Suppression of Hydrophobic Recovery in Photo-Initiated Chemical Vapor Deposition
Abstract
1. Introduction
2. Results
2.1. Surface Modification
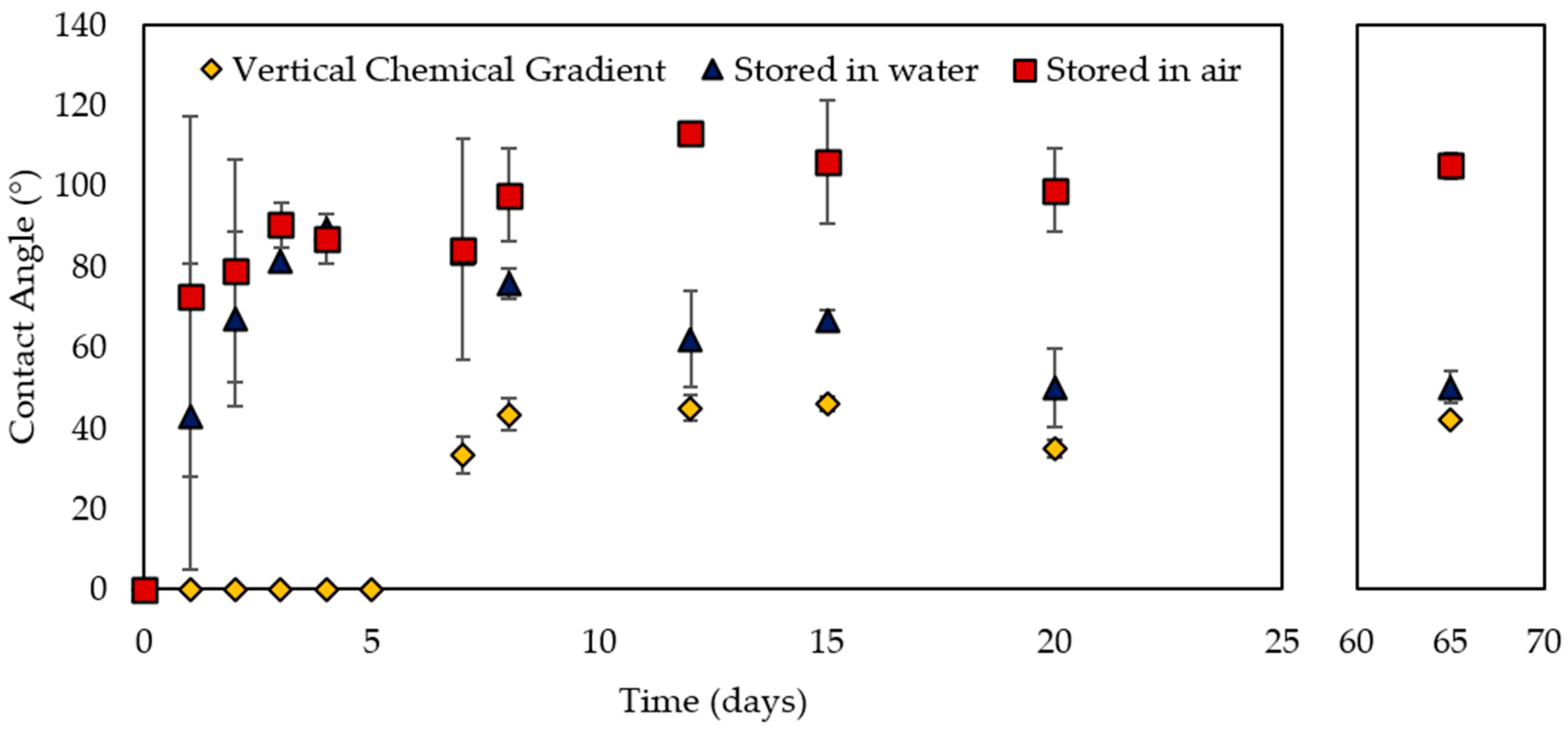
2.2. Effect of Storage Conditions
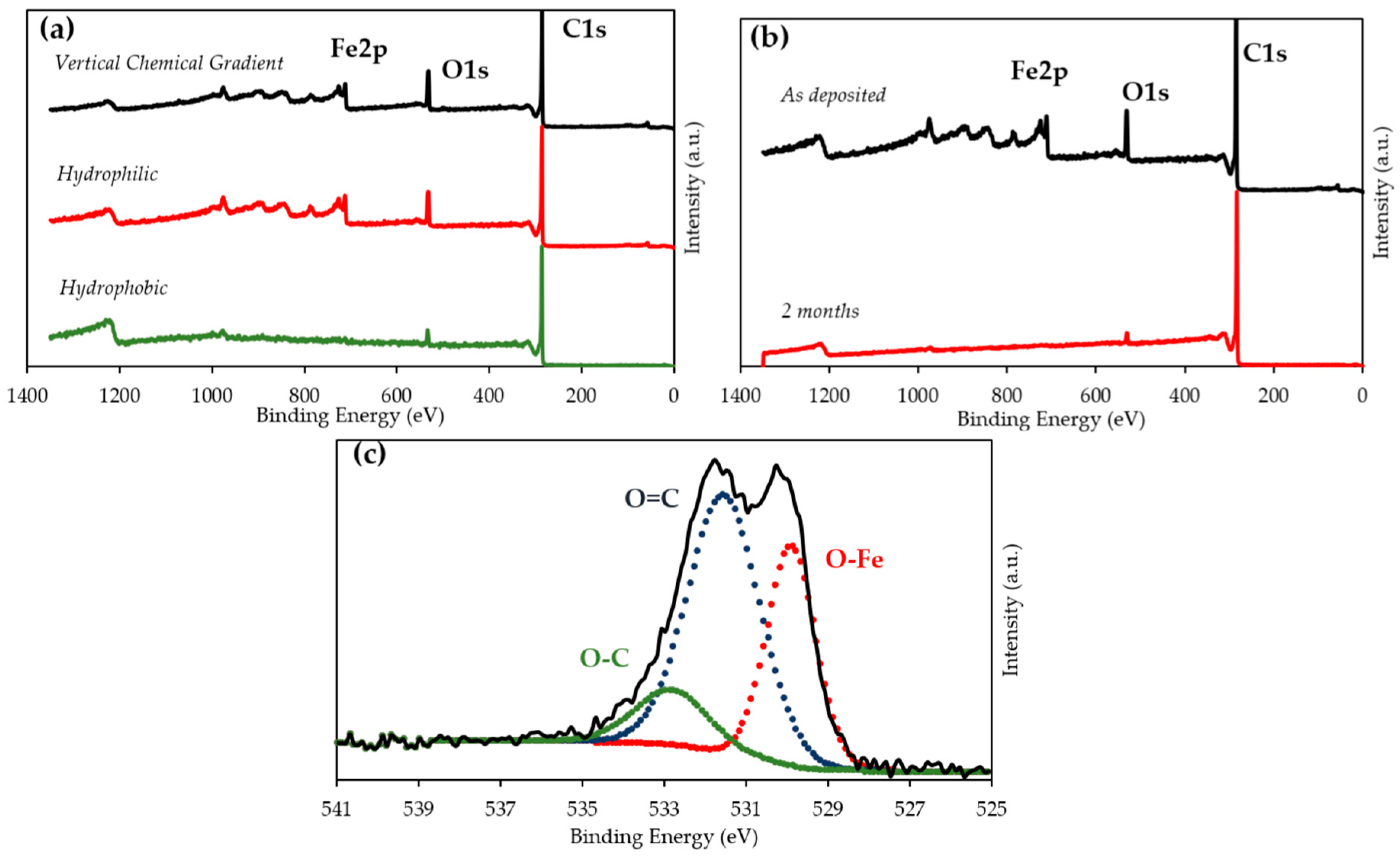
2.3. Effect of Surface Chemistry
3. Discussion
4. Materials and Methods
4.1. Carbon Nanotube (CNT) Growth
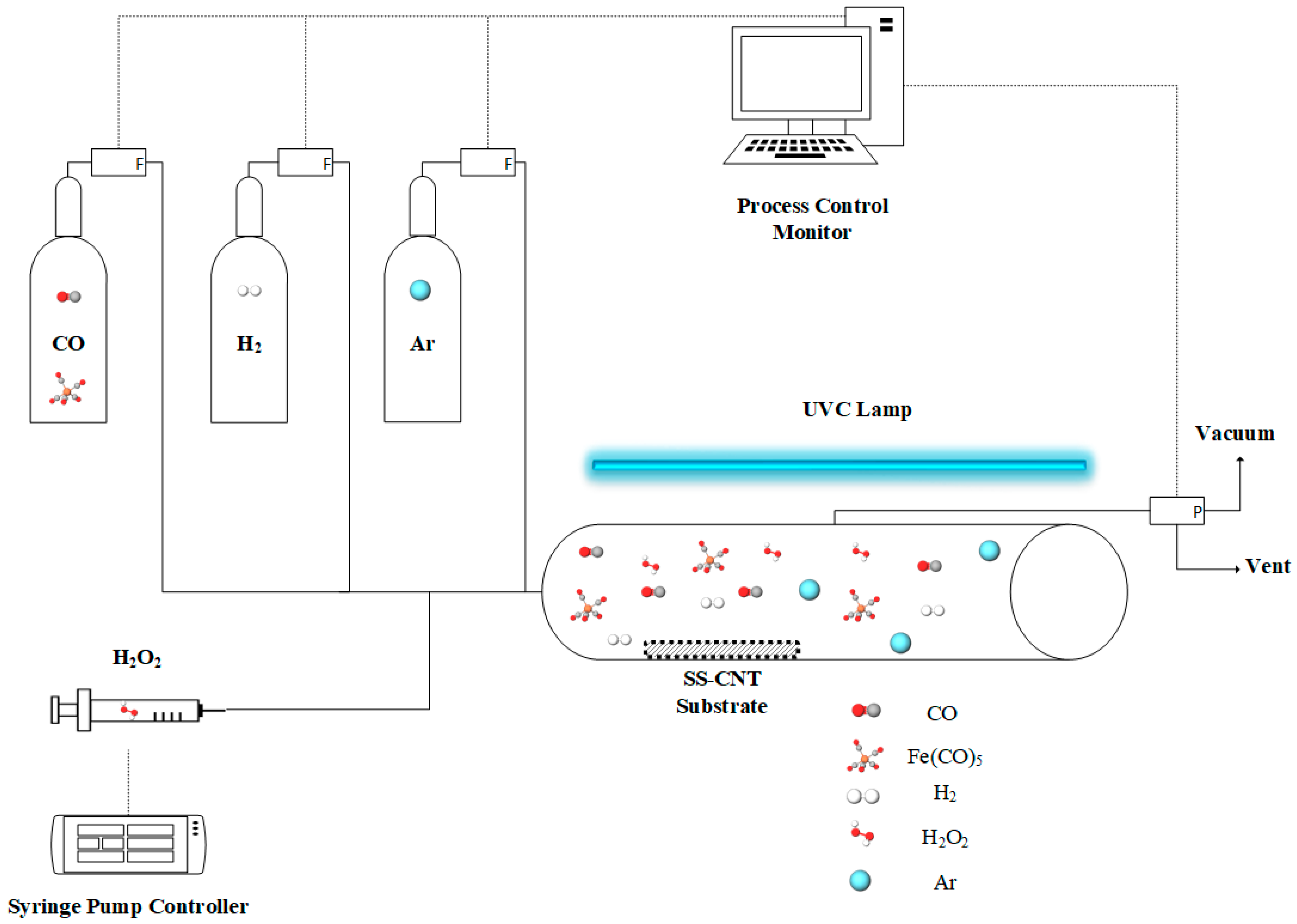
4.2. Photo-Initiated Chemical Vapor Deposition (PICVD)
4.3. Surface Characterization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, S.; Huang, J.; Chen, Z.; Lai, Y. Bioinspired Special Wettability Surfaces: From Fundamental Research to Water Harvesting Applications. Small 2017, 13, 1–28. [Google Scholar] [CrossRef]
- Drelich, J.; Chibowski, E.; Meng, D.D.; Terpilowski, K. Hydrophilic and superhydrophilic surfaces and materials. Soft Matter 2011, 7, 9804–9828. [Google Scholar] [CrossRef]
- Ueda, E.; Levkin, P.A. Emerging applications of superhydrophilic-superhydrophobic micropatterns. Adv. Mater. 2013, 25, 1234–1247. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.; Dong, Z.; Jiang, L. Bioinspired Designs of Superhydrophobic and Superhydrophilic Materials. ACS Cent. Sci. 2018, 4, 1102–1112. [Google Scholar] [CrossRef] [PubMed]
- Otitoju, T.A.; Ahmad, A.L.; Ooi, B.S. Superhydrophilic (superwetting) surfaces: A review on fabrication and application. J. Ind. Eng. Chem. 2017, 47, 19–40. [Google Scholar] [CrossRef]
- Yu, Z.; Yun, F.F.; Wang, Y.; Yao, L.; Dou, S.; Liu, K.; Jiang, L.; Wang, X. Desert Beetle-Inspired Superwettable Patterned Surfaces for Water Harvesting. Small 2017, 13, 1–6. [Google Scholar] [CrossRef]
- Salta, M.; Wharton, J.A.; Stoodley, P.; Dennington, S.P.; Goodes, L.R.; Werwinski, S.; Mart, U.; Wood, R.J.K.; Stokes, K.R. Designing biomimetic antifouling surfaces. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 4729–4754. [Google Scholar] [CrossRef]
- Nurioglu, A.G.; Esteves, A.C.C.; De With, G. Non-toxic, non-biocide-release antifouling coatings based on molecular structure design for marine applications. J. Mater. Chem. B 2015, 3, 6547–6570. [Google Scholar] [CrossRef] [PubMed]
- Thomas, K.V.; Brooks, S. The environmental fate and effects of antifouling paint biocides. Biofouling 2010, 26, 73–88. [Google Scholar] [CrossRef]
- Chiavarini, S.; Ubaldi, C.; Cannarsa, S. Biocides in antifouling paints: Environmental concentration levels and distribution. Energia Ambiente e Innovazione 2014, 52–56. [Google Scholar] [CrossRef]
- Majhy, B.; Iqbal, R.; Sen, A.K. Facile fabrication and mechanistic understanding of a transparent reversible superhydrophobic – superhydrophilic surface. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhao, N.; Xu, J. Fabrication and application of superhydrophilic surfaces: A review. J. Adhes. Sci. Technol. 2014, 28, 769–790. [Google Scholar] [CrossRef]
- Andrade, J.D.; Smith, L.M.; Gregonis, D.E. The Contact Angle and Interface Energetics. In Surface and Interfacial Aspects of Biomedical Polymers; Plenum Press: New York, NY, USA, 1985; pp. 249–292. [Google Scholar]
- Everaert, E.P.; Van Der Mei, H.C.; Busscher, H.J. Hydrophobic recovery of repeatedly plasma-treated silicone rubber. Part 2. A comparison of the hydrophobic recovery in air, water, or liquid nitrogen. J. Adhes. Sci. Technol. 1996, 10, 351–359. [Google Scholar] [CrossRef]
- Granick, S. Polymer surface dynamics. MRS Bull. 1996, 21, 33–36. [Google Scholar] [CrossRef]
- Morra, M.; Occhiello, E.; Garbassi, F. Contact Angle Hysteresis in Oxygen Plasma Treated Poly(tetrafluoroethylene). Langmuir 1989, 5, 872–876. [Google Scholar] [CrossRef]
- Van Os, M.T. Surface Modification by Plasma Polymerization: Film Deposition, Tailoring of Surface Properties and Biocompatibility; Universiteit Twente: Enschede, The Netherlands, 2000. [Google Scholar]
- Labonté, V.; Marion, A.; Virgilio, N.; Tavares, J.R. Gas-phase surface engineering of polystyrene beads used to challenge automated particle inspection systems. Ind. Eng. Chem. Res. 2016, 55, 7362–7372. [Google Scholar] [CrossRef]
- Borcia, C.; Punga, I.L.; Borcia, G. Surface properties and hydrophobic recovery of polymers treated by atmospheric-pressure plasma. Appl. Surf. Sci. 2014, 317, 103–110. [Google Scholar] [CrossRef]
- Bormashenko, E.; Chaniel, G.; Grynyov, R. Towards understanding hydrophobic recovery of plasma treated polymers: Storing in high polarity liquids suppresses hydrophobic recovery. Appl. Surf. Sci. 2013, 273, 549–553. [Google Scholar] [CrossRef]
- Vandenbossche, M.; Hegemann, D. Recent approaches to reduce aging phenomena in oxygen- and nitrogen-containing plasma polymer films: An overview. Curr. Opin. Solid State Mater. Sci. 2018, 22, 26–38. [Google Scholar] [CrossRef]
- Hegemann, D.; Michlíček, M.; Blanchard, N.E.; Schütz, U.; Lohmann, D.; Vandenbossche, M.; Zajíčková, L.; Drábik, M. Deposition of Functional Plasma Polymers Influenced by Reactor Geometry in Capacitively Coupled Discharges. Plasma Process. Polym. 2016, 13, 279–286. [Google Scholar] [CrossRef]
- Hegemann, D.; Lorusso, E.; Butron-Garcia, M.I.; Blanchard, N.E.; Rupper, P.; Favia, P.; Heuberger, M.; Vandenbossche, M. Suppression of Hydrophobic Recovery by Plasma Polymer Films with Vertical Chemical Gradients. Langmuir 2016, 32, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Holly, F.J.; Refojo, M.F. Wettability of hydrogels I. Poly(2-hydroxyethyl methacrylate). J. Biomed. Mater. Res. 1975, 9, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Li, Y.; Feng, F.; Zhang, H. Study on wettability, mechanical property and biocompatibility of electrospun gelatin/zein nanofibers cross-linked by glucose. Food Hydrocoll. 2019, 87, 1–10. [Google Scholar] [CrossRef]
- Spruell, J.M.; Wolffs, M.; Leibfarth, F.A.; Stahl, B.C.; Heo, J.; Connal, L.A.; Hu, J.; Hawker, C.J. Reactive, multifunctional polymer films through thermal cross-linking of orthogonal click groups. J. Am. Chem. Soc. 2011, 133, 16698–16706. [Google Scholar] [CrossRef] [PubMed]
- Owen, M.J.; Smith, P.J. Plasma treatment of polydimethylsiloxane. J. Adhes. Sci. Technol. 1994, 8, 1063–1075. [Google Scholar] [CrossRef]
- Everaert, E.P.; van der Mei, H.C.; De Vries, J.; Busscher, H.J. Hydrophobic recovery of repeatedly plasma-treated silicone rubber. Part 1. Storage in air. J. Adhes. Sci. Technol. 1995, 9, 1263–1278. [Google Scholar] [CrossRef]
- Marquez, A.; Daniel, C.; Sanz, J.F. The vacuum-ultraviolet spectrum of Fe(CO)5: An experimental analysis supported by a CASSCF CCI study of the Rydberg states. J. Phys. Chem. 1992, 96, 121–123. [Google Scholar] [CrossRef]
- Zhang, Y.; Kobayashi, I.; Neves, M.A.; Uemura, K.; Nakajima, M. Effects of surface treatment and storage conditions of silicon microchannel emulsification plates on their surface hydrophilicity and preparation of soybean oil-in-water emulsion droplets. J. Food Eng. 2015, 167, 106–113. [Google Scholar] [CrossRef]
- Lavielle, L.; Schultz, J. Surface properties of graft polyethylene in contact with water. I. Orientation phenomena. J. Colloid Interface Sci. 1985, 106, 438–445. [Google Scholar] [CrossRef]
- Vergelati, C.; Perwuelz, A.; Vovelle, L.; Romero, M.A.; Holl, Y. Poly(ethylene terephthalate) surface dynamics in air and water studied by tensiometry and molecular modelling. Polymer (Guildf). 1994, 35, 262–270. [Google Scholar] [CrossRef]
- Rupper, P.; Vandenbossche, M.; Bernard, L.; Hegemann, D.; Heuberger, M. Composition and Stability of Plasma Polymer Films Exhibiting Vertical Chemical Gradients. Langmuir 2017, 33, 2340–2352. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Dai, X.J.; Xu, H.S.; Zhao, J.H.; Yang, P.; Maurdev, G.; Du Plessis, J.; Lamb, P.R.; Fox, B.L.; Michalski, W.P. Combined continuous wave and pulsed plasma modes: For more stable interfaces with higher functionality on metal and semiconductor surfaces. Plasma Process. Polym. 2009, 6, 615–619. [Google Scholar] [CrossRef]
- Yousif, E.; Haddad, R. Photodegradation and photostabilization of polymers, especially polystyrene: Review. Springerplus 2013, 2, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Hedenqvist, M.S. Barrier packaging materials. In Handbook of Environmental Degradation of Materials; William Andrew Publishing: Norwich, NY, USA, 2005; pp. 547–563. [Google Scholar]
- Galant, O.; Davidovich-Pinhas, M.; Diesendruck, C.E. The Effect of Intramolecular Cross-Linking on Polymer Interactions in Solution. Macromol. Rapid Commun. 2018, 39, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Dorval Dion, C.A.; Raphael, W.; Tong, E.; Tavares, J.R. Photo-initiated chemical vapor deposition of thin films using syngas for the functionalization of surfaces at room temperature and near-atmospheric pressure. Surf. Coatings Technol. 2014, 244, 98–108. [Google Scholar] [CrossRef]
- Chan, K.; Gleason, K.K. Photoinitiated chemical vapor deposition of polymeric thin films using a volatile photoinitiator. Langmuir 2005, 21, 11773–11779. [Google Scholar] [CrossRef]
- McMahon, B.J.; Pfluger, C.A.; Sun, B.; Ziemer, K.S.; Burkey, D.D.; Carrier, R.L. Photoinitiated chemical vapor deposition of cytocompatible poly(2-hydroxyethyl methacrylate) films. J. Biomed. Mater. Res. - Part A 2014, 102, 2375–2382. [Google Scholar] [CrossRef]
- Baxamusa, S.H. Photoinitiated Chemical Vapor Depostion [sic]: Mechanism and Applications. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 2009. [Google Scholar]
- Zhao, J.; Wang, M.; Gleason, K.K. Stabilizing the Wettability of Initiated Chemical Vapor Deposited (iCVD) Polydivinylbenzene Thin Films by Thermal Annealing. Adv. Mater. Interfaces 2017, 4, 1–9. [Google Scholar] [CrossRef]
- Lari, H.N.; Chaouki, J.; Tavares, J.R. Continuous aerosol photopolymerization to coat de-agglomerated nanoparticles. Chem. Eng. J. 2020, 390, 124526. [Google Scholar] [CrossRef]
- Nasri Lari, H.; Farhanian, D.; Boffito, D.C.; Patience, G.S.; De Crescenzo, G.; Chaouki, J.; Tavares, J.R. Shedding light on iron pentacarbonyl photochemistry through a CVD case study. Catal. Commun. 2017, 100, 19–23. [Google Scholar] [CrossRef]
- Hosseininasab, S.; Faucheux, N.; Soucy, G.; Tavares, J.R. Full range of wettability through surface modification of single-wall carbon nanotubes by photo-initiated chemical vapour deposition. Chem. Eng. J. 2017, 325, 101–113. [Google Scholar] [CrossRef][Green Version]
- Berard, A.; Patience, G.S.; Chouinard, G.; Tavares, J.R. Photo Initiated Chemical Vapour Deposition to Increase Polymer Hydrophobicity. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Farhanian, D.; De Crescenzo, G.; Tavares, J.R. Kinetics, Chemistry, and Morphology of Syngas Photoinitiated Chemical Vapor Deposition. Langmuir 2017, 33, 1780–1791. [Google Scholar] [CrossRef] [PubMed]
- Petruczok, C.D.; Armagan, E.; Ince, G.O.; Gleason, K.K. Initiated chemical vapor deposition and light-responsive cross-linking of poly(vinyl cinnamate) thin films. Macromol. Rapid Commun. 2014, 35, 1345–1350. [Google Scholar] [CrossRef]
- Senzai, T.; Fujikawa, S. Fast hydrophobicity recovery of the surface-hydrophilic poly(dimethylsiloxane) films caused by rechemisorption of dimethylsiloxane derivatives. Langmuir 2019, 35, 9747–9752. [Google Scholar] [CrossRef]
- Yasuda, H.; Sharma, A.K.; Yasuda, T. Effect of Orientation and Mobility of Polymer Molecules At Surfaces on Contact Angle and Its Hysteresis. J. Polym. Sci. Part A-2 Polym. Phys. 1981, 19, 1285–1291. [Google Scholar] [CrossRef]
- Tseng, K.C.; Turro, N.J.; Durning, C.J. Molecular mobility in polymer thin films. Phys. Rev. E Stat. Phys. Plasmas Fluids Relat. Interdiscip. Top. 2000, 61, 1800–1811. [Google Scholar] [CrossRef]
- Briggs, D. Handbook of X-ray Photoelectron Spectroscopy; Wanger, C.D., Riggs, W.M., Davis, L.E., Moulder, J.F., Muilenberg, G.E., Eds.; Perkin-Elmer Corp., Physical Electronics Division: Eden Prairie, MN, USA, 1979; pp. 190–195. [Google Scholar]
- Grosvenor, A.P.; Kobe, B.A.; Biesinger, M.C.; McIntyre, N.S. Investigation of multiplet splitting of Fe 2p XPS spectra and bonding in iron compounds. Surf. Interface Anal. 2004, 36, 1564–1574. [Google Scholar] [CrossRef]
- Oswald, S. X-Ray Photoelectron Spectroscopy in Analysis of Surfaces. In Encyclopedia of Analytical Chemistry: Applications, Theory, and Instrumentation; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013; ISBN 9780470027318. [Google Scholar]
- Kerle, T.; Lin, Z.; Kim, H.C.; Russell, T.P. Mobility of polymers at the air/polymer interface. Macromolecules 2001, 34, 3484–3492. [Google Scholar] [CrossRef]
- Hu, W.; Wang, Z.; Xiao, Y.; Zhang, S.; Wang, J. Advances in crosslinking strategies of biomedical hydrogels. Biomater. Sci. 2019, 7, 843–855. [Google Scholar] [CrossRef]
- Andrzejewska, E. Photopolymerization kinetics of multifunctional monomers. Prog. Polym. Sci. 2001, 26, 605–665. [Google Scholar] [CrossRef]
- Hunger, K.; Schmeling, N.; Jeazet, H.B.T.; Janiak, C.; Staudt, C.; Kleinermanns, K. Investigation of cross-linked and additive containing polymer materials for membranes with improved performance in pervaporation and gas separation. Membranes (Basel). 2012, 2, 727–763. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.S.; Won, J.; Park, H.C.; Kim, U.Y.; Kang, Y.S.; Lee, Y.M. Morphology control of asymmetric membranes by UV irradiation on polyimide dope solution. J. Memb. Sci. 2000, 169, 229–235. [Google Scholar] [CrossRef]
- Boersma, A.; Cangialosi, D.; Picken, S.J. Mobility and solubility of antioxidants and oxygen in glassy polymers II. Influence of physical ageing on antioxidant and oxygen mobility. Polym. Degrad. Stab. 2003, 79, 427–438. [Google Scholar] [CrossRef]
- Vlachopoulou, M.E.; Petrou, P.S.; Kakabakos, S.E.; Tserepi, A.; Beltsios, K.; Gogolides, E. Effect of surface nanostructuring of PDMS on wetting properties, hydrophobic recovery and protein adsorption. Microelectron. Eng. 2009, 86, 1321–1324. [Google Scholar] [CrossRef]
- Myrstad, T.; Fredriksen, G.R. Removal of Fe(CO)5 from CO gas as detected by FTIR spectroscopy. Chem. Eng. Technol. 1998, 21, 297–299. [Google Scholar] [CrossRef]
- Williams, T.C.; Shaddix, C.R. Contamination of carbon monoxide with metal carbonyls: Implications for combustion research. Combust. Sci. Technol. 2007, 179, 1225–1230. [Google Scholar] [CrossRef]
- Carlton, H.E.; Oxley, J.H. Kinetics of the heterogeneous decomposition of iron pentacarbonyl. AIChE J. 1965, 11, 79–84. [Google Scholar] [CrossRef]
- Poliakoff, M.; Weitz, E. Shedding Light on Organometallic Reactions: The Characterization of Fe(CO)4, a Prototypical Reaction Intermediate. Acc. Chem. Res. 1987, 20, 408–414. [Google Scholar] [CrossRef]
- van Leeuwen, P.W.N.M. Homogeneous Catalysis; Springer: Cham, The Netherlands, 2004; Volume 3, ISBN 9781461329602. [Google Scholar]
- Herrick, D.E.; Tierney, J.W.; Wender, I.; Huffman, G.P.; Huggins, F.E. Activity and Characterization of Coprocessing Catalysts Produced from an Iron Pentacarbonyl Precursor. Energy Fuels 1990, 4, 231–236. [Google Scholar] [CrossRef]
- Smith, T.W.; Wychlck, D. Colloidal iron dispersions prepared via the polymer-catalyzed decomposition of iron pentacarbonyl. J. Phys. Chem. 1980, 84, 1621–1629. [Google Scholar] [CrossRef]
- Davidson, J.L. ChemInform Abstract: Homogeneous Catalysis. Chemischer Informationsdienst 1978, 9. [Google Scholar] [CrossRef]
- King, A.D.; King, R.B.; Yang, D.B. Homogeneous Catalysis of the Water Gas Shift Reaction Using Iron Pentacarbonyl. J. Am. Chem. Soc. 1980, 102, 1028–1032. [Google Scholar] [CrossRef]
- Pignatello, J.J.; Oliveros, E.; MacKay, A. Advanced oxidation processes for organic contaminant destruction based on the fenton reaction and related chemistry. Crit. Rev. Environ. Sci. Technol. 2006, 36, 1–84. [Google Scholar] [CrossRef]
- George, S.C.; Thomas, S. Transport phenomena through polymeric systems. Prog. Polym. Sci. 2001, 26, 985–1017. [Google Scholar] [CrossRef]
- Faupel, F.; Thran, A.; Kiene, M.; Strunskus, T.; Zaporojtchenko, V.; Behnke, K. Diffusion Of Metals In Polymers And During Metal/Polymer Interface Formation. In Low Dielectric Constant Materials for IC Applications; Springer: Berlin/Heidelberg, Germany, 2003; pp. 221–251. [Google Scholar]
- Faupel, F.; Willecke, R.; Thran, A. Diffusion of metals in polymers. Mater. Sci. Eng. R Rep. 1998, 22, 1–55. [Google Scholar] [CrossRef]
- Sonnenberg, M.; Gustus, R.; Sedelmeier, S.; Wegewitz, L.; Höfft, O.; Wieser, J.; Maus-Friedrichs, W. Polymer-induced metal diffusion during plastic processing: A reason for deposit formation. J. Polym. Eng. 2019, 39, 472–480. [Google Scholar] [CrossRef]
- Hordy, N.; Coulombe, S.; Meunier, J.L. Plasma functionalization of carbon nanotubes for the synthesis of stable aqueous nanofluids and poly(vinyl alcohol) nanocomposites. Plasma Process. Polym. 2013, 10, 110–118. [Google Scholar] [CrossRef]
- Baddour, C.E.; Fadlallah, F.; Nasuhoglu, D.; Mitra, R.; Vandsburger, L.; Meunier, J.L. A simple thermal CVD method for carbon nanotube synthesis on stainless steel 304 without the addition of an external catalyst. Carbon N. Y. 2009, 47, 313–318. [Google Scholar] [CrossRef]
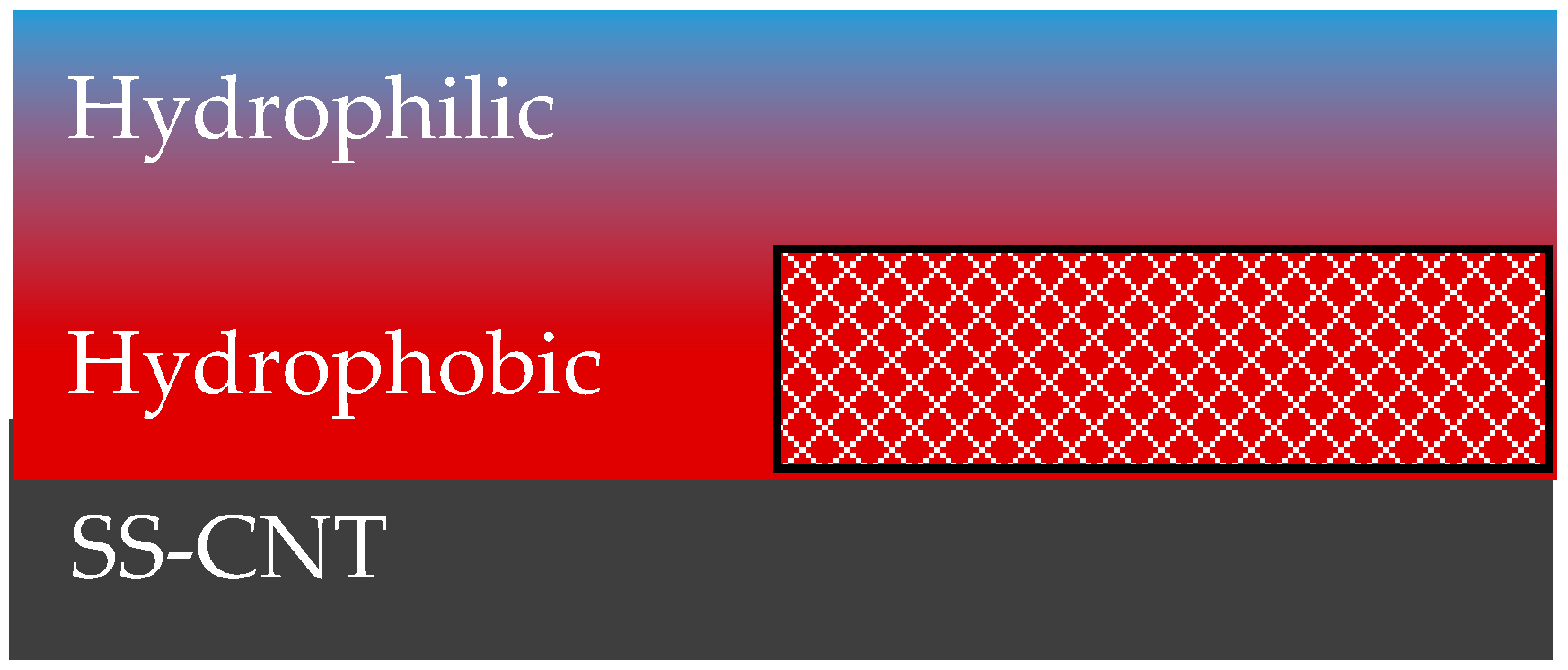
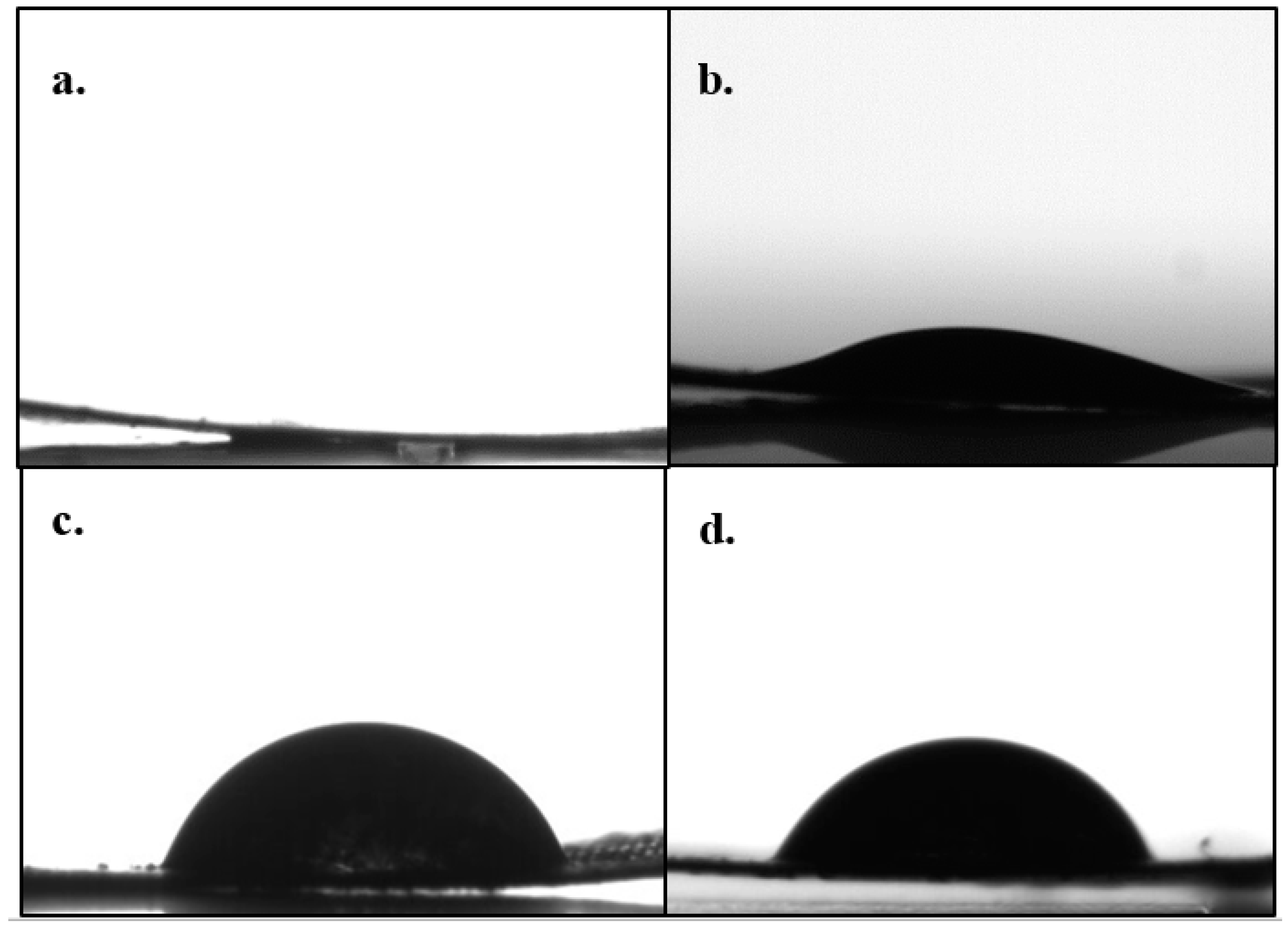
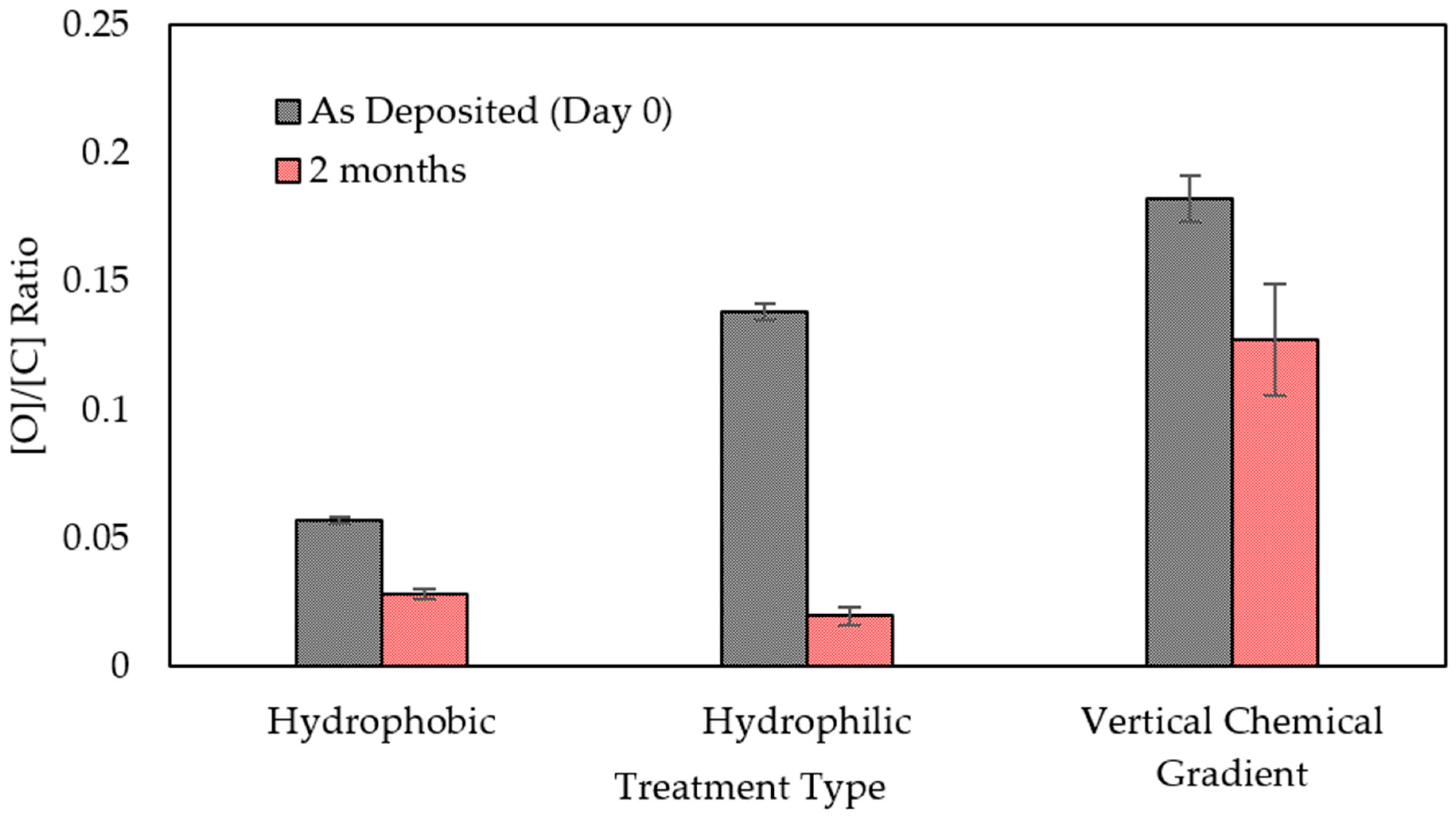
| Process Parameter | Effect on Wettability |
|---|---|
| Pressure (P) | Increase in pressure decreases wettability ( [45] |
| Photo-initiator (nPI) | Presence of a number density of photo-initiator increases wettability () [45] |
| Position (x) | Wettability is increased when the sample position is near the reactor inlet () [45,46] |
| Treatment duration (t) | Increase in treatment duration decreases wettability () [45] |
| Gas Ratio (R) | Increase in gas ratio decreases wettability () [45] |
| Wettability | Pressure (kPa) | Flow Rate (mL/min) | Gas Ratio (H2/CO) | Time (min) | H2O2 | Immediate Average Contact Angle |
|---|---|---|---|---|---|---|
| Hydrophobic | 15 | 600 | 0.14 | 60 | No | 125 ± 3° |
| Hydrophilic | −15 | 600 | 0.14 | 60 | Yes | <5° |
| VCG | 15/−15 | 600 | 0.14 | 180 | No/Yes | <5° |
| Sample | Contact Angle after 2 Months (°) |
|---|---|
| Bare SS-CNT | 123 ± 3° |
| Air-stored hydrophilic SS-CNT | 105 ± 7° |
| Water-stored hydrophilic SS-CNT | 50 ± 4° |
| VCG SS-CNT | 42 ± 1° |
| Sample | C1s | O1s | Fe2p | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Day 0 | Day 1 | 2 months | Day 0 | Day 1 | 2 months | Day 0 | Day 1 | 2 months | |
| Hydrophobic | 94.3% | - | 97.2% | 5.3% | - | 2.71% | 0.4% | - | 0.11% |
| Hydrophilic | 84.8% | - | 97.6% | 11.7% | - | 2.38% | 3.5% | - | 0.07% |
| VCG | 81.9% | 79.6% | 87.7% | 14.9% | 17.8% | 11.13% | 3.2% | 2.6% | 1.14% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aufoujal, A.; Legrand, U.; Meunier, J.-L.; Tavares, J.R. Suppression of Hydrophobic Recovery in Photo-Initiated Chemical Vapor Deposition. Catalysts 2020, 10, 534. https://doi.org/10.3390/catal10050534
Aufoujal A, Legrand U, Meunier J-L, Tavares JR. Suppression of Hydrophobic Recovery in Photo-Initiated Chemical Vapor Deposition. Catalysts. 2020; 10(5):534. https://doi.org/10.3390/catal10050534
Chicago/Turabian StyleAufoujal, Alessio, Ulrich Legrand, Jean-Luc Meunier, and Jason Robert Tavares. 2020. "Suppression of Hydrophobic Recovery in Photo-Initiated Chemical Vapor Deposition" Catalysts 10, no. 5: 534. https://doi.org/10.3390/catal10050534
APA StyleAufoujal, A., Legrand, U., Meunier, J.-L., & Tavares, J. R. (2020). Suppression of Hydrophobic Recovery in Photo-Initiated Chemical Vapor Deposition. Catalysts, 10(5), 534. https://doi.org/10.3390/catal10050534





