Pyridyl-Anchored Type BODIPY Sensitizer-TiO2 Photocatalyst for Enhanced Visible Light-Driven Photocatalytic Hydrogen Production
Abstract
1. Introduction
2. Results and Discussion
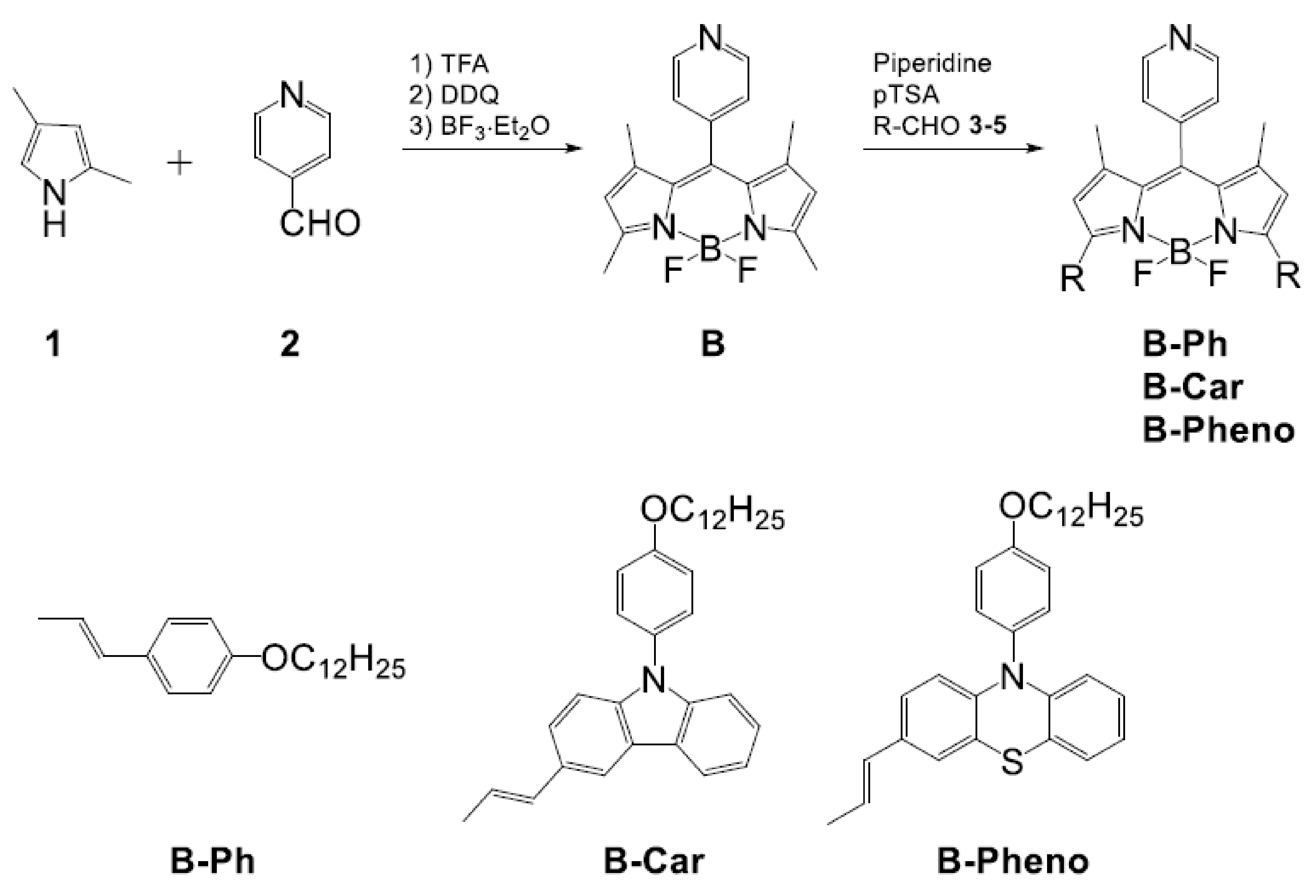
2.1. Synthesis
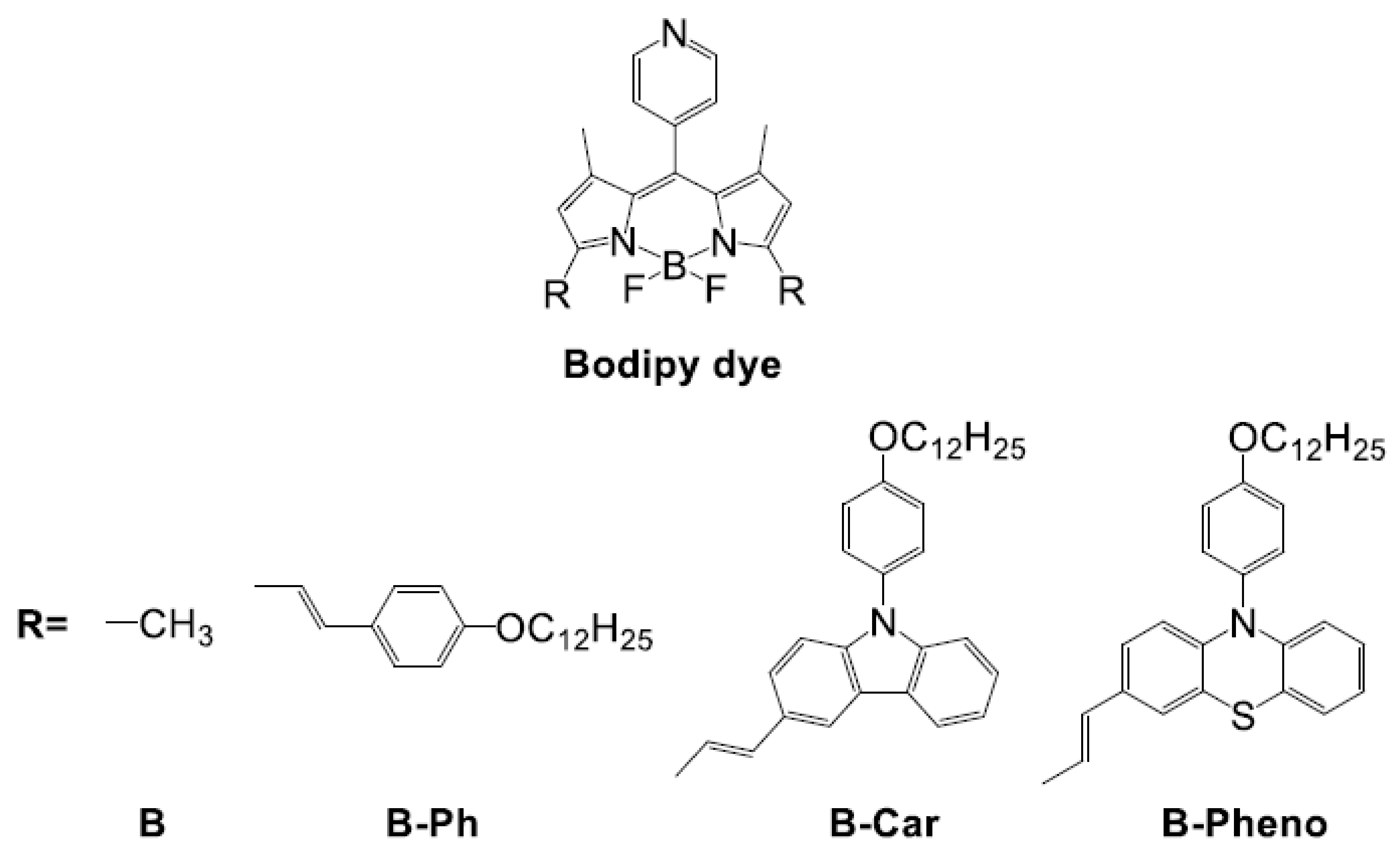
2.2. Physical/Theoretical Properties of BODIPY Dyes B, B-Ph, B-Car, and B-Pheno
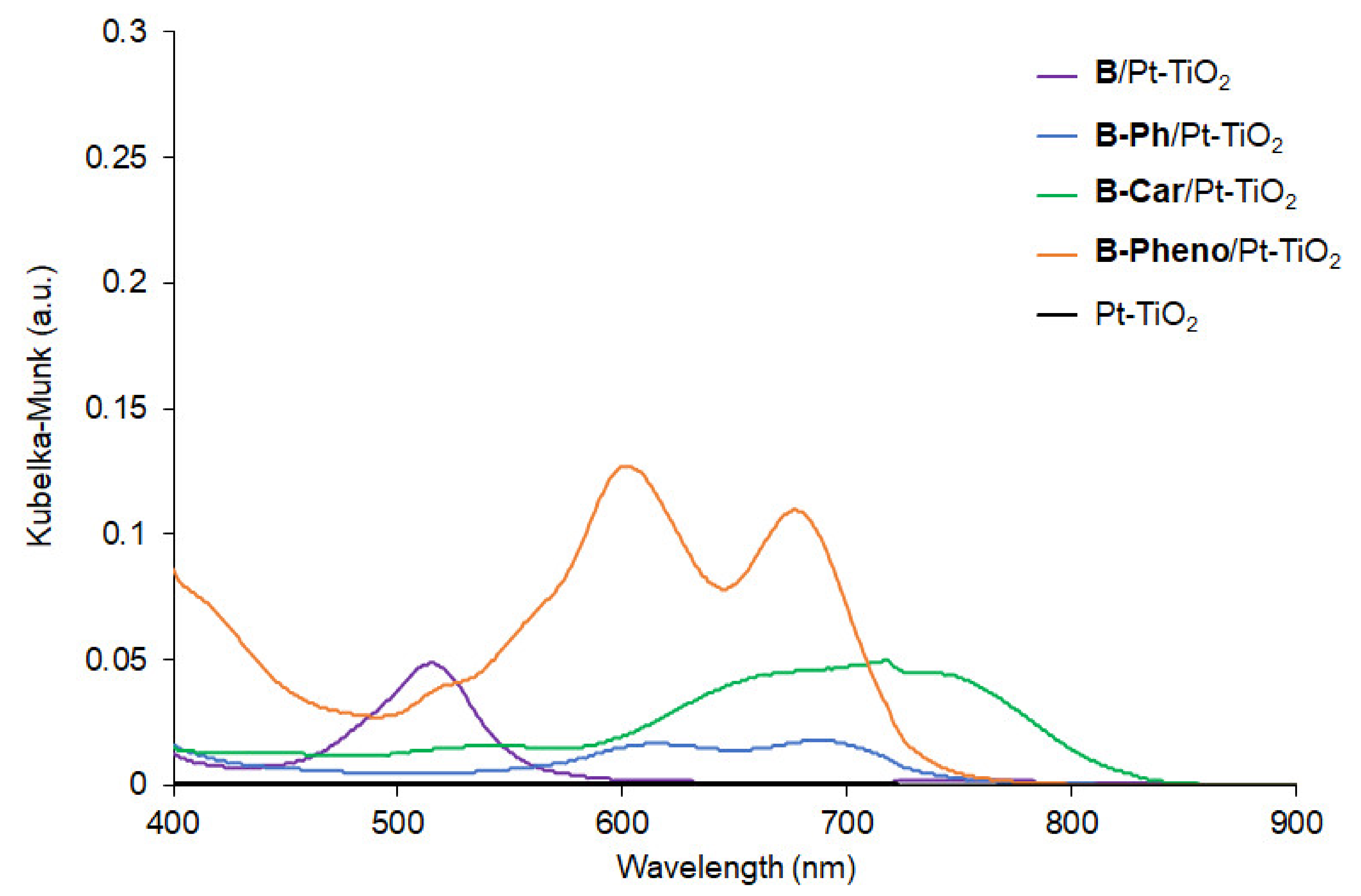
2.3. Photocatalytic Reaction
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Staffell, I.; Scamman, D.; Abad, A.V.; Balcombe, P.; Dodds, P.E.; Ekins, P.; Shah, N.; Ward, K.R. The role of hydrogen and fuel cells in the global energy system. Energy Environ. Sci. 2019, 12, 463–491. [Google Scholar] [CrossRef]
- Staykov, A.; Lyth, S.M.; Watanabe, M. Photocatalytic Water Splitting. In Hydrogen Energy Engineering. A Japanese Perspective; Sasaki, K., Li, H.-W., Hayashi, A., Yamabe, J., Ogura, T., Lyth, S.M., Eds.; Springer: Minato city, Tokyo, Japan, 2016; pp. 159–174. [Google Scholar]
- Barber, J. Hydrogen derived from water as a sustainable solar fuel: Learning from biology. Sustain. Energy Fuels 2018, 2, 927–935. [Google Scholar] [CrossRef]
- Takata, T.; Domen, K. Particulate Photocatalysts for Water Splitting: Recent Advances and Future Prospects. ACS Energy Lett. 2019, 4, 542–549. [Google Scholar] [CrossRef]
- Chen, S.; Takata, T.; Domen, K. Particulate photocatalysts for overall water splitting. Nat. Rev. 2017, 2, 17050. [Google Scholar] [CrossRef]
- Ashford, D.L.; Gish, M.K.; Vannucci, A.K.; Brennaman, M.K.; Templeton, J.L.; Papanikolas, J.M.; Meyer, T.J. Molecular Chromophore—Catalyst Assemblies for Solar Fuel Applications. Chem. Rev. 2015, 115, 13006–13049. [Google Scholar] [CrossRef]
- Wang, Q.; Domen, K. Particulate Photocatalysts for Light-Driven Water Splitting: Mechanisms, Challenges, and Design Strategies. Chem. Rev. 2020, 120, 919–985. [Google Scholar] [CrossRef]
- Watanabe, M. Dye-sensitized photocatalyst for effective water splitting catalyst. Sci. Tech. Adv. Mater. 2017, 18, 705–723. [Google Scholar] [CrossRef]
- Zhang, X.; Peng, T.; Song, S. Recent advances in dye-sensitized semiconductor systems for photocatalytic hydrogen production. J. Mater. Chem. A 2016, 4, 2365–2402. [Google Scholar] [CrossRef]
- Loudet, A.; Burgess, K. BODIPY Dyes and Their Derivatives: Syntheses and Spectroscopic Properties. Chem. Rev. 2007, 107, 4891–4932. [Google Scholar] [CrossRef]
- Boens, N.; Verbelen Ortiz, M.J.; Jiao, L.; Dehaen, W. An unexpected coupling–reduction tandem reaction for the synthesis of alkenyl-substituted BODIPYs. Coord. Chem. Rev. 2019, 399, 213024. [Google Scholar] [CrossRef]
- Shimizu, S. aza-BODIPY synthesis towards vis/NIR functional chromophores based on a Schiff base forming reaction protocol using lactams and heteroaromatic amines. Chem. Commun. 2019, 55, 8722–8743. [Google Scholar] [CrossRef] [PubMed]
- Ho, D.; Ozdemir, R.; Kim, H.; Earmme, T.; Usta, H.; Kim, C. BODIPY-Based Semiconducting Materials for Organic Bulk Heterojunction Photovoltaics and Thin-Film Transistors. ChemPlusChem 2019, 84, 18–37. [Google Scholar] [CrossRef] [PubMed]
- Kowada, T.; Maeda, H.; Kikuchi, K. BODIPY-based probes for the fluorescence imaging of biomolecules in living cells. Chem. Soc. Rev. 2015, 44, 4953–4972. [Google Scholar] [CrossRef] [PubMed]
- Farràs, P.; Cucinotta, F. Recent advances in artificial photosynthetic systems at Newcastle University. C.R. Chimie 2017, 20, 272–282. [Google Scholar] [CrossRef]
- Zheng, B.; Sabatini, R.P.; Fu, W.-F.; Eum, M.-S.; Brennessel, W.W.; Wang, L.; McCamant, D.W.; Eisenberg, R. Light-driven generation of hydrogen: New chromophore dyads for increased activity based on Bodipy dye and Pt(diimine)(dithiolate) complexes. PNAS 2015, 112, E3987–E3996. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Guo, S.; Wang, H.-J.; Chen, K.-K.; Zhang, N.; Zhang, Z.-M.; Lu, T.-B. A broadband and strong visible-light-absorbing photosensitizer boosts hydrogen evolution. Nat. Commun. 2019, 10, 3155. [Google Scholar] [CrossRef]
- Yang, H.; Wang, J.; Ma, J.; Yang, H.; Zhang, J.; Lv, K.; Wen, L.; Peng, T. A novel BODIPY-based MOF photocatalyst for efficient visible-light-driven hydrogen evolution. J. Mater. Chem. A 2019, 7, 10439–10445. [Google Scholar] [CrossRef]
- Yang, H.; Zhao, M.; Zhang, J.; Ma, J.; Wu, P.; Liu, W.; Wen, L. A noble-metal-free photocatalyst system obtained using BODIPY-based MOFs for highly efficient visible-light-driven H2 evolution. J. Mater. Chem. A 2019, 7, 20742–20749. [Google Scholar] [CrossRef]
- Ye, C.; Wang, X.-Z.; Li, J.-X.; Li, Z.-J.; Li, X.-B.; Zhang, L.-P.; Chen, B.; Tung, C.-H.; Wu, L.-Z. Protonated Graphitic Carbon Nitride with Surface Attached Molecule as Hole Relay for Efficient Photocatalytic O2 Evolution. ACS Catal. 2016, 6, 8336–8341. [Google Scholar] [CrossRef]
- Yadav, R.-K.; Baeg, J.-O.; Kumar, A.; Kong, K.; Oh, G.H.; Park, N.-J. Graphene—BODIPY as a photocatalyst in the photocatalytic—Biocatalytic coupled system for solar fuel production from CO2. J. Mater. Chem. A 2014, 2, 5068–5076. [Google Scholar] [CrossRef]
- Huang, L.; Zhao, J.; Guo, S.; Zhang, C.; Ma, J. Bodipy Derivatives as Organic Triplet Photosensitizers for Aerobic Photoorganocatalytic Oxidative Coupling of Amines and Photooxidation of Dihydroxylnaphthalenes. J. Org. Chem. 2013, 78, 5627–5637. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wagner, G.W.; Lu, A.X.; Nguyen, D.L.; Buchanan, J.H.; McNutt, P.M.; Karwacki, C.J. Photocatalytic Oxidation of Sulfur Mustard and Its Simulant on BODIPY-Incorporated Polymer Coatings and Fabrics. ACS Appl. Mater. Interfaces 2018, 10, 18771–18777. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhou, Z.; Li, Y.; Yang, W. Synthesis and properties of BODIPY polymers and their photocatalytic performance for aerobic oxidation of benzylamine. Catal. Commun. 2015, 64, 96–100. [Google Scholar] [CrossRef]
- Liras, M.; Pintado-Sierra, M.; Iglesias, M.; Sánchez, F. A deprotection strategy of a BODIPY conjugated porous polymer to obtain a heterogeneous (dipyrrin)(bipyridine)ruthenium (II) visible light Photocatalyst. J. Mater. Chem. A 2016, 4, 17274–17278. [Google Scholar] [CrossRef]
- Tachikawa, T.; Yamashita, S.; Majima, T. Evidence for Crystal-Face-Dependent TiO2 Photocatalysis from Single-Molecule Imaging and Kinetic Analysis. J. Am. Chem. Soc. 2011, 133, 7197–7204. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-G.; Tan, P.; Wang, H.; Song, M.; Pan, J.; Kuang, G.-C. BODIPY modified g-C3N4 as a highly efficient photocatalyst for degradation of Rhodamine B under visible light irradiation. J. Solid State Chem. 2018, 267, 22–27. [Google Scholar] [CrossRef]
- Suryani, O.; Higashino, Y.; Sato, H.; Kubo, Y. Visible-to-Near-Infrared Light-Driven Photocatalytic Hydrogen Production Using Dibenzo-BODIPY and Phenothiazine Conjugate as Organic Photosensitizer. ACS Appl. Energy Mater. 2019, 2, 448–458. [Google Scholar]
- Erten-Ela, S.; Yilmaz, M.; Icli, D.; Dede, Y.; Icli, S.; Akkaya, E.U. A Panchromatic Boradiazaindacene (BODIPY) Sensitizer for Dye-Sensitized Solar Cells. Org. Lett. 2008, 10, 3299–3302. [Google Scholar] [CrossRef]
- Qin, C.; Mirloup, A.; Leclerc, N.; Islam, A.; El-Shafei, A.; Han, L.; Ziessel, R. Molecular Engineering of New Thienyl-Bodipy Dyes for Highly Effi cient Panchromatic Sensitized Solar Cells. Adv. Energy Mater. 2014, 4, 1400085. [Google Scholar] [CrossRef]
- Watanabe, M.; Sun, S.; Ishihara, T.; Kamimura, T.; Nishimura, M.; Tani, F. Visible Light-Driven Dye-Sensitized Photocatalytic Hydrogen Production by Porphyrin and its Cyclic Dimer and Trimer: Effect of Multi-Pyridyl-Anchoring Groups on Photocatalytic Activity and Stability. ACS Appl. Energy Mater. 2018, 1, 6072–6081. [Google Scholar] [CrossRef]
- Zhang, L.; Cole, J.M. Anchoring Groups for Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2015, 7, 3427–3455. [Google Scholar] [CrossRef] [PubMed]
- Parry, E.P. An infrared study of pyridine adsorbed on acidic solids. Characterization of surface acidity. J. Catal. 1963, 2, 371–379. [Google Scholar] [CrossRef]
- Watanabe, M.; Hagiwara, H.; Ogata, Y.; Staykov, A.; Bishop, S.R.; Perry, N.H.; Chang, Y.J.; Ida, S.; Tanaka, K.; Ishihara, T. Impact of alkoxy chain length on carbazole-based, visible light-driven, dye sensitized photocatalytic hydrogen production. J. Mater. Chem. A 2015, 3, 21713–21721. [Google Scholar] [CrossRef]
- Yang, W.; Prabhakar, R.R.; Tan, J.; Tilley, S.D.; Moon, J. Strategies for enhancing the photocurrent, photovoltage, and stability of photoelectrodes for photoelectrochemical water splitting. Chem. Soc. Rev. 2019, 48, 4979–5015. [Google Scholar] [CrossRef] [PubMed]
- Hongxing, D.; Qiuoing, L.; Yuehui, H. Preparation of nanoporous BiVO4/TiO2/Ti film through electrodeposition for photoelectrochemical water splitting. R. Soc. Open Sci. 2018, 5, 180728. [Google Scholar] [CrossRef] [PubMed]
- Pellegrin, Y.; Odobel, F. Sacrificial electron donor reagents for solar fuel production. C.R. Chim. 2017, 20, 283–295. [Google Scholar] [CrossRef]
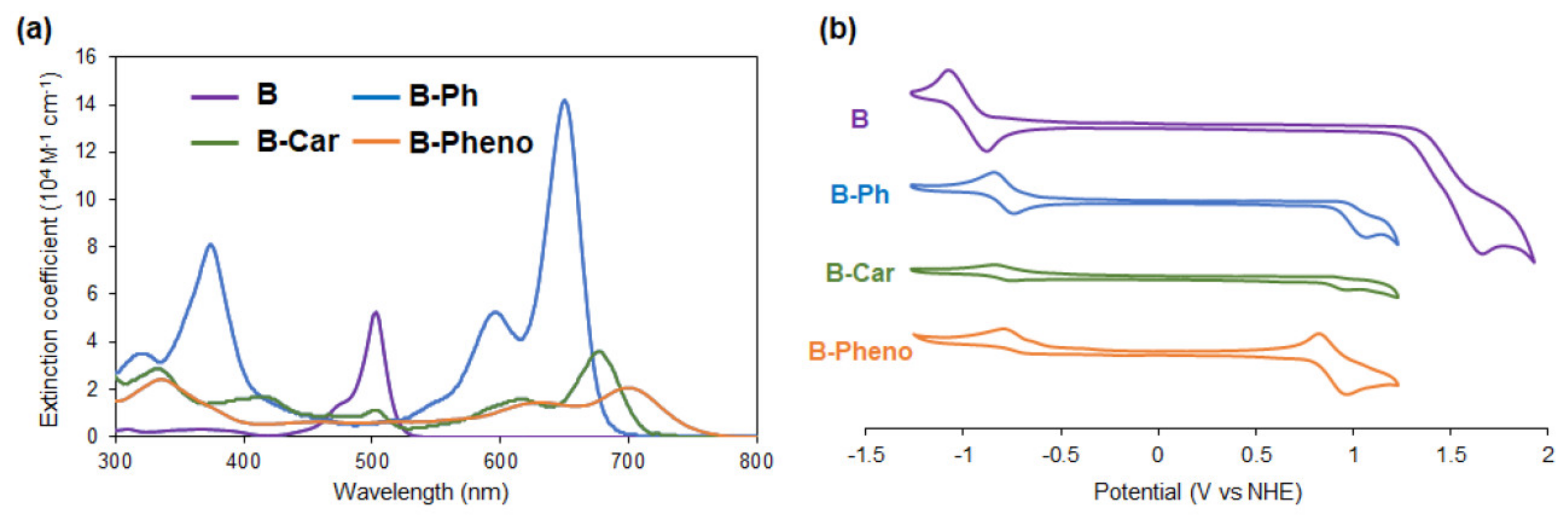

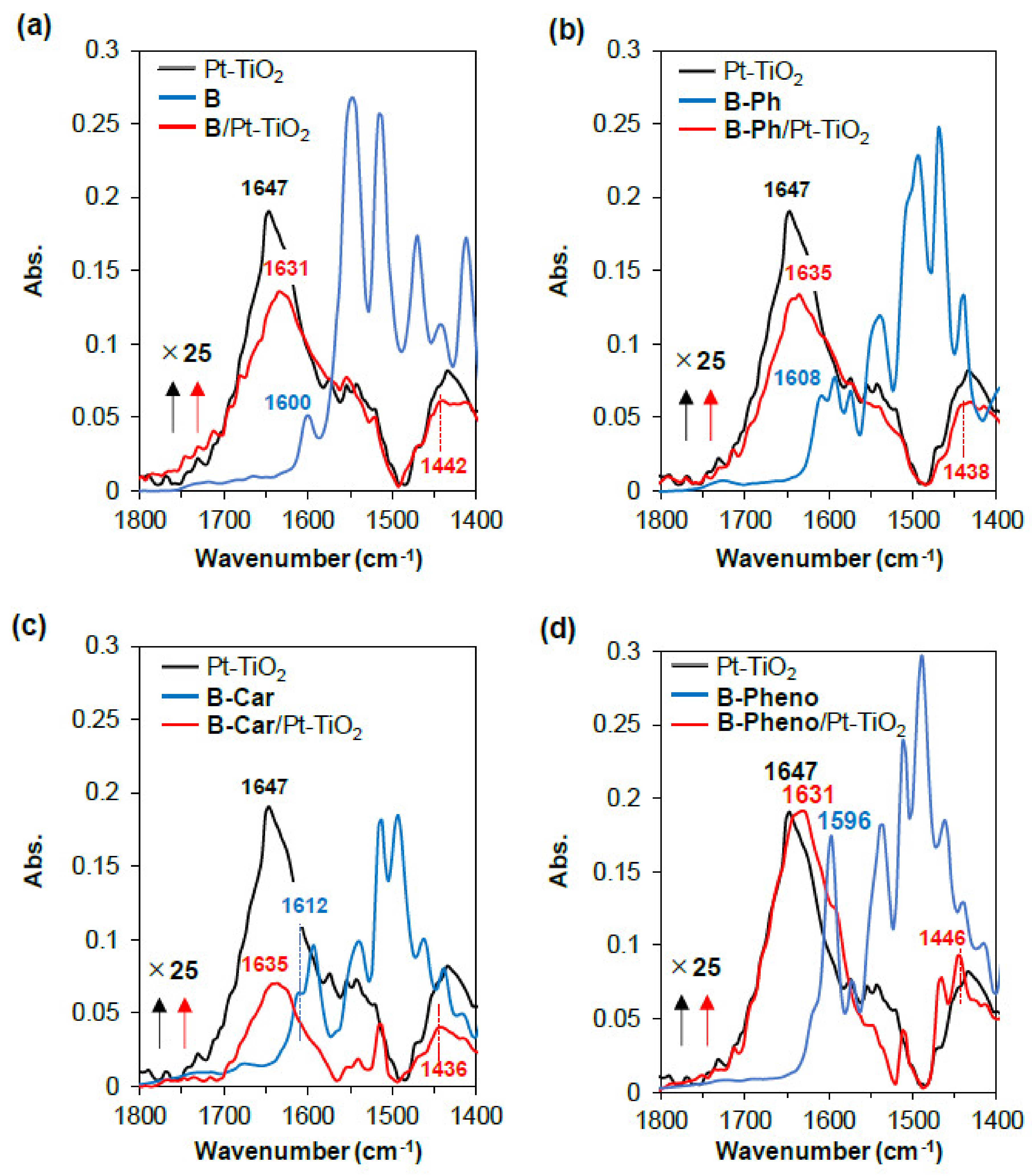
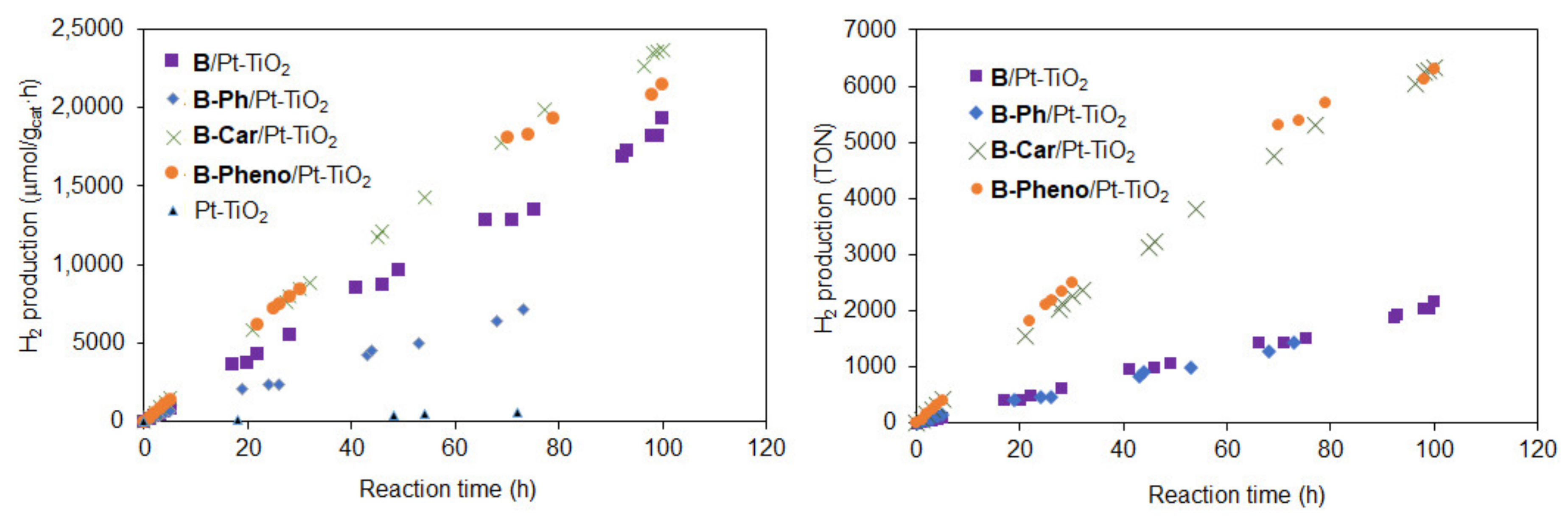
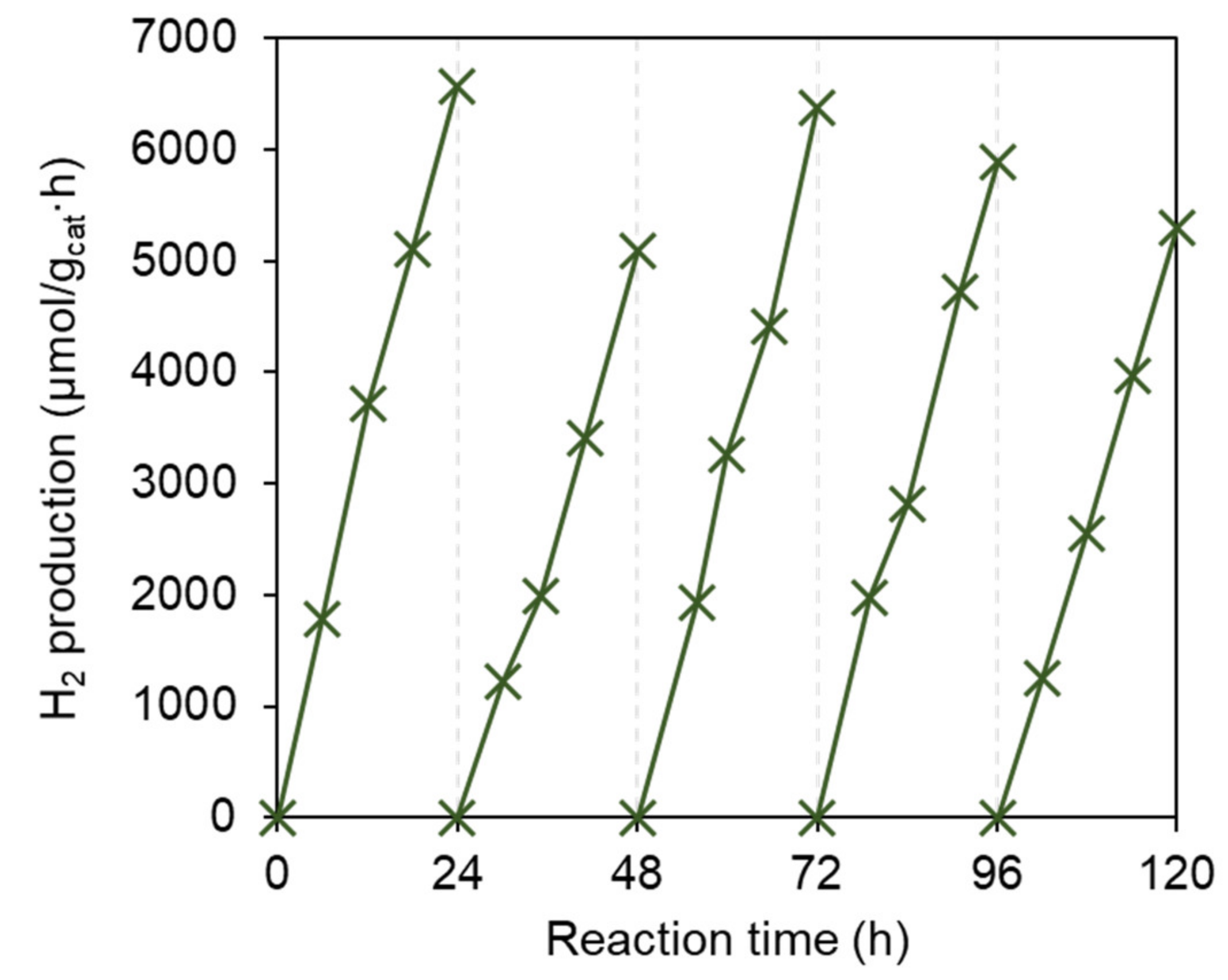


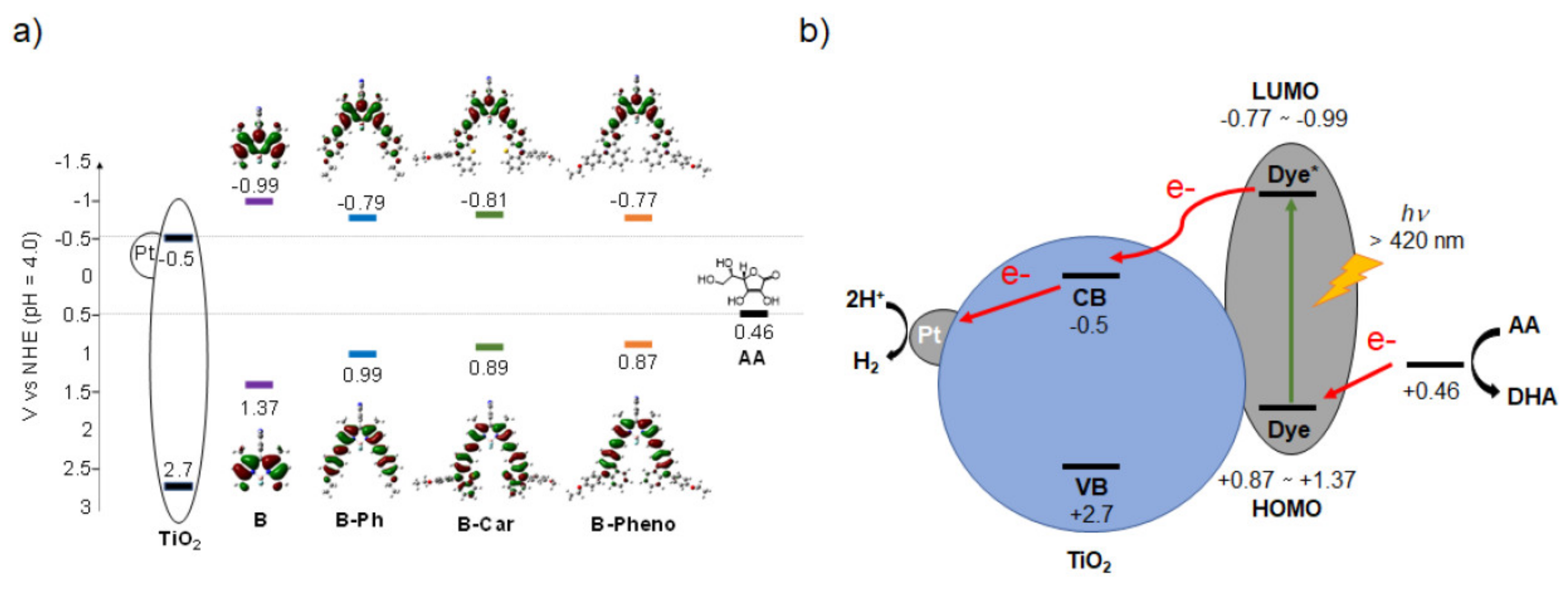
| Sample | Abs. at soln. a (nm/ε) | Abs. on Pt-TiO2 b (nm) | Oxidation Potential a (V vs NHE) | Reduction Potential a (V vs NHE) | Energy Gap d (V vs NHE) | HOMOv e (eV) | LUMOv e (eV) | Energy Gapv e (eV) |
|---|---|---|---|---|---|---|---|---|
| B | 503 / 5,2300 | 516 | - c +1.37 d | −0.98 c −0.99 d | 2.36 | −5.51 | −2.53 | 2.99 |
| B-Ph | 650 /14,1000 | 689 | - c +0.99 d | −0.78 c −0.79 d | 1.78 | −4.69 | −2.55 | 2.14 |
| B-Car | 676 / 3,6000 | 709 | +0.94 c +0.89 d | −0.76 c −0.81 d | 1.70 | −4.51 | −2.43 | 2.08 |
| B-Pheno | 699 / 2,1000 | 692 | +0.85 c +0.87 d | - c −0.77 d | 1.64 | −4.47 | −2.49 | 1.98 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, X.-F.; Watanabe, M.; Takagaki, A.; Song, J.T.; Ishihara, T. Pyridyl-Anchored Type BODIPY Sensitizer-TiO2 Photocatalyst for Enhanced Visible Light-Driven Photocatalytic Hydrogen Production. Catalysts 2020, 10, 535. https://doi.org/10.3390/catal10050535
Shen X-F, Watanabe M, Takagaki A, Song JT, Ishihara T. Pyridyl-Anchored Type BODIPY Sensitizer-TiO2 Photocatalyst for Enhanced Visible Light-Driven Photocatalytic Hydrogen Production. Catalysts. 2020; 10(5):535. https://doi.org/10.3390/catal10050535
Chicago/Turabian StyleShen, Xiao-Feng, Motonori Watanabe, Atsushi Takagaki, Jun Tae Song, and Tatsumi Ishihara. 2020. "Pyridyl-Anchored Type BODIPY Sensitizer-TiO2 Photocatalyst for Enhanced Visible Light-Driven Photocatalytic Hydrogen Production" Catalysts 10, no. 5: 535. https://doi.org/10.3390/catal10050535
APA StyleShen, X.-F., Watanabe, M., Takagaki, A., Song, J. T., & Ishihara, T. (2020). Pyridyl-Anchored Type BODIPY Sensitizer-TiO2 Photocatalyst for Enhanced Visible Light-Driven Photocatalytic Hydrogen Production. Catalysts, 10(5), 535. https://doi.org/10.3390/catal10050535







