1. Introduction
The use of catalysts to enhance the efficiency of ozonation can be observed as a recent trend. Catalytic advanced oxidation processes (AOPs) have become willingly explored methods for pollutant removal from aquatic environments. The urgent issue of micropollutants attracts scientific activity with the utmost attention. As far as pharmaceuticals, preservatives, and personal care products are commonly considered as the main sources of micropollutants in the environment [
1,
2,
3,
4,
5,
6], and new groups of micropollutants can be suspected to appear. One of the potential sources of micropollutants, which has not been sighted, can be the textile industry because of the use of an enormous number of chemicals. Among them, dyes appear as the most burdensome pollutants within the textile industry. Because of high light absorption even at low concentration, they can disturb the aquatic life in the biosphere [
7]. Dyes, as a numerous and varied group of complex, high-molecular-weight (mostly nitrogen-based compounds), cannot be assigned as micropollutants themselves. However, the by-products of their decomposition, aniline derivatives, and naphthalic acids [
8], with high probability can accumulate in the environment which makes them possible micropollutants. Therefore, the monitoring of the dye by-products presence should be cultivated. Unfortunately, the knowledge about dye by-products is rather poor. Even though there is a large spectrum of literature reports devoted to textile wastewater treatment, most of them are focused on color removal [
9]. Only a few papers on dye by-products have appeared during recent years [
10,
11,
12]. While, our previous paper on ozone-based AOPs showed that the chromophore disintegration, which results in color removal, is only the first stage of dye molecule decomposition [
13]. Consequently, the colorless by-products stay and accumulate in wastewater [
14]. Thus, the wastewater treatment can remove color and at the same time contribute the micropollutants production. Catalytic ozonation can be an alternative to a need for more efficient removal of colorless dye by-products.
Catalytic ozonation is widely concerned as an advanced oxidation process, which suggests the production of hydroxyl radicals in the process. As far as this statement cannot be denied, the hydroxyl radical’s production in catalytic ozonation is not a sufficient explanation of the crux of this process. Consequently, to motivate the selection of the catalyst a dipper discussion on the mechanisms of catalytic ozonation should be raised.
Generally, a substance, which enhances the overall reaction rate can be considered as a catalyst. However, the activity of catalysts is selective which means that only a specific stage of the reaction can be enhanced in a complex chemical system. For catalytic ozonation, the ozone self-decomposition resulting in hydroxyl radicals (HO) production was referred to a region of the catalytic action of catalysts most often [
15]. However, the basis of this action should be referred to the type of catalyst.
Two systems of catalysis, homogenic and heterogenic, are possible for catalytic ozonation. In the homogeneous systems, the transition metals cations such as Cr
3+, Mn
2+, Fe
2+, Ni
2+, and Zn
2+ are the catalysts and they are responsible for HO
• generation from ozone decomposition. Both metal cation and ozone are in the liquid phase. The most possible catalytic activity of metal cations was referred as indirect ozone decomposition on the complex of metal–pollutant [
15]. This means that the metal cations (dissolved in the bulk) attract the organics through electro-potential, which results in building of a temporary complex. Consequently, the ozone self-decomposition improves through its adsorption on this temporary complex. On the other hand, the direct activity of the metal cation on the ozone molecule was reported as a driving force of chain reactions leading to ozone self-decomposition, as well [
15].
In the heterogenic system of catalytic ozonation, the catalyst is a solid body. In this system, the adsorption ability of the catalyst has a key because at least one of the reagents must be adsorbed on the catalyst surface. Correspondingly, the catalytic action within the process is possible when one of the presented actions takes place: ozone is adsorbed on the catalyst surface, a molecule of a pollutant is adsorbed on the catalyst surface or both ozone and pollutant are adsorbed on the surface. The main groups of substances used as heterogenic catalysts are metal oxides, minerals, varieties of carbon and metals on the support [
16]. The most often supports are oxides [
17] or activated carbon [
18], but some novel structural materials can act as carriers, as well [
19,
20].
The most common representatives of metal oxide catalysts are TiO
2, MnO
2, and Fe
2O
3 (the transition metal oxides). The use of Al
2O
3 is also popular in catalytic ozonation [
19]. The catalytic action of metal oxides is based on the adsorptive properties related to active centers on oxides surface of Lewis acid character. At the same time, the hydroxyls, which cover the metal oxides surface, are also considered as adsorption centers because of their ion-exchange properties [
15].
Two routs of catalytic activity have been proposed for metals on the support. One is pollutant (organic molecule) adsorption on the catalyst active center and then its oxidation by ozone or HO
•. The second is ozone decomposition by electron transfer through reduced or oxidized metal site deposited on the surface of supporting material [
16].
As far as carbon materials are concerned the specific intermolecular effects can be shown as a driving force of reagent-catalyst interaction. The organic adsorption and ozone decomposition on activated carbon take place through the basic centers [
21]. In the case of graphene, the electrostatic attraction and π–π interaction contribute to the adsorption process [
22].
Since Arslan-Alaton and her co-workers have examined one of the first applications of catalytic ozonation for real industrial textile wastewater [
23,
24], only a few groups had investigated the issue [
25,
26,
27,
28,
29,
30,
31]. Whereas, only Malik [
25] and Wu [
28] and their co-workers investigated the possible by-products in wastewater. Followed the recent review on catalytic ozonation of textile wastewater more attention should be given to mineralization and detoxification, not only to color removal [
32]. Although a large number of studies can be found in the literature most of them are limited only to laboratory experiments on synthetic wastewater (single dye solution in water) [
32]. Only a few studies dealt with a process scale-up [
27,
31]. Moreover the contribution of wastewater constitution, beside Na
2CO
3 influence [
29], has never been checked. This short literature overview help to form the novelty of the presented study. It was clearly shown that despite a large number of literature reports there still is a visible gap in the knowledge about real industrial wastewater treatment and the constituents which build the wastewater matrix.
The mentioned above aniline derivatives, and naphthalic acid-based compounds, which are the most expected by-products of dyes decomposition are reported to be hardly oxidized by molecular ozone [
16]. Likewise, the aldehydes and carboxylic acids, which are secondary dyes decomposition by-products are poorly oxidized by ozone [
16]. Therefore, the catalytic ozonation was employed in this study to enhance ozonation effectiveness in color and refractory compounds removal by the catalytic activity of three types of heterogenic catalysts. A metal oxide, TiO
2 (P25, rutile–anatase mixture), a metal on the support, platinum on activated carbon, and activated carbon were used in the experiment. The objective of the study was to investigate the activity of the catalysts for industrial textile ozone treatment. The catalyst type, pH effect, and wastewater composition were studied to explain the probable mechanisms of the process. The changes of UV–VIS spectra, total organic carbon (TOC), and chemical oxygen demand (COD) were investigated to evaluate the dye and by-products removal. The toxicity assay was conducted to conclude if the use of the catalysts improves this factor.
2. Results and Discussion
The catalytic abilities of three types of heterogeneous catalysts were checked for real industrial textile wastewater. The catalysts tested for ozonation were titanium oxide (TiO2), activated carbon (AC), platinum supported on activated carbon (Pt–AC). The color removal and colorless refractory by-products removal were investigated for catalysts’ effectiveness. Two types of the wastewater originated in industrial dyeing were examined, the raw one and the wastewater after electrocoagulation (EC) pretreatment. The probable mechanism of the catalytic action of each catalyst in specific conditions of the textile wastewater matrix was studied and discussed.
2.1. Catalytic Action for Color Removal
2.1.1. Color Removal Directly from Raw Industrial Wastewater
Figure 1 presents the results of ozonation and catalytic ozonation of the raw textile wastewater. As can be seen, none of the catalysts, TiO
2, AC, or Pt–AC resulted in any visible catalytic activity. The experimental data were collected for all catalysts as absorbance values at 596 nm, which corresponded to the color of the Reactive Black 5 dye (RB5), could be roughly approximated by the same trendline (
Figure 1a). Correspondingly, the values of the pseudo-first-order rate calculated for color removal were almost equal for all the catalysts and not higher than for single ozonation (
Figure 1b). For the lack of catalytic action of any catalyst, can be explained by the textile wastewater matrix. The common denominator of the issue is the adsorptive abilities of the catalysts. Even though each of the tested catalysts was characterized by different types of active centers of adsorption all of them could be deactivated by residuals of the surfactant which is commonly used in the operations of industrial dyeing of textiles as an assistant agent and builds the matrix of this wastewater.
2.1.2. Residual Color Removal after EC
In contrary to raw wastewater catalytic ozonation, this process was efficient for the wastewater pretreated by electrocoagulation (EC). Data presented in
Figure 2 revealed additive effectiveness when catalysts were used for color removal, but not for all catalysts. Consequently, two issues come to mind. Firstly, what was the reason for unblocking of catalytic activity of some catalysts when the pretreated wastewater was investigated? Secondly, why were some catalysts were more active than others? To answer these two questions the interactions between the wastewater matrix and the catalysts on the molecular level must be taken under consideration.
It has to be burned in mind that the textile wastewater, the dyeing effluent, is one of the most polluted wastewater stream appearing in textile processing [
33]. In this study, the wastewater from the reactive dyeing of cotton was examined. It means that extremely high salinity and alkalinity can be expected in this wastewater. Moreover, the use of the surfactant (dispersant, anti-stain agent) is necessary during the exhaustion-fixation dyeing method which is widespread in the industrial processing of cellulosic textiles. The constitution of the wastewater matrix (characterized in
Section 2.1), which has little in common to laboratory dye solution, seems to be crucial for the crux of the catalytic action within catalytic ozonation. The lack of catalytic activity could be observed in the previous section which described the raw wastewater. The EC pretreatment did not result in visible alkalinity decrease and NaCl content was not significantly changed. However, the EC contributed to the efficient coagulation of the surfactant. The Fe
2+ ions electrochemically eluted from steel anode material could have taken over the surfactant molecules and effectively removed them by flock formation and precipitation. Consequently, after decreasing the surfactant concentration below the critical micelle concentration (CMC) value it lost the micelle formation abilities and could not cover the surface of the catalyst.
As can be observed in
Figure 2a,b, not all the catalysts were active in the process. Even though the surfactant effect was minimized by EC pretreatment there was no visible activity of TiO
2 in catalytic ozonation of pretreated wastewater. TiO
2 is a semiconducting material, known mostly as a UVA-activated electron transferring body which can generate reactive oxygen species (ROS) through irradiation [
17]. However, the use of TiO
2 without light irradiation is also noticeable, then the active centers of adsorption settled in the crystallography structure are used during catalytic ozonation [
34]. In the case of this experiment, the active centers must have been inaccessible for effective ozone or dye adsorption. Therefore, the conditions for the catalytic action of TiO
2 was not accomplished, and consequently, the additive effect in color removal was not observed. Likewise, the catalytic activity of the platinum supported on AC and Pt–AC, while more explicit than TiO
2, but still not too significant in this experiment for color removal. Platinum is a highly reactive transition metal that was probably blocked and lost the activity. The most promising results were observed for the use of AC catalyst. The color removal was more significant than for single ozonation (
Figure 2a) and the color removal rate was higher (
Figure 2b). It can be a premise to expect AC did not lose adsorption abilities during an experiment. The presented results can be correlated with similar work of Faria and co-workers [
29], who used cerium oxide, AC and cerium oxide supported on AC.
Because the catalytic activity of the catalysts could be observed only for EC pretreated wastewater the further part of the investigation was focused on this kind of wastewater.
2.2. Catalytic Action for Colorless By-Product Removal
Our previous study on RB5 ozonation kinetics gave us information on the integral role of the by-products occurrence during the oxidation process [
35]. It was found that decolorization was the first step of dye decomposition and it was caused by azo chromophore oxidation. The secondary dye decomposition occurred immediately and resulted in colorless by-products appearance which influenced the kinetics of oxidation. Consequently, the conclusion was that the role of the secondary colorless by-products cannot be neglected. Moreover, in our next study [
36] we found that colorless by-products are not removed but they accumulated in the wastewater while multi-recycling loop. When the possible RB5 degradation pathway was considered by us the naphthol and phenol derivatives were taken under consideration as the most probable by-products resistive to ozone oxidation [
37]. Therefore, one of the objectives of the study was to investigate the possible catalytic activity for enhanced colorless by-product removal. The analysis of UV–VIS spectra proceeded for indirect evaluation of some possible colorless by-products and constituents of the wastewater matrix. The possible catalytic, non-catalytic and adsorptive actions for the removal of the colorless by-products were considered. The analysis was conducted by comparison between the spectra of the wastewater (
Figure 3a) and the spectra of the purified model substances: the possible by-products, namely H-acid and p-ester and the surfactant, Perigen LDR (
Figure 3b). For all treatments the same catalyst dose, 0.5 g/L, and the same ozone dose (in the case of ozone application)—0.9 g O
3/L was used.
When compares the spectra presented in
Figure 3a,b some characteristic peaks could be observed. Even though the analysis of the complex matrix such as the wastewater cannot be clear, some indirect information was concluded. Firstly, the characteristic peak in the visible region of the wastewater spectrum, between 596 and 610 nm, was found to be responsible for the dye chromophore and correlated to color. It could be observed that both EC and O
3 resulted in a significant decrease in this peak which means color removal. In the case of catalytic ozonation, the catalytic effect could be observed at most for AC which was in agreement with data presented in
Section 2.1.2. The adsorptive abilities were found to be poor for color removal, likewise in the abovementioned Faria and co-workers study [
29].
In contrast to the peak correlated with the color of the dye, peaks of the UV spectral region were much more resistant to the EC and O
3 treatments. The UV spectral region is related to the strong absorption of UV light caused mostly by aromatic organics which are colorless for a human eye [
38]. This can be a suggestion that colorless aromatic and non-aromatic compounds are partially resistant to basic treatments such as ozonation or EC. Nevertheless, some characteristic phenomena could be observed for the UV spectral region for single treatments O
3 and EC as well as for catalytic ozonation. Moreover, the adsorptive action of catalysts was also visible in the UV region.
The reduction in absorbance at 310 nm occurred after EC, but not after single ozonation. It can be explained by the above mentioned effective coagulation of surfactant by Fe2+. Consequently, the UV peaks at 210 and 250 nm were decreased after the EC, as well, which must have been related to partial coagulation of colorless compounds from the wastewater.
At the same time, rather poor adsorptive action could be observed. The adsorption was the step after EC and was compared to this data (not to the spectrum of the raw wastewater). The decrease of UV peak at 260 nm was the most significant, probably related to aniline derivatives adsorption on AC.
The last significant observation was that the use of catalysts gave effect not only in RB5 removal (which was responsible for color) but in the removal of colorless compounds as well. Probably, the colorless substances detected in the UV spectral region could more effective oxidized during catalytic oxidation, especially in the case of AC use. These results are in agreement with previous works of Malik [
25], Wu [
28], Faria [
29], and their co-workers, who showed that colorless refractory compounds can be much more effectively removed by catalytic process than non-catalytic (single ozonation).
Unfortunately, the UV spectra of single compounds overlap when complex mixture such as wastewater is investigated which can be observed in
Figure 3a. However, some characteristic peaks could be observed in the UV region the deeper analysis was not possible (especially the quantitative). Therefore, the more accurate chromatographic analysis is planned in the future to identify the specific by-products.
2.3. Mineralization in Catalytic and Non-Catalytic Processes
As can be observed in
Section 2.2 the analysis of the specific constituents of the wastewater can be considered as a demanding task. As far as the complex matrix of the wastewater was concerned the global indicators such as total organic carbon (TOC), chemical oxygen demand (COD), and 5-day biochemical oxygen demand (BOD) were helpful. The treatments were analyzed in three groups: non-catalytic processes (O
3, EC, and EC + O
3), adsorptive processes (EC + AC, EC + AC-Pt, and EC + TiO
2) and catalytic processes (EC+ O
3AC, EC+ O
3AC-Pt, and EC+ O
3TiO
2).
In
Figure 4a, the data of non-catalytic processes (O
3, EC, and EC + O
3) were presented. In contrast to the satisfying results of decolorization achieved after O
3 and EC (when proceeded as a single treatment), rather poor TOC and COD removal could be observed. The phenomenon can be correlated to the abovementioned poor removal of colorless compounds. This result is in agreement with previous works in the field [
9]. Much more satisfying result could be observed when EC and O
3 were used jointly. Then the colorless constituents of the wastewater matrix were removed more effectively and resulted in lower TOC/TOC
0 and COD/COD
0 values. The BOD/BOD
0 ratio raised only when oxidative ozone treatment was used, but the BOD after EC remained almost unchanged (
Figure 4a). The reason could be an occurrence of more biodegradable by-products of lower molecular weight during O
3, while they could be coagulated during EC.
While adsorptive abilities of the catalysts observed (
Figure 4b) it can be considered that they cannot be neglected. All catalysts revealed adsorptive abilities mostly to colorless compounds (mentioned in
Section 2.2) which resulted in a decrease in TOC/TOC
0 and COD/COD
0 values. The high adsorptive ability of carbon material has been reported before [
39]. However, it is hard to compare the results of simulated and real textile wastewater. The BOD/BOD
0 values noted for adsorptive processes were about 1.0 so the change BOD was rather insignificant and hard to discuss.
In
Figure 4c, the results of catalytic ozonation were presented. The catalytic action of all catalysts was proved by a significant decrease in TOC/TOC
0 and COD/COD
0. Even though the additional color removal was not visible for catalytic ozonation with the use of AC–Pt and TiO
2, but only for AC, taking into account the global parameters (TOC and COD) the enhancement in colorless compounds removal could be assumed. The BOD/BOD
0 ratio increased for all catalysts as well. Therefore, more efficient oxidation resulting in the production of more biodegradable compounds can be expected. The AC was found as the most efficient catalyst for mineralization and biodegradability enhancement during catalytic ozonation.
2.4. Toxicity in Catalytic and Non-Catalytic Processes
The toxicity assessment was evaluated as a screening test resulting in the effective coefficient (EC
50) values. The EC
50 is an indicator, which gives information on what concentration of the sample will cause the death of half of the population (indicator organisms) after the specified exposure time. In this study, the marine bacteria,
Vibrio fischeri, was used because of their physiological living conditions in a salty environment, which was in some correlation to the wastewater. The EC
50 values were collected after 15 min of exposure. As can be observed in
Figure 5, the EC treatment had a negligible effect on the toxicity of the wastewater, the EC
50 was the same as for the raw wastewater. An oxidative treatment by O
3 increased EC
50 probably due to the decomposition of some toxic compounds (mentioned in
Section 2.2, naphthol and aniline derivatives). However, using O
3 as a polishing step after EC treatment gave adverse effects. Even though the decrease in BOD has not been disclosed (
Figure 4a) it must be concluded that toxic by-products appeared. The adsorption on the carbonic materials (AC and AC–Pt) gave positive results in toxicity removal which must have been caused by catching by-products. This effect was not observed for TiO
2, which may suggest lower adsorption compering to carbon-based materials. In the case of catalytic ozonation respectively lower EC
50 values were detected. Firstly, because of partial desorption of low-molecular-weight by-products. Secondly, catalytic ozonation resulted in more efficient by-products occurrence. These by-products should be detected and investigated separately for their toxicity. Therefore, more scientific attention should be directed to by-products testing, to avoid increasing the toxicity of the wastewater after the treatment. Globally, the toxicity after catalytic ozonation using carbon-based catalysts resulted in lower toxicity than this disclosed for the raw wastewater. Unfortunately, for the use of TiO
2 as a catalyst, the final toxicity was higher than before treatment. Even though these results are partially in agreement with some previous works, e.g., initial EC
50 value [
40] and decrease in toxicity after combined use of AC and ozone [
41], not all have a reflection in the literature. The investigation of TiO
2 needs more attention to find a deeper explanation and it should be kept in mind how few studies were carried out for real industrial wastewater.
2.5. Basis of Catalytic Action
Three types of catalysts (metal oxide, metal on the support and activated carbon) and two types of wastewater (raw and pre-treated) were investigated for catalytic ozonation. Generally, two main issues should be taken into consideration when the catalytic action is disused:
- (1)
The wastewater matrix which determines process conditions,
- (2)
The catalyst type, namely the type of interaction between pollutants (organics) or ozone and the catalyst.
Nawrocki, in his work [
15], points out the outstanding role of the experimental conditions, especially: the pH value, adsorption of the substrate (pollutant) on the catalyst surface, adsorption of the ozonation by-products on the catalyst surface, ions occurring in the experimental solution. Undoubtedly, these conditions are directly or indirectly determined by the wastewater matrix. In this study, the wastewater after textile dyeing was the object. Therefore, the extremely high alkalinity (pH 11.29) and salinity (NaCl conc. 59.9 g/L) occurred. Moreover, the high surfactant content can be expected in this wastewater, because it was used as a leavening agent (0.5 g/L) in dyeing. Consequently, high concentration of Na
+ and Cl
− ions from dissociated salt, the OH
− ion from water and alkalis, as well as, the high content of polar-non-polar surfactant molecules build the wastewater matrix beside the dye.
As mentioned in
Section 2.1, a surfactant disturbed color removal during catalytic ozonation. However, when the surfactant was partially removed by EC, the catalytic action in color removal could be observed (
Figure 2). The explanation is the critical micelle concentration, CMC, which is the concentration of surfactant causing the spontaneous building of the mono-molecular adsorptive layer on any interphase surface. Probably this adsorptive layer of the surfactant blocked the catalyst independently from its type. Consequently, the additive action of catalysts could not have been observed for color removal from raw wastewater.
When the type of catalyst is considered the active centers on the surface must be discussed because at least one of above-mentioned action must occur for catalytic ozonation: ozone is adsorbed on the catalyst surface, a molecule of a pollutant is adsorbed on the catalyst surface or both ozone and pollutant are adsorbed on the surface. The high pH value and the ion content of the wastewater influenced on the catalysts surface and the activity of centers.
In this study, the lowest activity was observed for TiO
2. Due to high alkalinity all catalysts, including TiO
2, worked above the pH
PZC (the P25 pH
PZC is 6.26 by producer, measured by the salt method according to [
42] was 4.5). Moreover, TiO
2 was used without UV light. Therefore, it could act only as an adsorbent and electron excited centers could not occur on the catalyst surface in this case. Correspondingly, TiO
2 could act only as transition metal oxide which was characterized by Lewis acid active centers. This type of active center builds electron donor–acceptor complex by electron acceptance from Lewis bases. However, the center localized on single atom (Ti
4+) might have been blocked by chlorides which could disturb adsorption in this experiment and resulted in the low catalytic activity of this catalyst type [
43,
44]. Moreover, the surface of TiO
2 must have been covered by adsorbed water, more precisely by HO
− ions, which generates proton Bronsted centers [
44]. Consequently, the TiO
2 surface expectedly covered by TiOH (possibly TiO
− in a more dissociated form) which might be considered as centers for ozone decomposition initiators. However, the adsorption of our main pollutant, RB5, could be difficult, because it is negatively charged. It is more likely that the catalytic activity occurred through the initiation of the ozone decomposition and, consequently, oxidation of organics by ROS in the bulk rather than on the catalyst surface.
The catalytic activity of Pt–AC catalyst is determined mostly by highly reactive platinum molecules. The catalytic action of metal on the support was referred to as a hydroxyl radical generation form ozone through the electron transfer from reduced form of the metal. Subsequently, the oxidized metal could temporarily adsorb the organics. The organics could be next oxidized by ozone or hydroxyl radical and then the by-product was desorbed living the metal in the reduced form, waiting for the next cycle [
16]. In our experimental conditions, the highly reactive platinum particles were probably immediately blocked by Cl
− ions and their activity were dramatically decreased. The catalytic activity which could be observed in
Section 2.1 and
Section 2.2 was probably the result of the AC carrier adsorption.
The highest efficiency was noted for the AC. Such high AC effectivity could be observed for color removal and colorless compounds (by-products), as well. It can be noted that AC support during ozonation was referred as giving additive results. However, in most cases, these results were referred to non-complex objects such as phenol or organic acids [
21].
Dual AC action, involving surface and bulk reaction, is the most probable in the case of our study when the complex wastewater matrix was investigated. The surface action can be promoted due to AC active center, which is of Lewis basis character, and is not blocked in the presence of HO
− and Cl
− ions. These centers are referred to as e.g., pyrones or chromone which are reach in delocalized π-electron systems [
34]. Therefore, the ozone can be held by these active centers and react with organics. As it was previously showed by Wawrzkiewicz and coworkers [
45], the electrostatic π–π interaction could also be significant for the adsorption process of dye molecules. Therefore, the electrostatic action can be significant for the AC catalytic activity by keeping organics near the active surface.
The hypothesis of an important role of π–π interaction of AC can be as far significant as it can be assumed that they are likely renewable. The same AC could be used during four sequential cycles without a significant loss in activity (
Figure S1, Supplementary Materials).
Moreover, the active centers on AC surface through the donor-acceptor complexes with H
2O molecules can act as ozone decomposition initiators, especially in the alkaline environment [
46]. Afterward, the initiation step on AC, and then homogeneous propagation steps take place in the bulk leading to ROS formation (in the bulk). The following reactions of ROS with organics takes place in the bulk. Nevertheless, Biernacki and coworkers, in their recent study [
47], proposed the mechanism of catalytic ozone decomposition resulting in hydroxyl radical adsorbed on the AC surface. Furthermore, they suggested radical reactions occurrence on the AC surface claiming that the rate of those reactions is higher than the rate of radical desorption. Taking into consideration how complex is the experimental solution (the wastewater) none of abovementioned hypothesis cannot be excluded and it can be expected that they all participate in the overall catalytic effect. In the case of our study, the suggestion for more active ROS generation by AC was the visible enhancement in colorless refractory by-products removal (discussed in
Section 2.2) which are unlikely decomposed by molecular ozone.
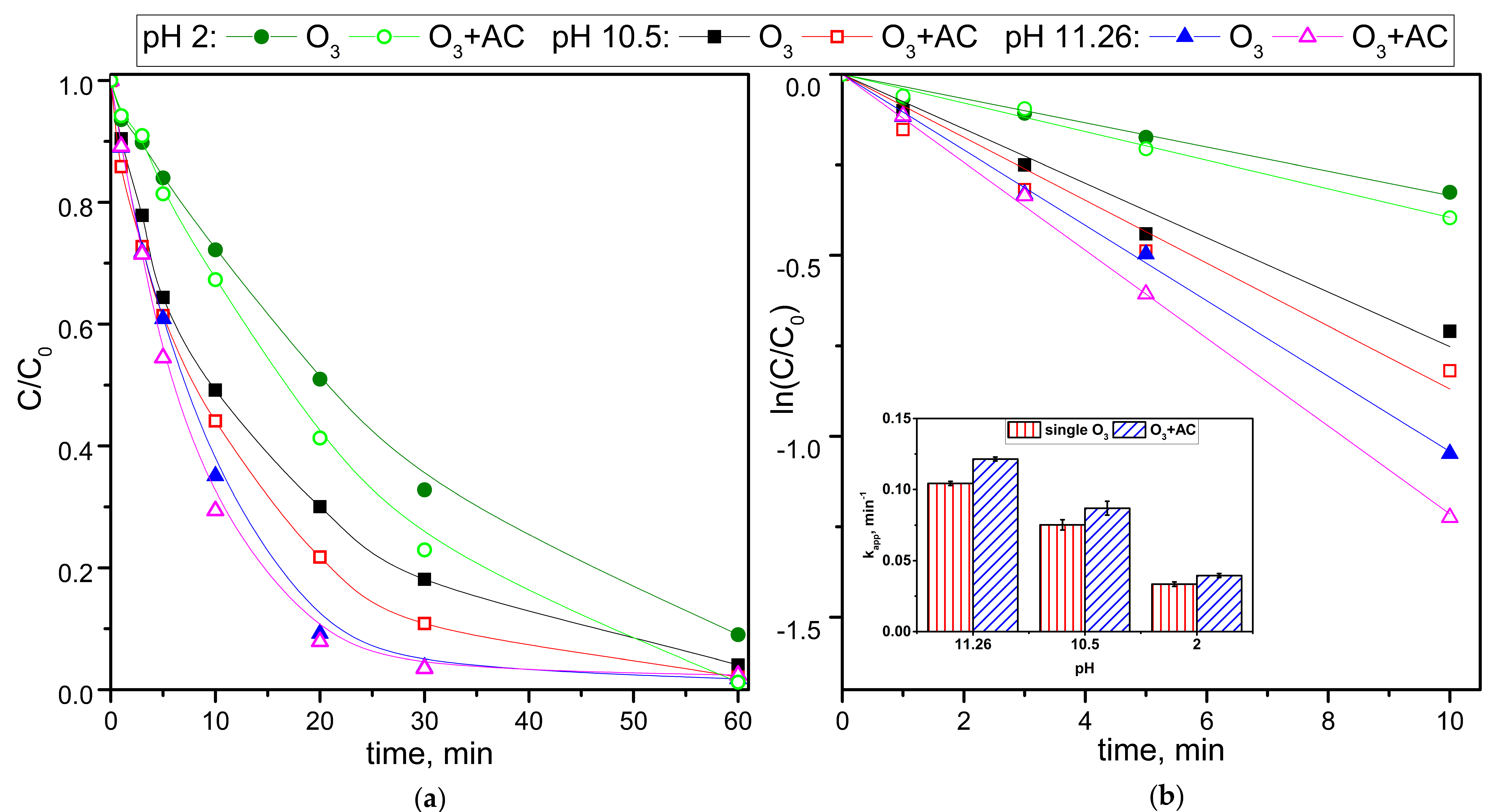
Figure 6 presents the catalytic activity of AC exanimated for a model solution of RB5 at various pH: 2, 10.5, and 11.26. Our further study showed that during RB5 ozonation the oxidation through radical action could be observed at relatively low pH equal 4 and contribution of radical oxidation raised with increasing pH [
35]. Therefore, the additional catalytic AC action at high alkalinity could be questionable. However, it could be observed that independently from pH value decolorization rate was always higher for AC catalyzed ozonation than for single ozonation. It was even higher in the alkaline medium which is in agreement with results presented by Beltran and coworkers [
46].
3. Materials and Methods
3.1. Materials
In the study two types of real industrial textile wastewater (emanate from the dyeing of cotton fabric) were used. The base industrial textile wastewater was untreated-raw (the first) or pretreated by electrocoagulation (EC) (second). In wastewater the dye and the auxiliaries were used (all in technical grade). As a dye the industrial product named Remazol Ultraschwarz NN (DyStar (worldwide Co.), (product based on Reactive Black 5 (RB5), Singapore). Perigen LDR was applied as an industrial dyeing assistant—(SAA–naphthalenesulfonic acid and carboxylates mixture (Textilchemie Dr. Petry Co. Reutlingen, Germany)). Moreover, NaCl, NaOH, and Na
2CO
3 were used (provided by Solino, Warsaw, Poland and Jarbur, Szydłowiec, Poland, respectively). To obtain the wastewater homogeneity the average wastewater from several dyeing operations was stored in the equalization reservoir. The wastewater after EC was taken from collection tank on EC installation. The characteristic of both, the raw wastewater and EC-pretreated wastewater were presented in
Table 1.
As a catalyst: activated carbon (AC), platinum supported on activated carbon, 1% w/w of Pt (Pt–AC) and titanium dioxide (TiO2) were applied. The catalysts based on activated carbon were provided by Sigma-Aldrich®. AC from charcoal in a micro-powder form of 149 μm grain size (100 US Mesh) was used. TiO2, a commercial product P25 from Degussa (rutile: anatase/85:15, 99.9% purity, 20 nm grains) was used.
P-Ester (sodium salt of 4-((2-sulfatoethyl) sulfonyl) aniline) and sodium 5-amino-4-hydroxy-7-sulfonaphthalene-2-sulfonate, which is H-acid was used as the possible generated by-products during the treatment (both of the analytical grade, (Boruta, Poland)).
3.2. Methods
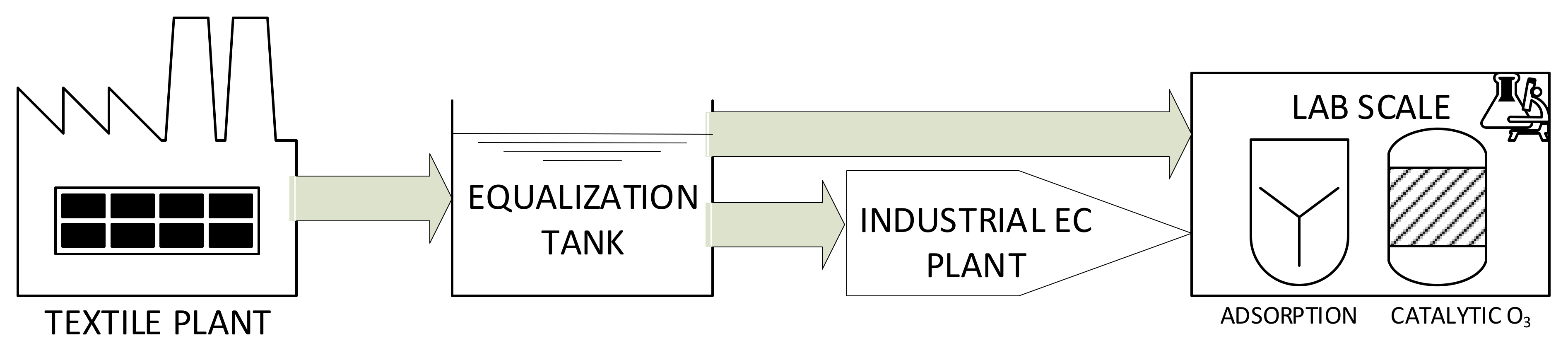
The textile wastewater originated from the textile plant was stored in the equalization tank and transferred to an industrial-scale EC treatment plant. Afterward the catalytic ozonation, preceded by the adsorption phase, was carried out in the laboratory as it is presented in
Figure 7.
Electrocoagulation Treatment: The EC plant was purchased from PFTechnology Co. (Poland). The industrial EC plant was equipped in 300 L electrochemical reactor (the input volumetric flow was equal to 2200 L/h) was used. The iron electrodes (steel) were used. In the process the 50 electrodes with the overall area of 17.5 m2 (5 parallel sections of 10 electrodes in each package) were applied. The current was set on 87.5 A (current density equal to 5 A/m2). After EC reactor the mixing tank (for residual hydrogen removal) and a horizontal-flow settler and a filter (1 μm) were used in the system for sludge removal. The pretreated wastewater was collected without changing the pH value.
Ozonation Treatment: The experiments were performed in the lab-scale (heterogeneous gas–liquid system). A semi-batch glass reactor (1 L) with the cooling jacket was used. Ozone was pumped into the reaction solution via the porous plate located at the bottom of the reactor. Additional mixing was performed by magnetic stirrer (set at 200 rpm, Wigo ES 21(Poland)). Ozonek Ozone Generator (Poland) was used for ozone production. The O3 was generated from the oxygen supplied from a compressed gas cylinder (O2 purity 99.5%). The inlet and outlet O3 concentration was measured by BMT 963 Vent ozone analyzer. During the experiments the temperature and pH were monitored by Elmetron C411 device (Poland).
Single Adsorption: Before catalytic ozonation, for all catalysts the adsorption experiments were carried out for 30 min. The catalyst was added directly into wastewater. The adsorption was carried out in the same reactor as ozonation treatment but without ozone inlet to maintain the uniform experimental conditions. All experimental condition was the same as during ozonation treatment. The temperature was kept stable and was equal to 23 ± 1 °C.
Catalytic Ozonation: The same reactor as for adsorption and ozonation was used for catalytic ozonation. All experimental condition was the same as during ozonation treatment. For all catalysts the catalyst concentration was 0.5 g/L for each time. After reaction the filtration (0.45 μm filter) of the catalysts was made.
3.3. Analytical Methods
Absorbance: The spectrophotometer (Helios Thermo) was used for measurement of the spectra of the samples collected at time intervals). To determine the color removal the absorbance in the visible absorption region (λmax 596 nm) was investigated.
Chemical Oxygen Demand (COD) was obtained by the dichromate (VI)–LCK 514 and 314 tests using the standard method with a HACH-LANGE apparatus (DR 3800). Total Organic Carbon (TOC) was measured a HACH IL 550TOC-TN apparatus
Before COD and TOC tests the samples were diluted to avoid the influence of the salts.
5-day Biochemical Oxygen Demand (BOD) determined using low-volume LCK 555 tests measured with a HACH-LANGE apparatus (DR 3800).
Toxicity assessment was performed using the marine luminescent bacteria
Vibrio fischeri according to ISO 11348–3 [
48]. The procedure was followed by the methodology 81.9% basic test protocol (MicrotoxOmni 4.2, Modern Water Inc.) provided by Microtox Model 500 Analyzer (Modern Water Inc., Newark, Delaware, USA). As very high toxicity was expected, the effluents were diluted 1:10 with MQ water. Toxicity after 15 min. of exposure was expressed as EC
50.
4. Conclusions
A wide study on the catalytic activity of three types of catalysts (metal oxide, metal on the support, and activated carbon) for the ozone treatment of two types of wastewater (raw and pre-treated) was conducted. The experiment led us to the main conclusion that the catalytic action can be seriously limited by the unfavorable conditions of the wastewater matrix. When the raw wastewater was considered the blocking of the surface of the catalyst could be observed. Consequently, the color removal was not higher than this observed for single ozone treatment. The main cause of the phenomenon was found in the mono-molecular layer formation form the surfactant which was used as a leveling agent in dyeing operation. As far as the surfactant that was present in the wastewater was in a concentration above the CMC value the ability to spontaneous micelle formation was unavoidable. When the surfactant content was reduced by EC pretreatment the catalytic activity of the catalysts could be observed in color removal, colorless organics removal, higher mineralization, and lower toxicity. However, depending on the catalyst type, the various catalytic effect could be observed. The lowest catalytic activity was observed for TiO2. The activity of the adsorption centers of Lewis acid origins was probably highly limited by high alkalinity and ion content of the wastewater. In case of the Pt–AC catalyst, it was highly probable that the metal (Pt) related active centers were blocked by Cl-ions (from dissociated NaCl) and the observed catalytic effect was caused by AC carrier. The most significant catalytic activity was shown for AC. This activity was relevant in color removal which means that high-molecular weight RB5 dye molecules could be more effectively decomposed comparing to single ozonation. But the low-molecular-weight colorless by-products could be removed more efficiently, as well. The catalytic activity of AC was assigned as a basic center activity. The double role of these basic centers can be assumed, the can contribute to organics adsorption and ozone decomposition initiators. The electrostatic π–π interaction could also be significant for AC catalytic activity. Based on the presented results the AC catalytic action should be concerned as a complex mechanism where the additional ROS generation was possible, but, the adsorptive AC abilities were significant, as well. Nor the surface oxidation or bulk oxidation cannot have been excluded, and it was highly possible that both occurred and were significant.
This preliminary study on the catalytic action of various types of catalysts gave a piece of information on how complex issues the industrial wastewater treatment of dyeing effluents can be. Further study is encouraged to be continued to learn more about the basis of catalytic action and oxidation of specific by-products.
Summarizing the discussion on probable mechanisms of catalytic activity of various types of catalyst, the inhibition of the catalytic abilities was the main problem. Two main levels of blocking might be considered:
Morphological: the interface surface catalyst-bulk covered by surfactant layer (of the character of Langmuir–Blodgett film).
Deactivation (Pt–AC) or limitation of activity (TiO2) of the adsorption centers of the catalysts.