In-Depth Structural and Optical Analysis of Ce-modified ZnO Nanopowders with Enhanced Photocatalytic Activity Prepared by Microwave-Assisted Hydrothermal Method
Abstract
1. Introduction
2. Results and Discussion
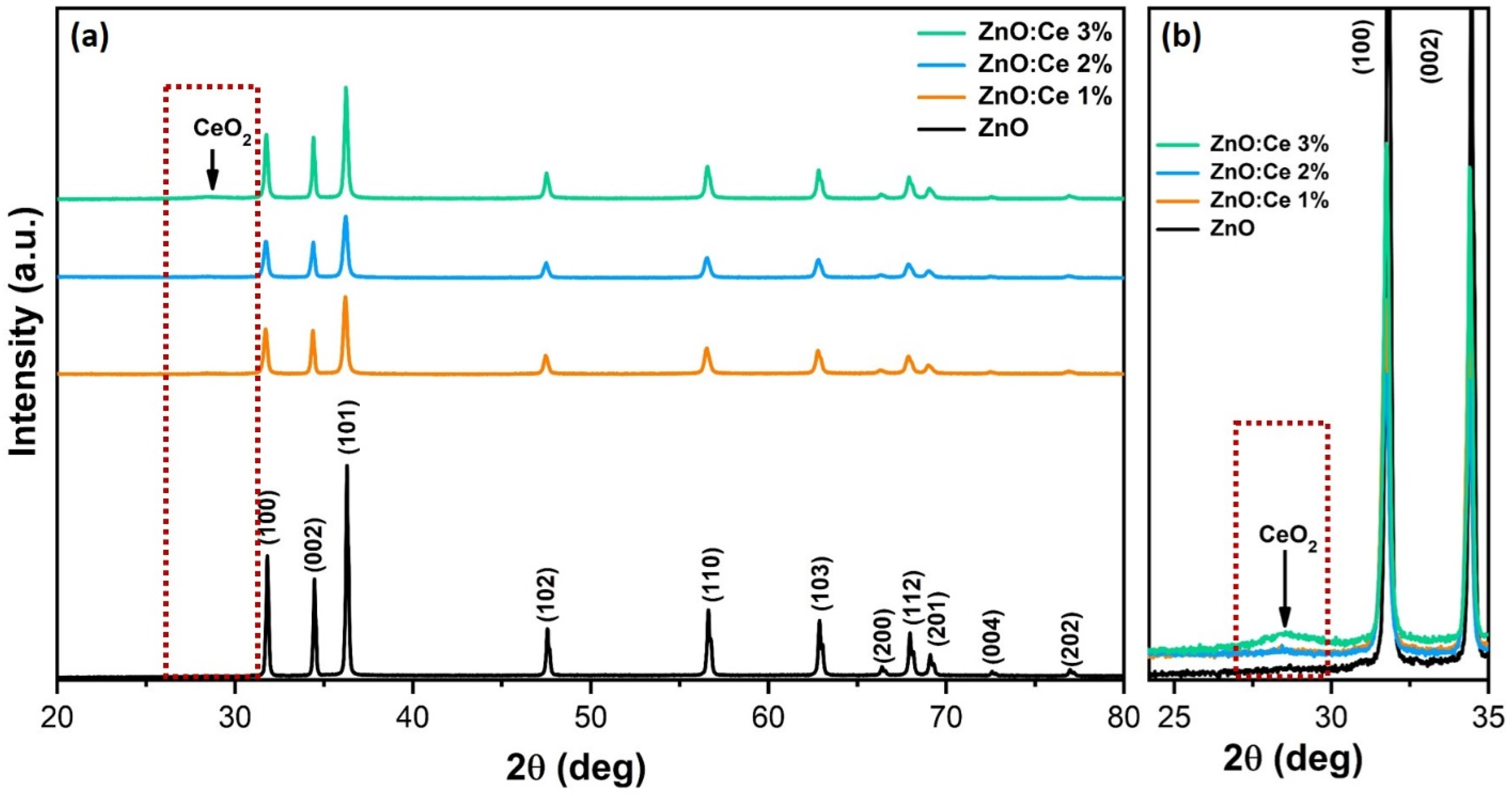
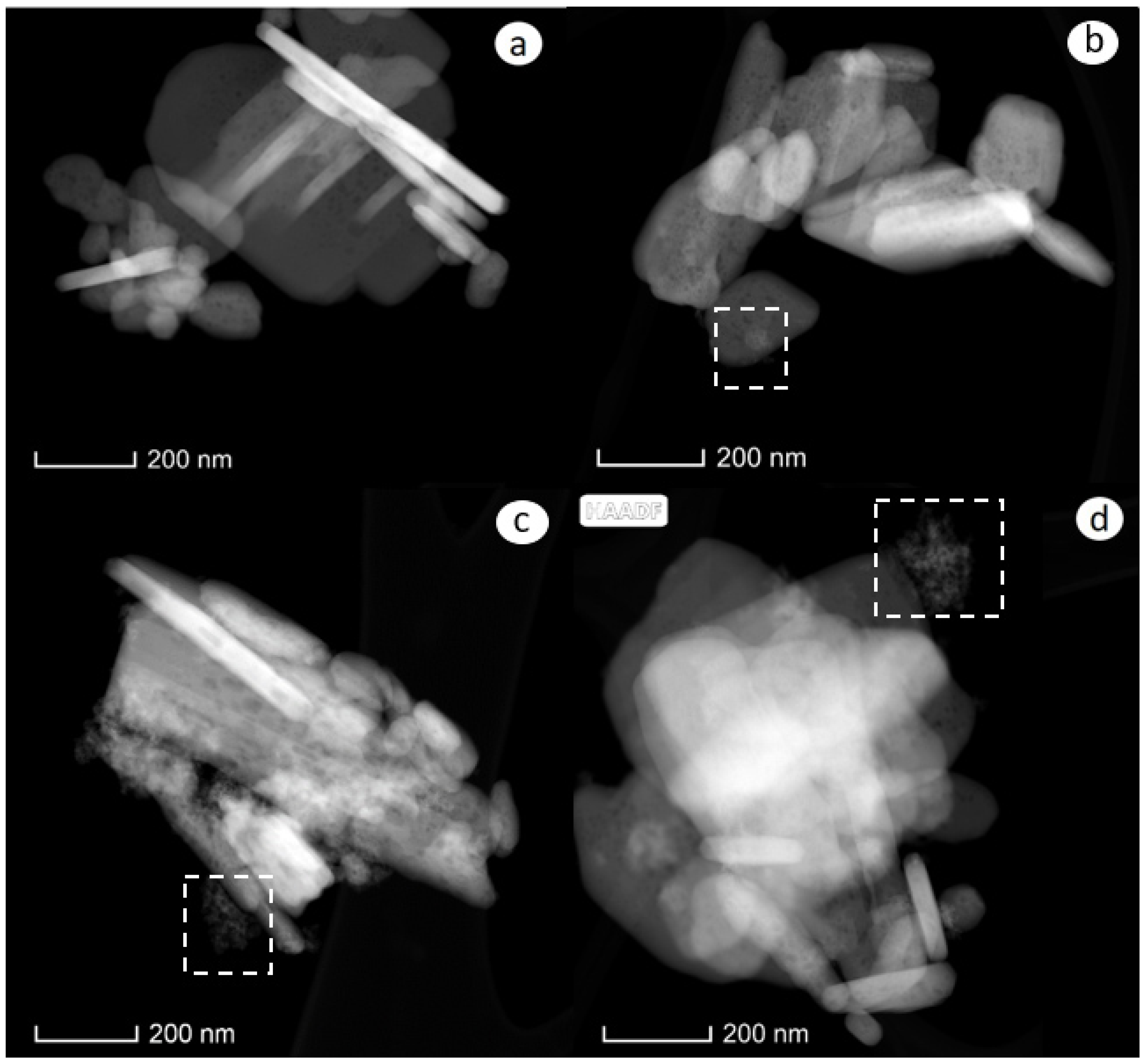
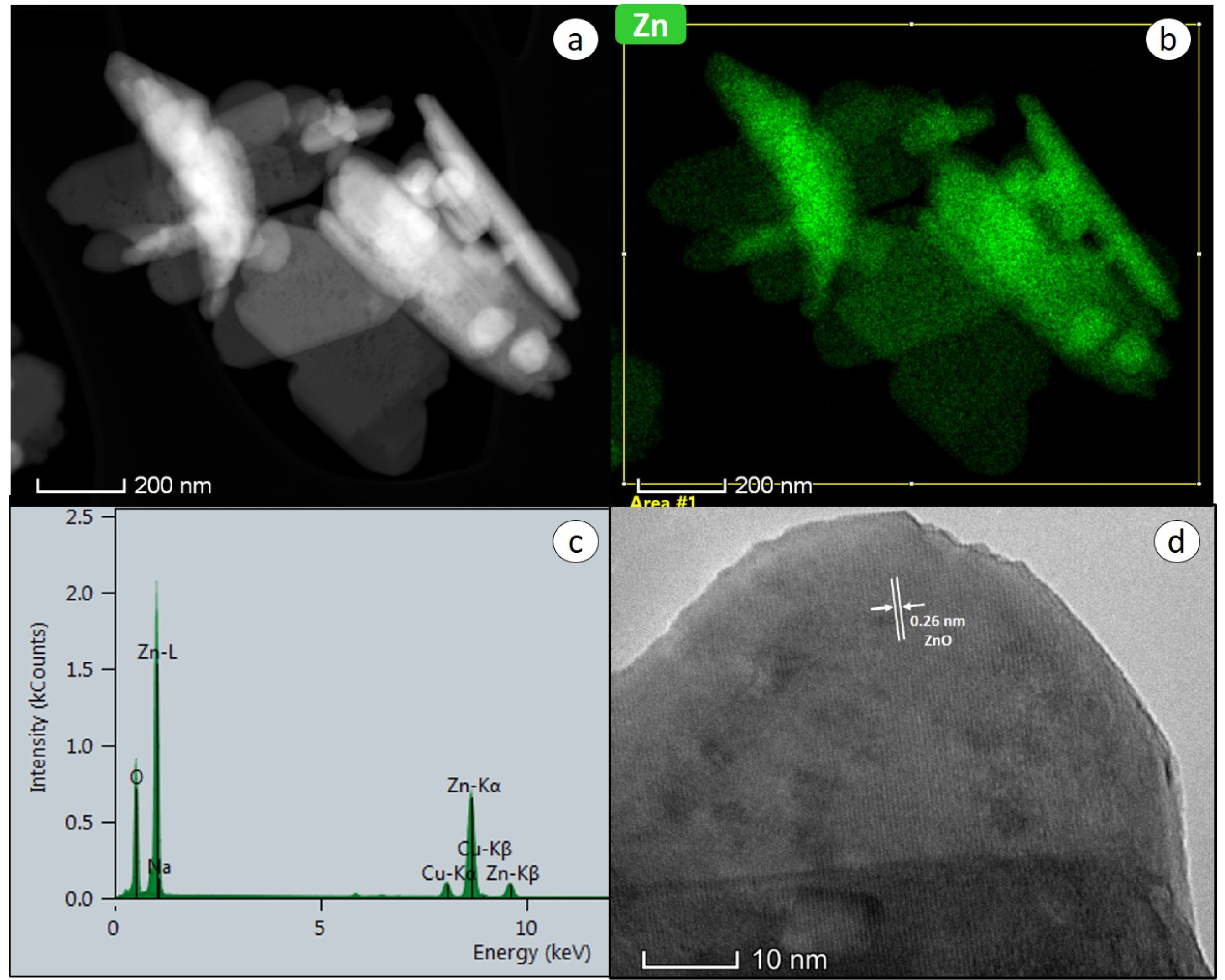
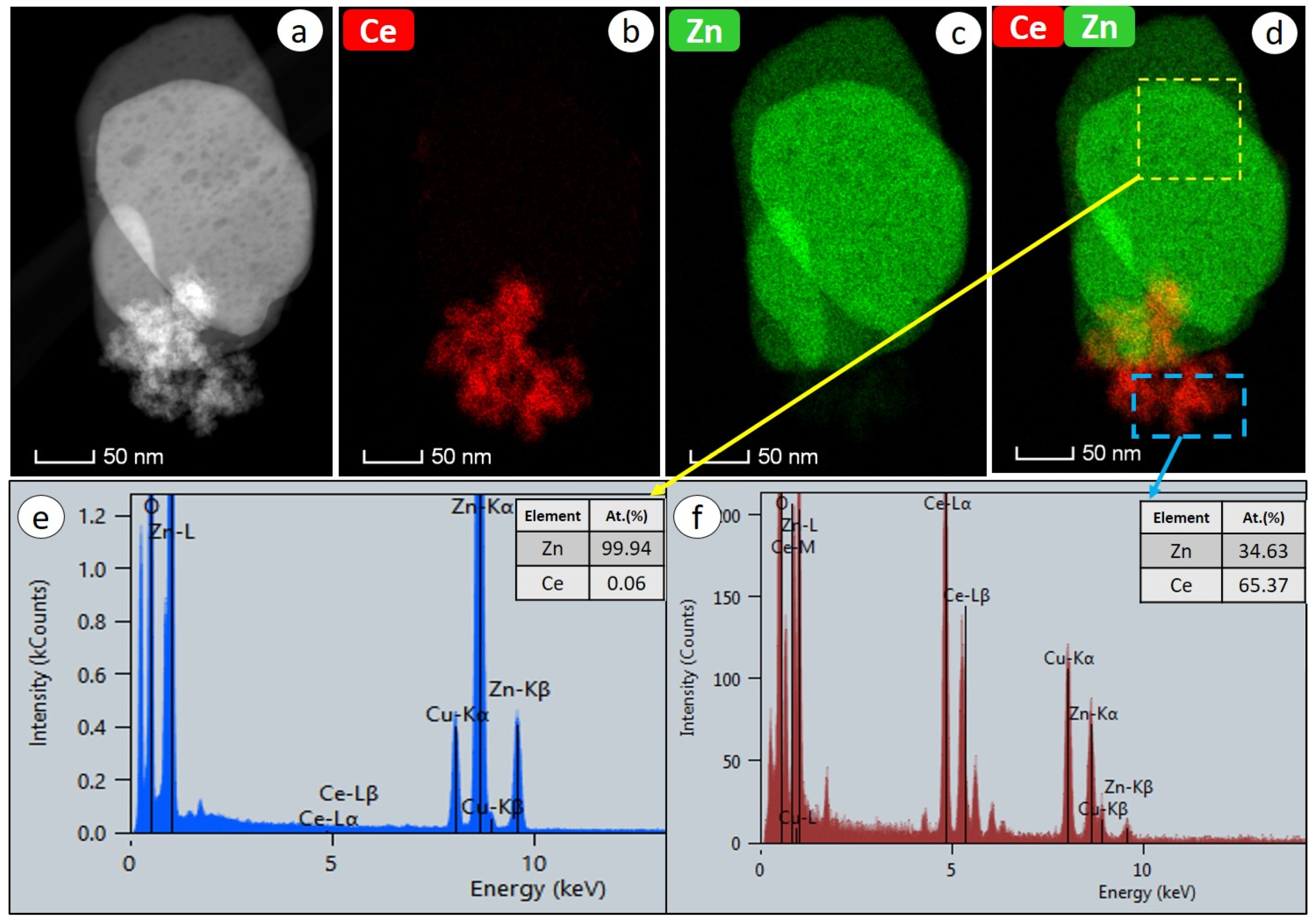
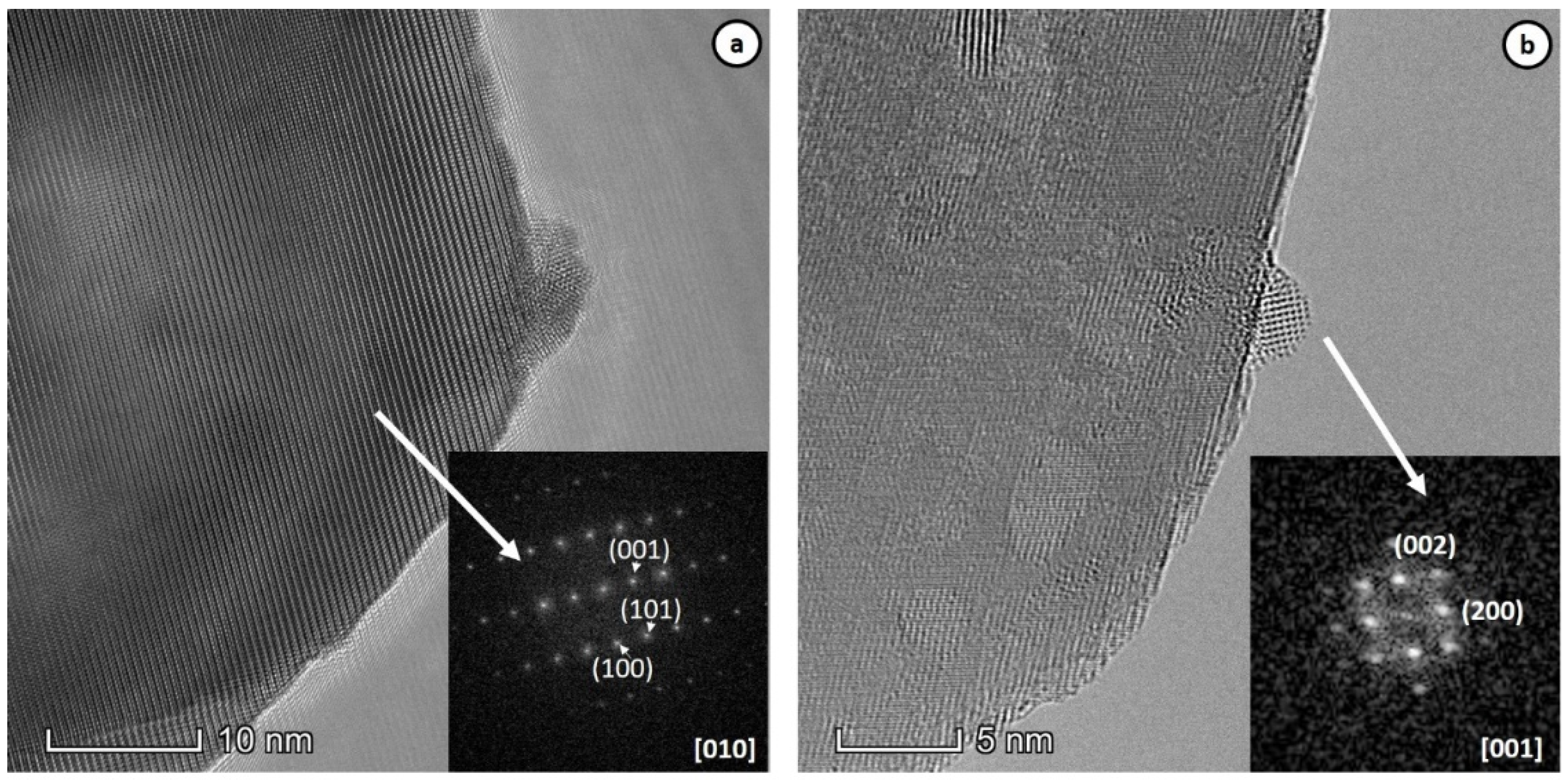
2.1. Structural Properties
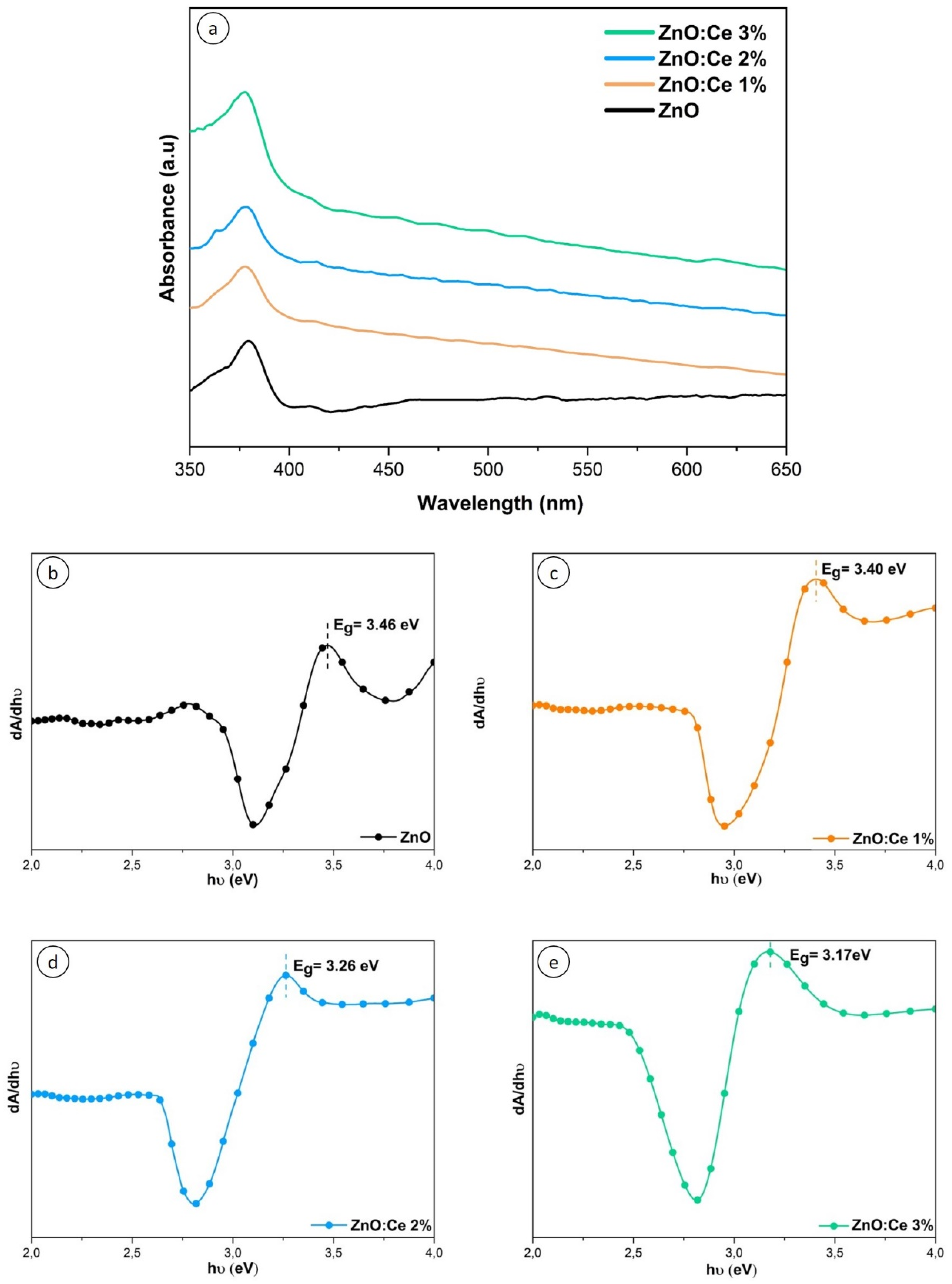
2.2. Optical Absorption
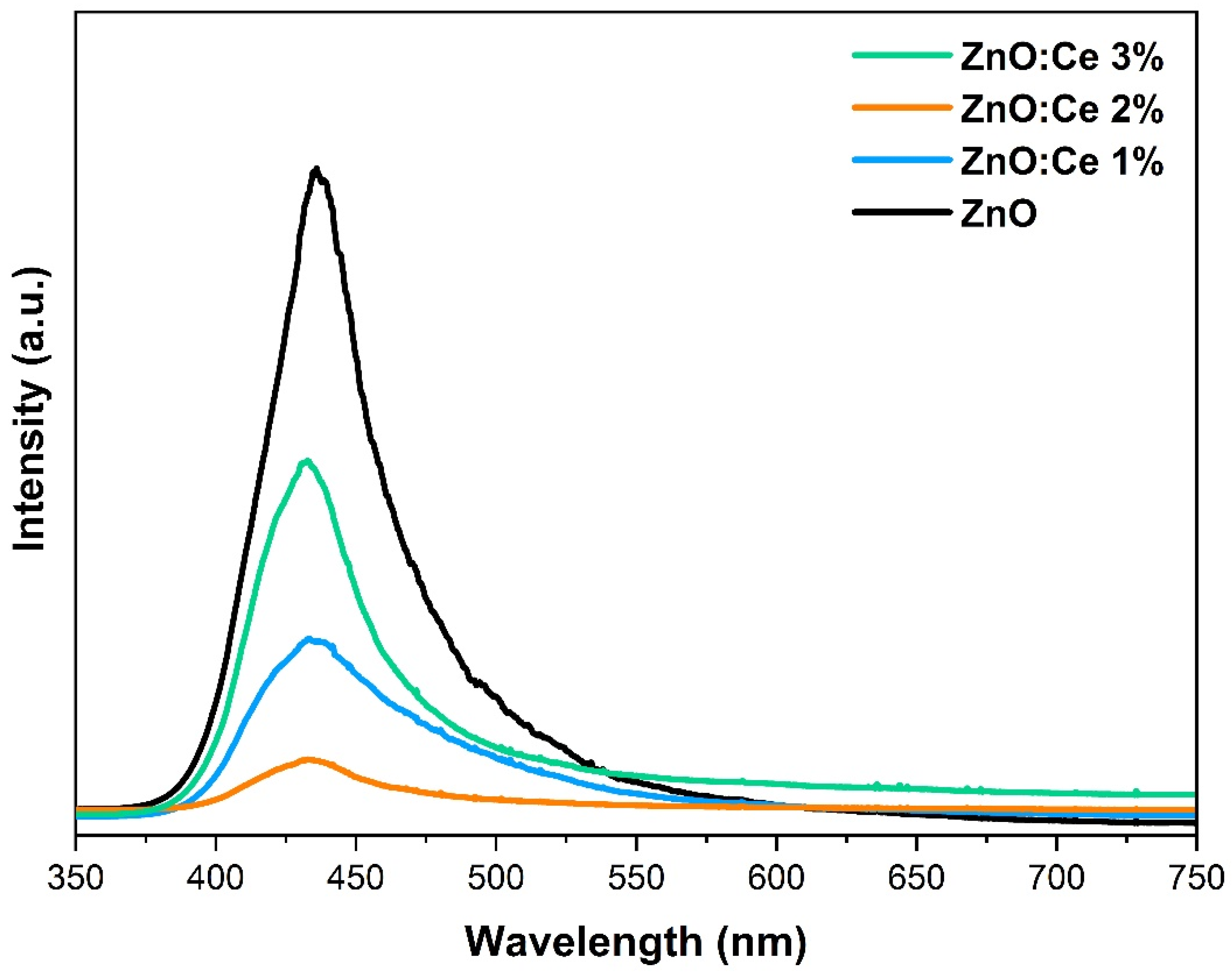
2.3. PL Spectroscopy
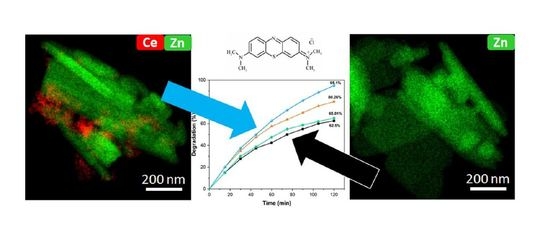
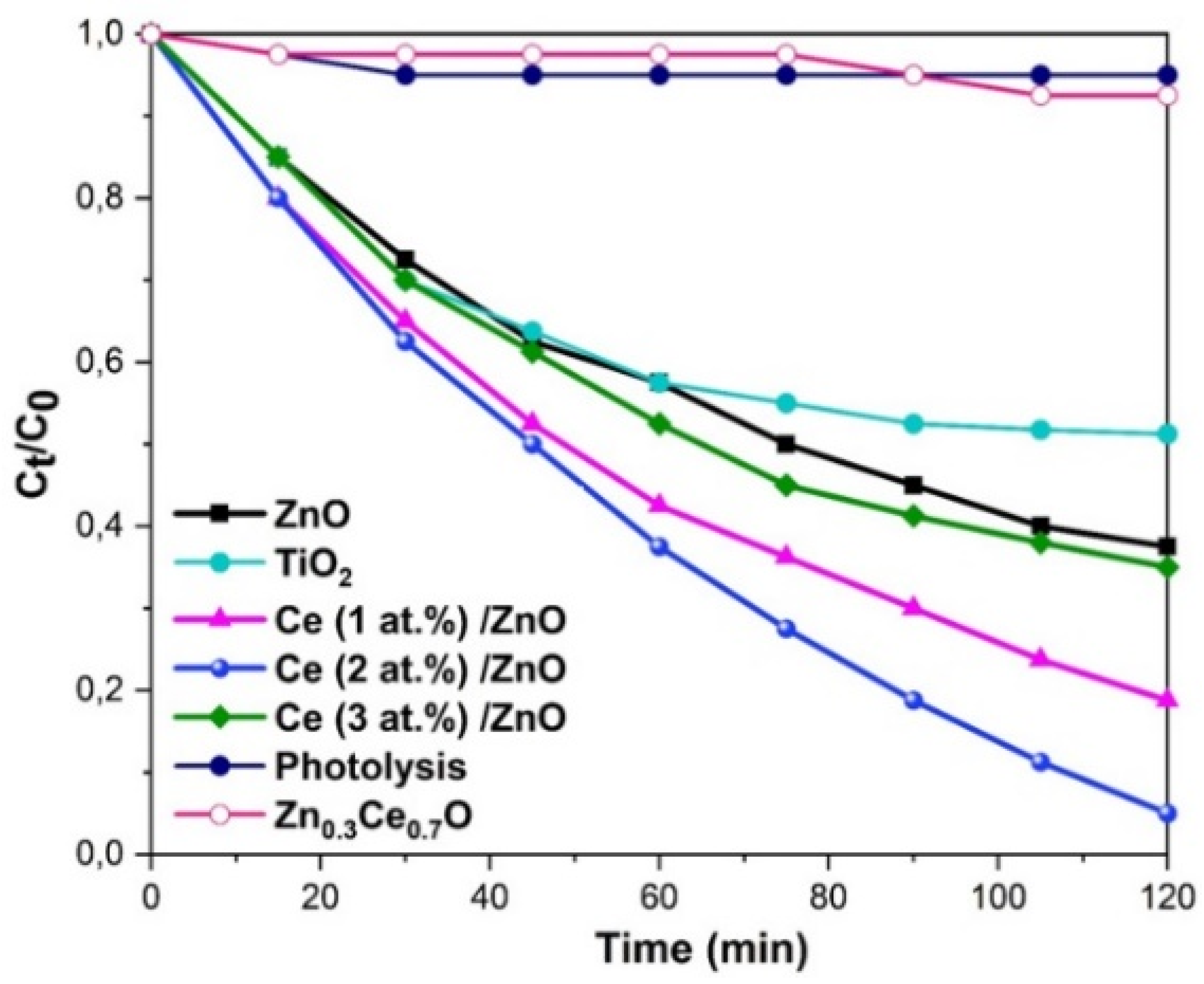
2.4. Photocatalytic Degradation of Methylene Blue by ZnO:Ce Photocatalysts
3. Experimental
3.1. Materials
3.2. Preparation of Pure and Ce-Modified ZnO Photocatalysts
3.3. Characterization
3.4. Photodegradation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kostedt IV, W.L.; Ismail, A.A.; Mazyc, D.W. Impact of heat treatment and composition of ZnO-TiO2 nanoparticles for photocatalytic oxidation of an azo dye. Ind. Eng. Chem. Res. 2008, 47, 1483–1487. [Google Scholar] [CrossRef]
- Abu Tariq, M.; Faisal, M.; Muneer, M. Semiconductor-mediated photocatalysed degradation of two selected azo dye derivatives, amaranth and bismarck brown in aqueous suspension. J. Hazard. Mater. 2005, 127, 172–179. [Google Scholar] [CrossRef]
- Faisal, M.; Khan, S.B.; Rahman, M.M.; Jamal, A.; Asiri, A.M.; Abdullah, M.M. Synthesis, characterizations, photocatalytic and sensing studies of ZnO nanocapsules. Appl. Surf. Sci. 2011, 258, 672–677. [Google Scholar] [CrossRef]
- Zhang, H.H.; Pan, X.H.; Li, Y.; Ye, Z.Z.; Lu, B.; Chen, W.; Huang, J.Y.; Ding, P.; Chen, S.S.; He, H.P.; et al. The role of band alignment in p-type conductivity of Na-doped ZnMgO: Polar versus non-polar. Appl. Phys. Lett. 2014, 104. [Google Scholar] [CrossRef]
- Masuda, Y.; Kato, K. Aqueous synthesis of ZnO rod arrays for molecular sensor. Cryst. Growth Des. 2009, 9, 3083–3088. [Google Scholar] [CrossRef]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms and applications. Renew. Sustain. Energy Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Bazta, O.; Urbieta, A.; Piqueras, J.; Fernández, P.; Addou, M.; Calvino, J.J.; Hungría, A.B. Enhanced UV emission of Li–Y co-doped ZnO thin films via spray pyrolysis. J. Alloy. Compd. 2019, 808, 151710. [Google Scholar] [CrossRef]
- Xing, X.; Chen, T.; Li, Y.; Deng, D.; Xiao, X.; Wang, Y. Flash synthesis of Al-doping macro-/nanoporous ZnO from self-sustained decomposition of Zn-based complex for superior gas-sensing application to n-butanol. Sens. Actuators B Chem. 2016, 237, 90–98. [Google Scholar] [CrossRef]
- Minami, T.; Miyata, T.; Ihara, K.; Minamino, Y.; Tsukada, S. Effect of ZnO film deposition methods on the photovoltaic properties of ZnO-Cu2O heterojunction devices. Thin Solid Film. 2006, 494, 47–52. [Google Scholar] [CrossRef]
- Bazta, O.; Urbieta, A.; Piqueras, J.; Fernández, P.; Addou, M.; Calvino, J.J.; Hungría, A.B. Influence of yttrium doping on the structural, morphological and optical properties of nanostructured ZnO thin films grown by spray pyrolysis. Ceram. Int. 2018. [Google Scholar] [CrossRef]
- Heo, Y.W.; Norton, D.P.; Tien, L.C.; Kwon, Y.; Kang, B.S.; Ren, F.; Pearton, S.J.; Laroche, J.R. ZnO nanowire growth and devices. Mater. Sci. Eng. R Rep. 2004, 47, 1–47. [Google Scholar] [CrossRef]
- Wahab, R.; Tripathy, S.K.; Shin, H.S.; Mohapatra, M.; Musarrat, J.; Al-Khedhairy, A.A.; Kumar Kaushik, N. Photocatalytic oxidation of acetaldehyde with ZnO-quantum dots. Chem. Eng. J. 2013, 226, 154–160. [Google Scholar] [CrossRef]
- Lu, Y.; Lin, Y.; Wang, D.; Wang, L.; Xie, T.; Jiang, T. A high performance cobalt-doped ZnO visible light photocatalyst and its photogenerated charge transfer properties. Nano Res. 2011, 4, 1144–1152. [Google Scholar] [CrossRef]
- Neppolian, B. Solar/UV-induced photocatalytic degradation of three commercial textile dyes. J. Hazard. Mater. 2002, 89, 303–317. [Google Scholar] [CrossRef]
- Kong, J.-Z.; Li, A.-D.; Li, X.-Y.; Zhai, H.-F.; Zhang, W.-Q.; Gong, Y.-P.; Li, H.; Wu, D. Photo-degradation of methylene blue using Ta-doped ZnO nanoparticle. J. Solid State Chem. 2010, 183, 1359–1364. [Google Scholar] [CrossRef]
- Teng, X.M.; Fan, H.T.; Pan, S.S.; Ye, C.; Li, G.H. Influence of annealing on the structural and optical properties of ZnO: Tb thin films. J. Appl. Phys. 2006, 100, 053507. [Google Scholar] [CrossRef]
- Zong, Y.; Li, Z.; Wang, X.; Ma, J.; Men, Y. Synthesis and high photocatalytic activity of Eu-doped ZnO nanoparticles. Ceram. Int. 2014, 40, 10375–10382. [Google Scholar] [CrossRef]
- Khatamian, M.; Khandar, A.A.; Divband, B.; Haghighi, M.; Ebrahimiasl, S. Heterogeneous photocatalytic degradation of 4-nitrophenol in aqueous suspension by Ln (La 3+, Nd 3+ or Sm 3+) doped ZnO nanoparticles. J. Mol. Catal. A Chem. 2012, 365, 120–127. [Google Scholar] [CrossRef]
- Sin, J.C.; Lam, S.M.; Lee, K.T.; Mohamed, A.R. Preparation and photocatalytic properties of visible light-driven samarium-doped ZnO nanorods. Ceram. Int. 2013, 39, 5833–5843. [Google Scholar] [CrossRef]
- Chouchene, B.; Chaabane, T.B.; Balan, L.; Girot, E.; Mozet, K.; Medjahdi, G.; Schneider, R. High performance Ce-doped ZnO nanorods for sunlight-driven photocatalysis. Beilstein J. Nanotechnol. 2016, 7, 1338–1349. [Google Scholar] [CrossRef]
- Tan, W.K.; Abdul Razak, K.; Lockman, Z.; Kawamura, G.; Muto, H.; Matsuda, A. Photoluminescence properties of rod-like Ce-doped ZnO nanostructured films formed by hot-water treatment of sol-gel derived coating. Opt. Mater. 2013, 35, 1902–1907. [Google Scholar] [CrossRef]
- Ahmad, M.; Ahmed, E.; Zafar, F.; Khalid, N.R.; Niaz, N.A.; Hafeez, A.; Ikram, M.; Khan, M.A.; Hong, Z. Enhanced photocatalytic activity of Ce-doped ZnO nanopowders synthesized by combustion method. J. Rare Earths 2015, 33, 255–262. [Google Scholar] [CrossRef]
- Faisal, M.; Ismail, A.A.; Ibrahim, A.A.; Bouzid, H.; Al-Sayari, S.A. Highly efficient photocatalyst based on Ce doped ZnO nanorods: Controllable synthesis and enhanced photocatalytic activity. Chem. Eng. J. 2013, 229, 225–233. [Google Scholar] [CrossRef]
- Shi, Q.; Wang, C.; Li, S.; Wang, Q.; Zhang, B.; Wang, W.; Zhang, J.; Zhu, H. Enhancing blue luminescence from Ce-doped ZnO nanophosphor by Li doping. Nanoscale Res. Lett. 2014, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Subash, B.; Krishnakumar, B.; Velmurugan, R.; Swaminathan, M.; Shanthi, M. Synthesis of Ce co-doped Ag-ZnO photocatalyst with excellent performance for NBB dye degradation under natural sunlight illumination. Catal. Sci. Technol. 2012, 2, 2319–2326. [Google Scholar] [CrossRef]
- Liang, Y.; Guo, N.; Li, L.; Li, R.; Ji, G.; Gan, S. Preparation of porous 3D Ce-doped ZnO microflowers with enhanced photocatalytic performance. RSC Adv. 2015, 5, 59887–59894. [Google Scholar] [CrossRef]
- Cerrato, E.; Gionco, C.; Paganini, M.C.; Giamello, E.; Albanese, E.; Pacchioni, G. Origin of Visible Light Photoactivity of the CeO 2 /ZnO Heterojunction. ACS Appl. Energy Mater. 2018, 1, 4247–4260. [Google Scholar] [CrossRef]
- Zhu, L.; Li, H.; Xia, P.; Liu, Z.; Xiong, D. Hierarchical ZnO Decorated with CeO2 Nanoparticles as the Direct Z-Scheme Heterojunction for Enhanced Photocatalytic Activity. ACS Appl. Mater. Interfaces 2018, 10, 39679–39687. [Google Scholar] [CrossRef]
- St John, J.; Coffer, J.L.; Chen, Y.; Pinizzotto, R.F. Synthesis and characterization of discrete luminescent erbium-doped silicon nanocrystals. J. Am. Chem. Soc. 1999, 121, 1888–1892. [Google Scholar] [CrossRef]
- Sarro, M.; Gule, N.P.; Laurenti, E.; Gamberini, R.; Paganini, M.C.; Mallon, P.E.; Calza, P. ZnO-based materials and enzymes hybrid systems as highly efficient catalysts for recalcitrant pollutants abatement. Chem. Eng. J. 2018, 334, 2530–2538. [Google Scholar] [CrossRef]
- Karunakaran, C.; Gomathisankar, P.; Manikandan, G. Preparation and characterization of antimicrobial Ce-doped ZnO nanoparticles for photocatalytic detoxification of cyanide. Mater. Chem. Phys. 2010, 123, 585–594. [Google Scholar] [CrossRef]
- Duclľre, J.R.; Doggett, B.; Henry, M.O.; McGlynn, E.; Rajendra Kumar, R.T.; Mosnier, J.P.; Perrin, A.; Guilloux-Viry, M. (20–23) ZnO thin films grown by pulsed laser deposition on Ce O2 -buffered r -sapphire substrate. J. Appl. Phys. 2007, 101. [Google Scholar] [CrossRef]
- Yousefi, M.; Amiri, M.; Azimirad, R.; Moshfegh, A.Z. Enhanced photoelectrochemical activity of Ce doped ZnO nanocomposite thin films under visible light. J. Electroanal. Chem. 2011, 661, 106–112. [Google Scholar] [CrossRef]
- Kellici, S.; Gong, K.; Lin, T.; Brown, S.; Clark, R.J.H.; Vickers, M.; Cockcroft, J.K.; Middelkoop, V.; Barnes, P.; Perkins, J.M.; et al. High-throughput continuous hydrothermal flow synthesis of Zn-Ce oxides: Unprecedented solubility of Zn in the nanoparticle fluorite lattice. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 4331–4349. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Fan, H.; Wang, Z. Enhanced optical properties of heterostructured ZnO/CeO2 nanocomposite fabricated by one-pot hydrothermal method: Fluorescence and ultraviolet absorption and visible light transparency. Opt. Mater. 2014, 38, 145–153. [Google Scholar] [CrossRef]
- Jeanguillaume, C.; Colliex, C. Spectrum-image: The next step in EELS digital acquisition and processing. Ultramicroscopy 1989, 28, 252–257. [Google Scholar] [CrossRef]
- Qin, H.; Li, W.; Xia, Y.; He, T. Photocatalytic activity of heterostructures based on ZnO and N-doped ZnO. ACS Appl. Mater. Interfaces 2011, 3, 3152–3156. [Google Scholar] [CrossRef]
- Othman, A.A.; Ali, M.A.; Ibrahim, E.M.M.; Osman, M.A. Influence of Cu doping on structural, morphological, photoluminescence, and electrical properties of ZnO nanostructures synthesized by ice-bath assisted sonochemical method. J. Alloy. Compd. 2016, 683, 399–411. [Google Scholar] [CrossRef]
- Kaneva, N.; Bojinova, A.; Papazova, K.; Dimitrov, D. Photocatalytic purification of dye contaminated sea water by lanthanide (La 3+, Ce 3+, Eu 3+) modified ZnO. Catal. Today 2015, 252, 113–119. [Google Scholar] [CrossRef]
- Hiley, C.I.; Fisher, J.M.; Thompsett, D.; Kashtiban, R.J.; Sloan, J.; Walton, R.I. Incorporation of square-planar Pd2+ in fluorite CeO2: Hydrothermal preparation, local structure, redox properties and stability. J. Mater. Chem. A 2015, 3, 13072–13079. [Google Scholar] [CrossRef]
- Houas, A.; Lachheb, H.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J.-M. Photocatalytic degradation pathway of methylene blue in water. Appl. Catal. B Environ. 2001, 31, 145–157. [Google Scholar] [CrossRef]
- Sin, J.C.; Lam, S.M.; Lee, K.T.; Mohamed, A.R. Preparation of cerium-doped ZnO hierarchical micro/nanospheres with enhanced photocatalytic performance for phenol degradation under visible light. J. Mol. Catal. A Chem. 2015, 409, 1–10. [Google Scholar] [CrossRef]
- Yayapao, O.; Thongtem, S.; Phuruangrat, A.; Thongtem, T. Sonochemical synthesis, photocatalysis and photonic properties of 3% Ce-doped ZnO nanoneedles. Ceram. Int. 2013, 39, 563–568. [Google Scholar] [CrossRef]
- Lang, J.; Wang, J.; Zhang, Q.; Li, X.; Han, Q.; Wei, M.; Sui, Y.; Wang, D.; Yang, J. Chemical precipitation synthesis and significant enhancement in photocatalytic activity of Ce-doped ZnO nanoparticles. Ceram. Int. 2016, 42, 14175–14181. [Google Scholar] [CrossRef]
- Kumar, K.V. Langmuir—Hinshelwood kinetics—A theoretical study. Catal. Commun. 2008, 9, 82–84. [Google Scholar] [CrossRef]




| Sample | d (nm)–ZnO |
|---|---|
| ZnO | 65 |
| ZnO-1%Ce | 47 |
| ZnO-2%Ce | 40 |
| ZnO-3%Ce | 69 |
| Sample | Eg (eV) | Eex (eV) |
|---|---|---|
| ZnO | 3.46 | 3.26 |
| ZnO:Ce 1 at.% | 3.40 | 3.28 |
| ZnO:Ce 2 at.% | 3.26 | 3.28 |
| ZnO:Ce 3 at.% | 3.17 | 3.29 |
| Sample | k (M. min−1) |
|---|---|
| ZnO | 5.2 × 10−3 |
| P25 | 1.6 × 10−3 |
| ZnO:Ce 1 at.% | 1.3 × 10−2 |
| ZnO:Ce 2 at.% | 3.9 × 10−2 |
| ZnO:Ce 3 at.% | 5.8 × 10−3 |
| Sample | Surface Area m2.g−1) |
|---|---|
| ZnO | 10 |
| ZnO:Ce 1 at.% | 10 |
| ZnO:Ce 2 at.% | 22 |
| ZnO:Ce 3 at.% | 15 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bazta, O.; Urbieta, A.; Trasobares, S.; Piqueras, J.; Fernández, P.; Addou, M.; Calvino, J.J.; Hungría, A.B. In-Depth Structural and Optical Analysis of Ce-modified ZnO Nanopowders with Enhanced Photocatalytic Activity Prepared by Microwave-Assisted Hydrothermal Method. Catalysts 2020, 10, 551. https://doi.org/10.3390/catal10050551
Bazta O, Urbieta A, Trasobares S, Piqueras J, Fernández P, Addou M, Calvino JJ, Hungría AB. In-Depth Structural and Optical Analysis of Ce-modified ZnO Nanopowders with Enhanced Photocatalytic Activity Prepared by Microwave-Assisted Hydrothermal Method. Catalysts. 2020; 10(5):551. https://doi.org/10.3390/catal10050551
Chicago/Turabian StyleBazta, Otman, Ana Urbieta, Susana Trasobares, Javier Piqueras, Paloma Fernández, Mohammed Addou, Jose Juan Calvino, and Ana Belén Hungría. 2020. "In-Depth Structural and Optical Analysis of Ce-modified ZnO Nanopowders with Enhanced Photocatalytic Activity Prepared by Microwave-Assisted Hydrothermal Method" Catalysts 10, no. 5: 551. https://doi.org/10.3390/catal10050551
APA StyleBazta, O., Urbieta, A., Trasobares, S., Piqueras, J., Fernández, P., Addou, M., Calvino, J. J., & Hungría, A. B. (2020). In-Depth Structural and Optical Analysis of Ce-modified ZnO Nanopowders with Enhanced Photocatalytic Activity Prepared by Microwave-Assisted Hydrothermal Method. Catalysts, 10(5), 551. https://doi.org/10.3390/catal10050551