Nanostructured Magnéli-Phase W18O49 Thin Films for Photoelectrochemical Water Splitting
Abstract
1. Introduction
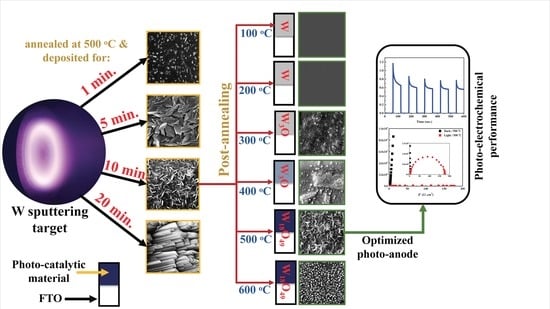
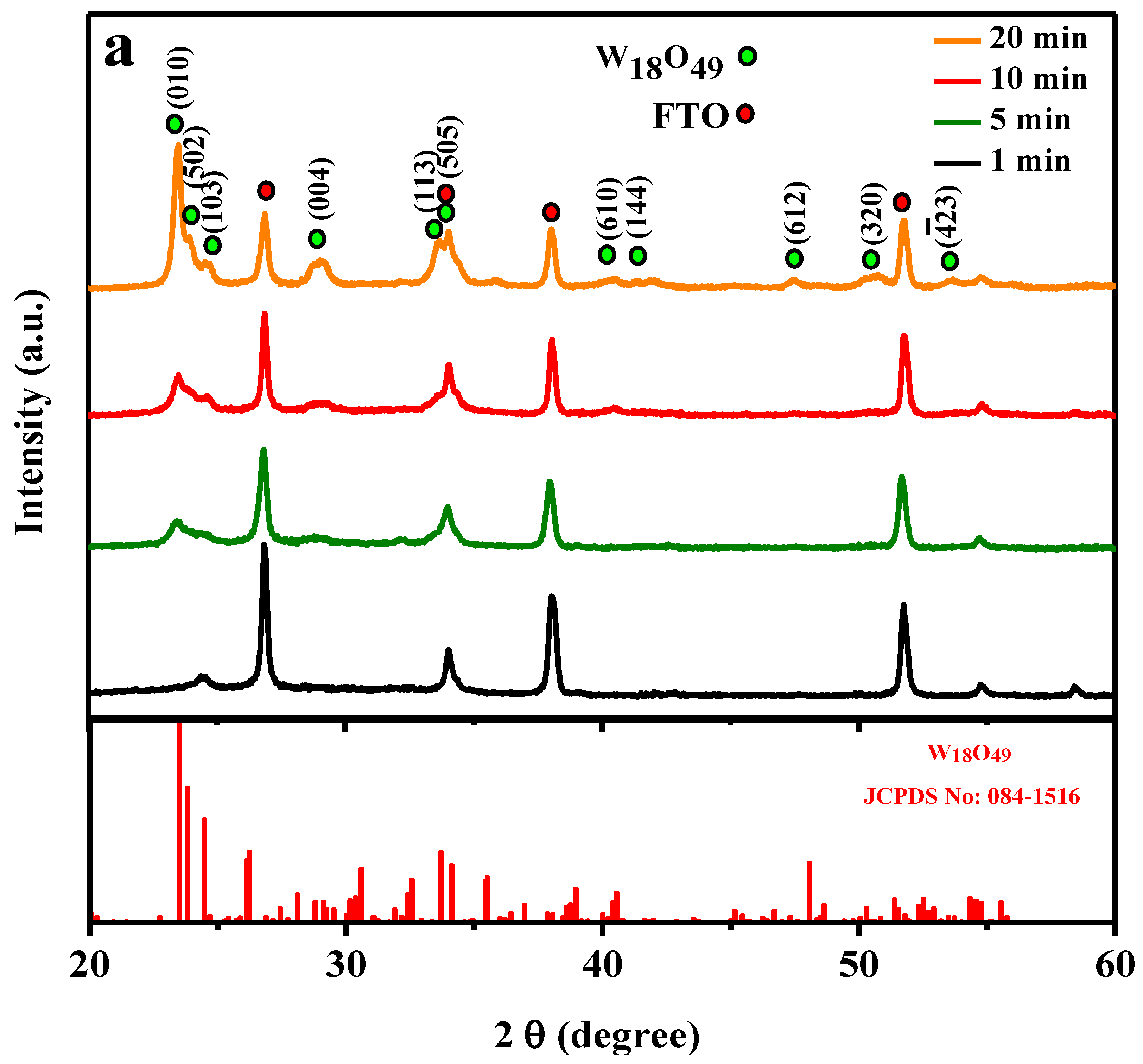
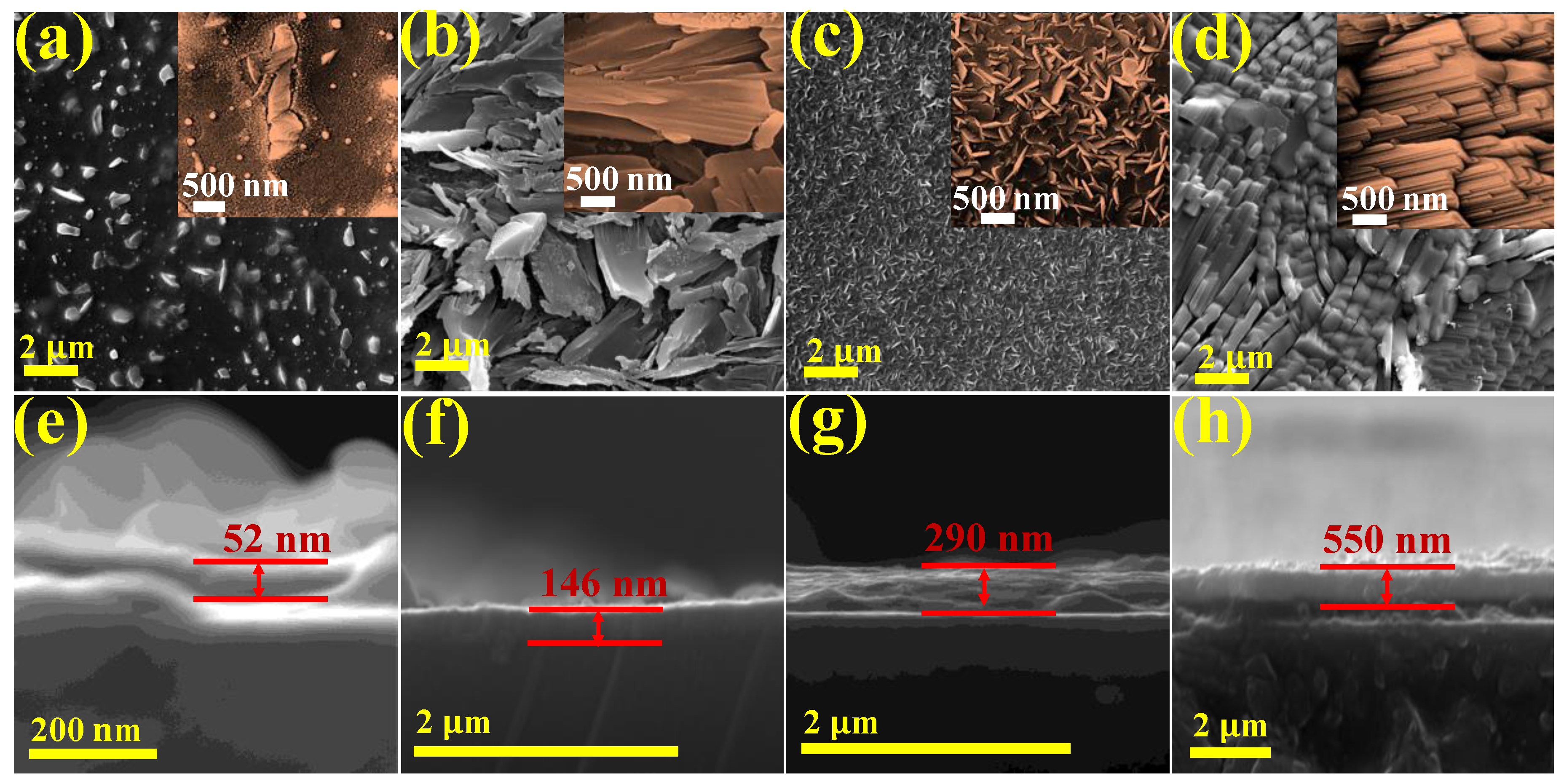
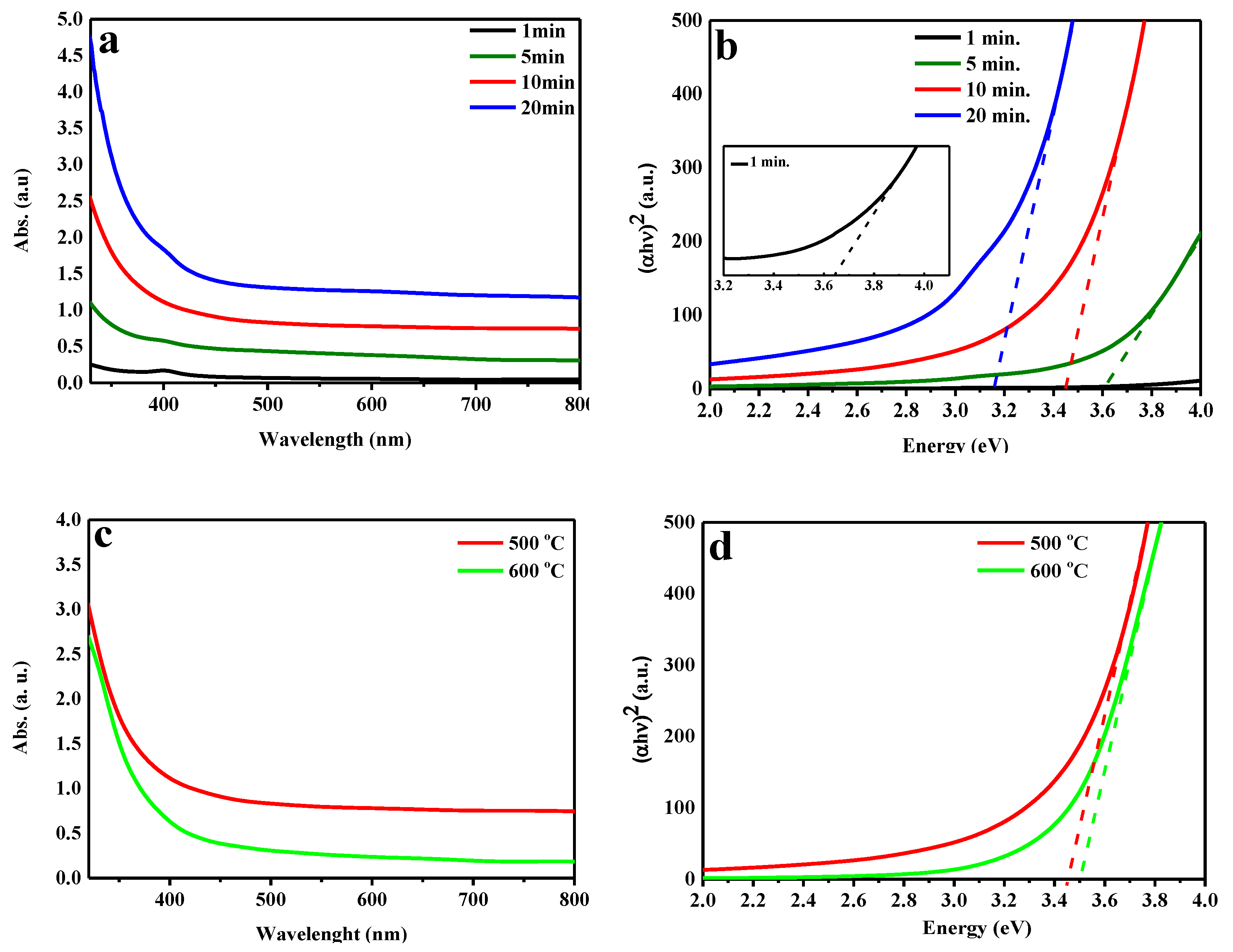
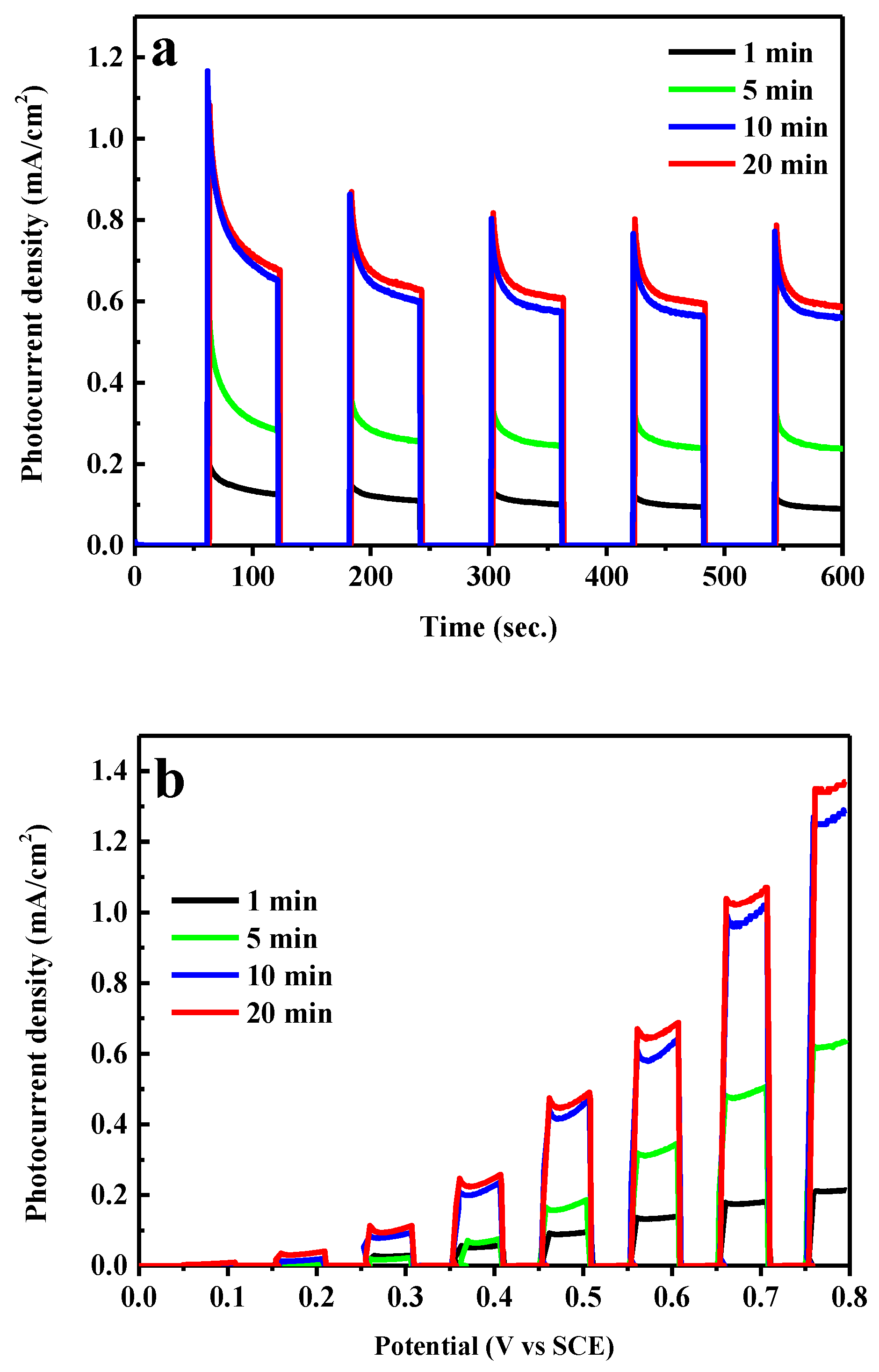
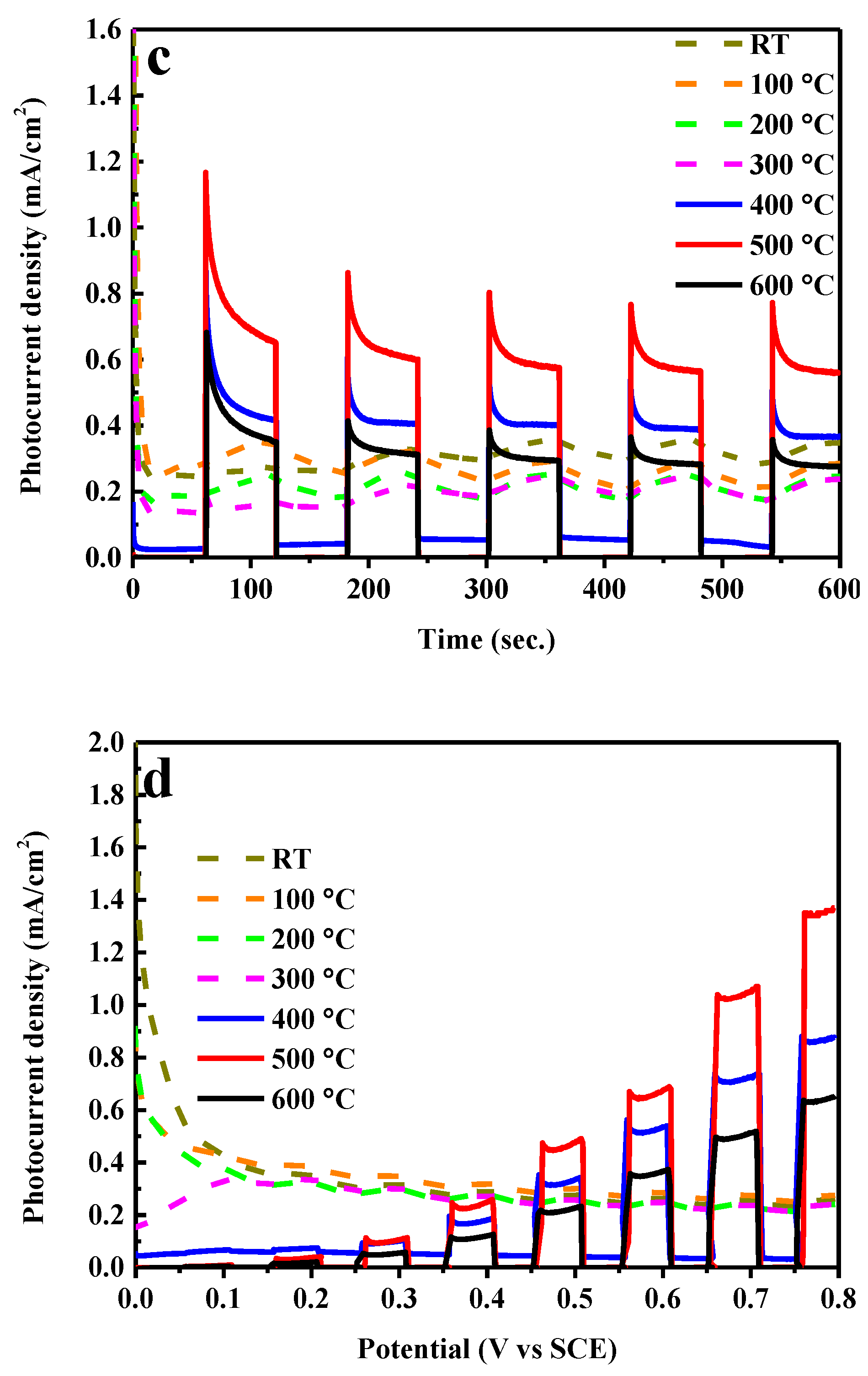
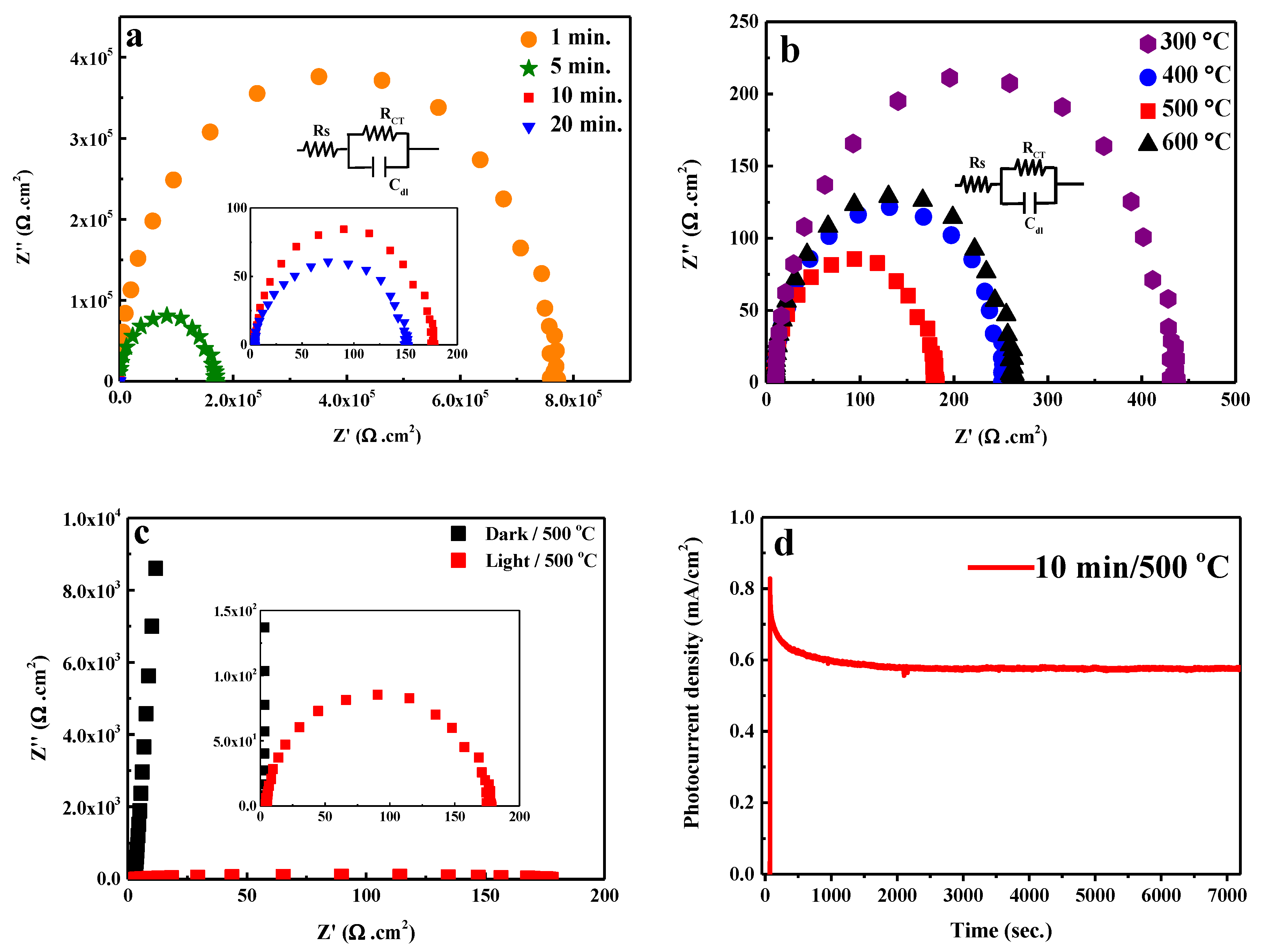
2. Results and Discussion
3. Experimental Details
3.1. Preparation of W18O49 Thin-Film Electrodes
3.2. Thin Films’ Characterization
3.3. Evaluation of Photo-Electrochemical (PEC) Activity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Green, M.A. High-efficiency silicon solar cells. In Proceedings of the Seventh E.C. Photovoltaic Solar Energy Conference, Sevilla, Spain, 27–31 October 1986. [Google Scholar]
- Grimes, C.; Varghese, O.; Ranjan, S. Light, Water, Hydrogen: The Solar Generation of Hydrogen by Water Photoelectrolysis; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Eisenstadt, M.; Cox, K. Hydrogen production from solar energy. Sol. Energy 1975, 17, 59–65. [Google Scholar] [CrossRef]
- Çelik, D.; Yıldız, M. Investigation of hydrogen production methods in accordance with green chemistry principles. Int. J. Hydrog. Energy 2017, 42, 23395–23401. [Google Scholar] [CrossRef]
- Scott, K. Chapter 11. Conclusions: Electrolytic Hydrogen Production and Sustainable Routes. In Electrochemical Methods for Hydrogen Production; Royal Society of Chemistry: London, UK, 2019; Volume 25, pp. 392–404. [Google Scholar]
- Peter, L.M.; Wijayantha, K.G.U. Photoelectrochemical Water Splitting at Semiconductor Electrodes: Fundamental Problems and New Perspectives. Chem. Phys. Chem. 2014, 15, 1983–1995. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Qin, J.; Xue, Y.; Yu, P.; Zhang, B.; Wang, L.; Liu, R. Effect of aspect ratio and surface defects on the photocatalytic activity of ZnO nanorods. Sci. Rep. 2014, 4, 4596. [Google Scholar] [CrossRef]
- Weon, S.; Choi, W. TiO2 Nanotubes with Open Channels as Deactivation-Resistant Photocatalyst for the Degradation of Volatile Organic Compounds. Environ. Sci. Technol. 2016, 50, 2556–2563. [Google Scholar] [CrossRef]
- Artioli, G.A.; Mancini, A.; Barbieri, V.R.; Quattrini, M.C.; Quartarone, E.; Mozzati, M.C.; Drera, G.; Sangaletti, L.; Gombac, V.; Fornasiero, P.; et al. Correlation between Deposition Parameters and Hydrogen Production in CuO Nanostructured Thin Films. Langmuir 2016, 32, 1510–1520. [Google Scholar] [CrossRef]
- Bhattacharya, C.; Lee, H.C.; Bard, A.J. Rapid Screening by Scanning Electrochemical Microscopy (SECM) of Dopants for Bi2WO6 Improved Photocatalytic Water Oxidation with Zn Doping. J. Phys. Chem. C 2013, 117, 9633–9640. [Google Scholar] [CrossRef]
- Ma, C.; Zhou, J.; Zhu, H.; Yang, W.; Liu, J.; Wang, Y.; Zou, Z. Constructing a high-efficiency MoO3/polyimide hybrid photocatalyst based on strong interfacial interaction. ACS Appl. Mater. Interfaces 2015, 7, 14628–14637. [Google Scholar]
- Wu, M.; Lin, X.; Hagfeldt, A.; Ma, T. A novel catalyst of WO2 nanorod for the counter electrode of dye-sensitized solar cells. Chem. Commun. 2011, 47, 4535. [Google Scholar] [CrossRef]
- Liu, Y.; Wygant, B.R.; Kawashima, K.; Mabayoje, O.; Hong, T.E.; Lee, S.; Lin, J.; Kim, J.; Yubuta, K.; Li, W.; et al. Facet effect on the photoelectrochemical performance of a WO3/BiVO4 heterojunction photoanode. Appl. Catal. B Environ. 2019, 245, 227–239. [Google Scholar] [CrossRef]
- Zhang, N.; Jalil, A.; Wu, D.; Chen, S.; Liu, Y.; Gao, C.; Ye, W.; Qi, Z.; Ju, H.; Wang, C.; et al. Refining Defect States in W18O49 by Mo Doping: A Strategy for Tuning N2 Activation towards Solar-Driven Nitrogen Fixation. J. Am. Chem. Soc. 2018, 140, 9434–9443. [Google Scholar] [CrossRef] [PubMed]
- Seabold, J.A.; Choi, K.-S. Effect of a Cobalt-Based Oxygen Evolution Catalyst on the Stability and the Selectivity of Photo-Oxidation Reactions of a WO3 Photoanode. Chem. Mater. 2011, 23, 1105–1112. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Kieslich, G.; Tremel, W. Magnéli oxides as promising n-type thermoelectrics. AIMS Mater. Sci. 2014, 1, 184–190. [Google Scholar] [CrossRef]
- Bai, H.; Yi, W.; Liu, J.-Y.; Lv, Q.; Zhang, Q.; Ma, Q.; Yang, H.; Xi, G. Large-scale synthesis of ultrathin tungsten oxide nanowire networks: An efficient catalyst for aerobic oxidation of toluene to benzaldehyde under visible light. Nanoscale 2016, 8, 13545–13551. [Google Scholar] [CrossRef]
- Jia, C.; Zhang, X.; Matras-Postolek, K.; Huang, B.; Yang, P. Z-scheme reduced graphene oxide/TiO2-Bronze/W18O49 ternary heterostructure towards efficient full solar-spectrum photocatalysis. Carbon 2018, 139, 415–426. [Google Scholar] [CrossRef]
- Huang, Z.-F.; Song, J.; Pan, L.; Lv, F.; Wang, Q.; Zou, J.-J.; Zhang, X.; Wang, L. Mesoporous W18O49 hollow spheres as highly active photocatalysts. Chem. Commun. 2014, 50, 10959. [Google Scholar] [CrossRef]
- Guo, C.; Yin, S.; Huang, Y.; Dong, Q.; Sato, T. Synthesis of W18O49Nanorod via Ammonium Tungsten Oxide and Its Interesting Optical Properties. Langmuir 2011, 27, 12172–12178. [Google Scholar] [CrossRef]
- Guo, C.; Yin, S.; Yan, M.; Kobayashi, M.; Kakihana, M.; Sato, T. Morphology-Controlled Synthesis of W18O49 Nanostructures and Their Near-Infrared Absorption Properties. Inorg. Chem. 2012, 51, 4763–4771. [Google Scholar] [CrossRef]
- Hai, G.; Huang, J.; Jie, Y.; Cao, L.; Wang, L.; Fu, C.; Xiao, T.; Niu, M.; Feng, L. Unveiling the relationships between (010) facets-orientation growth and photocatalytic activity in W18O49 nanowires. J. Alloys Compd. 2020, 820, 153127. [Google Scholar] [CrossRef]
- Chen, P.; Qin, M.; Chen, Z.; Jia, B.; Qu, X. Solution combustion synthesis of nanosized WOx: Characterization, mechanism and excellent photocatalytic properties. RSC Adv. 2016, 6, 83101–83109. [Google Scholar] [CrossRef]
- Song, P.K.; Akao, H.; Kamei, M.; Shigesato, Y.; Yasui, I. Preparation and Crystallization of Tin-doped and Undoped Amorphous Indium Oxide Films Deposited by Sputtering. Jpn. J. Appl. Phys. 1999, 38, 5224–5226. [Google Scholar] [CrossRef]
- Maillé, L.; Sant, C.; Le Paven-Thivet, C.; Legrand-Buscema, C.; Garnier, P. Structure and morphological study of nanometer W and W3O thin films. Thin Solid Film. 2003, 428, 237–241. [Google Scholar] [CrossRef]
- Tauc, J. Optical Properties of Amorphous Semiconductors. In Amorphous and Liquid Semiconductors; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 1974; pp. 159–220. [Google Scholar]
- Al-Kuhaili, M. Electromodulated transmittance of optical transitions in tungsten oxide. J. Phys. Chem. Solids 2020, 139, 109317. [Google Scholar] [CrossRef]
- Abdelrahman, A.; Yunus, W.; Arof, A.K. Optical properties of tin sulphide (SnS) thin film estimated from transmission spectra. J. Non Cryst. Solids 2012, 358, 1447–1451. [Google Scholar] [CrossRef]
- Vani, V.P.G.; Reddy, M.R.V.; Reddy, K.T.R. Influence of Source—Substrate Distance of Cu4SnS4 Thin Films Grown by Co-Evaporation. Adv. Mater. Res. 2013, 768, 103–108. [Google Scholar] [CrossRef]
- Wong, H.; Zhou, J.; Zhang, J.; Jin, H.; Kakushima, K.; Iwai, H. The interfaces of lanthanum oxide-based subnanometer EOT gate dielectrics. Nanoscale Res. Lett. 2014, 9, 472. [Google Scholar] [CrossRef]
- Xie, F.; Gong, L.; Liu, X.; Tao, Y.; Zhang, W.; Chen, S.; Meng, H.; Chen, J. XPS studies on surface reduction of tungsten oxide nanowire film by Ar+ bombardment. J. Electron Spectrosc. Relat. Phenom. 2012, 185, 112–118. [Google Scholar] [CrossRef]
- Zhong, Q.; Macharia, D.K.; Zhong, W.; Liu, Z.; Chen, Z. Synthesis of hydrophobic W18O49 nanorods for constructing UV/NIR-shielding and self-cleaning film. Ceram. Int. 2020. [Google Scholar] [CrossRef]
- Lv, Z.; Liu, H.; Zhang, X.; Shen, X.; Liu, S.; Wu, X.; Fang, M.; Liu, Y.-G.; Min, X.; Huang, Z. Solvothermally synthesized highly porous W18O49 nanowires for UV, humidity and pH detection. Phys. E Low Dimens. Syst. Nanostruct. 2020, 117, 113719. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Z.; Zhai, G.; Men, Y. Boosting photocatalytic activity of WO3 nanorods with tailored surface oxygen vacancies for selective alcohol oxidations. Appl. Surf. Sci. 2018, 462, 760–771. [Google Scholar] [CrossRef]
- Park, Y.; McDonald, K.J.; Choi, K.-S. Progress in bismuth vanadate photoanodes for use in solar water oxidation. Chem. Soc. Rev. 2013, 42, 2321–2337. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhou, H.; Pei, S.; Xie, S.; You, S. Oxygen-deficient WO3−x nanoplate array film photoanode for efficient photoelectrocatalytic water decontamination. Chem. Eng. J. 2020, 381, 122740. [Google Scholar] [CrossRef]
- Hosseini, M.G.; Sefidi, P.Y.; Aydin, Z.; Kinayyigit, S. Toward enhancing the photoelectrochemical water splitting efficiency of organic acid doped polyaniline-WO3 photoanode by photo-assisted electrochemically reduced graphene oxide. Electrochim. Acta 2020, 333, 135475. [Google Scholar] [CrossRef]
- Qamar, M.; Merzougui, B.; Ahmed, M.I.; Yamani, Z.H. Broad Solar Spectrum-Responsive and Highly Efficient Photoanode of Nonstoichiometric TiO2 Nanoplates/Reduced Graphene Oxide. ACS Sustain. Chem. Eng. 2017, 6, 2112–2121. [Google Scholar] [CrossRef]
- Mai, M.; Ma, X.; Zhou, H.; Ye, M.; Li, T.; Ke, S.; Lin, P.; Zeng, X. Effect of oxygen pressure on pulsed laser deposited WO3 thin films for photoelectrochemical water splitting. J. Alloys Compd. 2017, 722, 913–919. [Google Scholar] [CrossRef]
- Claudino, C.H.; Kuznetsova, M.; Rodrigues, B.S.; Chen, C.; Wang, Z.; Sardela, M.; Souza, J.S. Facile one-pot microwave-assisted synthesis of tungsten-doped BiVO4/WO3 heterojunctions with enhanced photocatalytic activity. Mater. Res. Bull. 2020, 125, 110783. [Google Scholar] [CrossRef]
- Wang, R.; Shen, J.; Zhang, W.; Liu, Q.; Zhang, M.; Zulfiqar; Tang, H. Build-in electric field induced step-scheme TiO2/W18O49 heterojunction for enhanced photocatalytic activity under visible-light irradiation. Ceram. Int. 2020, 46, 23–30. [Google Scholar] [CrossRef]
- Zhang, T.; Paulose, M.; Neupane, R.; Schaffer, L.A.; Rana, D.B.; Su, J.; Guo, L.; Varghese, O.K. Nanoporous WO3 films synthesized by tuning anodization conditions for photoelectrochemical water oxidation. Sol. Energy Mater. Sol. Cells 2020, 209, 110472. [Google Scholar] [CrossRef]
- Markhabayeva, A.A.; Moniruddin; Dupre, R.; Abdullin, K.A.; Nuraje, N. Designing of WO3@Co3O4 Heterostructures to Enhance Photoelectrochemical Performances. J. Phys. Chem. A 2019, 124, 486–491. [Google Scholar] [CrossRef]
- Zhao, L.-D.; Zhang, Q.; Fan, J.-B.; Yin, L.-Q.; Qi, P.-W.; Yao, H.-C.; Li, Z.-J. Improvement of the photoelectrochemical performance of vertically aligned WO3 nanosheet array film with a disordered surface layer by electroreduction. J. Solid State Electrochem. 2019, 23, 1621–1630. [Google Scholar] [CrossRef]
- Kalanur, S.S.; Seo, H. Aligned nanotriangles of tantalum doped tungsten oxide for improved photoelectrochemical water splitting. J. Alloys Compd. 2019, 785, 1097–1105. [Google Scholar] [CrossRef]
- Acosta, M.; Méndez, R.A.; Riech, I.; Rodríguez-Pérez, M.; Gattorno, G.R. Structural, optical and photoelectrochemical properties of tungsten oxide thin films grown by non-reactive RF-sputtering. Superlattices Microstruct. 2019, 127, 123–127. [Google Scholar] [CrossRef]
- Pedroni, M.; Canetti, M.; Chiarello, G.L.; Cremona, A.; Inzoli, F.; Luzzati, S.; Pietralunga, S.; Tagliaferri, A.; Zani, M.; Vassallo, E. Tungsten oxide thin film photo-anodes by reactive RF diode sputtering. Thin Solid Film. 2016, 616, 375–380. [Google Scholar] [CrossRef]
- Pedroni, M.; Chiarello, G.L.; Vassallo, E.; Selli, E. Multilayer WO3/BiVO4 Photoanodes for Solar-Driven Water Splitting Prepared by RF-Plasma Sputtering. Surfaces 2020, 3, 10. [Google Scholar] [CrossRef]
- Cole, B.; Marsen, B.; Miller, E.; Yan, Y.; To, B.; Jones, K.; Al-Jassim, M. Evaluation of Nitrogen Doping of Tungsten Oxide for Photoelectrochemical Water Splitting. J. Phys. Chem. C 2008, 112, 5213–5220. [Google Scholar] [CrossRef]
- Li, W.; Liu, C.; Yang, Y.; Li, J.; Chen, Q.; Liu, F. Platelike WO3 from hydrothermal RF sputtered tungsten thin films for photoelectrochemical water oxidation. Mater. Lett. 2012, 84, 41–43. [Google Scholar] [CrossRef]
- Valerini, D.; Hernández, S.; Di Benedetto, F.; Russo, N.; Saracco, G.; Rizzo, A. Sputtered WO3 films for water splitting applications. Mater. Sci. Semicond. Process. 2016, 42, 150–154. [Google Scholar] [CrossRef]
- Wiriyachailerd, C.; Horprathum, M.; Eiamchai, P.; Ponchio, C. Improved the chrage transfer for highly efficient photoelectrochemical water oxidation: The case of WO3 and BiVO4. Mater. Today Proc. 2018, 5, 13874–13878. [Google Scholar] [CrossRef]



| Preparation Method | Structure | Photo-Current mA/cm2 | Bias Voltage | Electrolyte | Light Source | Ref. |
|---|---|---|---|---|---|---|
| solvothermal | plates-like | 0.20 | 0.6 V vs. Ag/AgCl | 0.5 M Na2SO4 | solar simulation A.M. 1.5 G spectrum with the intensity of 100 mW·cm−2 | [41] |
| solvothermal | spindle-like | 0.20 | Ag/AgCl | 0.5 M Na2SO4 | - | [42] |
| tungsten foil anodization | nanoporous layer | 0.40 | 0.6 V vs. Ag/AgCl | 0.5 M Na2SO4 | 300 W Xe lamp | [43] |
| Spin coating | Nano particles | 0.25 | 1.25 V vs. RHE | 0.1 M Na2SO4 | 300 W Xenon lamp | [38] |
| electrodeposition | Nano-islands | 0.02 | 0.7 V vs. Ag/AgCl | 0.5 M H2SO4 | Xenon solar simulator (AM 1.5 G, 100 mW/cm2) | [44] |
| hydrothermal method | Nanosheets | 0.9 | 1.2 V vs. RHE | 0.5 M Na2SO4 | LED lamp coupled with an AM 1.5 G filter (100 mW cm−2, CEL-LED 100) | [45] |
| hydrothermal method | Nanosheets | 0.3 | 1.2 V vs. RHE | 0.5 M Na2SO4 | 1000 W xenon arc lamp | [46] |
| RF-sputtering | Rough film | 0.01 | 1 V vs. Ag/AgCl | 1 M HClO4 | 400 W ozone-free Xe arc lamp (AM 1.5 G filter) | [47] |
| RF-sputtering | film | 0.9 | 0.6 V vs. SCE | 0.5 M Na2SO4 | mercury arc lamp 350 nm < λ < 450 nm | [48] |
| RF-sputtering | film | 0.3 | 0.6 V vs. SCE | 0.5 M Na2SO4 | 300 W LOT-qd Xe lamp, equipped with an AM 1.5 G filter | [49] |
| RF-sputtering | Nanosheets | <0.5 | 0.6 V vs. SCE | 0.33 molar H3PO | AM 1.5 G illumination generated by solar simulator | [50] |
| RF-sputtering | film | 0.2 | 0.6 V vs. Ag/AgCl | 0.5 mol/L H2SO4 | A 500 W Xe lamp/A 400 nm cutoff filter | [51] |
| RF-sputtering | film | 0 | 0.6 V vs. RHE | 0.1 M Na2SO4 | Xe lamp (150 W)/wavelengths range from 300 to 570 nm | [52] |
| DC-sputtering | film | 0.05 | 1.0 V vs. Ag/AgCl | 0.5 M Na2SO4 | visible light irradiation (λ > 420 nm) | [53] |
| DC-sputtering | Nanosheets | 0.6 | 0.6 V vs. SCE | 0.5 M Na2SO4 | 300 W Xenon lamp UV-visible (300–600 nm) | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamedkhair, A.K.; Drmosh, Q.A.; Qamar, M.; Yamani, Z.H. Nanostructured Magnéli-Phase W18O49 Thin Films for Photoelectrochemical Water Splitting. Catalysts 2020, 10, 526. https://doi.org/10.3390/catal10050526
Mohamedkhair AK, Drmosh QA, Qamar M, Yamani ZH. Nanostructured Magnéli-Phase W18O49 Thin Films for Photoelectrochemical Water Splitting. Catalysts. 2020; 10(5):526. https://doi.org/10.3390/catal10050526
Chicago/Turabian StyleMohamedkhair, A. K., Q. A. Drmosh, Mohammad Qamar, and Z. H. Yamani. 2020. "Nanostructured Magnéli-Phase W18O49 Thin Films for Photoelectrochemical Water Splitting" Catalysts 10, no. 5: 526. https://doi.org/10.3390/catal10050526
APA StyleMohamedkhair, A. K., Drmosh, Q. A., Qamar, M., & Yamani, Z. H. (2020). Nanostructured Magnéli-Phase W18O49 Thin Films for Photoelectrochemical Water Splitting. Catalysts, 10(5), 526. https://doi.org/10.3390/catal10050526