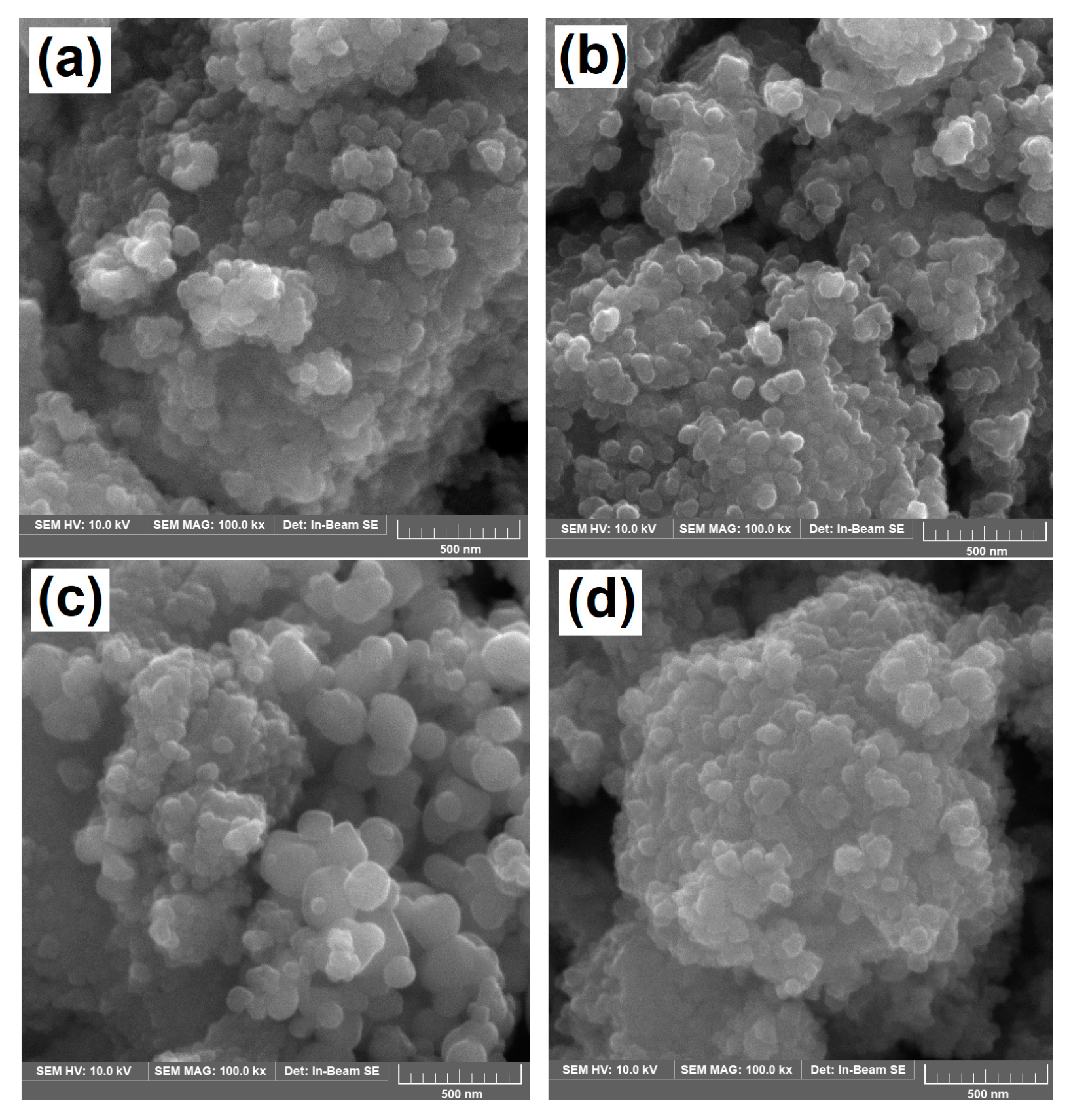
2.1. Morphology
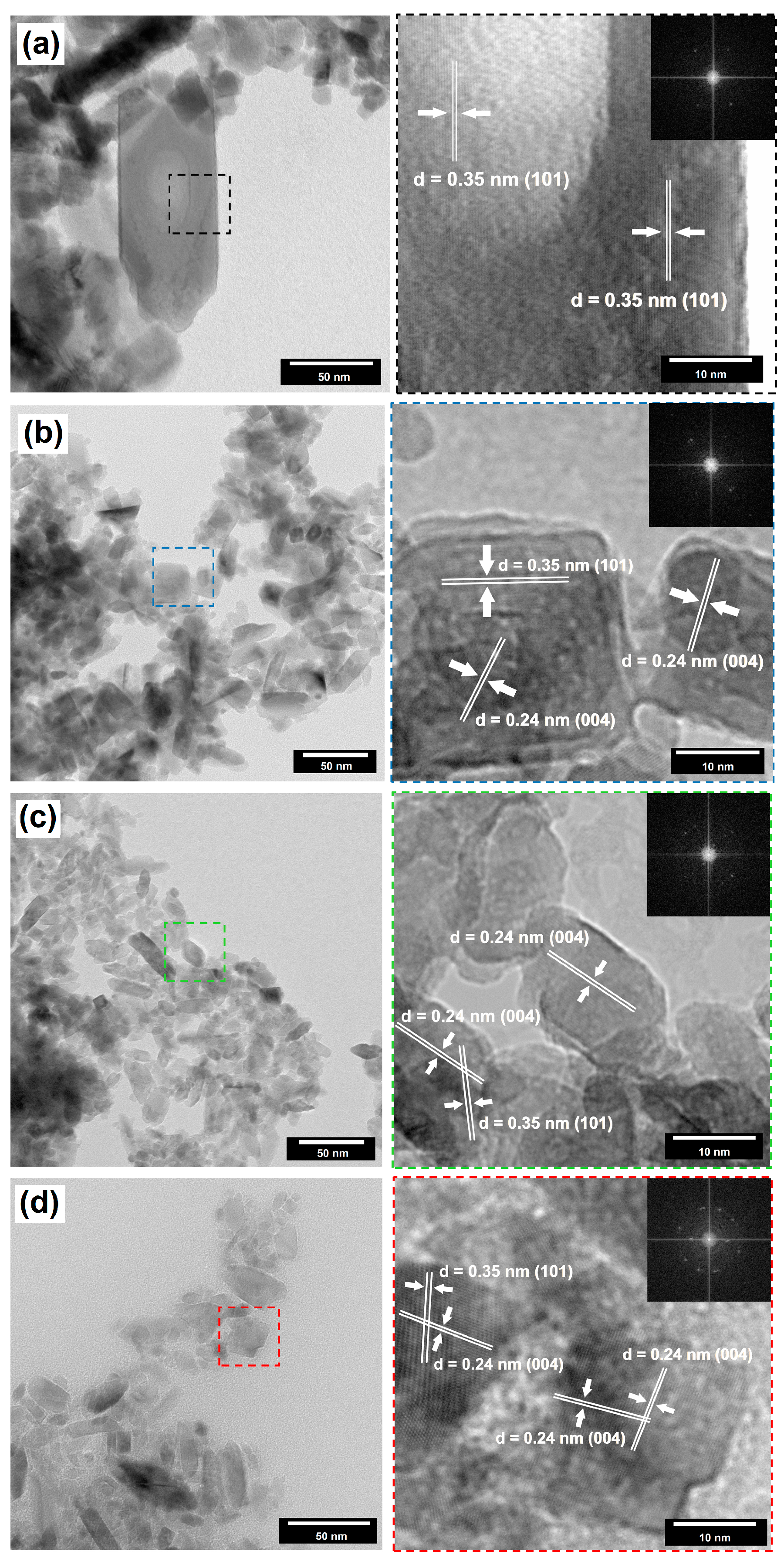
2.2. Crystalline Structure
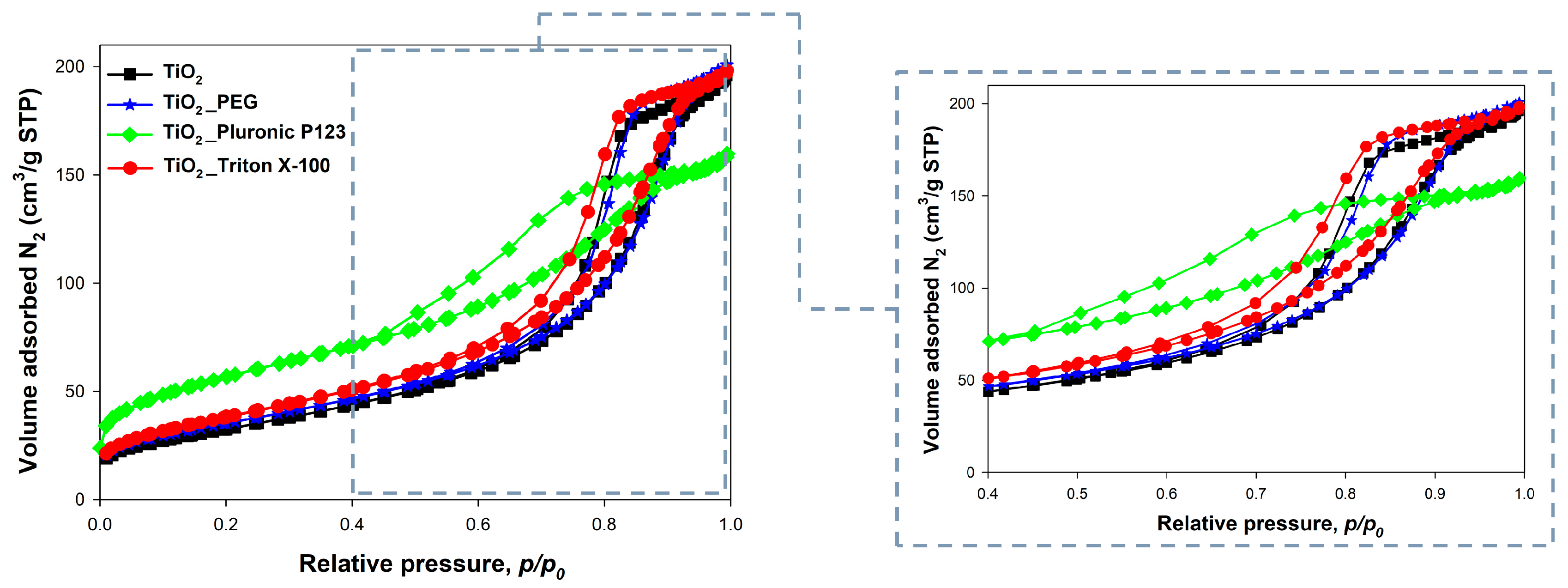
2.3. Analysis of Parameters of the Porous Structure
2.4. Diffuse Reflectance Spectroscopy (DRS)
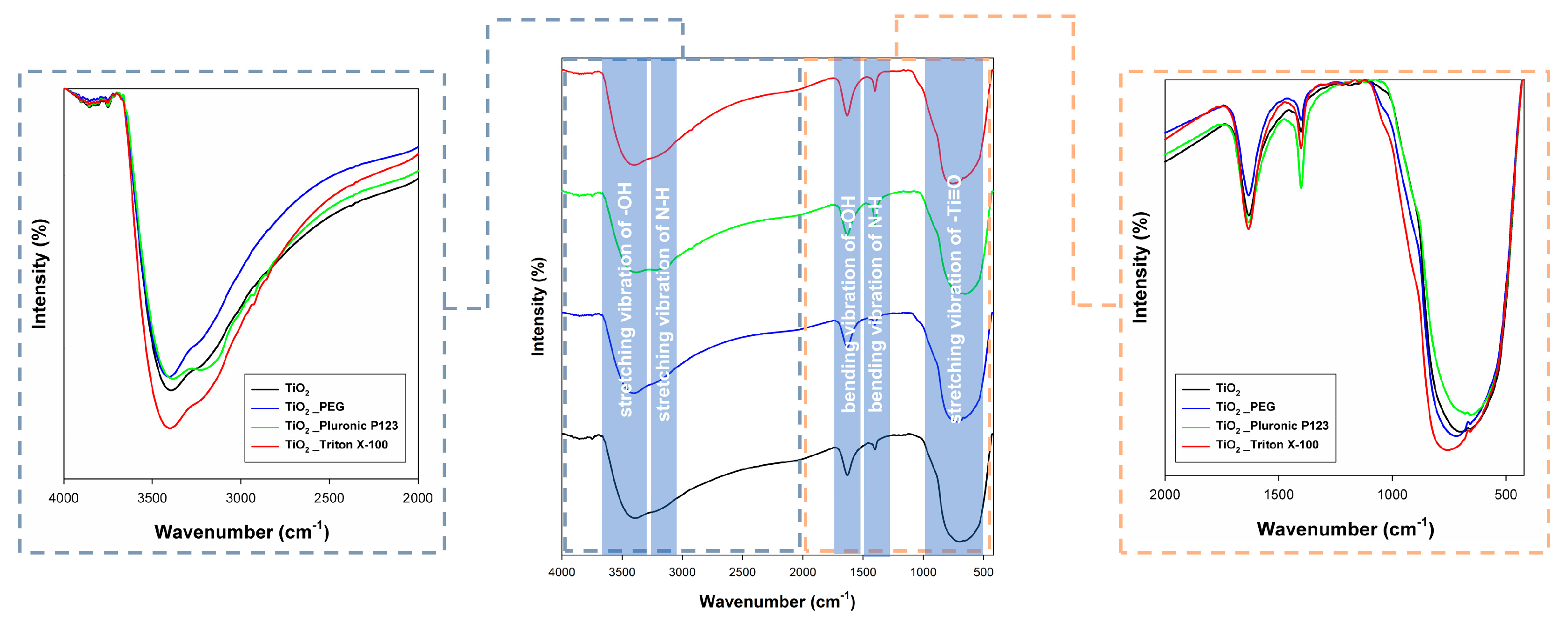
2.5. FTIR Analysis
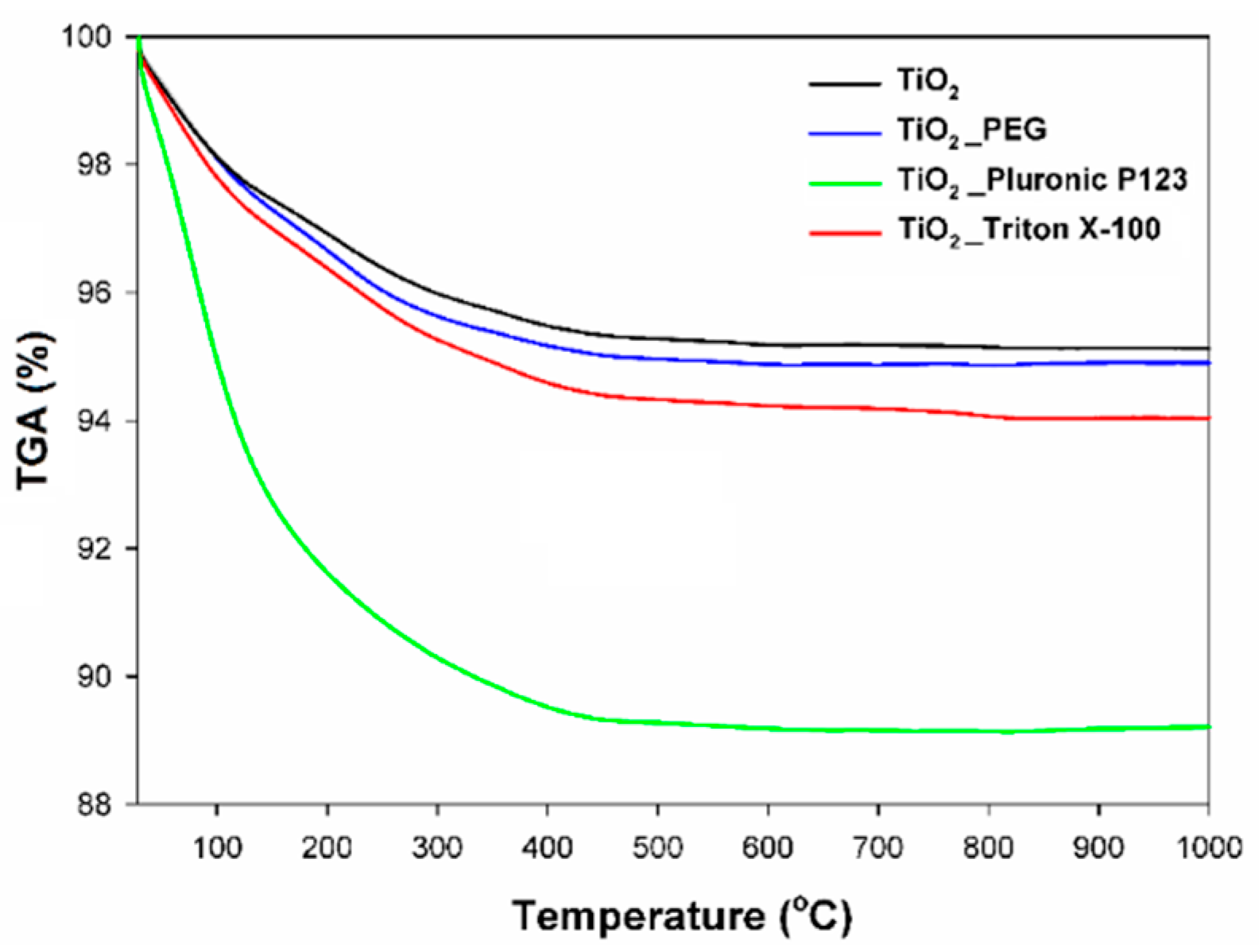
2.6. Thermogravimetric Analysis
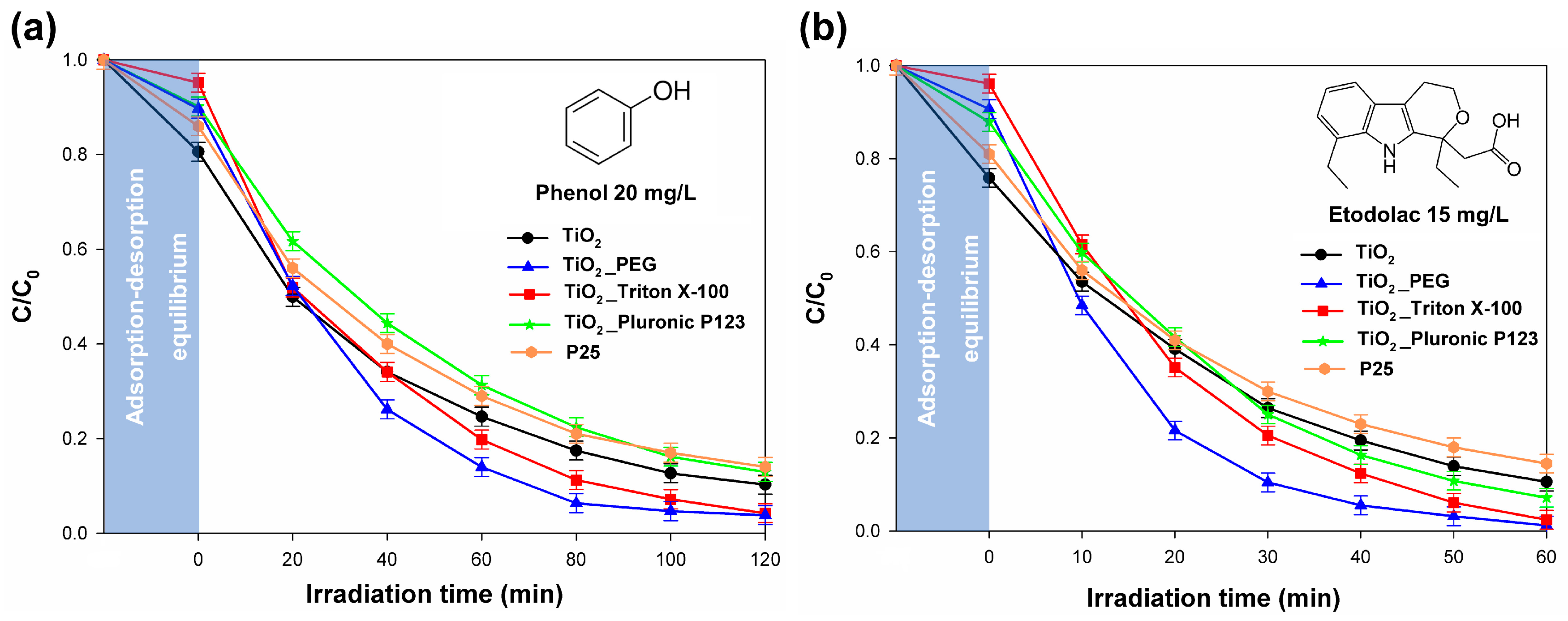
2.7. Photocatalytic Activity
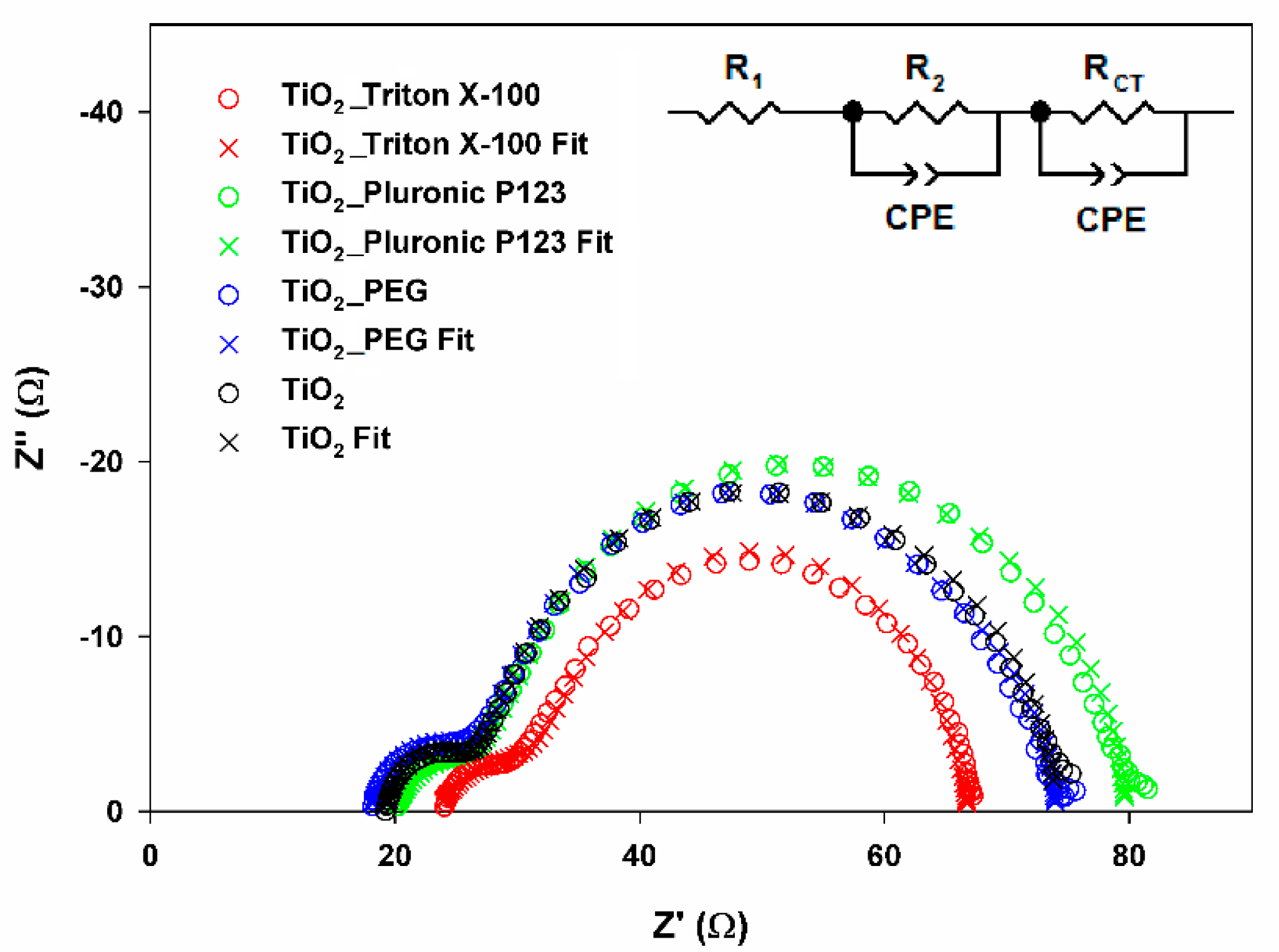
2.8. Photovoltaic Properties
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Titania Nanoparticles
3.3. Analysis of Materials
3.4. Photocatalytic Activity
3.5. Photovoltaic Properties
3.5.1. Preparation of DSSC
3.5.2. Dye Loading Determination
3.5.3. Characteristics of Obtained DSSC’s
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Sample | JSC (mA/cm2) | VOC (mV) | FF (%) | η (%) |
|---|---|---|---|---|
| TiO2_1 | 10.3 | 734 | 72.2 | 5.45 |
| TiO2_2 | 11.4 | 736 | 70.5 | 5.93 |
| TiO2_3 | 12.0 | 729 | 70.3 | 6.16 |
| TiO2_4 | 11.4 | 750 | 71.3 | 6.11 |
| TiO2_5 | 12.3 | 711 | 71.4 | 6.15 |
| TiO2_PEG_1 | 10.9 | 710 | 70.5 | 5.46 |
| TiO2_PEG_2 | 10.1 | 726 | 72.3 | 5.30 |
| TiO2_PEG_3 | 9.9 | 690 | 72.0 | 4.88 |
| TiO2_PEG_4 | 10.0 | 739 | 72.1 | 5.35 |
| TiO2_PEG_5 | 10.0 | 729 | 71.2 | 5.20 |
| TiO2_Pluronic P123_1 | 10.3 | 726 | 71.3 | 5.32 |
| TiO2_Pluronic P123_2 | 9.8 | 732 | 71.0 | 5.10 |
| TiO2_Pluronic P123_3 | 9.9 | 729 | 69.9 | 2.76 |
| TiO2_Pluronic P123_4 | 9.5 | 709 | 70.1 | 4.71 |
| TiO2_Pluronic P123_5 | 9.0 | 718 | 71.9 | 4.65 |
| TiO2_Triton X−100_1 | 13.5 | 734 | 69.1 | 6.84 |
| TiO2_Triton X−100_2 | 12.9 | 704 | 68.1 | 6.71 |
| TiO2_Triton X−100_3 | 12.9 | 680 | 67.0 | 6.20 |
| TiO2_Triton X−100_4 | 12.7 | 698 | 68.6 | 6.64 |
| TiO2_Triton X−100_5 | 12.9 | 714 | 68.6 | 6.78 |
| Sample | R1 (Ω) | R2 (Ω) | RCT (Ω) | τ (ms) |
|---|---|---|---|---|
| TiO2_1 | 17.3 | 11.7 | 43.2 | 12.8 |
| TiO2_2 | 20.2 | 8.9 | 42.8 | 12.8 |
| TiO2_3 | 19.2 | 7.3 | 36.8 | 12.8 |
| TiO2_4 | 22.5 | 8.9 | 40.0 | 12.8 |
| TiO2_5 | 16.6 | 8.6 | 40.3 | 12.8 |
| TiO2_PEG_1 | 18.0 | 9.1 | 39.0 | 12.8 |
| TiO2_PEG_2 | 18.0 | 10.5 | 44.8 | 12.8 |
| TiO2_PEG_3 | 17.9 | 10.4 | 32.9 | 10.2 |
| TiO2_PEG_4 | 17.8 | 9.5 | 34.7 | 12.8 |
| TiO2_PEG_5 | 18.0 | 8.2 | 36.4 | 10.2 |
| TiO2_Pluronic P123_1 | 19.9 | 7.8 | 40.0 | 12.8 |
| TiO2_Pluronic P123_2 | 22.2 | 6.6 | 42.5 | 16.0 |
| TiO2_Pluronic P123_3 | 23.6 | 5.7 | 67.6 | 12.8 |
| TiO2_Pluronic P123_4 | 32.0 | 7.1 | 44.2 | 12.8 |
| TiO2_Pluronic P123_5 | 20.1 | 6.6 | 47.3 | 12.8 |
| TiO2_Triton X−100_1 | 23.4 | 9.6 | 33.8 | 12.8 |
| TiO2_Triton X−100_2 | 17.6 | 9.8 | 27.6 | 12.8 |
| TiO2_Triton X−100_3 | 24.9 | 7.2 | 27.4 | 12.8 |
| TiO2_Triton X−100_4 | 18.2 | 8.4 | 27.4 | 12.8 |
| TiO2_Triton X−100_5 | 19.0 | 7.6 | 24.9 | 16.0 |
References
- Rockström, J.; Steffen, W.; Noone, K.; Persson, A.; Chapin, F.S.; Lambin, E.F.; Lenton, T.M.; Scheffer, M.; Folke, C.; Schellnhuber, H.J.; et al. A safe operation space for humanity. Nature 2009, 461, 472–475. [Google Scholar] [CrossRef] [PubMed]
- Gust, D.; Moore, T.A.; Moore, A.L. Solar fuels via artificial photosynthesis. Acc. Chem. Res. 2009, 42, 1890–1898. [Google Scholar] [CrossRef] [PubMed]
- Canadell, J.G.; Le Quéré, C.; Raupach, M.R.; Field, C.B.; Buitenhuis, E.T.; Ciais, P.; Conway, T.J.; Gillett, N.P.; Houghton, R.A.; Marland, G. Contributions to accelerating atmospheric CO2 growth from economic activity, carbon intensity, and efficiency of natural sinks. Proc. Natl. Acad. Sci. USA 2007, 104, 18866–18870. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.K.; Moghaddam, R.S.; Lai, M.L.; Docampo, P.; Higler, R.; Deschler, F.; Price, M.; Sadhanala, A.; Pazos, L.M.; Credgington, D.; et al. Bright light-emitting diodes based on organometal halide perovskite. Nat. Nanotechnol. 2014, 9, 687–692. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Yu, P.Y.; Mao, S.S. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef]
- Wadhwa, S.; Mathur, A.; Pendurthi, R.; Singhal, U.; Khanuja, M.; Roy, S.S. Titania-based porous nanocomposites for potential environmental applications. Bull. Mater. Sci. 2020, 43, 47. [Google Scholar] [CrossRef]
- Bai, F.Q.; Li, W.; Zhang, H.X. Theoretical studies of titanium dioxide for dye-sensitized solar cell and photocatalytic reaction. In Titananium Dioxide; Janus, M., Ed.; InterOpen: London, UK, 2017; pp. 229–248. [Google Scholar] [CrossRef]
- Ohtani, B. Photocatalysis A to Z-What we know and what we do not know in a scientific sense. J. Photochem. Photobiol. C Photochem. Rev. 2010, 11, 157–178. [Google Scholar] [CrossRef]
- Ohtani, B. Titania photocatalysis beyond recombination: A critical review. Catalysts 2013, 3, 942–953. [Google Scholar] [CrossRef]
- Ola, O.; Maroto-Valer, M.M. Review of material design and reactor engineering on TiO2 photocatalysis for CO2 reduction. J. Photochem. Photobiol. C Photochem. Rev. 2015, 24, 16–42. [Google Scholar] [CrossRef]
- Lodgejr, J. WHO regional publication, air quality guidelines for Europe. Environ. Sci. Pollut. Res. 1996, 23. [Google Scholar] [CrossRef]
- World Health Organization Regional Office for Europe Copenhagen. Air Quality Guidelines for Europe, 2nd ed.; WHO: Geneva, Switzerland, 2000; Volume 35, pp. 812–815. [Google Scholar]
- Fleischer, N.L.; Merialdi, M.; van Donkelaar, A.; Vadillo-Ortega, F.; Martin, R.V.; Betran, A.P.; Souza, J.P.; O’Neill, M.S. Outdoor air pollution, preterm birth, and low birth weight: Analysis of the world health organization global survey on maternal and perinatal health. Environ. Health Perspect. 2014, 122, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Hüsken, G.; Hunger, M.; Brouwers, H.J.H. Experimental study of photocatalytic concrete products for air purification. Build. Environ. 2009, 44, 2463–2474. [Google Scholar] [CrossRef]
- Olmo, N.R.S.; Saldiva, P.H.N.; Braga, A.L.F.; Lin, C.A.; Santos, U.P.; Pereirai, L.A.A. A review of low-level air pollution and adverse effects on human health: Implications for epidemiological studies and public policy. Clinics 2011, 66, 681–690. [Google Scholar] [CrossRef] [PubMed]
- European Union. European council directive 1999/30/EC of 22 April 1999 relating to limit values for sulphur dioxide, nitrogen dioxide and oxides of nitrogen, particulate matter and lead in ambient air C directive. Off. J. Eur. Commun. 1999, 41–60. [Google Scholar]
- Olivier, J.G.J.; Peters, J.A.H.W. Trends in Global CO2 and Total Greenhouse Gas Emissions; 2019 Report; Netherlands Environmental Assessment Agency: Hague, The Netherlands, 2012; pp. 12–28. [Google Scholar]
- Gautam, D.; Bolia, N.B. Air pollution: Impact and interventions. Air Qual. Atmos. Health 2020, 13, 209–223. [Google Scholar] [CrossRef]
- Ni, M.; Leung, M.K.H.; Leung, D.Y.C.; Sumathy, K. A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production. Renew. Sustain. Energy Rev. 2007, 11, 401–425. [Google Scholar] [CrossRef]
- Leong, S.; Razmjou, A.; Wang, K.; Hapgood, K.; Zhang, X.; Wang, H. TiO2 based photocatalytic membranes: A review. J. Memb. Sci. 2014, 472, 167–184. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, X. Photocatalytic oxidation for indoor air purification: A literature review. Build. Environ. 2003, 38, 645–654. [Google Scholar] [CrossRef]
- Ohko, Y.; Utsumi, Y.; Niwa, C.; Tatsuma, T.; Kobayakawa, K.; Satoh, Y.; Kubota, Y.; Fujishima, A. Self-sterilizing and self-cleaning of silicone catheters coated with TiO2 photocatalyst thin films: A preclinical work. J. Biomed. Mater. Res. 2001, 58, 97–101. [Google Scholar] [CrossRef]
- Sarkar, J.; Bhattacharyya, S. Status and outlook of sensitizers/dyes used in dye sensitized solar cells (DSSC): A review. Int. J. Energy Res. 2012, 33, 23–40. [Google Scholar] [CrossRef]
- Shakeel, A.M.; Pandey, A.K.; Abd-Rahim, N. Advancements in the development of TiO2 photoanodes and its fabrication methods for dye sensitized solar cell (DSSC) applications. A review. Renew. Sustain. Energy Rev. 2017, 77, 89–108. [Google Scholar] [CrossRef]
- Green, M.A. Thin-film solar cells: Review of materials, technologies and commercial status. J. Mater. Sci. Mater. Electron. 2007, 18, 15–19. [Google Scholar] [CrossRef]
- Vittal, R.; Ho, K.C. Zinc oxide based dye-sensitized solar cells: A review. Renew. Sustain. Energy Rev. 2017, 70, 920–935. [Google Scholar] [CrossRef]
- Prabavathy, N.; Shalini, S.; Balasundaraprabhu, R.; Velauthapillai, D.; Prasanna, S.; Muthukumarasamy, N. Enhancement in the photostability of natural dyes for dye-sensitized solar cell (DSSC) applications: A review. Int. J. Energy Res. 2017, 41, 1372–1396. [Google Scholar] [CrossRef]
- Kumara, N.T.R.N.; Lim, A.; Lim, C.M.; Petra, M.I.; Ekanayake, P. Recent progress and utilization of natural pigments in dye sensitized solar cells: A review. Renew. Sustain. Energy Rev. 2017, 78, 301–317. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Ma, D.K.; Zhang, Y.G.; Chen, W.; Huang, S.M. N-doped carbon quantum dots for TiO2-based photocatalysts and dye-sensitized solar cells. Nano Energy 2013, 2, 545–552. [Google Scholar] [CrossRef]
- Xu, J.; Ao, Y.; Fu, D.; Yuan, C. Low-temperature preparation of F-doped TiO2 film and its photocatalytic activity under solar light. Appl. Surf. Sci. 2008, 254, 3033–3038. [Google Scholar] [CrossRef]
- Zhang, X.; Thavasi, V.; Mhaisalkar, S.G.; Ramakrishna, S. Novel hollow mesoporous 1D TiO2 nanofibers as photovoltaic and photocatalytic materials. Nanoscale 2012, 4, 1707–1716. [Google Scholar] [CrossRef]
- Yang, R.; Cai, J.; Lv, K.; Wu, X.; Wang, W.; Xu, Z.; Li, M.; Li, Q.; Xu, W. Fabrication of TiO2 hollow microspheres assembly from nanosheets (TiO2-HMSs-NSs) with enhanced photoelectric conversion efficiency in DSSCs and photocatalytic activity. Appl. Catal. B Environ. 2017, 210, 184–193. [Google Scholar] [CrossRef]
- Canevali, C.; Polizzi, S.; Testino, A.; Bellobono, I.R.; Buscaglia, V. Optimizing the photocatalytic properties of hydrothermal TiO2 by the control of phase composition and particle morphology. A systematic approach. J. Am. Chem. Soc. 2007, 129, 3564–3575. [Google Scholar] [CrossRef]
- Nam, W.S.; Han, G.Y. A photocatalytic performance of TiO2 photocatalyst prepared by the hydrothermal method. Korean J. Chem. Eng. 2003, 20, 180–184. [Google Scholar] [CrossRef]
- Kolahalam, L.A.; Kasi Viswanath, I.V.; Diwakar, B.S.; Govindh, B.; Reddy, V.; Murthy, Y.L.N. Review on nanomaterials: Synthesis and applications. Mater. Today Proc. 2019, 18, 2182–2190. [Google Scholar] [CrossRef]
- Yu, H.; Wang, J.; Yan, X.; Wang, J.; Cheng, P.; Xia, C.J. Effect of surfactants on the morphology and photocatocatalytic properties of ZnO nanostructures. Optik 2019, 185, 990–996. [Google Scholar] [CrossRef]
- Zielińska-Jurek, A.; Wei, Z.; Janczarek, M.; Wysocka, I.; Kowalska, E. Size-controlled synthesis of Pt particles on TiO2 surface: Physicochemical characteristic and photocatalytic activity. Catalysts 2019, 8, 940. [Google Scholar] [CrossRef]
- Janczarek, M.; Kowalska, E. On the origin of enhanced photocatalytic activity of copper-modified titania in the oxidative reaction systems. Catalysts 2017, 7, 317. [Google Scholar] [CrossRef]
- Hu, J.; Li, H.; Muhammad, S.; Wu, Q.; Zhao, Y.; Jiao, Q. Surfactant-assisted hydrothermal synthesis of TiO2/reduced graphene oxide nanocomposites and their photocatalytic performances. J. Solid State Chem. 2017, 253, 113–120. [Google Scholar] [CrossRef]
- Haider, A.J.; Jameel, Z.N.; Al-Hussaini, I.H.M. Review on: Titanium dioxide applications. Energy Proc. 2019, 157, 17–29. [Google Scholar] [CrossRef]
- Noman, M.T.; Ashraf, M.A.; Ali, A. Synthesis and applications of nano-TiO2: A review. Environ. Sci. Pollut. Res. 2019, 26, 3262–3291. [Google Scholar] [CrossRef]
- Wang, F.; Shi, Z.; Gong, F.; Jiu, J.; Adachi, M. Morphology control of anatase TiO2 by surfactant-assisted hydrothermal method. Chin. J. Chem. Eng. 2007, 15, 754–759. [Google Scholar] [CrossRef]
- Galkina, O.L.; Vinogradov, V.V.; Agafonov, A.V.; Vinogradov, A.V. Surfactant-assisted sol-gel synthesis of TiO2 with uniform particle size distribution. Int. J. Inorg. Chem. 2011, 2011, 1–8. [Google Scholar] [CrossRef]
- Chan, C.; Chang, C.; Hsu, W.; Wang, S.; Lin, J. Photocatalytic activities of Pd-loaded mesoporous TiO2 thin films. Chem. Eng. J. 2009, 152, 492–497. [Google Scholar] [CrossRef]
- Yuenyongsuwan, J.; Nithiyakorn, N.; Sabkird, P.; O’Rear, E.A.; Pongprayoon, T. Surfactant effect on phase-controlled synthesis and photocatalyst property of TiO2 nanoparticles. Mater. Chem. Phys. 2018, 214, 330–336. [Google Scholar] [CrossRef]
- Jun, Y.W.; Casula, M.F.; Sim, J.H.; Kim, S.Y.; Cheon, J.; Alivisatos, A.P. Surfactant-assisted elimination of a high energy facet as a means of controlling the shapes of TiO2 nanocrystals. J. Am. Chem. Soc. 2003, 125, 15981–15985. [Google Scholar] [CrossRef] [PubMed]
- Jang, I.; Leong, H.J.; Oh, S.G. Effects of surfactants on the preparation of TiO2 nanoparticles in microwave-assisted sol-gel process and their photocatalytic activity. Korean J. Chem. Eng. 2016, 33, 1647–1652. [Google Scholar] [CrossRef]
- Yener, H.B.; Şarkaya, S.; Helvacı, Ş.Ş. Nano-sized TiO2 synthesis in triton X-100 reverse micelles. In Trends in Colloid and Interface Science XXIII. Progress in Colloid and Polymer Science; Springer: Berlin, Germany, 2010; Volume 137. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.S.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Abdolahi-Sadatlu, M.A.; Mozaffari, N. Synthesis of mesoporous TiO2 structures through P123 copolymer as the structural directing agent and assessment of their performance in dye-sensitized solar cells. Sol. Energy 2016, 133, 24–34. [Google Scholar] [CrossRef]
- Liu, J.; An, T.; Li, G.; Bao, N.; Sheng, G.; Fu, J. Preparation and characterization of highly active mesoporous TiO2 photocatalysts by hydrothermal synthesis under weak acid conditions. Micropor. Mesopor. Mater. 2009, 124, 197–203. [Google Scholar] [CrossRef]
- Rathouský, J.; Slabová, M.; Macounová, K.; Zukal, A. Organized mesoporous titanium dioxide—A powerful photocatalyst for the removal of water pollutants. Stud. Surf. Sci. Catal. 2002, 141, 599–606. [Google Scholar] [CrossRef]
- Guo, W.; Li, H.; Ma, H.; Teng, W. Different surfactants-assisted hydrothermal fabrication and photocatalytic properties of Bi2MoO6 for methylene blue degradation under simulated sunlight irradiation. J. Chem. 2014, 2014, 2–7. [Google Scholar] [CrossRef]
- Suthakaran, S.; Dhanapandian, S.; Krishnakumar, N.; Ponpandian, N. Surfactants assisted SnO2 nanoparticles synthesized by a hydrothermal approach and potential applications in water purification and energy conversion. J. Mater. Sci. Mater. Electron. 2019, 30, 13174–13190. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Pigłowska, M.; Kurc, B.; Rymaniak, Ł.; Lijewski, P.; Fuć, P. Kinetics and thermodynamics of thermal degradation of different starches and estimation the OH group and H2O content on the surface by TG/DTG-DTA. Polymers 2020, 12, 357. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Y.; Tu, K.J.; Deng, J.P.; Lo, Y.S.; Wu, C.H. Markedly enhanced surface hydroxyl groups of TiO2 nanoparticles with superior water-dispersibility for photocatalysis. Materials 2017, 10, 566. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhao, X.; Zhao, Q.; Wang, G. Preparation and characterization of super-hydrophilic porous TiO2 coating films. Mater. Chem. Phys. 2001, 68, 253–259. [Google Scholar] [CrossRef]
- World Health Organization; International Labour Organization; United Nations Environment Programme. Phenol Health and Safety Guide; WHO Environmental Health Criteria: Geneva, Switzerland, 1994; Volume 161, pp. 151–185. [Google Scholar]
- Znaidi, L.; Séraphimova, R.; Bocquet, J.F.; Colbeau-Justin, C.; Pommier, C. A semi-continuous process for the synthesis of nanosize TiO2 powders and their use as photocatalysts. Mater. Res. Bull. 2001, 36, 811–825. [Google Scholar] [CrossRef]
- Di Paola, A.; Bellardita, M.; Palmisano, L.; Barbieriková, Z.; Brezová, V. Influence of crystallinity and OH surface density on the photocatalytic activity of TiO2 powders. J. Photochem. Photobiol. A Chem. 2014, 273, 59–67. [Google Scholar] [CrossRef]
- Liu, L.; Liu, H.; Zhao, Y.P.; Wang, Y.; Duan, Y.; Gao, G.; Ge, M.; Chen, W. Directed synthesis of hierarchical nanostructured TiO2 catalysts and their morphology-dependent photocatalysis for phenol degradation. Environ. Sci. Technol. 2008, 42, 2342–2348. [Google Scholar] [CrossRef]
- Gadade, D.D.; Pekamwar, S.S.; Lahoti, S.R.; Patni, S.D.; Sarode, M.C. Cocrystallization of etodolac: Prediction of cocrystallization, synthesis, solid state characterization and in vitro drug release. Marmara Pharm. J. 2016, 21, 78–88. [Google Scholar] [CrossRef]
- Saxena, D.; Damale, S.; Datar, A. Investigation of forced degradation products of etodolac by LC and LC-MS/MS. Int. J. Pharm. Pharm. Sci. 2016, 8, 127–135. [Google Scholar]
- Mrotek, E.; Dudziak, S.; Malinowska, I.; Pelczarski, D.; Ryżyńska, Z.; Zielińska-Jurek, A. Improved degradation of etodolac in the presence of core-shell ZnFe2O4/SiO2/TiO2 magnetic photocatalyst. Sci. Total Environ. 2020, 724, 138167. [Google Scholar] [CrossRef]
- Li, H.; Zeng, Y.; Huang, T.; Piao, L.; Yan, Z.; Liu, M. Hierarchical TiO2 nanospheres with dominant {001} facets: Facile synthesis, growth mechanism, and photocatalytic activity. Chem. Eur. J. 2012, 18, 7525–7532. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Yu, J.C.; Fu, X. Characterization and photocatalytic mechanism of nanosized CdS coupled TiO2 nanocrystals under visible light irradiation. J. Mol. Catal. Chem. 2006, 244, 25–32. [Google Scholar] [CrossRef]
- Augugliaro, V.; Coluccia, S.; Loddo, V.; Marchese, L.; Martra, G.; Palmisano, L.; Schiavello, M. Photocatalytic oxidation of gaseous toluene on anatase TiO2 catalyst: Mechanistic aspects and FT-IR investigation. Appl. Catal. B Environ. 1999, 20, 15–27. [Google Scholar] [CrossRef]
- Busca, G.; Berardinelli, S.; Resini, C.; Arrighi, L. Technologies for the removal of phenol from fluid streams: A short review of recent developments. J. Hazard. Mater. 2008, 160, 265–288. [Google Scholar] [CrossRef] [PubMed]
- Atitar, M.F.; Ismail, A.A.; Dillert, R.; Bahnemann, D.W. Photodegradation of herbicide imazapyr and phenol over mesoporous bicrystalline phases TiO2: A kinetic study. Catalysts 2019, 9, 640. [Google Scholar] [CrossRef]
- Colón, G.; Hidalgo, M.C.; Navío, J.A. Photocatalytic behaviour of sulphated TiO2 for phenol degradation. Appl. Catal. B Environ. 2003, 45, 39–50. [Google Scholar] [CrossRef]
- Ahmed, S.; Rasul, M.G.; Martens, W.N.; Brown, R.; Hashib, M.A. Heterogeneous photocatalytic degradation of phenols in wastewater: A review on current status and developments. Desalination 2010, 261, 3–18. [Google Scholar] [CrossRef]
- Hadj-Salah, N.; Bouhelassa, M.; Bekkouche, S.; Boultif, A. Study of photocatalytic degradation of phenol. Desalination 2004, 166, 347–354. [Google Scholar] [CrossRef]
- Neves, M.C.; Nogueira, J.M.F.; Trindade, T.; Mendonça, M.H.; Pereira, M.I.; Monteiro, O.C. Photosensitization of TiO2 by Ag2S and its catalytic activity on phenol photodegradation. J. Photochem. Photobiol. A Chem. 2009, 204, 168–173. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Boschloo, G.; Sun, L.; Kloo, L.; Pettersson, H. Dye-sensitized solar cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef]
- Subramanian, A.; Wang, H.W. Effect of hydroxyl group attachment on TiO2 films for dye-sensitized solar cells. Appl. Surf. Sci. 2012, 258, 7833–7838. [Google Scholar] [CrossRef]
- Jalali, M.; Moakhar, R.S.; Kushwaha, A.; Goh, G.K.L.; Sadrnezhaad, S.K.; Riahi-Noori, N. TiO2 surface nanostructuring for improved dye loading and light scattering in double-layered screen-printed dye-sensitized solar cells. J. Appl. Electrochem. 2015, 45, 831–838. [Google Scholar] [CrossRef]
- Sarker, S.; Ahammad, A.J.S.; Seo, H.W.; Kim, D.M. Electrochemical impedance spectra of dye-sensitized solar cells: Fundamentals and spreadsheet calculation. Int. J. Photoenergy 2014, 2014. [Google Scholar] [CrossRef]
- Adachi, M.; Sakamoto, M.; Jiu, J.; Ogata, Y.; Isoda, S. Determination of parameters of electron transport in dye-sensitized solar cells using electrochemical impedance spectroscopy. J. Phys. Chem. B 2006, 110, 13872–13880. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Yin, L.; Liu, H.; Ding, Y. Nanograss-structured counter electrode for dye-sensitized solar cells. J. Power Sources 2012, 218, 405–411. [Google Scholar] [CrossRef]
- Fakharuddin, A.; Ahmed, I.; Khalidin, Z.; Yusoff, M.M.; Jose, R. Charge transport through split photoelectrodes in dye-sensitized solar cells. J. Appl. Phys. 2014, 115, 164509. [Google Scholar] [CrossRef]
- Mozer, A.J.; Wagner, P.; Officer, D.L.; Wallace, G.G.; Campbell, W.M.; Miyashita, M.; Sunahara, K.; Mori, S. The origin of open circuit voltage of porphyrin-sensitised TiO2 solar cells. Chem. Commun. 2008, 4741–4743. [Google Scholar] [CrossRef] [PubMed]
- Randazzo, B.; Chemello, G.; Tortarolo, I.; Chiarello, G.L.; Zalas, M.; Santini, A.; Liberatore, M.; Liberatore, M.; Selli, E.; Olivotto, I. A novel photocatalytic purification system for fish culture. Zebrafish 2017, 14, 411–421. [Google Scholar] [CrossRef]
- Zalas, M.; Jelak, K. Optimization of platinum precursor concentration for new, fast and simple fabrication method of counter electrode for DSSC application. Optik 2020, 206, 164314. [Google Scholar] [CrossRef]
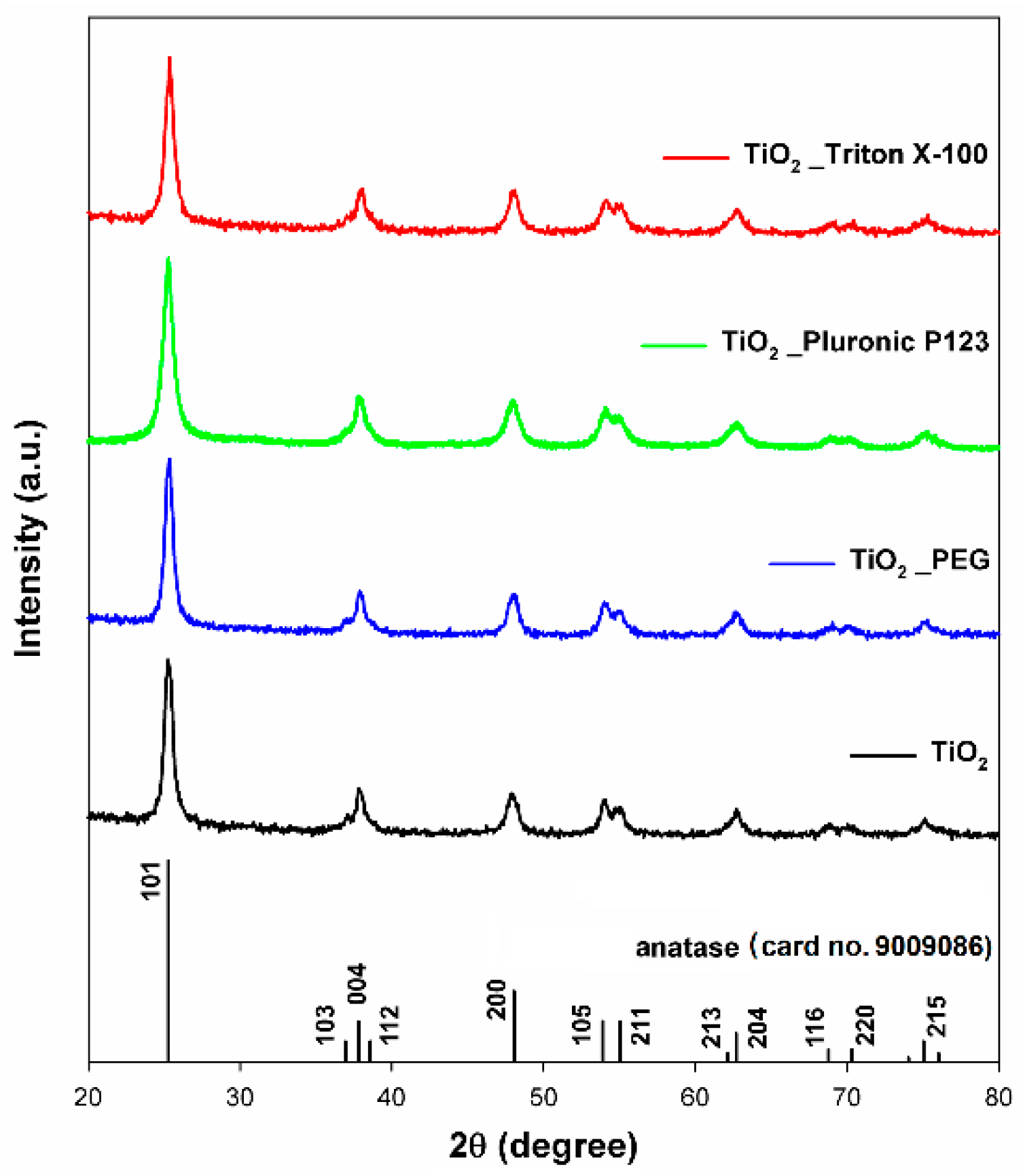


| Sample | Crystallite Size (nm) | Crystalline Phase of Anatase (%) |
|---|---|---|
| TiO2 | 15.2 (±0.1) | 100 |
| TiO2_PEG | 16.9 (±0.3) | |
| TiO2_Pluronic P123 | 9.8 (±0.1) | |
| TiO2_Triton X−100 | 17.4 (±0.2) |
| Sample | ABET (m2/g) | Vp (cm3/g) | Sp (nm) |
|---|---|---|---|
| TiO2 | 118 | 0.35 | 8.6 |
| TiO2_PEG | 128 | 0.31 | 8.7 |
| TiO2_Pluronic P123 | 200 | 0.21 | 5.1 |
| TiO2_Triton X−100 | 138 | 0.28 | 7.9 |
| Sample | TGA (%) | nOH (mmol/g TiO2) |
|---|---|---|
| TiO2 | 4.53 | 0.49 |
| TiO2_PEG | 5.01 | 0.58 |
| TiO2_Pluronic P123 | 10.67 | 1.21 |
| TiO2_Triton X−100 | 5.57 | 0.70 |
| Sample | Jsc (mA/cm2) | Voc (mV) | FF (%) | η (%) | Ndye (10−8 mol/cm2) |
|---|---|---|---|---|---|
| TiO2 | 12.0 | 729 | 70.3 | 6.16 | 3.31 |
| TiO2_PEG | 10.9 | 710 | 70.5 | 5.46 | 3.34 |
| TiO2_Pluronic P123 | 10.3 | 726 | 71.3 | 5.32 | 2.39 |
| TiO2_Triton X−100 | 13.5 | 734 | 69.1 | 6.84 | 3.41 |
| Sample | R1 (Ω) | R2 (Ω) | RCT (Ω) | τ (ms) |
|---|---|---|---|---|
| TiO2 | 19.2 | 7.3 | 36.8 | 12.8 |
| TiO2_PEG | 18.0 | 9.1 | 39.0 | 12.8 |
| TiO2_Pluronic P123 | 19.9 | 7.8 | 40.0 | 12.8 |
| TiO2_Triton_X−100 | 23.4 | 9.6 | 33.8 | 12.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubiak, A.; Bielan, Z.; Bartkowiak, A.; Gabała, E.; Piasecki, A.; Zalas, M.; Zielińska-Jurek, A.; Janczarek, M.; Siwińska-Ciesielczyk, K.; Jesionowski, T. Synthesis of Titanium Dioxide via Surfactant-Assisted Microwave Method for Photocatalytic and Dye-Sensitized Solar Cells Applications. Catalysts 2020, 10, 586. https://doi.org/10.3390/catal10050586
Kubiak A, Bielan Z, Bartkowiak A, Gabała E, Piasecki A, Zalas M, Zielińska-Jurek A, Janczarek M, Siwińska-Ciesielczyk K, Jesionowski T. Synthesis of Titanium Dioxide via Surfactant-Assisted Microwave Method for Photocatalytic and Dye-Sensitized Solar Cells Applications. Catalysts. 2020; 10(5):586. https://doi.org/10.3390/catal10050586
Chicago/Turabian StyleKubiak, Adam, Zuzanna Bielan, Aleksandra Bartkowiak, Elżbieta Gabała, Adam Piasecki, Maciej Zalas, Anna Zielińska-Jurek, Marcin Janczarek, Katarzyna Siwińska-Ciesielczyk, and Teofil Jesionowski. 2020. "Synthesis of Titanium Dioxide via Surfactant-Assisted Microwave Method for Photocatalytic and Dye-Sensitized Solar Cells Applications" Catalysts 10, no. 5: 586. https://doi.org/10.3390/catal10050586
APA StyleKubiak, A., Bielan, Z., Bartkowiak, A., Gabała, E., Piasecki, A., Zalas, M., Zielińska-Jurek, A., Janczarek, M., Siwińska-Ciesielczyk, K., & Jesionowski, T. (2020). Synthesis of Titanium Dioxide via Surfactant-Assisted Microwave Method for Photocatalytic and Dye-Sensitized Solar Cells Applications. Catalysts, 10(5), 586. https://doi.org/10.3390/catal10050586











