Hydrothermal Aging of Pd/LTA Monolithic Catalyst for Complete CH4 Oxidation
Abstract
1. Introduction
2. Results
2.1. Characterization
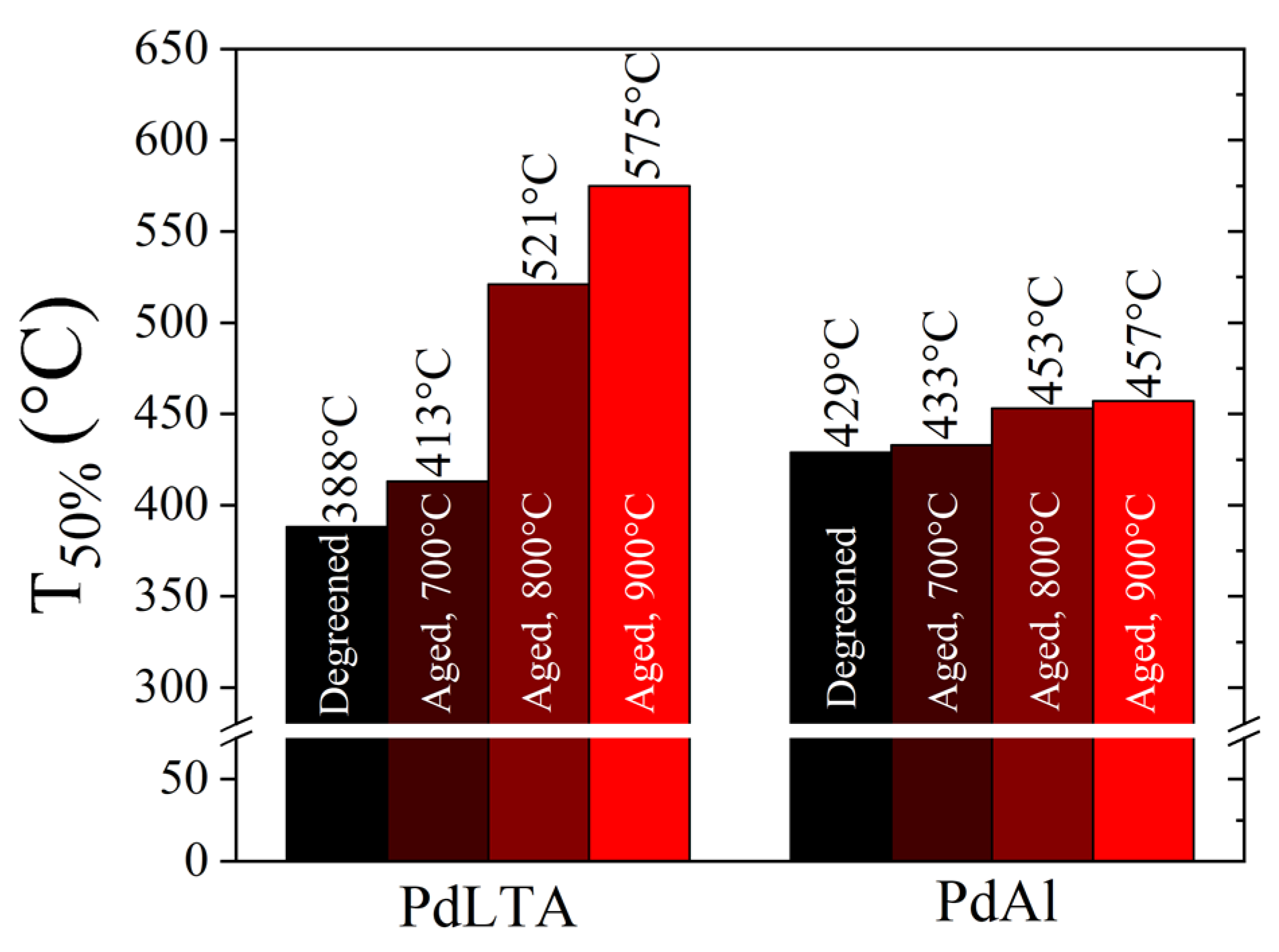
2.2. Catalytic Activity of Degreened and Aged Samples
3. Materials and Methods
3.1. Catalyst Preparation
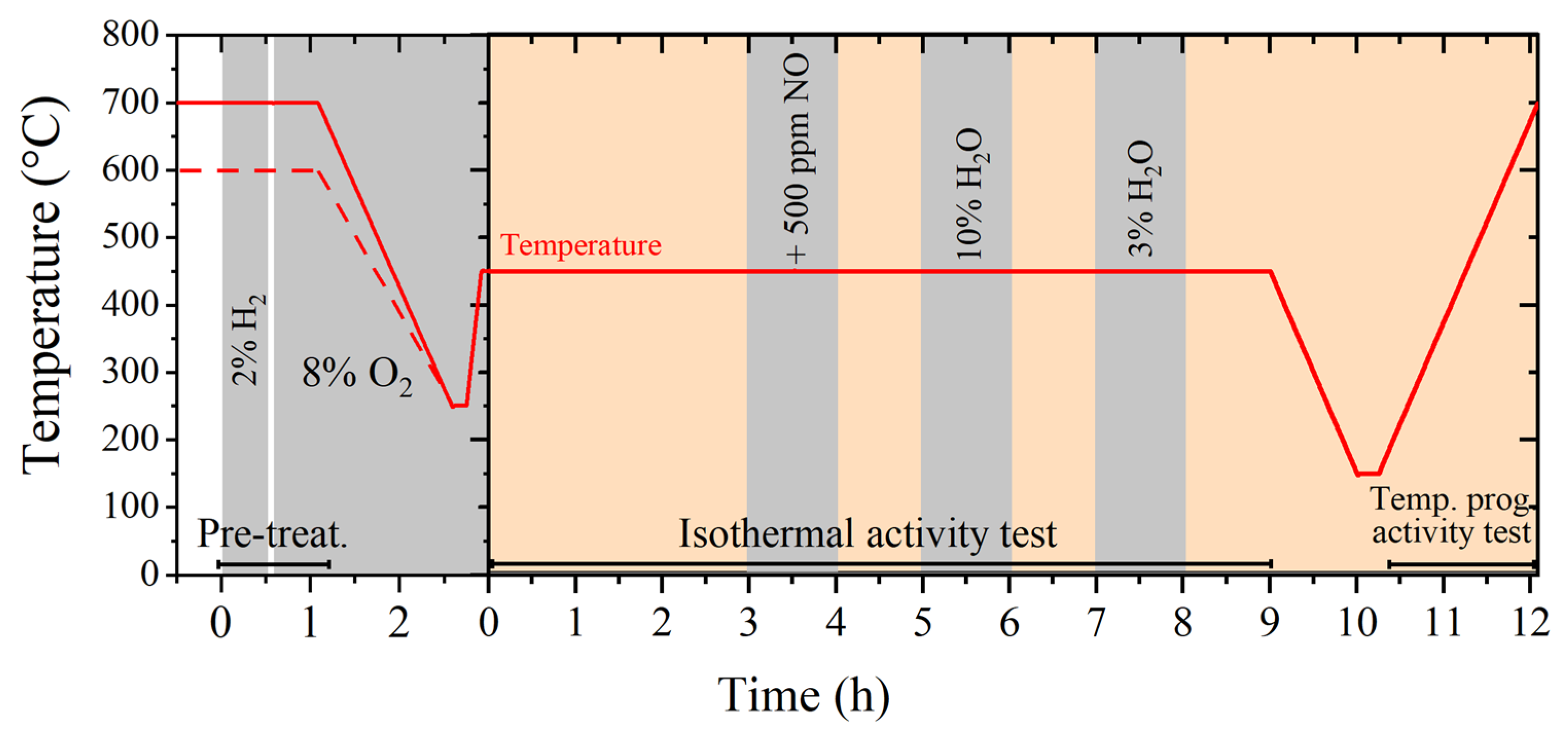
3.2. Catalytic Activity Tests and Aging
3.2.1. Degreening and Pre-Treatment
- 2% H2 at 500 °C (30 min)
- Wet reaction mixture at 600 °C (60 min)
- 2% H2 and 5% H2O at 600 °C (20 min)
- Wet reaction mixture at 600 °C (60 min)
- 2% H2 at 600 °C (30 min)
- 8% O2 at 600 °C (30 min)
- Cooling in 8% O2 to 250 °C
3.2.2. Catalytic Activity Tests
- Heating in 8% O2 to 450 °C
- Wet reaction mixture at 450 °C (3 h)
- Wet reaction mixture + 500 ppm NO at 450 °C (1 h)
- Wet reaction mixture at 450 °C (1 h)
- Wet reaction mixture but with 10% H2O at 450 °C (1 h)
- Wet reaction mixture at 450 °C (1 h)
- Wet reaction mixture but with 3% H2O at 450 °C (1 h)
- Wet reaction mixture at 450 °C (1 h)
- Cooling to 150 °C in wet reaction mixture
- Heating to 700 °C in wet reaction mixture (5 °C/min)
3.2.3. Hydrothermal Aging
- Degreening
- Pre-treatment 600 °C
- Activity test
- Aging 700 °C
- Pre-treatment 700 °C
- Activity test
- Aging 800 °C
- Pre-treatment 700 °C
- Activity test
- Aging 900 °C
- Pre-treatment 700 °C
- Activity test
3.2.4. Degreening and Hydrothermal Aging of Catalyst Powder for Characterization
3.3. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gelin, P.; Primet, M. Complete oxidation of methane at low temperature over noble metal based catalysts: A review. Appl. Catal. B Environ. 2002, 39, 1–37. [Google Scholar] [CrossRef]
- Gholami, R.; Alyani, M.; Smith, K.J. Deactivation of Pd catalysts by water during low temperature methane oxidation relevant to natural gas vehicle converters. Catalysts 2015, 5, 561–594. [Google Scholar] [CrossRef]
- Burch, R.; Urbano, F.J.; Loader, P.K. Methane combustion over palladium catalysts: The effect of carbon dioxide and water on activity. Appl. Catal. A Gen. 1995, 123, 173–184. [Google Scholar] [CrossRef]
- Schwartz, W.R.; Ciuparu, D.; Pfefferle, L.D. Combustion of methane over palladium-based catalysts: Catalytic deactivation and role of the support. J. Phys. Chem. C 2012, 116, 8587–8593. [Google Scholar] [CrossRef]
- Schwartz, W.R.; Pfefferle, L.D. Combustion of methane over palladium-based catalysts: Support interactions. J. Phys. Chem. C 2012, 116, 8571–8578. [Google Scholar] [CrossRef]
- Bartholomew, C.H. Mechanisms of catalyst deactivation. Appl. Catal. A Gen. 2001, 212, 17–60. [Google Scholar] [CrossRef]
- Friberg, I.; Sadokhina, N.; Olsson, L. The effect of Si/Al ratio of zeolite supported Pd for complete CH4 oxidation in the presence of water vapor and SO2. Appl. Catal. B Environ. 2019, 250, 117–131. [Google Scholar] [CrossRef]
- Okumura, K.; Shinohara, E.; Niwa, M. Pd loaded on high silica beta support active for the total oxidation of diluted methane in the presence of water vapor. Catal. Today 2006, 117, 577–583. [Google Scholar] [CrossRef]
- Petrov, A.W.; Ferri, D.; Krocher, O.; Van Bokhoven, J.A. Design of Stable Palladium-Based Zeolite Catalysts for Complete Methane Oxidation by Postsynthesis Zeolite Modification. ASC Catal. 2019, 9, 2303–2312. [Google Scholar] [CrossRef]
- Petrov, A.W.; Ferri, D.; Krumeich, F.; Nachtegaal, M.; Van Bokhoven, J.A.; Kröcher, O. Stable complete methane oxidation over palladium based zeolite catalysts. Nat. Commun. 2018, 9, 2545. [Google Scholar] [CrossRef]
- Petrov, A.; Ferri, D.; Tarik, M.; Krocher, O.; Bokhoven, J. Deactivation Aspects of Methane Oxidation Catalysts Based on Palladium and ZSM-5. Top. Catal. 2017, 60, 123–130. [Google Scholar] [CrossRef]
- Liu, S.-b.; Wu, J.-F.; Ma, L.-J.; Tsai, T.-C.; Wang, I. On the thermal stability of zeolite beta. J. Catal. 1991, 132, 432–439. [Google Scholar] [CrossRef]
- Ding, L.; Zheng, Y.; Hong, Y.; Ring, Z. Effect of particle size on the hydrothermal stability of zeolite beta. Microporous Mesoporous Mat. 2007, 101, 432–439. [Google Scholar] [CrossRef]
- Kwak, J.H.; Tran, D.; Burton, S.D.; Szanyi, J.; Lee, J.H.; Peden, C.H.F. Effects of hydrothermal aging on NH3-SCR reaction over Cu/zeolites. J. Catal. 2012, 287, 203–209. [Google Scholar] [CrossRef]
- Blakeman, P.G.; Burkholder, E.M.; Chen, H.-Y.; Collier, J.E.; Fedeyko, J.M.; Jobson, H.; Rajaram, R.R. The role of pore size on the thermal stability of zeolite supported Cu SCR catalysts. Catal. Today 2014, 231, 56–63. [Google Scholar] [CrossRef]
- Leistner, K.; Kumar, A.; Kamasamudram, K.; Olsson, L. Mechanistic study of hydrothermally aged Cu/SSZ-13 catalysts for ammonia-SCR. Catal. Today 2018, 307, 55–64. [Google Scholar] [CrossRef]
- Wang, A.Y.; Arora, P.; Bernin, D.; Kumar, A.; Kamasamudram, K.; Olsson, L. Investigation of the robust hydrothermal stability of Cu/LTA for NH3-SCR reaction. Appl. Catal. B Environ. 2019, 246, 242–253. [Google Scholar] [CrossRef]
- Ryu, T.; Ahn, N.H.; Seo, S.; Cho, J.; Kim, H.; Jo, D.; Park, G.T.; Kim, P.S.; Kim, C.H.; Bruce, E.L.; et al. Fully Copper-Exchanged High-Silica LTA Zeolites as Unrivaled Hydrothermally Stable NH3-SCR Catalysts. Angew. Chem. Int. Ed. 2017, 56, 3256–3260. [Google Scholar] [CrossRef]
- Lim, J.B.; Jo, D.; Hong, S.B. Palladium-exchanged small-pore zeolites with different cage systems as methane combustion catalysts. Appl. Catal. B Environ. 2017, 219, 155–162. [Google Scholar] [CrossRef]
- Lee, J.; Ryou, Y.; Hwang, S.; Kim, Y.; Cho, S.J.; Lee, H.; Kim, C.H.; Kim, D.H. Comparative study of the mobility of Pd species in SSZ-13 and ZSM-5, and its implication for their activity as passive NOx adsorbers (PNAs) after hydro-thermal aging. Catal. Sci. Technol. 2019, 9, 163–173. [Google Scholar] [CrossRef]
- Ryou, Y.; Lee, J.; Cho, S.J.; Lee, H.; Kim, C.H.; Kim, D.H. Activation of Pd/SSZ-13 catalyst by hydrothermal aging treatment in passive NO adsorption performance at low temperature for cold start application. Appl. Catal. B Environ. 2017, 212, 140–149. [Google Scholar] [CrossRef]
- Hernandez-Garrido, J.C.; Gomez, D.M.; Gaona, D.; Vidal, H.; Gatica, J.M.; Sanz, O.; Rebled, J.M.; Peiro, F.; Calvino, J.J. Combined (S)TEM-FIB Insight into the Influence of the Preparation Method on the Final Surface Structure of a Co3O4/La-Modified-CeO2 Washcoated Monolithic Catalyst. J. Phys. Chem. C 2013, 117, 13028–13036. [Google Scholar] [CrossRef]
- Thevenin, P.O.; Pocoroba, E.; Pettersson, L.J.; Karhu, H.; Vayrynen, I.J.; Jaras, S.G. Characterization and activity of supported palladium combustion catalysts. J. Catal. 2002, 207, 139–149. [Google Scholar] [CrossRef]
- Ogura, M.; Hayashi, M.; Kage, S.; Matsukata, M.; Kikuchi, E. Determination of active palladium species in ZSM-5 zeolite for selective reduction of nitric oxide with methane. Appl. Catal. B Environ. 1999, 23, 247–257. [Google Scholar] [CrossRef]
- Watson, J.M.; Ozkan, U.S. Adsorption characteristics of sol-gel Gd-Pd/TiO2 catalysts in reduction of nitric oxide with CH4: DRIFTS and TPD. J. Catal. 2002, 210, 295–312. [Google Scholar] [CrossRef]
- Chakarova, K.; Ivanova, E.; Hadjiivanov, K.; Klissurski, D.; Knozinger, H. Co-ordination chemistry of palladium cations in Pd-H-ZSM-5 as revealed by FTIR spectra of adsorbed and co-adsorbed probe molecules (CO and NO). Phys. Chem. Chem. Phys. 2004, 6, 3702–3709. [Google Scholar] [CrossRef]
- Chen, H.Y.; Collier, J.E.; Liu, D.X.; Mantarosie, L.; Duran-Martin, D.; Novak, V.; Rajaram, R.R.; Thompsett, D. Low Temperature NO Storage of Zeolite Supported Pd for Low Temperature Diesel Engine Emission Control. Catal. Lett. 2016, 146, 1706–1711. [Google Scholar] [CrossRef]
- Lonyi, F.; Solt, H.E.; Valyon, J.; Decolatti, H.; Gutierrez, L.B.; Miro, E. An operando DRIFTS study of the active sites and the active intermediates of the NO-SCR reaction by methane over In,H- and In,Pd,H-zeolite catalysts. Appl. Catal. B Environ. 2010, 100, 133–142. [Google Scholar] [CrossRef]
- Pommier, B.; Gelin, P. On the nature of Pd species formed upon exchange of H-ZSM5 with Pd(NH3)(4)(2+) and calcination in O-2. Phys. Chem. Chem. Phys. 1999, 1, 1665–1672. [Google Scholar] [CrossRef]
- Loiland, J.A.; Lobo, R.F. Oxidation of zeolite acid sites in NO/O-2 mixtures and the catalytic properties of the new site in NO oxidation. J. Catal. 2015, 325, 68–78. [Google Scholar] [CrossRef]
- Hadjiivanov, K.; Saussey, J.; Freysz, J.L.; Lavalley, J.C. FT-IR study of NO+O-2 co-adsorption on H-ZSM-5: Re-assignment of the 2133 cm(-1) band to NO+ species. Catal. Lett. 1998, 52, 103–108. [Google Scholar] [CrossRef]
- Chen, H.Y.; Wei, Z.H.; Kollar, M.; Gao, F.; Wang, Y.L.; Szanyi, J.; Peden, C.H.F. NO oxidation on zeolite supported Cu catalysts: Formation and reactivity of surface nitrates. Catal. Today 2016, 267, 17–27. [Google Scholar] [CrossRef]
- Ahrens, M.; Marie, O.; Bazin, P.; Daturi, M. Fe-H-BEA and Fe-H-ZSM-5 for NO2 removal from ambient air—A detailed in situ and operando FTIR study revealing an unexpected positive water-effect. J. Catal. 2010, 271, 1–11. [Google Scholar] [CrossRef]
- Auvray, X.; Olsson, L. Stability and activity of Pd-, Pt- and Pd-Pt catalysts supported on alumina for NO oxidation. Appl. Catal. B Environ. 2015, 168, 342–352. [Google Scholar] [CrossRef]
- Mihai, O.; Trandafilovic, L.; Wentworth, T.; Torres, F.F.; Olsson, L. The Effect of Si/Al Ratio for Pd/BEA and Pd/SSZ-13 Used as Passive NOx Adsorbers. Top. Catal. 2018, 61, 2007–2020. [Google Scholar] [CrossRef]
- Sadokhina, N.; Smedler, G.; Nylén, U.; Olofsson, M.; Olsson, L. The influence of gas composition on Pd-based catalyst activity in methane oxidation—Inhibition and promotion by NO. Appl. Catal. B Environ. 2017, 200, 351–360. [Google Scholar] [CrossRef]
- Sadokhina, N.; Ghasempour, F.; Auvray, X.; Smedler, G.; Nylén, U.; Olofsson, M.; Olsson, L. An Experimental and kinetic modelling study for methane oxidation over Pd-based catalyst: Inhibition by water. Catal. Lett. 2017, 147, 2360–2371. [Google Scholar] [CrossRef]
- Zheng, Y.; Kovarik, L.; Engelhard, M.H.; Wang, Y.; Wang, Y.; Gao, F.; Szanyi, J. Low-Temperature Pd/Zeolite Passive NOx Adsorbers: Structure, Performance, and Adsorption Chemistry. J. Phys. Chem. C 2017, 121, 15793–15803. [Google Scholar] [CrossRef]
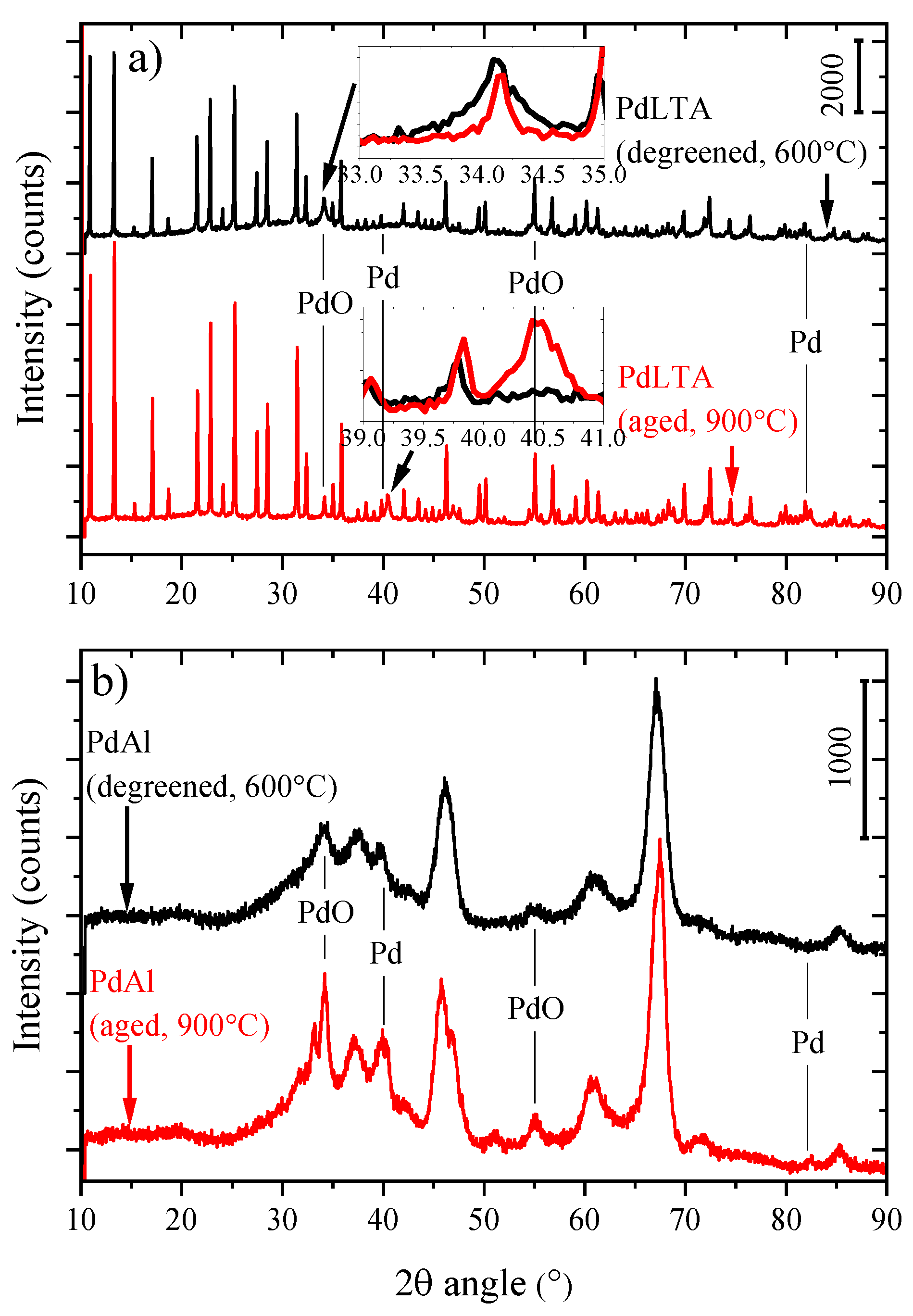
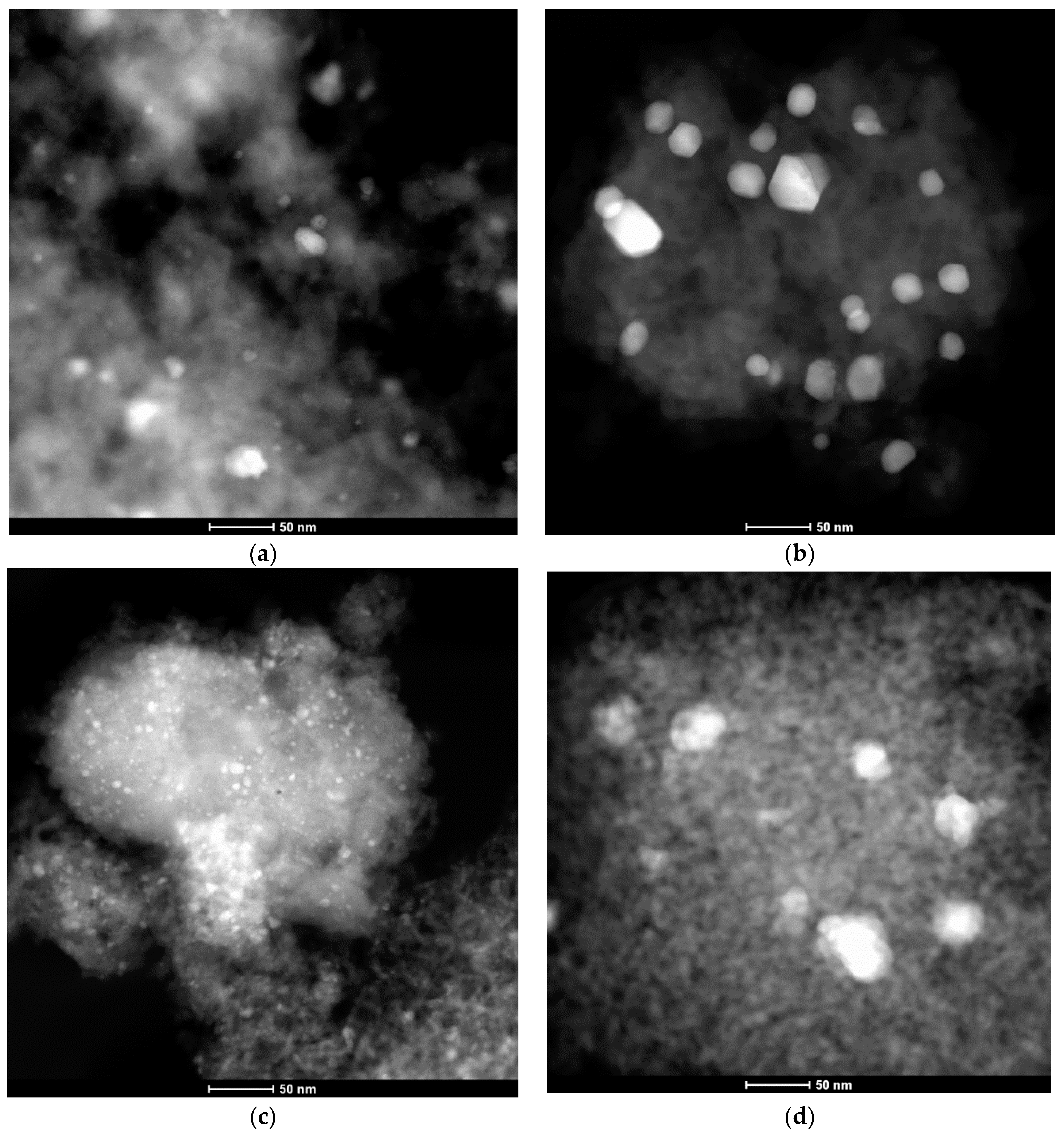
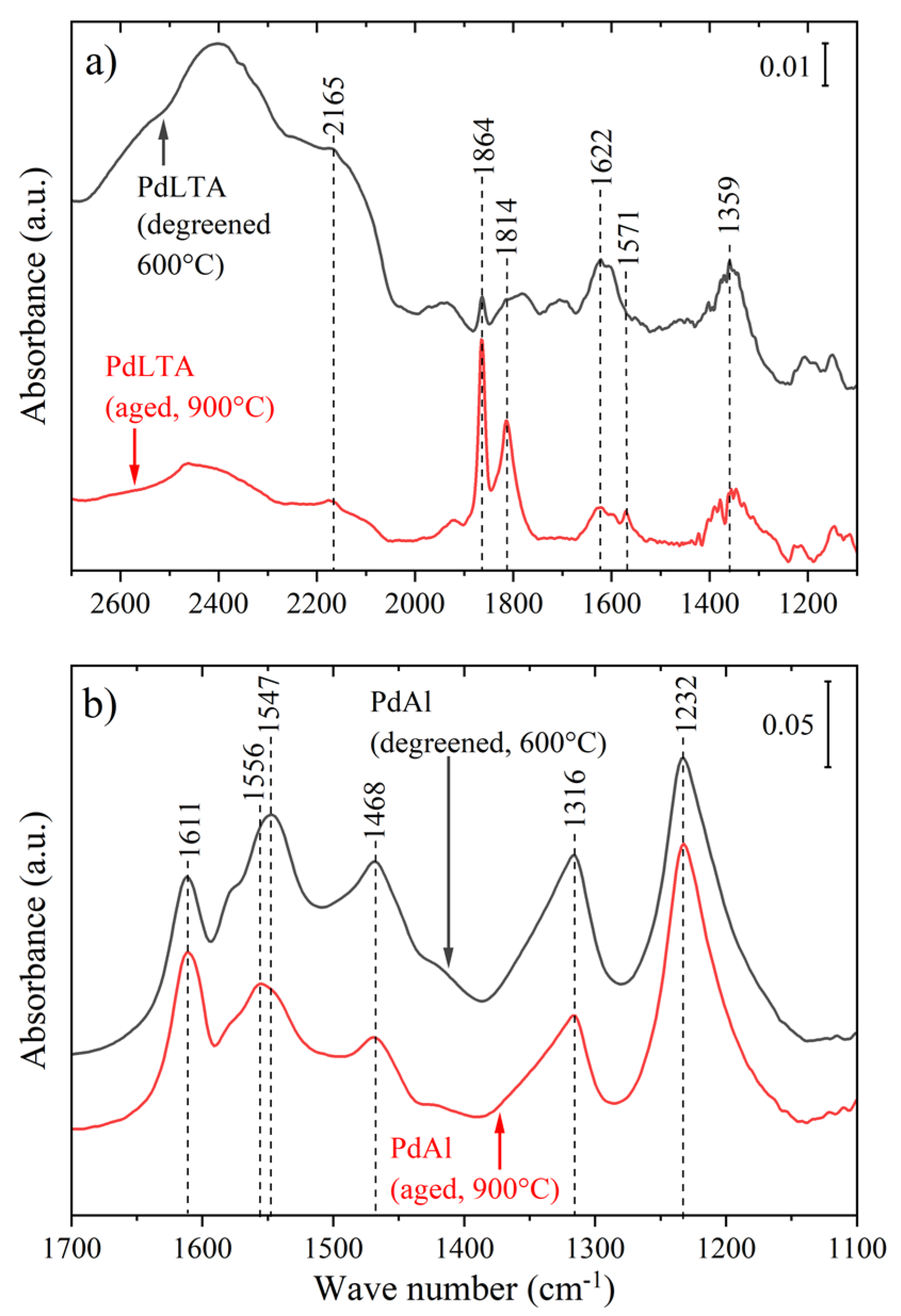

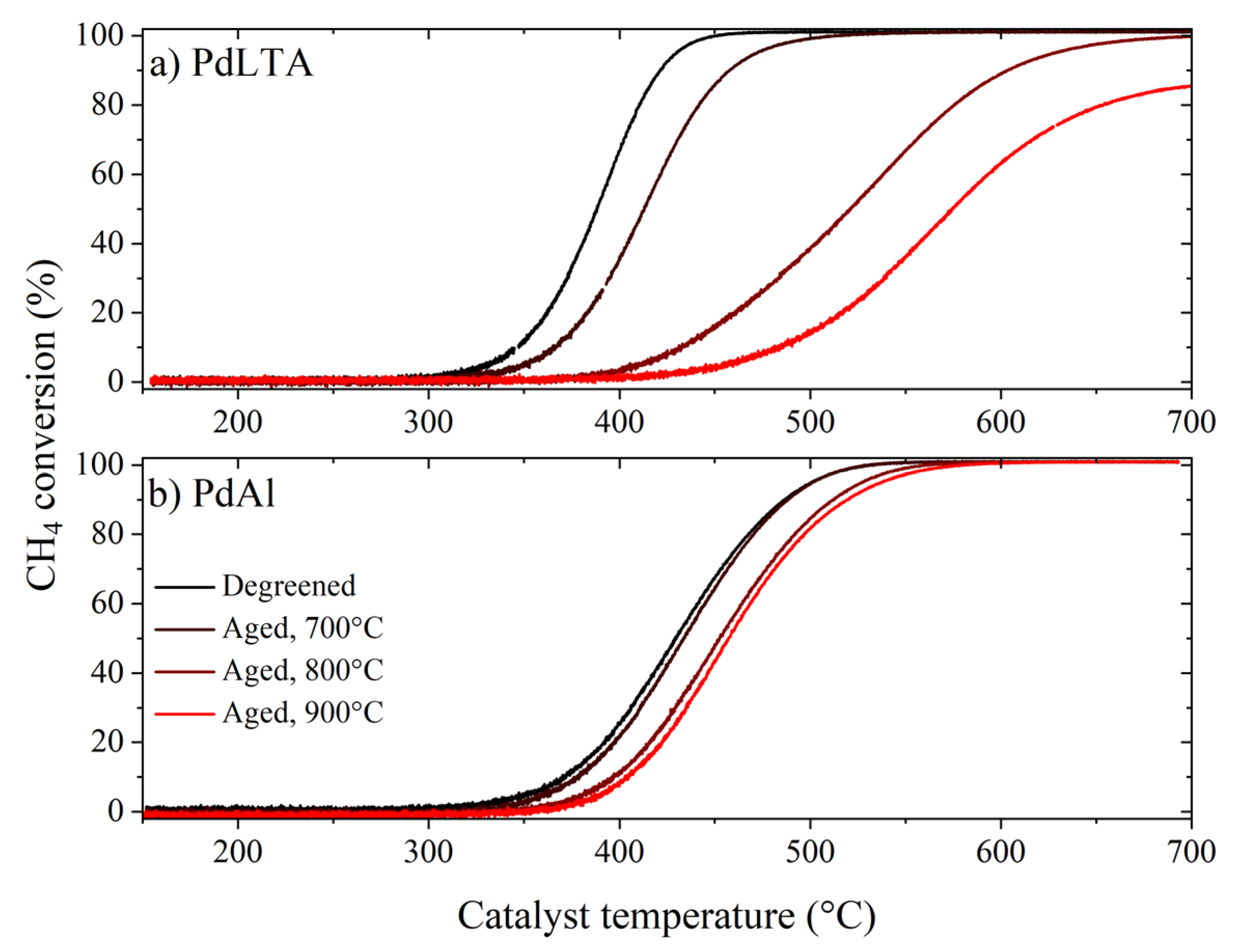
| Pd Content (wt %) | Si/Al Molar Ratio (-) | BET Surface Area (m2/g) | Micropore Volume (t-plot, cm3/g) | Pore Volume (BJH, cm3/g) | ||
|---|---|---|---|---|---|---|
| PdLTA (Pd/H-LTA) | 2.12 | 44 | Degreened (600 °C) | 500 | 0.18 | 0.35 |
| Aged (900 °C) | 464 | 0.17 | 0.32 | |||
| PdAl (Pd/γ-Al2O3) | 2.21 | - | Degreened (600 °C) | 170 | 0.00 | 0.50 |
| Aged (900 °C) | 123 | 0.00 | 0.44 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Friberg, I.; Wang, A.; Olsson, L. Hydrothermal Aging of Pd/LTA Monolithic Catalyst for Complete CH4 Oxidation. Catalysts 2020, 10, 517. https://doi.org/10.3390/catal10050517
Friberg I, Wang A, Olsson L. Hydrothermal Aging of Pd/LTA Monolithic Catalyst for Complete CH4 Oxidation. Catalysts. 2020; 10(5):517. https://doi.org/10.3390/catal10050517
Chicago/Turabian StyleFriberg, Ida, Aiyong Wang, and Louise Olsson. 2020. "Hydrothermal Aging of Pd/LTA Monolithic Catalyst for Complete CH4 Oxidation" Catalysts 10, no. 5: 517. https://doi.org/10.3390/catal10050517
APA StyleFriberg, I., Wang, A., & Olsson, L. (2020). Hydrothermal Aging of Pd/LTA Monolithic Catalyst for Complete CH4 Oxidation. Catalysts, 10(5), 517. https://doi.org/10.3390/catal10050517






