Promoting Effect of Ti Species in MnOx-FeOx/Silicalite-1 for the Low-Temperature NH3-SCR Reaction
Abstract
1. Introduction
2. Results and Discussion
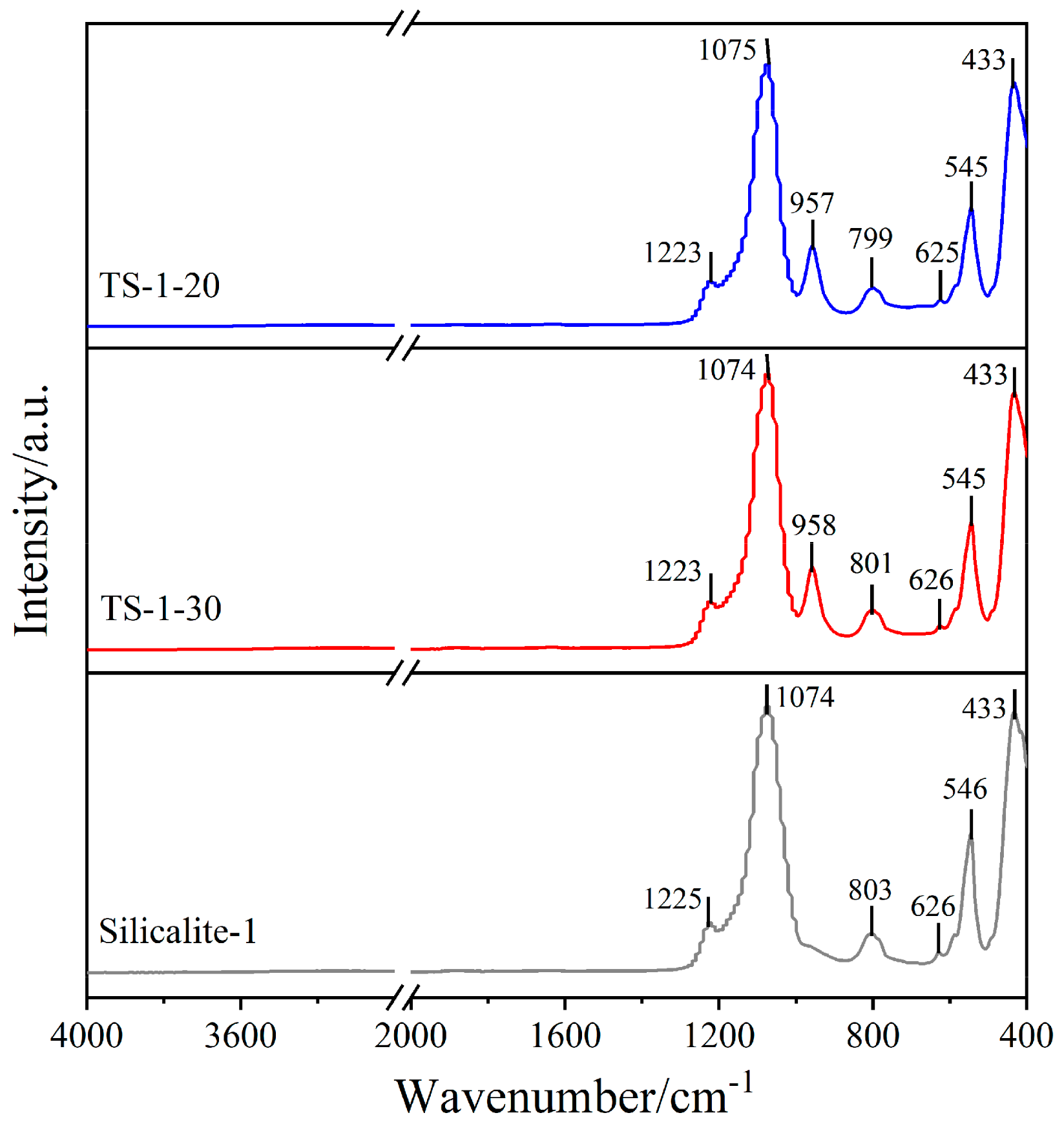
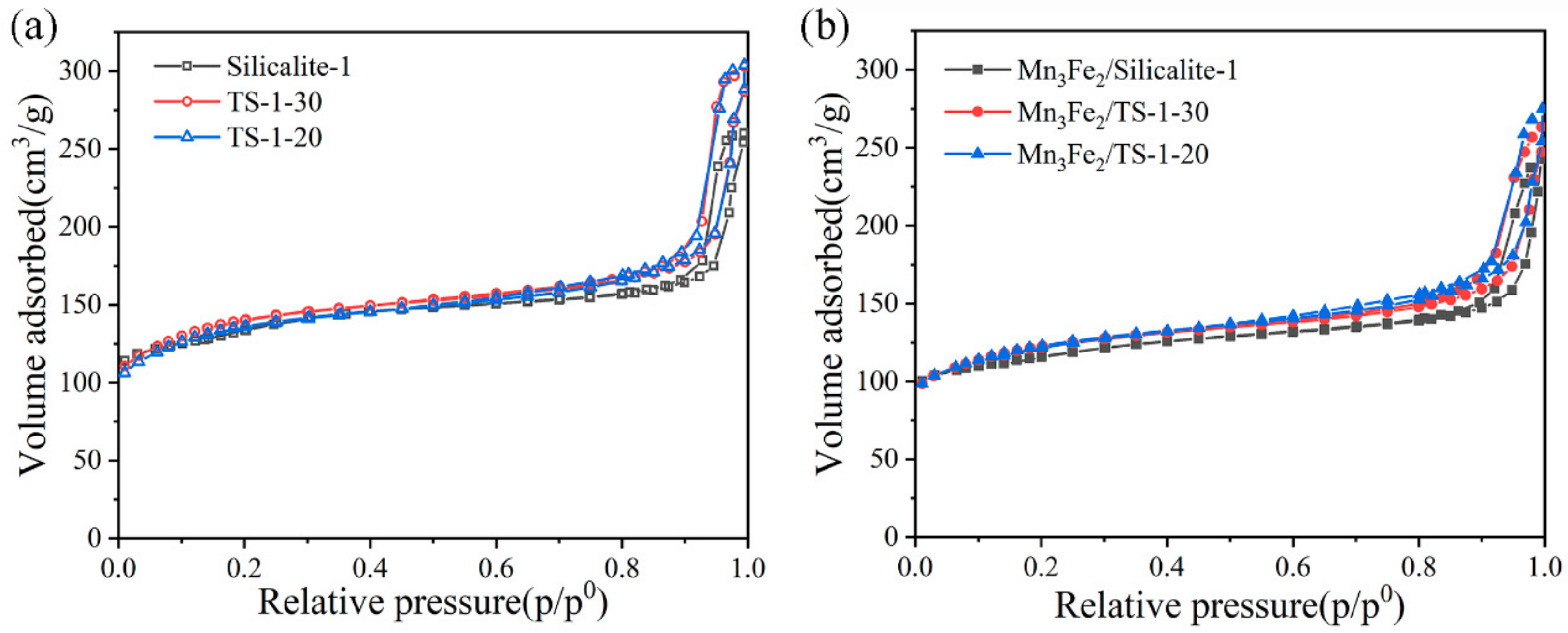
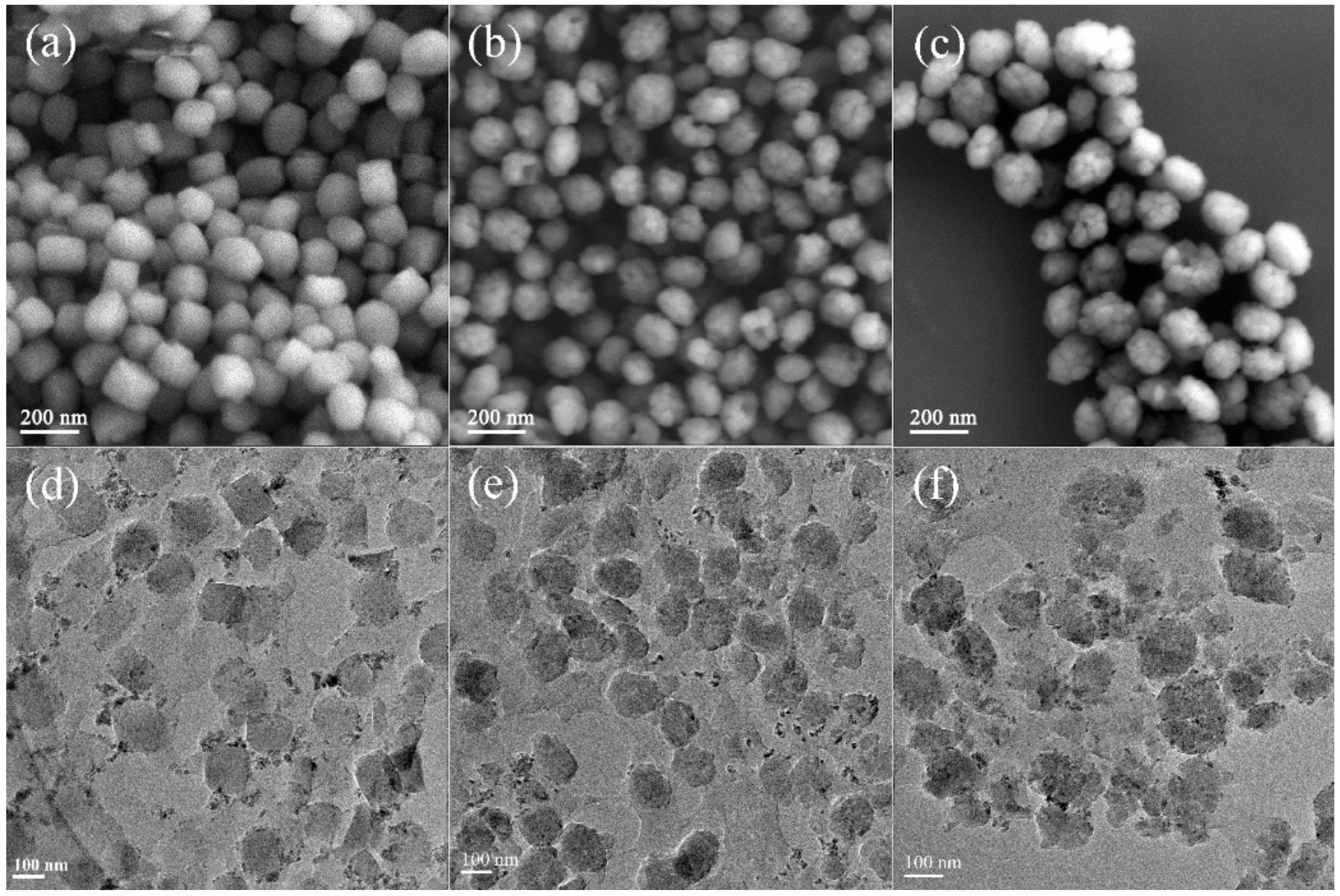
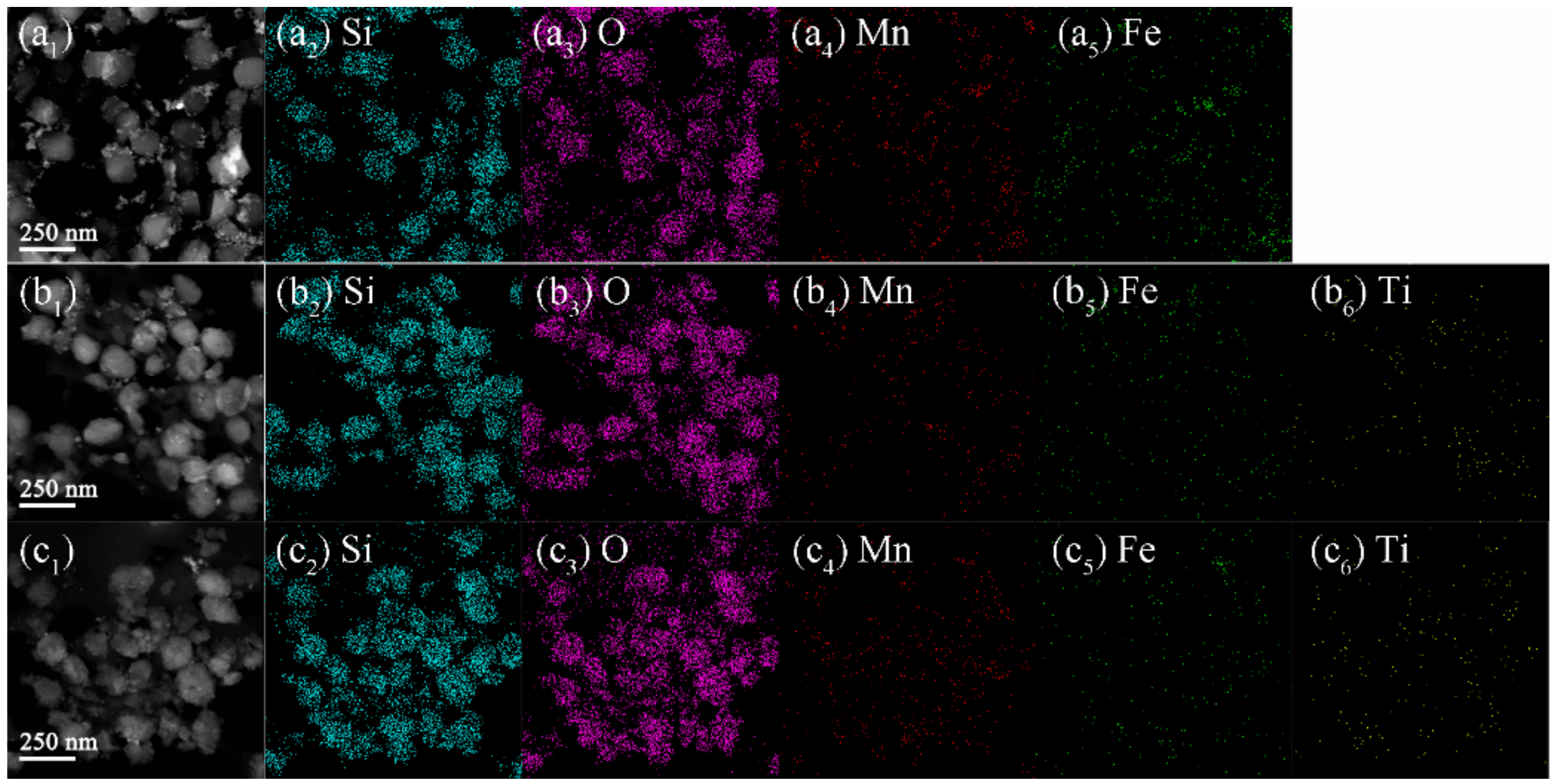
2.1. Chemical Compositions and Textural Properties of Samples
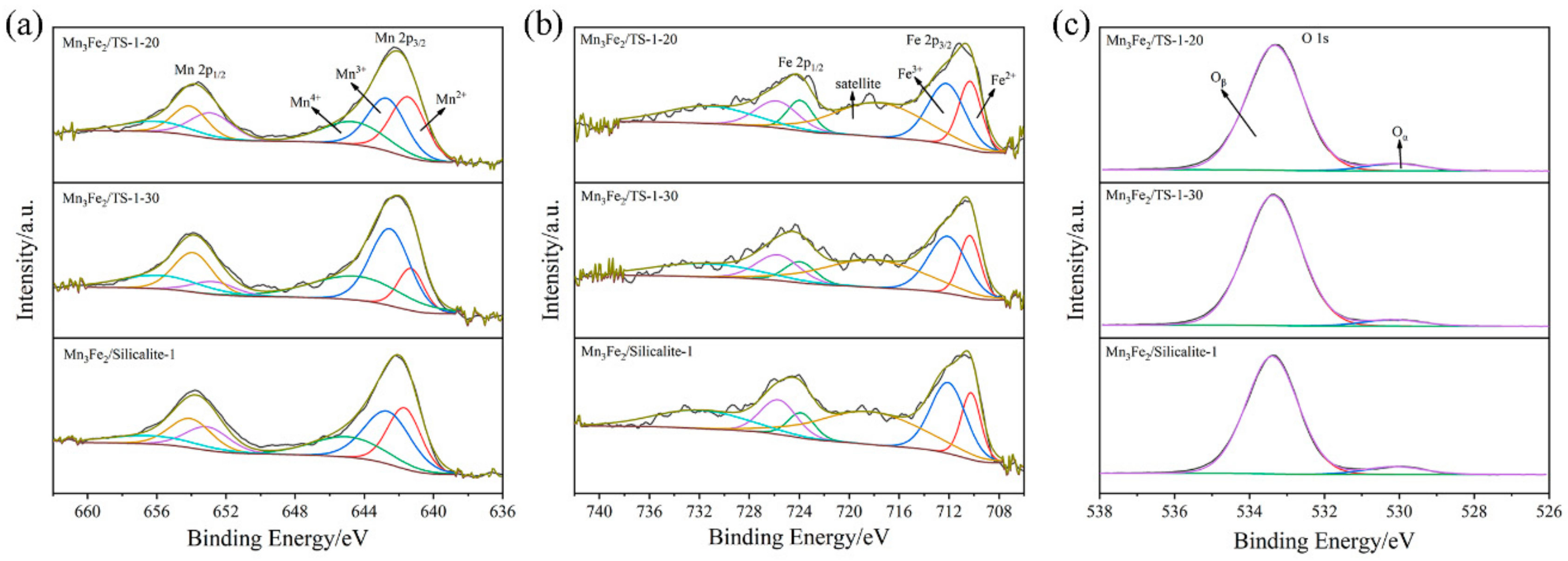
2.2. Surface Constituent and Chemical States of Samples
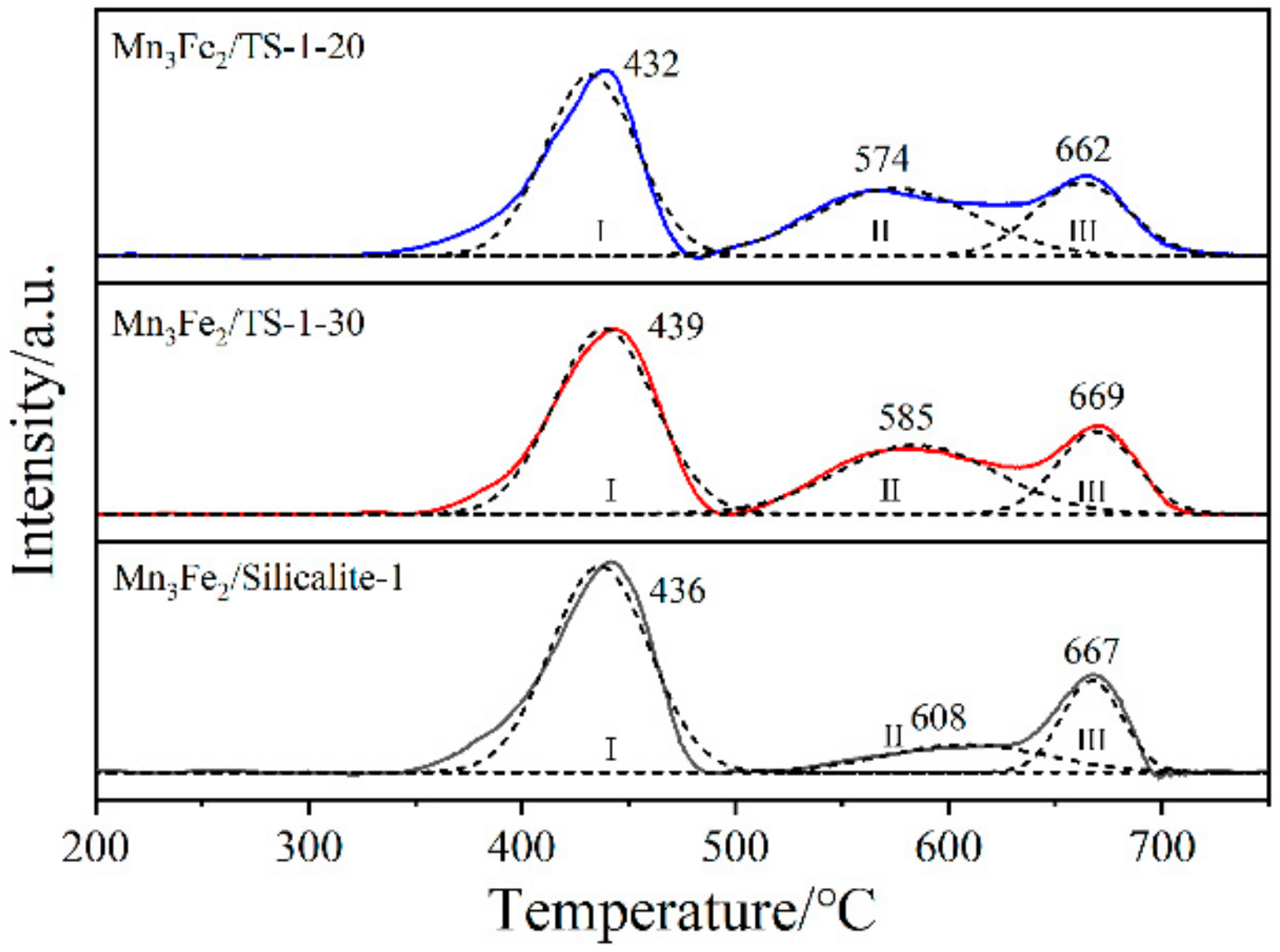
2.3. Redox Properties of Samples
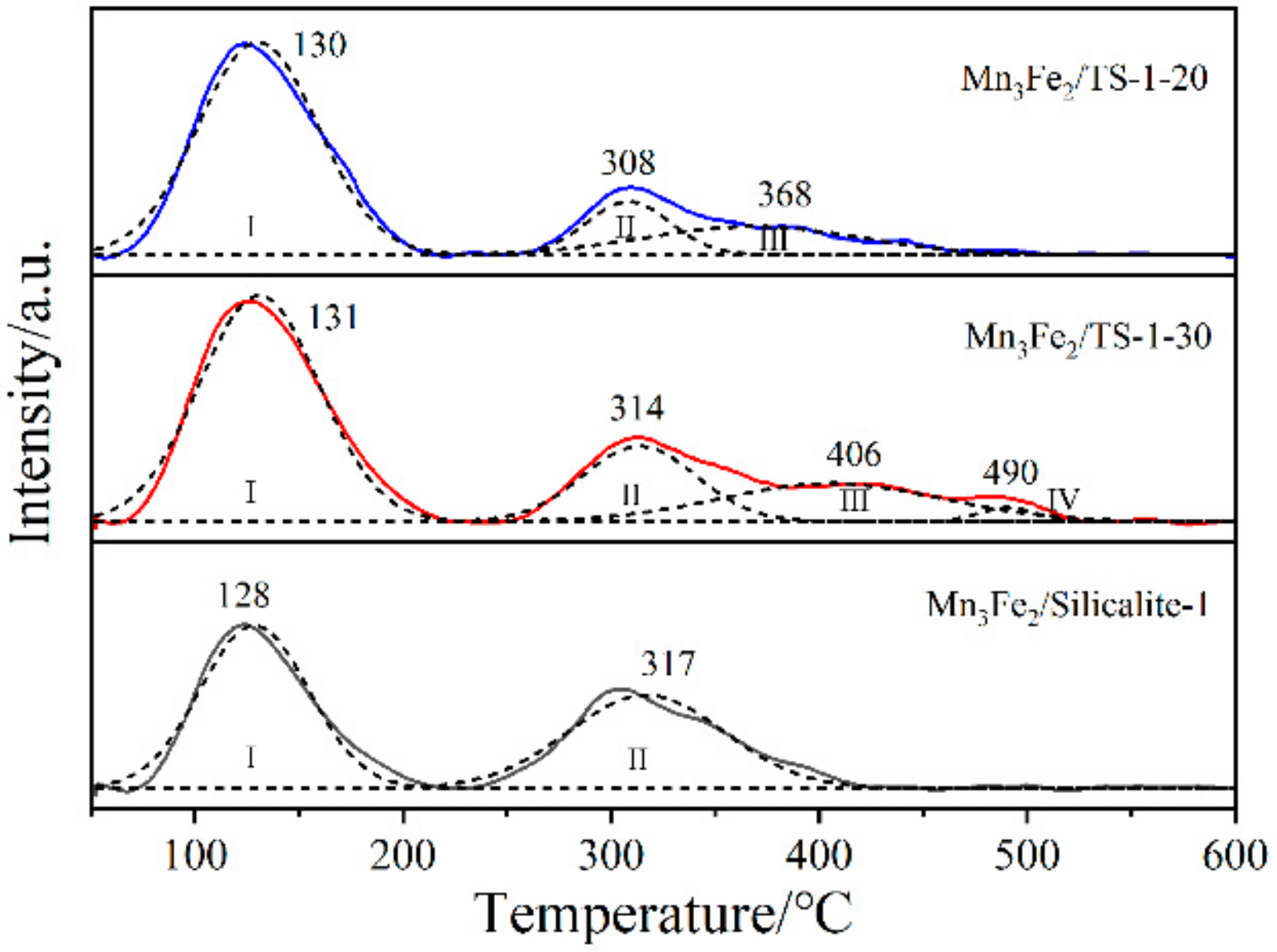
2.4. Surface Acidity of Samples
2.5. Catalytic Performance of Samples
3. Materials and Methods
3.1. Preparation of Samples
3.2. Characterization
3.3. NH3-SCR Activity Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Busca, G.; Lietti, L.; Ramis, G.; Berti, F. Chemical and mechanistic aspects of the selective catalytic reduction of NOx by ammonia over oxide catalysts: A review. Appl. Catal. B Environ. 1998, 18, 1–36. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Wu, Z. Highly active NbOPO4 supported Cu-Ce catalyst for NH3-SCR reaction with superior sulfur resistance. Chem. Eng. J. 2020, 382, 122941. [Google Scholar] [CrossRef]
- Paolucci, C.; Khurana, I.; Parekh, A.A.; Li, S.; Shih, A.J.; Li, H.; Di Iorio, J.R.; Albarracin-Caballero, J.D.; Yezerets, A.; Miller, J.T.; et al. Dynamic multinuclear sites formed by mobilized copper ions in NOx selective catalytic reduction. Science 2017, 357, 898–903. [Google Scholar] [CrossRef]
- Gillot, S.; Tricot, G.; Vezin, H.; Dacquin, J.-P.; Dujardin, C.; Granger, P. Development of stable and efficient CeVO4 systems for the selective reduction of NOx by ammonia: Structure-activity relationship. Appl. Catal. B Environ. 2017, 218, 338–348. [Google Scholar] [CrossRef]
- Mou, X.; Zhang, B.; Li, Y.; Yao, L.; Wei, X.; Su, D.S.; Shen, W. Rod-Shaped Fe2O3 as an Efficient Catalyst for the Selective Reduction of Nitrogen Oxide by Ammonia. Angew. Chem. Int. Ed. 2012, 51, 2989. [Google Scholar] [CrossRef]
- Chen, L.; Si, Z.; Wu, X.; Weng, D. DRIFT study of CuO-CeO2-TiO2 mixed oxides for NOx reduction with NH3 at low temperatures. ACS Appl. Mater. Interfaces 2014, 6, 8134–8145. [Google Scholar] [CrossRef]
- Gao, F.; Tang, X.; Yi, H.; Li, J.; Zhao, S.; Wang, J.; Chu, C.; Li, C. Promotional mechanisms of activity and SO2 tolerance of Co- or Ni-doped MnOx-CeO2 catalysts for SCR of NOx with NH3 at low temperature. Chem. Eng. J. 2017, 317, 20–31. [Google Scholar] [CrossRef]
- Fang, C.; Zhang, D.; Cai, S.; Zhang, L.; Huang, L.; Li, H.; Maitarad, P.; Shi, L.; Gao, R.; Zhang, J. Low-temperature selective catalytic reduction of NO with NH3 over nanoflaky MnOx on carbon nanotubes in situ prepared via a chemical bath deposition route. Nanoscale 2013, 5, 9199. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, Z.; Liu, L.; Mi, L.; Wang, X. In situ DRIFTS studies on MnOx nanowires supported by activated semi-coke for low temperature selective catalytic reduction of NOx with NH3. Appl. Surf. Sci. 2016, 366, 139–147. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, T.; Liu, H.; Chen, D.; Xu, B.; Qing, C. Low temperature SCR reaction over Nano-Structured Fe-Mn Oxides: Characterization, performance, and kinetic study. Appl. Surf. Sci. 2018, 457, 1116–1125. [Google Scholar] [CrossRef]
- Yang, J.; Ren, S.; Zhang, T.; Su, Z.; Long, H.; Kong, M.; Yao, L. Iron doped effects on active sites formation over activated carbon supported Mn-Ce oxide catalysts for low-temperature SCR of NO. Chem. Eng. J. 2020, 379, 122398. [Google Scholar] [CrossRef]
- Pan, W.-G.; Hong, J.-N.; Guo, R.-T.; Zhen, W.-L.; Ding, H.-L.; Jin, Q.; Ding, C.-G.; Guo, S.-Y. Effect of support on the performance of Mn–Cu oxides for low temperature selective catalytic reduction of NO with NH3. J. Ind. Eng. Chem. 2014, 20, 2224–2227. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, F.; Li, H.; Yang, Q.; Wang, L.; Li, X. Low-Temperature Selective Catalytic Reduction of NOx with NH3 over Fe–Mn Mixed-Oxide Catalysts Containing Fe3Mn3O8 Phase. Ind. Eng. Chem. Res. 2011, 51, 202–212. [Google Scholar] [CrossRef]
- Liu, Z.; Yi, Y.; Zhang, S.; Zhu, T.; Zhu, J.; Wang, J. Selective catalytic reduction of NOx with NH3 over Mn-Ce mixed oxide catalyst at low temperatures. Catal. Today 2013, 216, 76–81. [Google Scholar] [CrossRef]
- Li, J.H.; Chang, H.Z.; Ma, L.; Hao, J.M.; Yang, R.T. Low-temperature selective catalytic reduction of NOx with NH3 over metal oxide and zeolite catalysts-A review. Catal. Today 2011, 175, 147–156. [Google Scholar] [CrossRef]
- Liu, C.; Shi, J.-W.; Gao, C.; Niu, C. Manganese oxide-based catalysts for low-temperature selective catalytic reduction of NOx with NH3: A review. Appl. Catal. A Gen. 2016, 522, 54–69. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, L.; Wang, X.; Zou, W.; Cao, Y.; Sun, J.; Tang, C.; Gao, F.; Deng, Y.; Dong, L. Synthesis, characterization and catalytic performance of FeMnTiOx mixed oxides catalyst prepared by a CTAB-assisted process for mid-low temperature NH3-SCR. Appl. Catal. A Gen. 2015, 505, 235–242. [Google Scholar] [CrossRef]
- Cao, F.; Su, S.; Xiang, J.; Wang, P.; Hu, S.; Sun, L.; Zhang, A. The activity and mechanism study of Fe–Mn–Ce/γ-Al2O3 catalyst for low temperature selective catalytic reduction of NO with NH3. Fuel 2015, 139, 232–239. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J.; Yan, Z.; Liu, L.; Zhang, Z.; Wang, X. Promoting effect of Nd on the reduction of NO with NH3 over CeO2 supported by activated semi-coke: An in situ DRIFTS study. Catal. Sci. Technol. 2015, 5, 2251–2259. [Google Scholar] [CrossRef]
- Wang, J.; Yan, Z.; Liu, L.; Chen, Y.; Zhang, Z.; Wang, X. In situ DRIFTS investigation on the SCR of NO with NH3 over V2O5 catalyst supported by activated semi-coke. Appl. Surf. Sci. 2014, 313, 660–669. [Google Scholar] [CrossRef]
- Su, Y.; Fan, B.; Wang, L.; Liu, Y.; Huang, B.; Fu, M.; Chen, L.; Ye, D. MnOx supported on carbon nanotubes by different methods for the SCR of NO with NH3. Catal. Today 2013, 201, 115–121. [Google Scholar] [CrossRef]
- Lou, X.; Liu, P.; Li, J.; Li, Z.; He, K. Effects of calcination temperature on Mn species and catalytic activities of Mn/ZSM-5 catalyst for selective catalytic reduction of NO with ammonia. Appl. Surf. Sci. 2014, 307, 382–387. [Google Scholar] [CrossRef]
- Seo, C.-K.; Choi, B.; Kim, H.; Lee, C.-H.; Lee, C.-B. Effect of ZrO2 addition on de-NOx performance of Cu-ZSM-5 for SCR catalyst. Chem. Eng. J. 2012, 191, 331–340. [Google Scholar] [CrossRef]
- Lv, G.; Bin, F.; Song, C.; Wang, K.; Song, J. Promoting effect of zirconium doping on Mn/ZSM-5 for the selective catalytic reduction of NO with NH3. Fuel 2013, 107, 217–224. [Google Scholar] [CrossRef]
- Wang, T.; Wan, Z.; Yang, X.; Zhang, X.; Niu, X.; Sun, B. Promotional effect of iron modification on the catalytic properties of Mn-Fe/ZSM-5 catalysts in the Fast SCR reaction. Fuel Process. Technol. 2018, 169, 112–121. [Google Scholar] [CrossRef]
- Du, T.; Qu, H.; Liu, Q.; Zhong, Q.; Ma, W. Synthesis, activity and hydrophobicity of Fe-ZSM-5@silicalite-1 for NH3-SCR. Chem. Eng. J. 2015, 262, 1199–1207. [Google Scholar] [CrossRef]
- Vu, D.V.; Miyamoto, M.; Nishiyama, N.; Ichikawa, S.; Egashira, Y.; Ueyama, K. Catalytic activities and structures of silicalite-1/H-ZSM-5 zeolite composites. Microporous Mesoporous Mater. 2008, 115, 106–112. [Google Scholar] [CrossRef]
- Serrano, D.P.; Calleja, G.; Botas, J.A.; Gutierrez, F.J. Characterization of adsorptive and hydrophobic properties of silicalite-1, ZSM-5, TS-1 and Beta zeolites by TPD techniques. Sep. Purif. Technol. 2007, 54, 1–9. [Google Scholar] [CrossRef]
- Li, G.; Wang, X.; Guo, X.; Liu, S.; Zhao, Q.; Bao, X.; Lin, L. Titanium species in titanium silicalite TS-1 prepared by hydrothermal method. Mater. Chem. Phys. 2001, 71, 195–201. [Google Scholar] [CrossRef]
- Mu, W.; Zhu, J.; Zhang, S.; Guo, Y.; Su, L.; Li, X.; Li, Z. Novel proposition on mechanism aspects over Fe–Mn/ZSM-5 catalyst for NH3-SCR of NOx at low temperature: Rate and direction of multifunctional electron-transfer-bridge and in situ DRIFTs analysis. Catal. Sci. Technol. 2016, 6, 7532–7548. [Google Scholar] [CrossRef]
- Xia, Y.; Zhan, W.; Guo, Y.; Guo, Y.; Lu, G. Fe-Beta zeolite for selective catalytic reduction of NOx with NH3: Influence of Fe content. Chin. J. Catal. 2016, 37, 2069–2078. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, G.; Chen, H.; Zhang, G.; Han, Y.; Chang, Y.; Gong, P. Mn/beta and Mn/ZSM-5 for the low-temperature selective catalytic reduction of NO with ammonia: Effect of manganese precursors. Chin. J. Catal. 2018, 39, 118–127. [Google Scholar] [CrossRef]
- Cheng, K.; Liu, J.; Zhao, Z.; Wei, Y.; Jiang, G.; Duan, A. Direct synthesis of V–W–Ti nanoparticle catalysts for selective catalytic reduction of NO with NH3. RSC Adv. 2015, 5, 45172–45183. [Google Scholar] [CrossRef]
- Hu, H.; Cai, S.; Li, H.; Huang, L.; Shi, L.; Zhang, D. In Situ DRIFTs Investigation of the Low-Temperature Reaction Mechanism over Mn-Doped Co3O4 for the Selective Catalytic Reduction of NOx with NH3. J. Phys. Chem. C 2015, 119, 22924–22933. [Google Scholar] [CrossRef]
- Liu, F.; He, H.; Ding, Y.; Zhang, C. Effect of manganese substitution on the structure and activity of iron titanate catalyst for the selective catalytic reduction of NO with NH3. Appl. Catal. B Environ. 2009, 93, 194–204. [Google Scholar] [CrossRef]
- Reddy, A.; Gopinath, C.; Chilukuri, S. Selective ortho-methylation of phenol with methanol over copper manganese mixed-oxide spinel catalysts. J. Catal. 2006, 243, 278–291. [Google Scholar] [CrossRef]
- Xiong, Y.; Tang, C.; Yao, X.; Zhang, L.; Li, L.; Wang, X.; Deng, Y.; Gao, B.; Dong, L. Effect of metal ions doping (M=Ti4+, Sn4+) on the catalytic performance of MnOx/CeO2 catalyst for low temperature selective catalytic reduction of NO with NH3. Appl. Catal. A Gen. 2015, 495, 206–216. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, L.; Qu, H.; Zhong, Q. A study on chemisorbed oxygen and reaction process of Fe–CuOx/ZSM-5 via ultrasonic impregnation method for low-temperature NH3-SCR. J. Mol. Catal. A Chem. 2015, 409, 207–215. [Google Scholar] [CrossRef]
- Tan, P. Active phase, catalytic activity, and induction period of Fe/zeolite material in nonoxidative aromatization of methane. J. Catal. 2016, 338, 21–29. [Google Scholar] [CrossRef]
- Li, L.; Wu, Y.; Hou, X.; Chu, B.; Nan, B.; Qin, Q.; Fan, M.; Sun, C.; Li, B.; Dong, L.; et al. Investigation of Two-Phase Intergrowth and Coexistence in Mn–Ce–Ti–O Catalysts for the Selective Catalytic Reduction of NO with NH3: Structure–Activity Relationship and Reaction Mechanism. Ind. Eng. Chem. Res. 2018, 58, 849–862. [Google Scholar] [CrossRef]
- Meng, D.; Zhan, W.; Guo, Y.; Guo, Y.; Wang, L.; Lu, G. A Highly Effective Catalyst of Sm-MnOx for the NH3-SCR of NOx at Low Temperature: Promotional Role of Sm and Its Catalytic Performance. ACS Catal. 2015, 5, 5973–5983. [Google Scholar] [CrossRef]
- Yao, X.; Chen, L.; Cao, J.; Chen, Y.; Tian, M.; Yang, F.; Sun, J.; Tang, C.; Dong, L. Enhancing the deNO performance of MnOx/CeO2-ZrO2 nanorod catalyst for low-temperature NH3-SCR by TiO2 modification. Chem. Eng. J. 2019, 369, 46–56. [Google Scholar] [CrossRef]
- You, X.; Sheng, Z.; Yu, D.; Yang, L.; Xiao, X.; Wang, S. Influence of Mn/Ce ratio on the physicochemical properties and catalytic performance of graphene supported MnOx-CeO2 oxides for NH3-SCR at low temperature. Appl. Surf. Sci. 2017, 423, 845–854. [Google Scholar] [CrossRef]
- Zha, K.; Cai, S.; Hu, H.; Li, H.; Yan, T.; Shi, L.; Zhang, D. In Situ DRIFTs Investigation of Promotional Effects of Tungsten on MnOx-CeO2/meso-TiO2 Catalysts for NOx Reduction. J. Phys. Chem. C 2017, 121, 25243–25254. [Google Scholar] [CrossRef]
- Kang, M.; Park, E.D.; Kim, J.M.; Yie, J.E. Cu–Mn mixed oxides for low temperature NO reduction with NH3. Catal. Today 2006, 111, 236–241. [Google Scholar] [CrossRef]
- Chen, J.; Chen, X.; Chen, X.; Xu, W.; Xu, Z.; Jia, H.; Chen, J. Homogeneous introduction of CeOy into MnOx-based catalyst for oxidation of aromatic VOCs. Appl. Catal. B Environ. 2018, 224, 825–835. [Google Scholar] [CrossRef]
- Du, J.; Qu, Z.; Dong, C.; Song, L.; Qin, Y.; Huang, N. Low-temperature abatement of toluene over Mn-Ce oxides catalysts synthesized by a modified hydrothermal approach. Appl. Surf. Sci. 2018, 433, 1025–1035. [Google Scholar] [CrossRef]
- Lin, L.-Y.; Lee, C.-Y.; Zhang, Y.-R.; Bai, H. Aerosol-assisted deposition of Mn-Fe oxide catalyst on TiO2 for superior selective catalytic reduction of NO with NH3 at low temperatures. Catal. Commun. 2018, 111, 36–41. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, D.; Li, B.; Wang, C.; Li, J.; Crittenden, J.; Hao, J. Impacts of Pb and SO2 Poisoning on CeO2-WO3/TiO2-SiO2 SCR Catalyst. Environ. Sci. Technol. 2017, 51, 11943–11949. [Google Scholar] [CrossRef]
- Liu, Y.; Gu, T.; Weng, X.; Wang, Y.; Wu, Z.; Wang, H. DRIFT Studies on the Selectivity Promotion Mechanism of Ca-Modified Ce-Mn/TiO2 Catalysts for Low-Temperature NO Reduction with NH3. J. Phys. Chem. C 2012, 116, 16582–16592. [Google Scholar] [CrossRef]
- Ramis, G.; Yi, L.; Busca, G. Ammonia activation over catalysts for the selective catalytic reduction of NOx and the selective catalytic oxidation of NH3. An FT-IR study. Catal. Today 1996, 28, 373–380. [Google Scholar] [CrossRef]
- Lin, S.D.; Gluhoi, A.C.; Nieuwenhuys, B.E. Ammonia oxidation over Au/MO/γ-Al2O3—activity, selectivity and FTIR measurements. Catal. Today 2004, 90, 3–14. [Google Scholar] [CrossRef]
- Ettireddy, P.R.; Ettireddy, N.; Boningari, T.; Pardemann, R.; Smirniotis, P.G. Investigation of the selective catalytic reduction of nitric oxide with ammonia over Mn/TiO2 catalysts through transient isotopic labeling and in situ FT-IR studies. J. Catal. 2012, 292, 53–63. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, J.; Li, J.; Ma, L.; Woo, S.I. Novel Mn-Ce-Ti mixed-oxide catalyst for the selective catalytic reduction of NOx with NH3. ACS Appl. Mater. Interfaces 2014, 6, 14500–14508. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, B.; Liu, B.; Sun, S. A review of Mn-containing oxide catalysts for low temperature selective catalytic reduction of NOx with NH3: Reaction mechanism and catalyst deactivation. RSC Adv. 2017, 7, 26226–26242. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, R.; Bao, W.; Wang, H.; Wang, Z.; Wei, Y. Effective catalytic abatement of indoor formaldehyde at room temperature over TS-1 supported platinum with relatively low content. Catal. Today 2019. [Google Scholar] [CrossRef]
- Kuperman, A.; Nadimi, S.; Oliver, S.; Ozin, G.A.; Garces, J.M.; Olken, M.M. Non-aqueous Synthesis of Giant Crystal of Zeolites and Molecular Sieves. Nature 1993, 365, 239–242. [Google Scholar] [CrossRef]
- Zuo, Y.; Liu, M.; Zhang, T.; Hong, L.; Guo, X.; Song, C.; Chen, Y.; Zhu, P.; Jaye, C.; Fischer, D. Role of pentahedrally coordinated titanium in titanium silicalite-1 in propene epoxidation. RSC Adv. 2015, 5, 17897–17904. [Google Scholar] [CrossRef]



| Sample | The Mass Fraction of Metal Elements/wt.% | ||
|---|---|---|---|
| Mn | Fe | Ti | |
| Mn3Fe2/Silicalite-1 | 3.18 | 1.87 | - |
| Mn3Fe2/TS-1-30 | 3.17 | 1.97 | 2.23 |
| Mn3Fe2/TS-1-20 | 3.11 | 1.94 | 3.56 |
| Sample | BET Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Diameter (nm) |
|---|---|---|---|
| Silicalite-1 | 454.31 | 0.32 | 2.85 |
| TS-1-30 | 485.43 | 0.37 | 3.08 |
| TS-1-20 | 471.39 | 0.37 | 3.16 |
| Mn3Fe2/Silicalite-1 | 393.64 | 0.27 | 2.76 |
| Mn3Fe2/TS-1-30 | 422.14 | 0.32 | 3.08 |
| Mn3Fe2/TS-1-20 | 423.22 | 0.31 | 2.96 |
| Sample | Atomic Concentration/at% | ||||
|---|---|---|---|---|---|
| Si | O | Mn | Fe | Ti | |
| Mn3Fe2/TS-1-20 | 37.37 | 61.78 | 0.53 | 0.13 | 0.19 |
| Mn3Fe2/TS-1-30 | 37.33 | 61.86 | 0.50 | 0.15 | 0.16 |
| Mn3Fe2/Silicalite-1 | 37.07 | 62.22 | 0.52 | 0.19 | - |
| Samples | Atomic Ratio/% | ||
|---|---|---|---|
| Mn4+/(Mn4+ + Mn3+ + Mn2+) | Fe3+/(Fe3+ + Fe2+) | Oβ/(Oα + Oβ) | |
| Mn3Fe2/TS-1-20 | 25.6 | 59.1 | 94.37 |
| Mn3Fe2/TS-1-30 | 34.6 | 62.9 | 95.01 |
| Mn3Fe2/Silicalite-1 | 25.0 | 65.5 | 93.01 |
| Samples | Peak Temperature/°C | H2 Consumption/a.u. | |||||
|---|---|---|---|---|---|---|---|
| TⅠ | TⅡ | TⅢ | SⅠ | SⅡ | SⅢ | Stotal | |
| Mn3Fe2/TS-1-20 | 432 | 574 | 662 | 356 | 231 | 150 | 737 |
| Mn3Fe2/TS-1-30 | 439 | 585 | 669 | 400 | 236 | 140 | 776 |
| Mn3Fe2/Silicalite-1 | 436 | 608 | 667 | 436 | 96 | 119 | 651 |
| Samples | Peak Temperature/°C | Acid Amount/a.u. | |||||||
|---|---|---|---|---|---|---|---|---|---|
| TⅠ | TⅡ | TⅢ | TⅣ | SⅠ | SⅡ | SⅢ | SⅣ | Stotal | |
| Mn3Fe2/TS-1-20 | 130 | 308 | 368 | - | 373 | 65 | 88 | - | 526 |
| Mn3Fe2/TS-1-30 | 131 | 314 | 406 | 490 | 399 | 126 | 123 | 11 | 659 |
| Mn3Fe2/Silicalite-1 | 128 | 317 | - | - | 259 | 213 | - | - | 472 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, J.; Duan, R.; Chen, W.; Chen, Y.; Liu, L.; Wang, X. Promoting Effect of Ti Species in MnOx-FeOx/Silicalite-1 for the Low-Temperature NH3-SCR Reaction. Catalysts 2020, 10, 566. https://doi.org/10.3390/catal10050566
Gu J, Duan R, Chen W, Chen Y, Liu L, Wang X. Promoting Effect of Ti Species in MnOx-FeOx/Silicalite-1 for the Low-Temperature NH3-SCR Reaction. Catalysts. 2020; 10(5):566. https://doi.org/10.3390/catal10050566
Chicago/Turabian StyleGu, Jialiang, Rudi Duan, Weibin Chen, Yan Chen, Lili Liu, and Xidong Wang. 2020. "Promoting Effect of Ti Species in MnOx-FeOx/Silicalite-1 for the Low-Temperature NH3-SCR Reaction" Catalysts 10, no. 5: 566. https://doi.org/10.3390/catal10050566
APA StyleGu, J., Duan, R., Chen, W., Chen, Y., Liu, L., & Wang, X. (2020). Promoting Effect of Ti Species in MnOx-FeOx/Silicalite-1 for the Low-Temperature NH3-SCR Reaction. Catalysts, 10(5), 566. https://doi.org/10.3390/catal10050566




