Synthesis of Conducting Bifunctional Polyaniline@Mn-TiO2 Nanocomposites for Supercapacitor Electrode and Visible Light Driven Photocatalysis
Abstract
:1. Introduction
2. Results and discussion
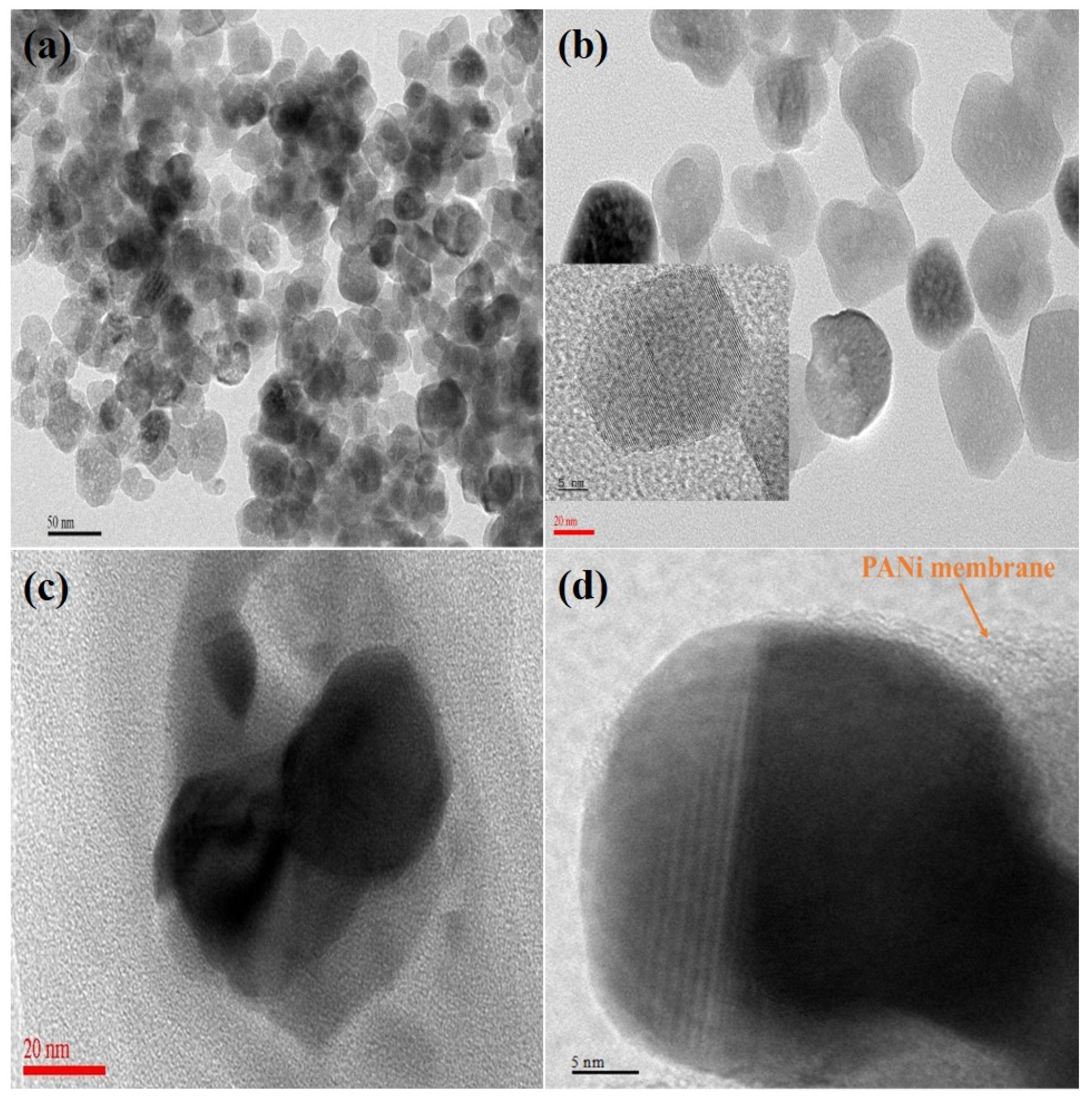
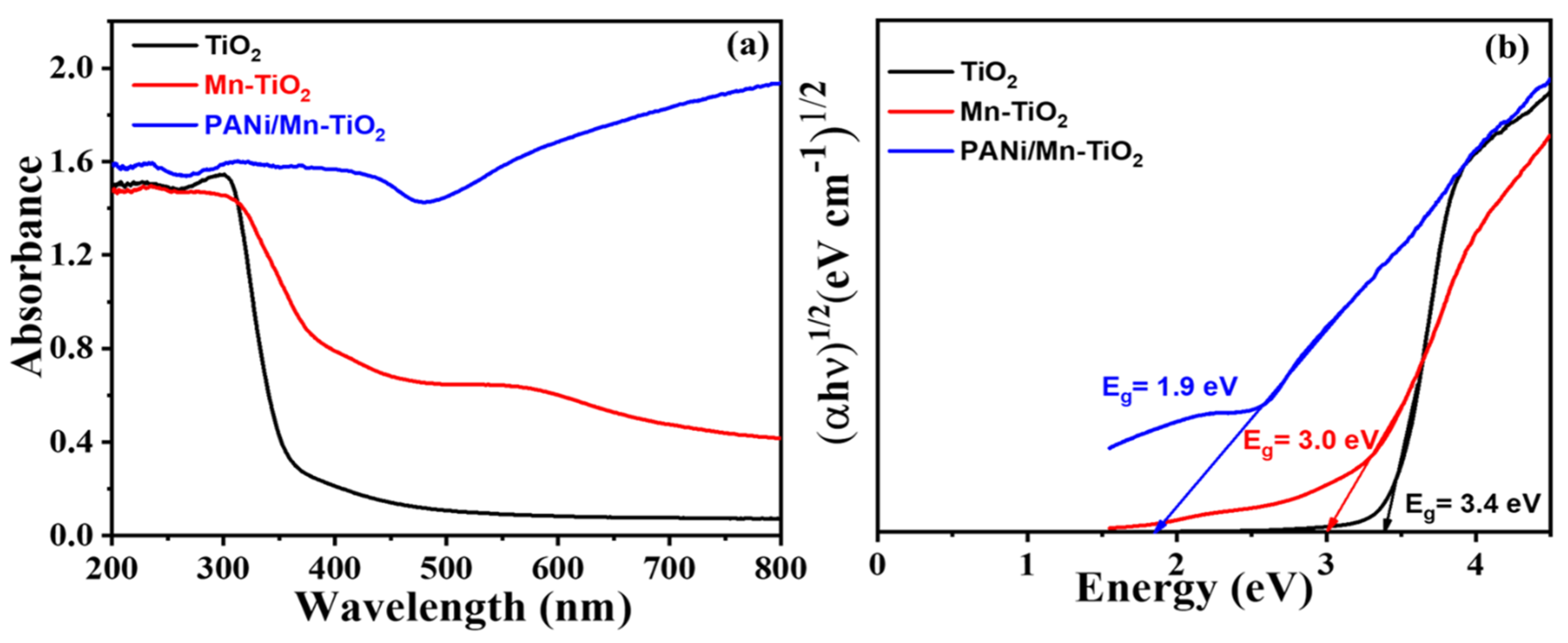
2.1. Physiochemical and Morphological Characterization
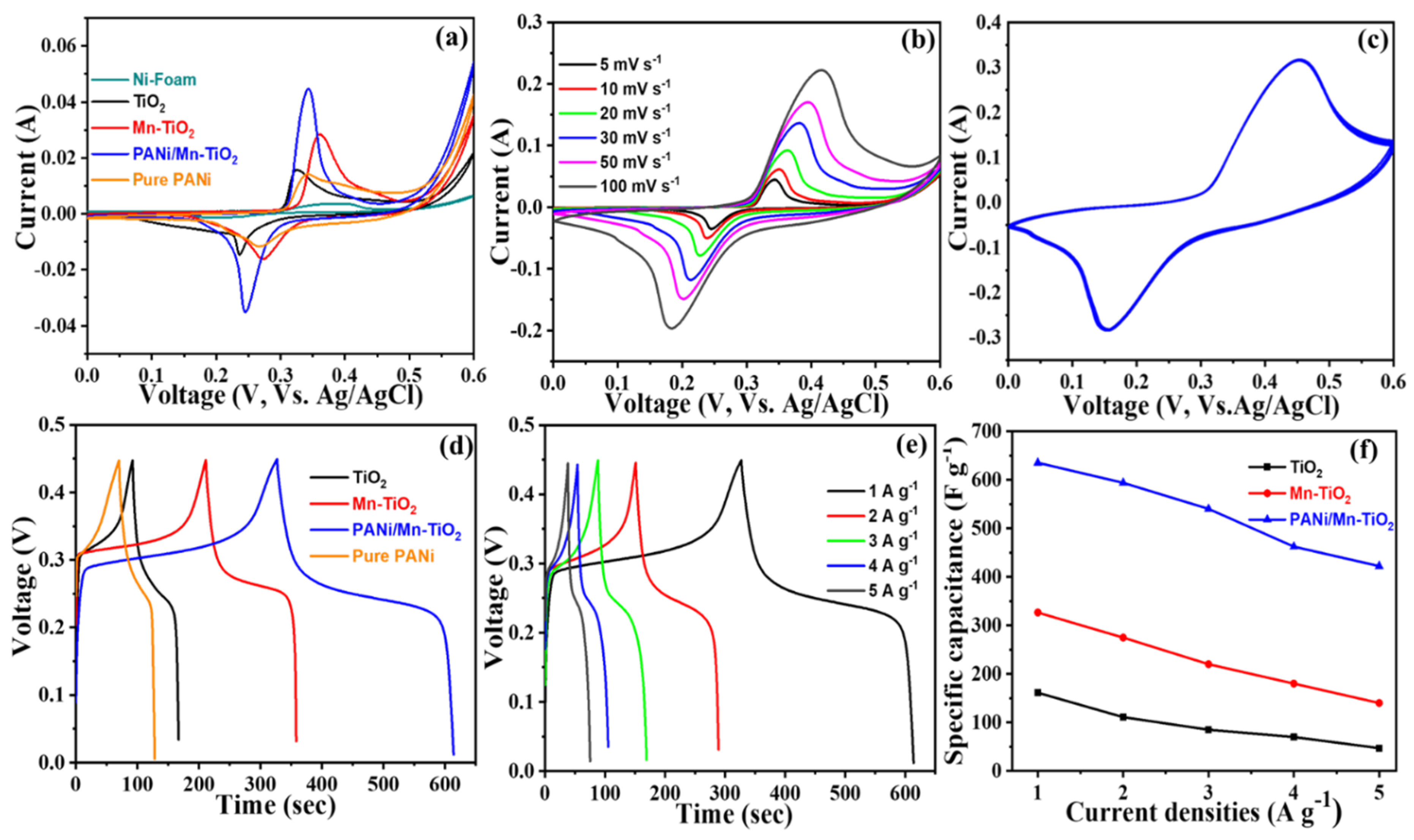
2.2. Electrochemical Performance
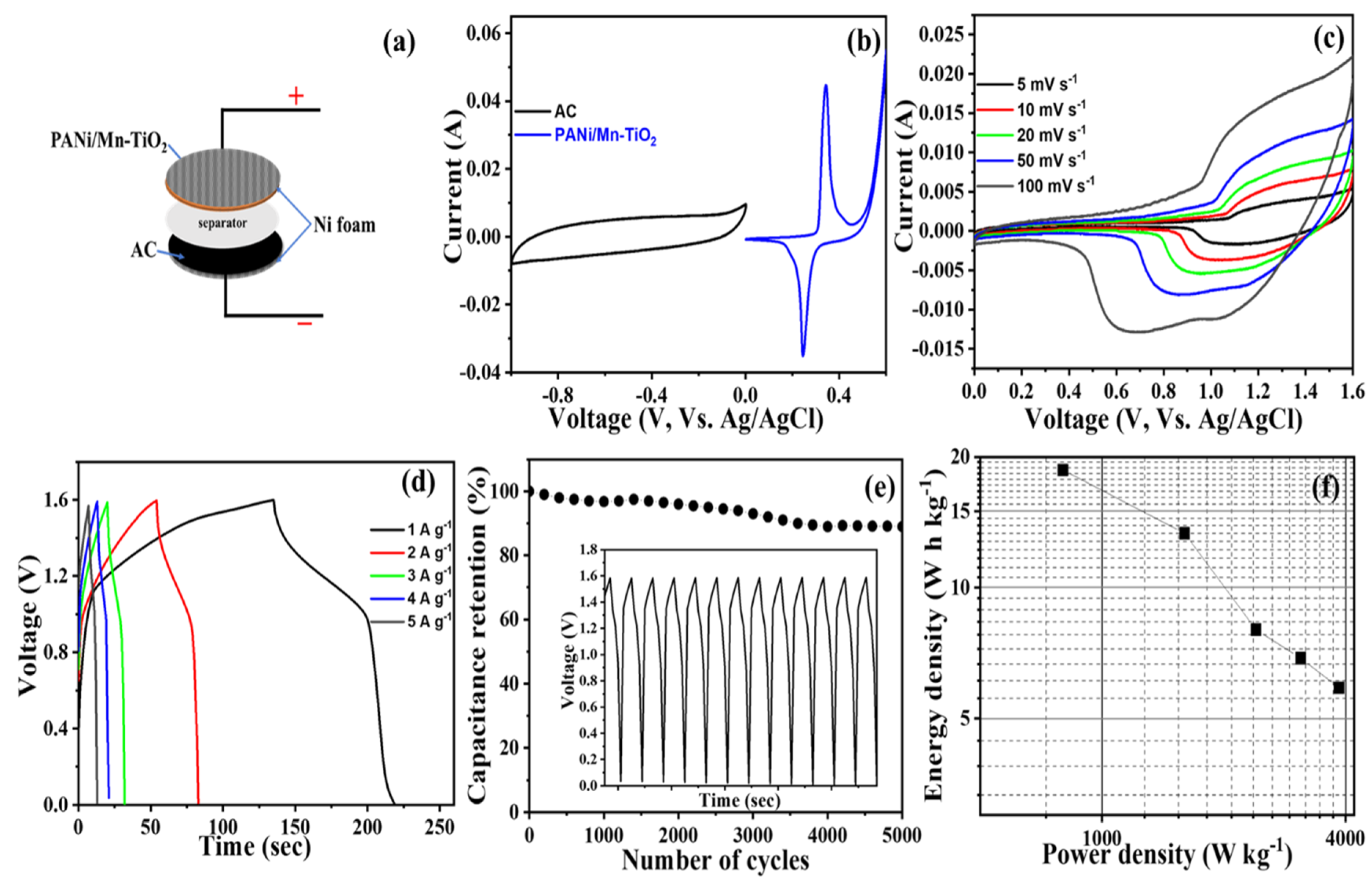
2.3. Asymmetric Supercapacitor
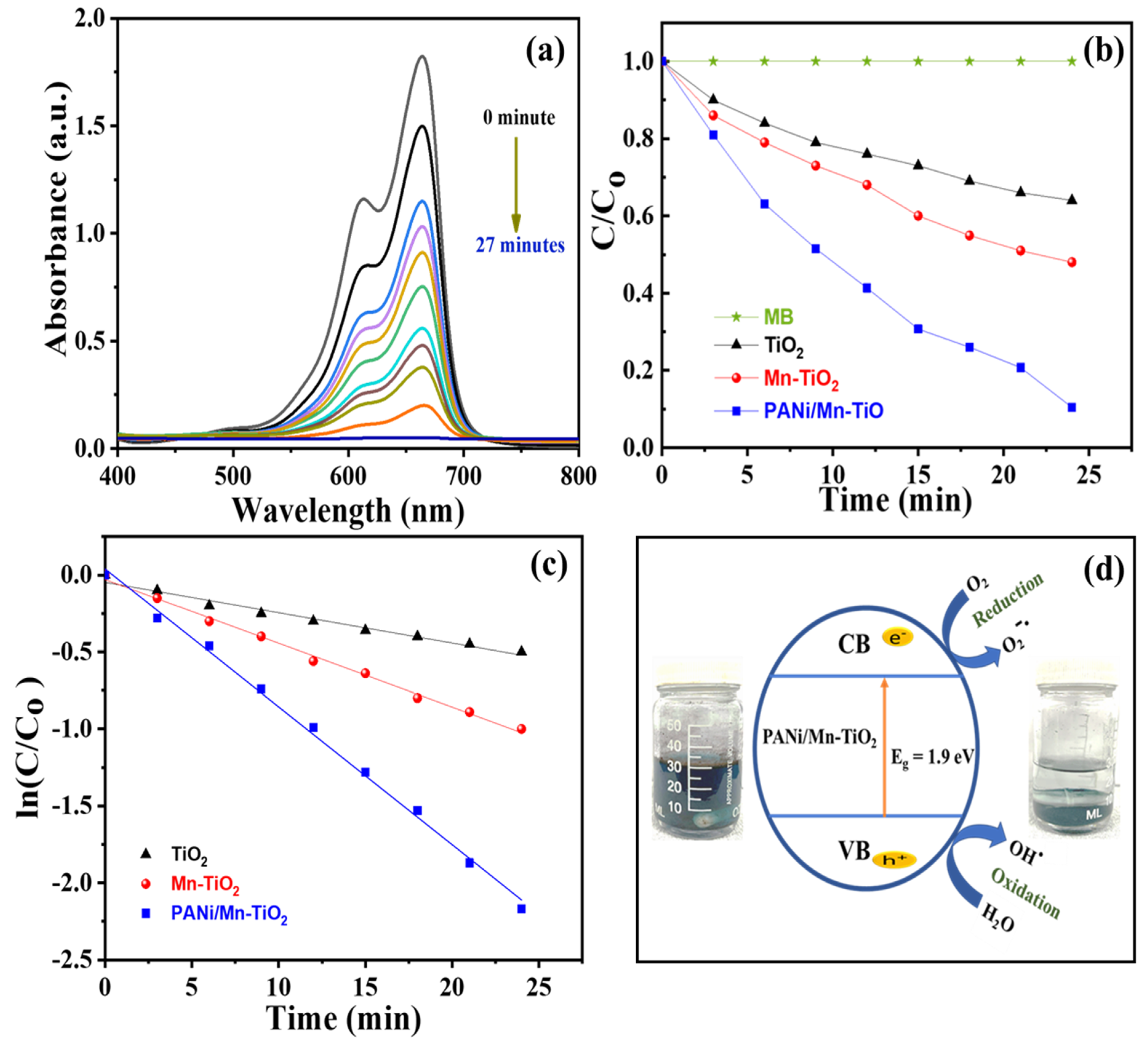
2.4. Photocatalytic Effect of PANi/Mn-TiO2 for Degradation of Methylene Blue
3. Materials and Methods
3.1. Materials and Reagents
3.2. Synthesis of Mn-TiO2
3.3. Preparation of PANi/Mn-TiO2
3.4. Characterization Techniques
3.5. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Z.-S.; Wang, D.-W.; Ren, W.; Zhao, J.; Zhou, G.; Li, F.; Cheng, H.-M. Anchoring Hydrous RuO2 on Graphene Sheets for High-Performance Electrochemical Capacitors. Adv. Funct. Mater. 2010, 20, 3595–3602. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y.; Dunn, B. Where Do Batteries End and Supercapacitors Begin? Science 2014, 343, 1210. [Google Scholar] [CrossRef] [Green Version]
- Ojha, G.P.; Pant, B.; Muthurasu, A.; Chae, S.-H.; Park, S.-J.; Kim, T.; Kim, H.-Y. Three-dimensionally assembled manganese oxide ultrathin nanowires: Prospective electrode material for asymmetric supercapacitors. Energy 2019, 188, 116066. [Google Scholar] [CrossRef]
- Karki, H.P.; Ojha, D.P.; Joshi, M.K.; Kim, H.J. Effective reduction of p-nitrophenol by silver nanoparticle loaded on magnetic Fe3O4/ATO nano-composite. Appl. Surf. Sci. 2018, 435, 599–608. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, C.; Li, J. Photocatalysis and wave-absorbing properties of polyaniline/TiO2 microbelts composite by in situ polymerization method. Appl. Surf. Sci. 2010, 257, 944–948. [Google Scholar] [CrossRef]
- Pant, B.; Park, M.; Park, S.-J. Recent Advances in TiO2 Films Prepared by Sol-Gel Methods for Photocatalytic Degradation of Organic Pollutants and Antibacterial Activities. Coatings 2019, 9, 613. [Google Scholar] [CrossRef] [Green Version]
- Sharotri, N.; Sharma, D.; Sud, D. Experimental and theoretical investigations of Mn-N-co-doped TiO2 photocatalyst for visible light induced degradation of organic pollutants. J. Mater. Res. Technol. 2019, 8, 3995–4009. [Google Scholar] [CrossRef]
- Anpo, M.; Takeuchi, M. The design and development of highly reactive titanium oxide photocatalysts operating under visible light irradiation. J. Catal. 2003, 216, 505–516. [Google Scholar] [CrossRef]
- Vásquez, G.C.; Peche-Herrero, M.A.; Maestre, D.; Cremades, A.; Ramírez-Castellanos, J.; González-Calbet, J.M.; Piqueras, J. Effects of Transition Metal Doping on the Growth and Properties of Rutile TiO2 Nanoparticles. J. Phys. Chem. C 2013, 117, 1941–1947. [Google Scholar] [CrossRef]
- Kale, G.; Arbuj, S.; Chothe, U.; Khore, S.; Nikam, L.; Kale, B. Highly Crystalline Ordered Cu-dopedTiO2Nanostructure by Paper Templated Method: Hydrogen Production and Dye Degradation under Natural Sunlight. J. Compos. Sci. 2020, 4, 48. [Google Scholar] [CrossRef]
- Pant, B.; Park, M.; Park, S.-J. MoS2/CdS/TiO2 ternary composite incorporated into carbon nanofibers for the removal of organic pollutants from water. Inorg. Chem. Commun. 2019, 102, 113–119. [Google Scholar] [CrossRef]
- Jedsukontorn, T.; Ueno, T.; Saito, N.; Hunsom, M. Narrowing band gap energy of defective black TiO2 fabricated by solution plasma process and its photocatalytic activity on glycerol transformation. J. Alloy. Compd. 2018, 757, 188–199. [Google Scholar] [CrossRef]
- Coronado, J.M.; Javier Maira, A.; Martínez-Arias, A.; Conesa, J.C.; Soria, J. EPR study of the radicals formed upon UV irradiation of ceria-based photocatalysts. J. Photochem. Photobiol. A: Chem. 2002, 150, 213–221. [Google Scholar] [CrossRef]
- Rudge, A.; Davey, J.; Raistrick, I.; Gottesfeld, S.; Ferraris, J.P. Conducting polymers as active materials in electrochemical capacitors. J. Power Sources 1994, 47, 89–107. [Google Scholar] [CrossRef]
- Monfared, A.H.; Jamshidi, M. Effects of photocatalytic activity of nano TiO2 and PAni/TiO2 nanocomposite on the physical/mechanical performances of acrylic pseudo paints. Prog. Org. Coat. 2019, 136, 105300. [Google Scholar] [CrossRef]
- Xu, D.; Yang, M.; Liu, Y.; Zhu, R.; Lv, X.; Zhang, C.; Liu, B. Fabrication of an innovative designed TiO2 nanosheets/CdSe/polyaniline/graphene quaternary composite and its application as in-situ photocathodic protection coatings on 304SS. J. Alloy. Compd. 2020, 822, 153685. [Google Scholar] [CrossRef]
- Ojha, D.P.; Poudel, M.B.; Kim, H.J. Investigation of electrochemical performance of a high surface area mesoporous Mn doped TiO2 nanoparticle for a supercapacitor. Mater. Lett. 2020, 264, 127363. [Google Scholar] [CrossRef]
- Lan, T.; Tu, J.; Zou, Q.; Zeng, X.; Zou, J.; Huang, H.; Wei, M. Synthesis of anatase TiO2 mesocrystals with highly exposed low-index facets for enhanced electrochemical performance. Electrochim. Acta 2019, 319, 101–109. [Google Scholar] [CrossRef]
- Deng, Q.R.; Xia, X.H.; Guo, M.L.; Gao, Y.; Shao, G. Mn-doped TiO2 nanopowders with remarkable visible light photocatalytic activity. Mater. Lett. 2011, 65, 2051–2054. [Google Scholar] [CrossRef]
- Ganesh, R.S.; Durgadevi, E.; Navaneethan, M.; Patil, V.L.; Ponnusamy, S.; Muthamizhchelvan, C.; Kawasaki, S.; Patil, P.S.; Hayakawa, Y. Low temperature ammonia gas sensor based on Mn-doped ZnO nanoparticle decorated microspheres. J. Alloy. Compd. 2017, 721, 182–190. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, J.; Wang, H.; Zhan, J.; Zhu, Y.; Zhang, Q.; Shen, Q.; Yang, H. TiO2-C nanowire arrays@polyaniline core-shell nanostructures on carbon cloth for high performance supercapacitors. Appl. Surf. Sci. 2019, 493, 1125–1133. [Google Scholar] [CrossRef]
- Katoch, A.; Burkhart, M.; Hwang, T.; Kim, S.S. Synthesis of polyaniline/TiO2 hybrid nanoplates via a sol–gel chemical method. Chem. Eng. J. 2012, 192, 262–268. [Google Scholar] [CrossRef]
- Quillard, S.; Louarn, G.; Lefrant, S.; Macdiarmid, A.G. Vibrational analysis of polyaniline: A comparative study of leucoemeraldine, emeraldine, and pernigraniline bases. Phys. Rev. B 1994, 50, 12496–12508. [Google Scholar] [CrossRef] [PubMed]
- Rahman, K.H.; Kar, A.K. Titanium-di-oxide (TiO2) concentration-dependent optical and morphological properties of PAni-TiO2 nanocomposite. Mater. Sci. Semicond. Process. 2020, 105, 104745. [Google Scholar] [CrossRef]
- Yang, J.; Yang, Y.; Lan, J.; Yu, Y.; Yang, X. Polyaniline-manganese dioxide-carbon nanofiber ternary composites with enhanced electrochemical performance for supercapacitors. J. Electroanal. Chem. 2019, 843, 22–30. [Google Scholar] [CrossRef]
- Wang, F.; Shen, B.; Zhu, S.; Wang, Z. Promotion of Fe and Co doped Mn-Ce/TiO2 catalysts for low temperature NH3-SCR with SO2 tolerance. Fuel 2019, 249, 54–60. [Google Scholar] [CrossRef]
- Shao, W.; Jamal, R.; Xu, F.; Ubul, A.; Abdiryim, T. The Effect of a Small Amount of Water on the Structure and Electrochemical Properties of Solid-State Synthesized Polyaniline. Materials 2012, 5, 1811–1825. [Google Scholar] [CrossRef] [Green Version]
- Su, S.-J.; Kuramoto, N. Processable polyaniline–titanium dioxide nanocomposites: Effect of titanium dioxide on the conductivity. Synth. Met. 2000, 114, 147–153. [Google Scholar] [CrossRef]
- Lee, H.-K.; Lee, S.-W. Template-sacrificial conversion of MnCO3 microspheres to fabricate Mn-doped TiO2 visible light photocatalysts. Mater. Des. 2020, 189, 108497. [Google Scholar] [CrossRef]
- Xia, H.; Wang, Q. Ultrasonic Irradiation: A Novel Approach To Prepare Conductive Polyaniline/Nanocrystalline Titanium Oxide Composites. Chem. Mater. 2002, 14, 2158–2165. [Google Scholar] [CrossRef]
- Huang, Y.-G.; Zhang, X.-H.; Chen, X.-B.; Wang, H.-Q.; Chen, J.-R.; Zhong, X.-X.; Li, Q.-Y. Electrochemical properties of MnO2-deposited TiO2 nanotube arrays 3D composite electrode for supercapacitors. Int. J. Hydrogen Energy 2015, 40, 14331–14337. [Google Scholar] [CrossRef]
- Kumar, R.; Rai, P.; Sharma, A. Facile synthesis of Cu2O microstructures and their morphology dependent electrochemical supercapacitor properties. RSC Adv. 2016, 6, 3815–3822. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Li, H.; Wu, H.; Wu, Y.; Shang, Y.; Pan, J.; Xiong, X. Fabrication of TiO2@PANI nanobelts with the enhanced absorption and photocatalytic performance under visible light. Mater. Lett. 2016, 172, 52–55. [Google Scholar] [CrossRef]
- Geng, J.; Ma, C.; Zhang, D.; Ning, X. Facile and fast synthesis of SnO2 quantum dots for high performance solid-state asymmetric supercapacitor. J. Alloy. Compd. 2020, 825, 153850. [Google Scholar] [CrossRef]
- Garcia-Segura, S.; Tugaoen, H.O.N.; Hristovski, K.; Westerhoff, P. Photon flux influence on photoelectrochemical water treatment. Electrochem. Commun. 2018, 87, 63–65. [Google Scholar] [CrossRef]
- Villaluz, F.J.A.; de Luna, M.D.G.; Colades, J.I.; Garcia-Segura, S.; Lu, M.-C. Removal of 4-chlorophenol by visible-light photocatalysis using ammonium iron(II) sulfate-doped nano-titania. Process Saf. Environ. Prot. 2019, 125, 121–128. [Google Scholar] [CrossRef]
- Dhanalakshmi, S.; Mathi Vathani, A.; Muthuraj, V.; Prithivikumaran, N.; Karuthapandian, S. Mesoporous Gd2O3/NiS2 microspheres: A novel electrode for energy storage applications. J. Mater. Sci. Mater. Electron. 2020, 31, 3119–3129. [Google Scholar] [CrossRef]
- Stoller, M.D.; Ruoff, R.S. Best practice methods for determining an electrode material’s performance for ultracapacitors. Energy Environ. Sci. 2010, 3, 1294–1301. [Google Scholar] [CrossRef]
- Samuel, E.; Kim, T.-G.; Park, C.-W.; Joshi, B.; Swihart, M.T.; Yoon, S.S. Supersonically Sprayed Zn2SnO4/SnO2/CNT Nanocomposites for High-Performance Supercapacitor Electrodes. ACS Sustain. Chem. Eng. 2019, 7, 14031–14040. [Google Scholar] [CrossRef]
- Suárez, L.; Centeno, T.A. Unravelling the volumetric performance of activated carbons from biomass wastes in supercapacitors. J. Power Sources 2020, 448, 227413. [Google Scholar] [CrossRef]
- Choudhary, N.; Li, C.; Moore, J.; Nagaiah, N.; Zhai, L.; Jung, Y.; Thomas, J. Asymmetric Supercapacitor Electrodes and Devices. Adv. Mater. 2017, 29, 1605336. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poudel, M.B.; Yu, C.; Kim, H.J. Synthesis of Conducting Bifunctional Polyaniline@Mn-TiO2 Nanocomposites for Supercapacitor Electrode and Visible Light Driven Photocatalysis. Catalysts 2020, 10, 546. https://doi.org/10.3390/catal10050546
Poudel MB, Yu C, Kim HJ. Synthesis of Conducting Bifunctional Polyaniline@Mn-TiO2 Nanocomposites for Supercapacitor Electrode and Visible Light Driven Photocatalysis. Catalysts. 2020; 10(5):546. https://doi.org/10.3390/catal10050546
Chicago/Turabian StylePoudel, Milan Babu, Changho Yu, and Han Joo Kim. 2020. "Synthesis of Conducting Bifunctional Polyaniline@Mn-TiO2 Nanocomposites for Supercapacitor Electrode and Visible Light Driven Photocatalysis" Catalysts 10, no. 5: 546. https://doi.org/10.3390/catal10050546
APA StylePoudel, M. B., Yu, C., & Kim, H. J. (2020). Synthesis of Conducting Bifunctional Polyaniline@Mn-TiO2 Nanocomposites for Supercapacitor Electrode and Visible Light Driven Photocatalysis. Catalysts, 10(5), 546. https://doi.org/10.3390/catal10050546





