A DFT Insight into the Tuning Effect of Potassium Promoter on the Formation of Carbon Atoms via Carburization Gases Dissociation on Iron-Based Catalysts
Abstract
1. Introduction
2. Results and Discussion
2.1. Adsorptions of C2H4, C2H2 and CO/H2—The Initial Step of Carburization
2.2. Surface Atomic Carbon Formation—The Second Step of Carburization
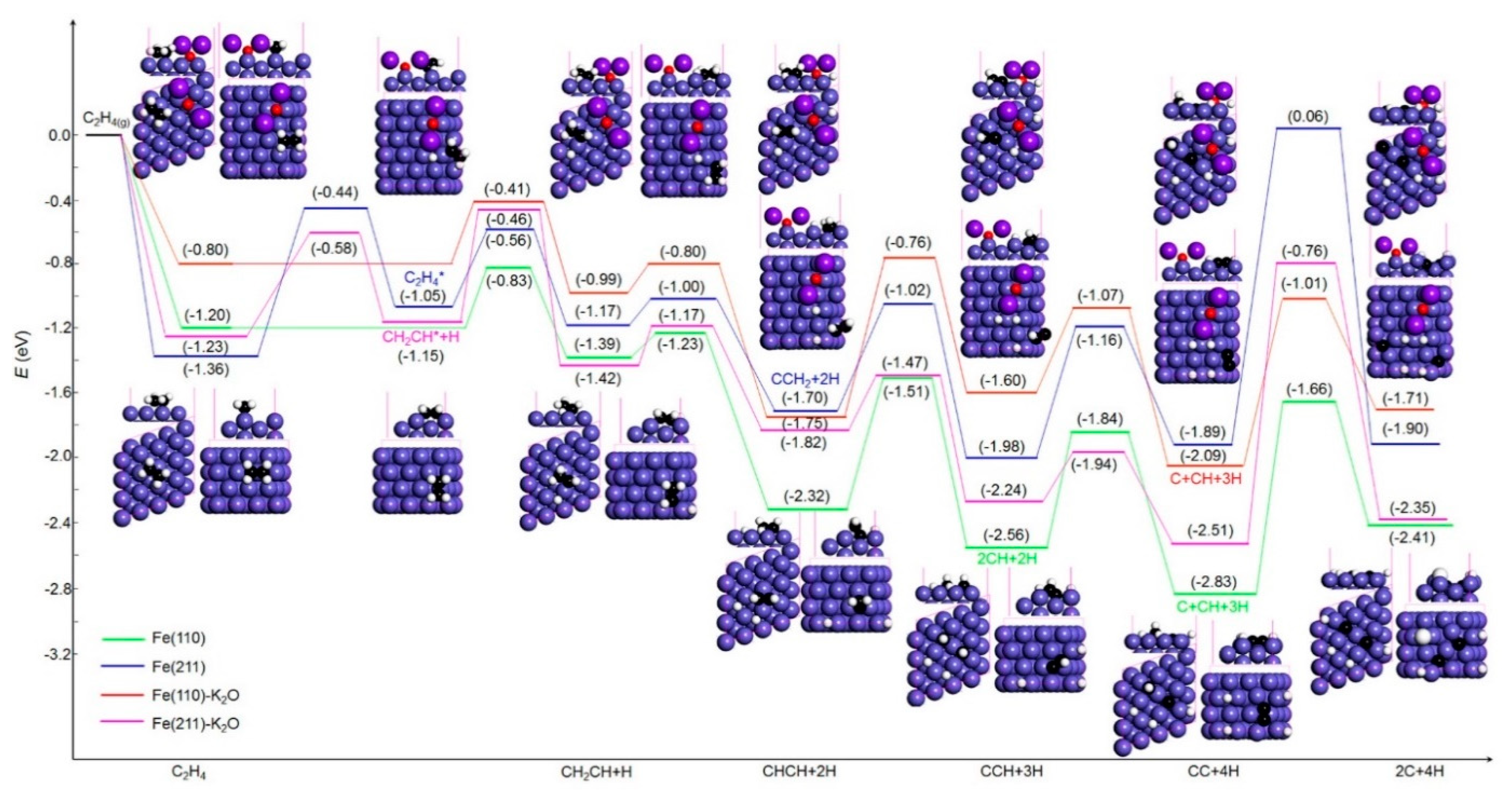
2.2.1. Surface Atomic Carbon Formation via the Dissociations of C2H4/C2H2
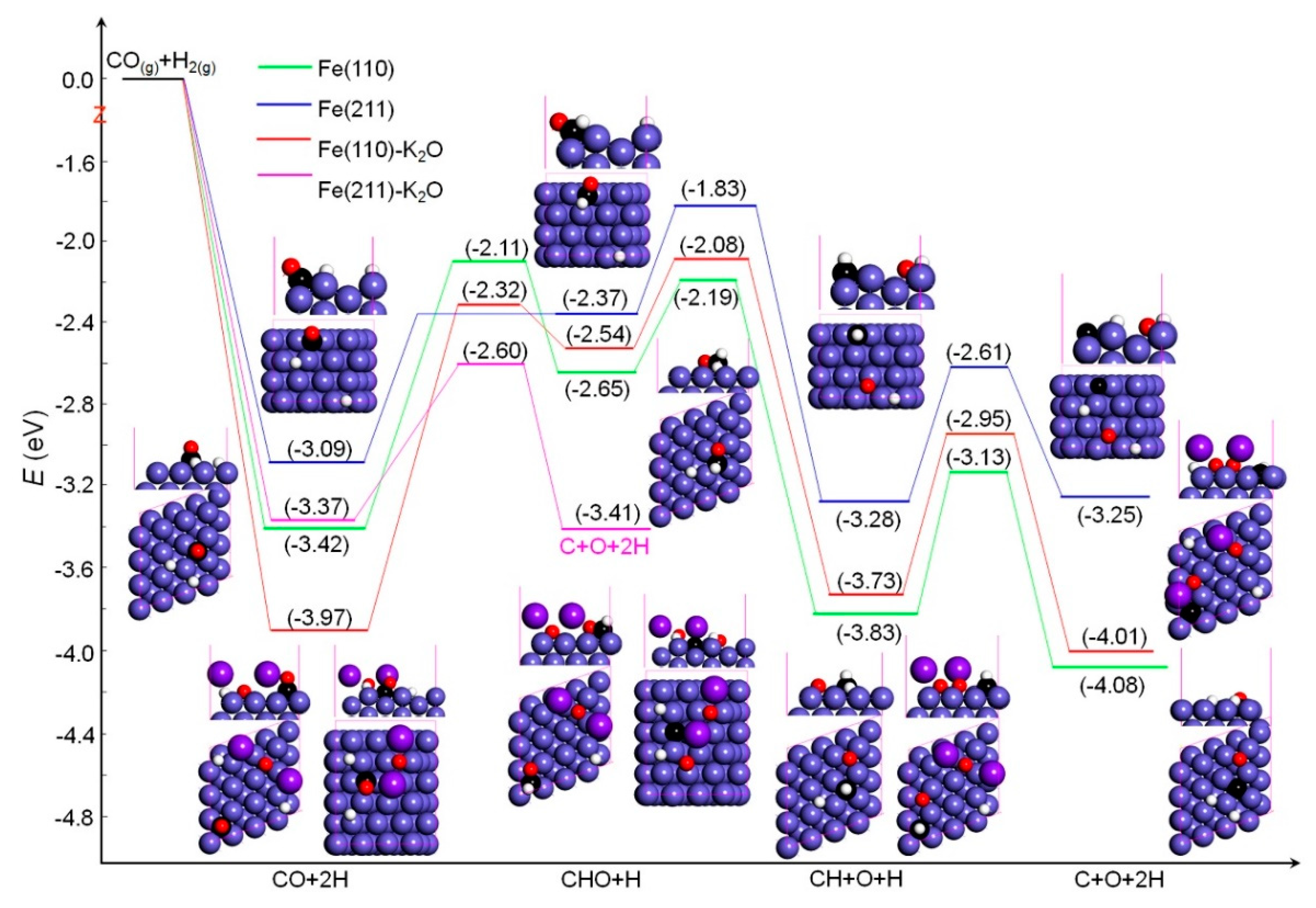
2.2.2. Surface Atomic Carbon Formation via the Dissociations of CO and CO/H2
2.3. Rate Constants of Elementary Steps
3. Methods and Models
3.1. Methods
3.2. Models
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yu, J.; Ju, Y.; Zhao, L.; Chu, X.; Yang, Z.; Tian, Y.; Sheng, F.; Lin, J.; Liu, F.; Dong, Y.; et al. Multistimuli-Regulated Photochemothermal Cancer Therapy Remotely Controlled via Fe5C2 Nanoparticles. ACS Nano 2015, 10, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Giordano, C.; Kraupner, A.; Wimbush, S.C.; Antonietti, M. Iron Carbide: An Ancient Advanced Material. Small 2010, 6, 1859–1862. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, J.; Guo, X.; Fan, H.; Wu, W.; Liu, H.; Wang, G. Fe3C@nitrogen doped CNT arrays aligned on nitrogen functionalized carbon nanofibers as highly efficient catalysts for the oxygen evolution reaction. J. Mater. Chem. A 2017, 5, 19672–19679. [Google Scholar] [CrossRef]
- Wirth, C.T.; Bayer, B.C.; Gamalski, A.D.; Esconjauregui, S.; Weatherup, R.; Ducati, C.; Baehtz, C.; Robertson, J.; Hofmann, S. The Phase of Iron Catalyst Nanoparticles during Carbon Nanotube Growth. Chem. Mater. 2012, 24, 4633–4640. [Google Scholar] [CrossRef]
- Chang, Q.; Zhang, C.H.; Liu, C.W.; Wei, Y.X.; Cheruvathur, A.V.; Dugulan, A.I.; Niemantsverdriet, J.W.; Liu, X.W.; He, Y.R.; Qing, M.; et al. Relationship between iron carbide phases (ε-Fe2C, Fe7C3, and χ-Fe5C2) and catalytic performances of Fe/SiO2 fischer-tropsch catalysts. ACS Catal. 2018, 8, 3304–3316. [Google Scholar] [CrossRef]
- De Smit, E.; Cinquini, F.; Beale, A.M.; Safonova, O.V.; van Beek, W.; Sautet, P.; Weckhuysen, B.M. Stability and reactivity of epsilon-chi-theta iron carbide catalyst phases in Fischer-Tropsch synthesis: Controlling µC. J. Am. Chem. Soc. 2010, 132, 14928–14941. [Google Scholar] [CrossRef]
- Petersen, M.A.; Berg, J.-A.V.D.; Van Rensburg, W.J. Role of Step Sites and Surface Vacancies in the Adsorption and Activation of CO on χ-Fe5C2 Surfaces. J. Phys. Chem. C 2010, 114, 7863–7879. [Google Scholar] [CrossRef]
- Xu, J.; Bartholomew, C.H. Temperature-Programmed Hydrogenation (TPH) and in Situ Mössbauer Spectroscopy Studies of Carbonaceous Species on Silica-Supported Iron Fischer−Tropsch Catalysts. J. Phys. Chem. B 2005, 109, 2392–2403. [Google Scholar] [CrossRef]
- Amelse, J.A.; Butt, J.B.; Schwartz, L.H. Carburization of supported iron synthesis catalysts. J. Phys. Chem. 1978, 82, 558–563. [Google Scholar] [CrossRef]
- Raupp, G.B.; Delgass, W.N. Delgass, Mossbauer investigation of supported Fe and FeNi catalysts: ‖. Carbides formed by Fischer-Tropsch synthesis. J. Catal. 1979, 58, 348–360. [Google Scholar] [CrossRef]
- Niemantsverdriet, J.W.; Van Der Kraan, A.M.; Van Dijk, W.L.; Van Der Baan, H.S. Behavior of metallic iron catalysts during Fischer-Tropsch synthesis studied with Mössbauer spectroscopy, x-ray diffraction, carbon content determination, and reaction kinetic measurements. J. Phys. Chem. 1980, 84, 3363–3370. [Google Scholar] [CrossRef]
- Lecaer, G.; Dubois, J.M.; Pijolat, M.; Perrichon, V.; Bussiere, P. Characterization by Mossbauer-spectroscopy of iron carbides formed by Fischer-Tropsch synthesis. J. Phys. Chem. 1982, 86, 4799–4808. [Google Scholar] [CrossRef]
- Mansker, L.D.; Jin, Y.; Bukur, D.B.; Datye, A. Characterization of slurry phase iron catalysts for Fischer–Tropsch synthesis. Appl. Catal. A Gen. 1999, 186, 277–296. [Google Scholar] [CrossRef]
- Li, S.; Meitzner, G.D.; Iglesia, E. Structure and Site Evolution of Iron Oxide Catalyst Precursors during the Fischer−Tropsch Synthesis. J. Phys. Chem. B 2001, 105, 5743–5750. [Google Scholar] [CrossRef]
- Arakawa, H.; Bell, A.T. Effects of potassium promotion on the activity and selectivity of iron Fischer-Tropsch catalysts. Ind. Eng. Chem. Process. Des. Dev. 1983, 22, 97–103. [Google Scholar] [CrossRef]
- Huo, C.-F.; Ren, J.; Li, Y.-W.; Wang, J.; Jiao, H. CO dissociation on clean and hydrogen precovered Fe(111) surfaces. J. Catal. 2007, 249, 174–184. [Google Scholar] [CrossRef]
- Erley, W.; Baro, A.M.; Ibach, H. Vibrational-spectra of acetylene and ethylene adsorbed on Fe(110). Surf. Sci. 1982, 120, 273–290. [Google Scholar] [CrossRef]
- Seip, U.; Tsai, M.C.; Kuppers, J.; Ertl, G. Interaction of acetylene and ethylene with an Fe(111) surface. Surf. Sci. 1984, 147, 65–88. [Google Scholar] [CrossRef]
- Hung, W.-H.; Bernasek, S. Adsorption and decomposition of ethylene and acetylene on Fe(100). Surf. Sci. 1995, 339, 272–290. [Google Scholar] [CrossRef]
- Booyens, S.; Gilbert, L.; Willock, D.; Bowker, M. The adsorption of ethene on Fe(111) and surface carbide formation. Catal. Today 2015, 244, 122–129. [Google Scholar] [CrossRef]
- Li, J.; Zhang, C.; Cheng, X.; Qing, M.; Xu, J.; Wu, B.; Yang, Y.; Li, Y. Effects of alkaline-earth metals on the structure, adsorption and catalytic behavior of iron-based Fischer–Tropsch synthesis catalysts. Appl. Catal. A Gen. 2013, 464, 10–19. [Google Scholar] [CrossRef]
- Li, J.; Cheng, X.; Zhang, C.; Yang, Y.; Li, Y. Effects of alkali on iron-based catalysts for Fischer-Tropsch synthesis: CO chemisorptions study. J. Mol. Catal. A Chem. 2015, 396, 174–180. [Google Scholar] [CrossRef]
- Li, J.; Cheng, X.; Zhang, C.; Chang, Q.; Wang, J.; Wang, X.; Lv, Z.; Dong, W.-S.; Yang, Y.; Li, Y. Effect of alkalis on iron-based Fischer-Tropsch synthesis catalysts: Alkali-FeOx interaction, reduction, and catalytic performance. Appl. Catal. A Gen. 2016, 528, 131–141. [Google Scholar] [CrossRef]
- Zhai, P.; Xu, C.; Gao, R.; Liu, X.; Li, M.; Li, W.; Fu, X.; Jia, C.; Xie, J.; Zhao, M.; et al. Highly Tunable Selectivity for Syngas-Derived Alkenes over Zinc and Sodium-Modulated Fe5C2Catalyst. Angew. Chem. Int. Ed. 2016, 55, 9902–9907. [Google Scholar] [CrossRef]
- Crowell, J.E.; Somorjai, G.A. The effect of potassium on the chemisorption of carbon monoxide on the Rh(111) crystal face. Appl. Surf. Sci. 1984, 19, 73–91. [Google Scholar] [CrossRef]
- Raje, A.P.; O’Brien, R.J.; Davis, B.H. Effect of Potassium Promotion on Iron-Based Catalysts for Fischer–Tropsch Synthesis. J. Catal. 1998, 180, 36–43. [Google Scholar] [CrossRef]
- Bukur, D.B.; Mukesh, D.; Patel, S.A. Promoter effects on precipitated iron catalysts for Fischer-Tropsch synthesis. Ind. Eng. Chem. Res. 1990, 29, 194–204. [Google Scholar] [CrossRef]
- Herzog, K.; Gaube, J. Kinetic studies for elucidation of the promoter effect of alkali in Fischer-Tropsch iron catalysts. J. Catal. 1989, 115, 337–346. [Google Scholar] [CrossRef]
- Gaube, J.; Klein, H.-F. The promoter effect of alkali in Fischer-Tropsch iron and cobalt catalysts. Appl. Catal. A Gen. 2008, 350, 126–132. [Google Scholar] [CrossRef]
- Dry, M. The correlation between catalyst surface basicity and hydrocarbon selectivity in the Fischer-Tropsch synthesis. J. Catal. 1968, 11, 18–24. [Google Scholar] [CrossRef]
- Dry, M. Heats of chemisorption on promoted iron surfaces and the role of alkali in Fischer-Tropsch synthesis. J. Catal. 1969, 15, 190–199. [Google Scholar] [CrossRef]
- Huo, C.-F.; Wu, B.-S.; Li, Y.-W.; Gao, P.; Yang, Y.; Jiao, H. The Mechanism of Potassium Promoter: Enhancing the Stability of Active Surfaces. Angew. Chem. Int. Ed. 2011, 50, 7403–7406. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Liu, X.-W.; Huo, C.-F.; Li, Y.-W.; Wang, J.; Jiao, H. The role of potassium promoter in surface carbon hydrogenation on Hägg carbide surfaces. Appl. Catal. A Gen. 2015, 493, 68–76. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, X.-W.; Huo, C.-F.; Wen, X.-D.; Guo, W.; Cao, D.; Yang, Y.; Li, Y.-W.; Wang, J.; Jiao, H. Morphology control of K2O promoter on Hägg carbide (χ-Fe5C2) under Fischer–Tropsch synthesis condition. Catal. Today 2016, 261, 93–100. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, X.-W.; Huo, C.-F.; Li, Y.-W.; Wang, J.; Jiao, H. Potassium promotion on CO hydrogenation on the χ-Fe 5 C 2 (111) surface with carbon vacancy. Appl. Catal. A Gen. 2017, 534, 22–29. [Google Scholar] [CrossRef]
- Niu, L.; Liu, X.; Liu, J.; Liu, X.; Wen, X.; Yang, Y.; Xu, J.; Li, Y. Tuning carburization behaviors of metallic iron catalysts with potassium promoter and CO/syngas/C2H4/C2H2 gases. J. Catal. 2019, 371, 333–345. [Google Scholar] [CrossRef]
- Lohitharn, N.; Goodwin, J.G.; Goodwinjr, J. Effect of K promotion of Fe and FeMn Fischer–Tropsch synthesis catalysts: Analysis at the site level using SSITKA. J. Catal. 2008, 260, 7–16. [Google Scholar] [CrossRef]
- Yang, Y.; Xiang, H.W.; Xu, Y.Y.; Bai, L.; Li, Y.W. Effect of potassium promoter on precipitated iron-manganese catalyst for Fischer-Tropsch synthesis. Appl. Catal. A Gen. 2004, 266, 181–194. [Google Scholar] [CrossRef]
- Frenking, G.; Fröhlich, N. The nature of the bonding in transition-metal compounds. Chem. Rev. 2000, 100, 717–774. [Google Scholar] [CrossRef]
- Eyring, H. The Activated Complex in Chemical Reactions. J. Chem. Phys. 1935, 3, 107. [Google Scholar] [CrossRef]
- Lu, J.; Behtash, S.; Faheem, M.; Heyden, A. Microkinetic modeling of the decarboxylation and decarbonylation of propanoic acid over Pd(111) model surfaces based on parameters obtained from first principles. J. Catal. 2013, 305, 56–66. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Blochl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Henkelman, G.; Uberuaga, B.P.; Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 2000, 113, 9901–9904. [Google Scholar] [CrossRef]
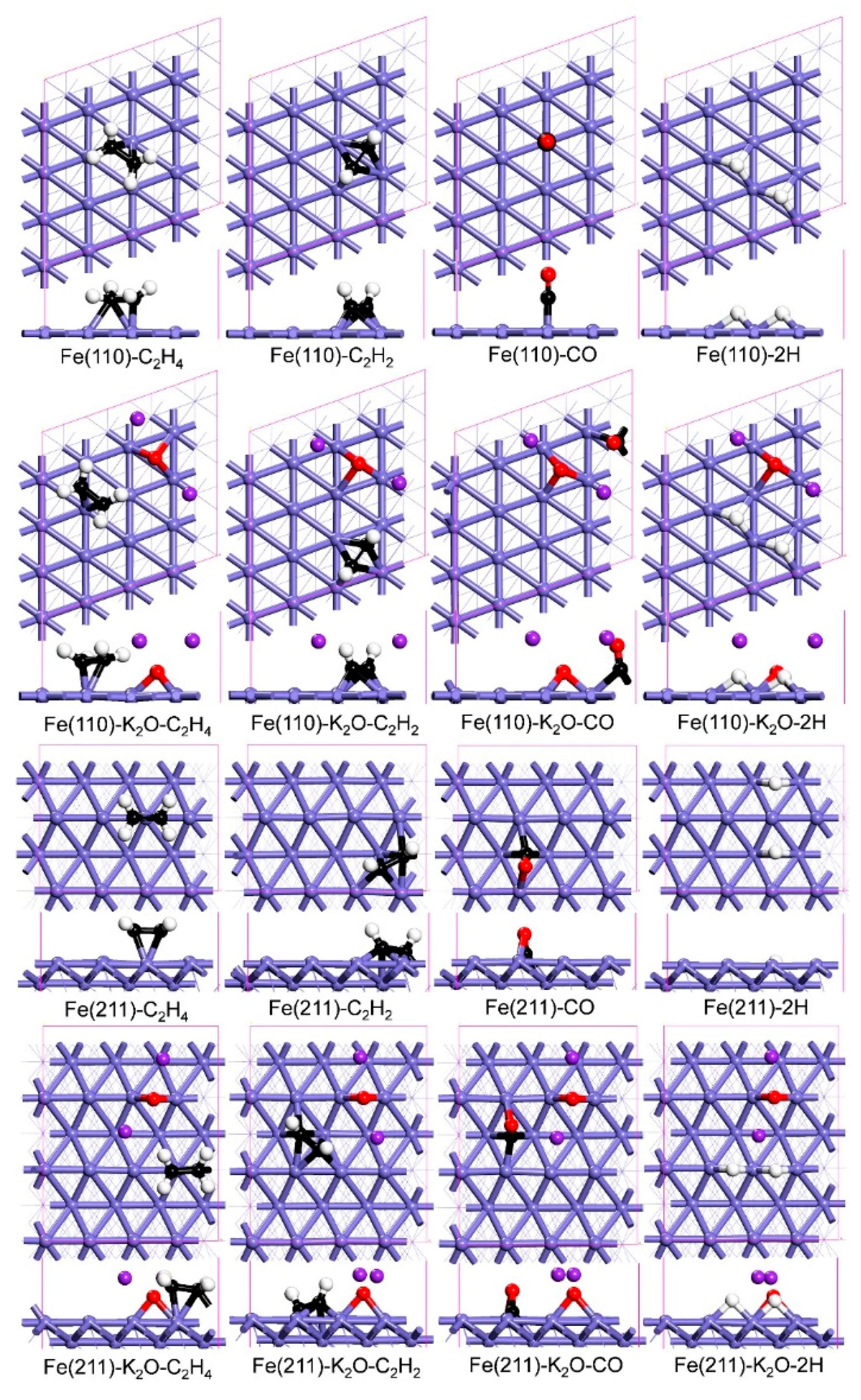

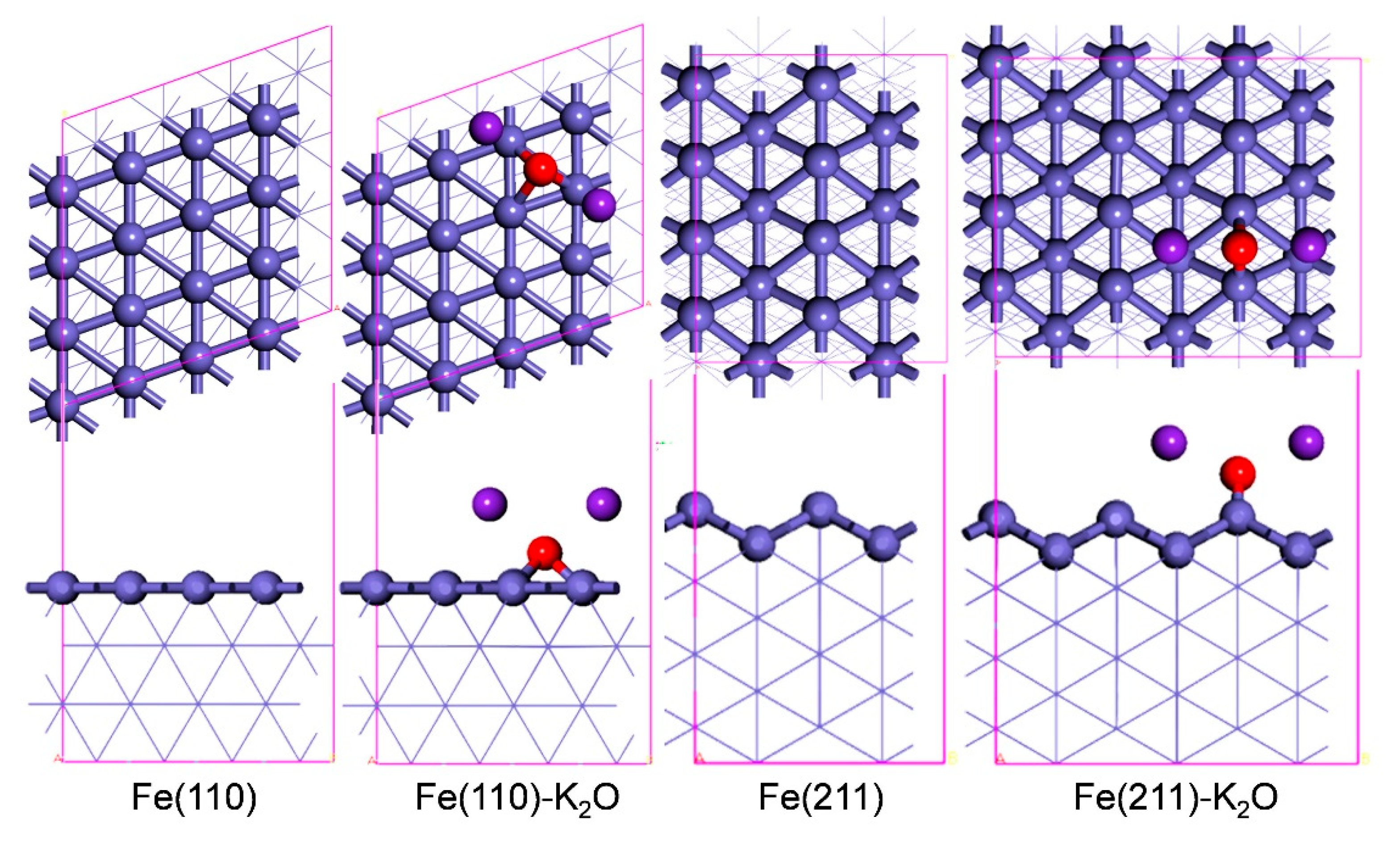
| Surfaces | Eads (eV) | |||
|---|---|---|---|---|
| CO | C2H4 | C2H2 | 2H | |
| Fe(110) | −2.00 | −1.20 | −3.39 | −1.52 |
| Fe(110)-K2O | −2.28 | −0.81 | −3.03 | −1.45 |
| Fe(211) | −1.98 | −1.36 | −2.58 | −1.14 |
| Fe(211)-K2O | −2.20 | −1.23 | −2.75 | −1.46 |
| Surfaces | ∆q | ||||
|---|---|---|---|---|---|
| C2H4 | C2H2 | CO | 2H | K2O | |
| Fe(110) | −0.49 | −0.88 | −0.41 | −0.71 | 0 |
| Fe(110)-K2O | −0.65 | −1.07 | −0.77 | −0.78 | 0.45 |
| Fe(211) | −0.48 | −1.07 | −0.97 | −0.68 | 0 |
| Fe(211)-K2O | −0.55 | −1.20 | −1.02 | −0.80 | 0.46 |
| Gases | Fe(110) | Fe(110)-K2O | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Element Steps | Ea | k | Eeff | keff | Element Steps | Ea | k | Eeff | keff | |
| C2H4 or C2H2 | C2H4 → C2H3 + H | 0.37 | 3.35 × 109 | 1.17 | 6.79 × 101 | C2H4 → C2H3 + H | 0.39 | 1.22 × 109 | 1.08 | 3.30 × 103 |
| C2H3 + H → CHCH + 2H | 0.16 | 4.32 × 1011 | C2H3 + H → CHCH + 2H | 0.19 | 6.48 × 1011 | |||||
| CHCH + 2H → 2CH + 2H | 0.81 | 2.42 × 105 | CHCH + 2H → CCH + 3H | 0.99 | 9.54 × 103 | |||||
| 2CH + 2H → C + CH + 3H | 0.72 | 2.41 × 105 | CCH + 2H → C + CH + 3H | 0.53 | 3.73 × 107 | |||||
| C + CH + 3H → 2C + 4H | 1.17 | 6.79 × 101 | C + CH + 3H → 2C + 4H | 1.08 | 3.30 × 103 | |||||
| CO/H2 | CO + 2H → CHO + H | 1.31 | 7.12 × 100 | 1.31 | 7.12 × 100 | CO + 2H → CHO + H | 1.65 | 1.51 × 10−2 | 1.89 | 2.52 × 10−5 |
| CHO + H → CH + O + H | 0.46 | 3.69 × 108 | CHO + H → CH + O + H | 0.45 | 5.08 × 108 | |||||
| CH + O + H → C + O + 2H | 0.70 | 2.24 × 106 | CH + O + H → C + O + 2H | 0.78 | 1.00 × 106 | |||||
| gases | Fe(211) | Fe(211)-K2O | ||||||||
| C2H4 or C2H2 | C2H4 transition | 0.92 | 4.33 × 103 | 2.04 | 1.70 × 10−6 | C2H4 → C2H3 + H | 0.65 | 2.00 × 107 | 1.75 | 3.03 × 10−4 |
| C2H4 → C2H3 + H | 0.49 | 2.49 × 108 | C2H3 transition | 0.69 | 1.90 × 106 | |||||
| C2H3 + H → CCH2 + 2H | 0.17 | 2.62 × 1011 | C2H3 + H → CHCH + 2H | 0.25 | 4.53 × 1010 | |||||
| CCH2 + 2H → CCH + 3H | 0.68 | 3.83 × 106 | CHCH + 2H → CCH + 3H | 0.35 | 4.23 × 109 | |||||
| CCH + 3H → CC + 4H | 0.82 | 4.57 × 105 | CCH + 3H → CC + 4H | 0.30 | 2.16 × 1010 | |||||
| CC + 4H → 2C + 4H | 1.95 | 4.11 × 10−6 | CC + 4H → 2C + 4H | 1.75 | 3.03 × 10−4 | |||||
| CO/H2 | CO + 2H → CHO + H | 0.72 | 1.05 × 107 | 1.26 | 3.79 × 101 | CO + 2H → C + O + 2H | 0.77 | 1.80 × 105 | 0.77 | 1.80 × 105 |
| CHO + H → CH + O + H | 0.54 | 4.09 × 107 | ||||||||
| CH + O + H → C + O + 2H | 0.67 | 5.27 × 106 | ||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, J.; Cao, C.; Sun, R.; Cui, L.; Gao, R.; Hao, H. A DFT Insight into the Tuning Effect of Potassium Promoter on the Formation of Carbon Atoms via Carburization Gases Dissociation on Iron-Based Catalysts. Catalysts 2020, 10, 527. https://doi.org/10.3390/catal10050527
Gong J, Cao C, Sun R, Cui L, Gao R, Hao H. A DFT Insight into the Tuning Effect of Potassium Promoter on the Formation of Carbon Atoms via Carburization Gases Dissociation on Iron-Based Catalysts. Catalysts. 2020; 10(5):527. https://doi.org/10.3390/catal10050527
Chicago/Turabian StyleGong, Juhui, Cheng Cao, Ruiqin Sun, Linxia Cui, Rui Gao, and Haigang Hao. 2020. "A DFT Insight into the Tuning Effect of Potassium Promoter on the Formation of Carbon Atoms via Carburization Gases Dissociation on Iron-Based Catalysts" Catalysts 10, no. 5: 527. https://doi.org/10.3390/catal10050527
APA StyleGong, J., Cao, C., Sun, R., Cui, L., Gao, R., & Hao, H. (2020). A DFT Insight into the Tuning Effect of Potassium Promoter on the Formation of Carbon Atoms via Carburization Gases Dissociation on Iron-Based Catalysts. Catalysts, 10(5), 527. https://doi.org/10.3390/catal10050527




