Kinetics of the Catalytic Thermal Degradation of Sugarcane Residual Biomass Over Rh-Pt/CeO2-SiO2 for Syngas Production
Abstract
1. Introduction
2. Results and Discussion
2.1. Biomass Characterization
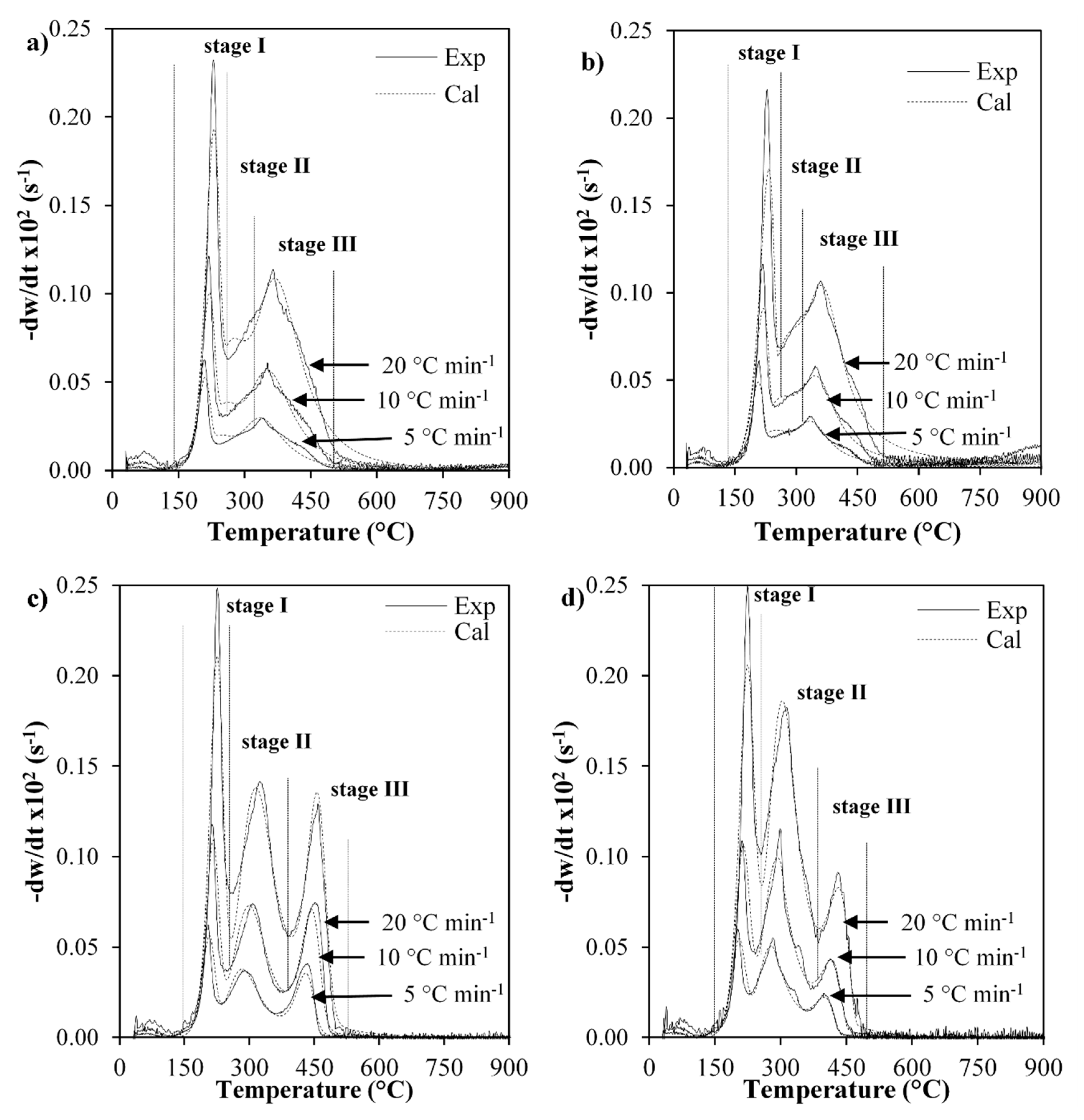
2.2. TG Analysis
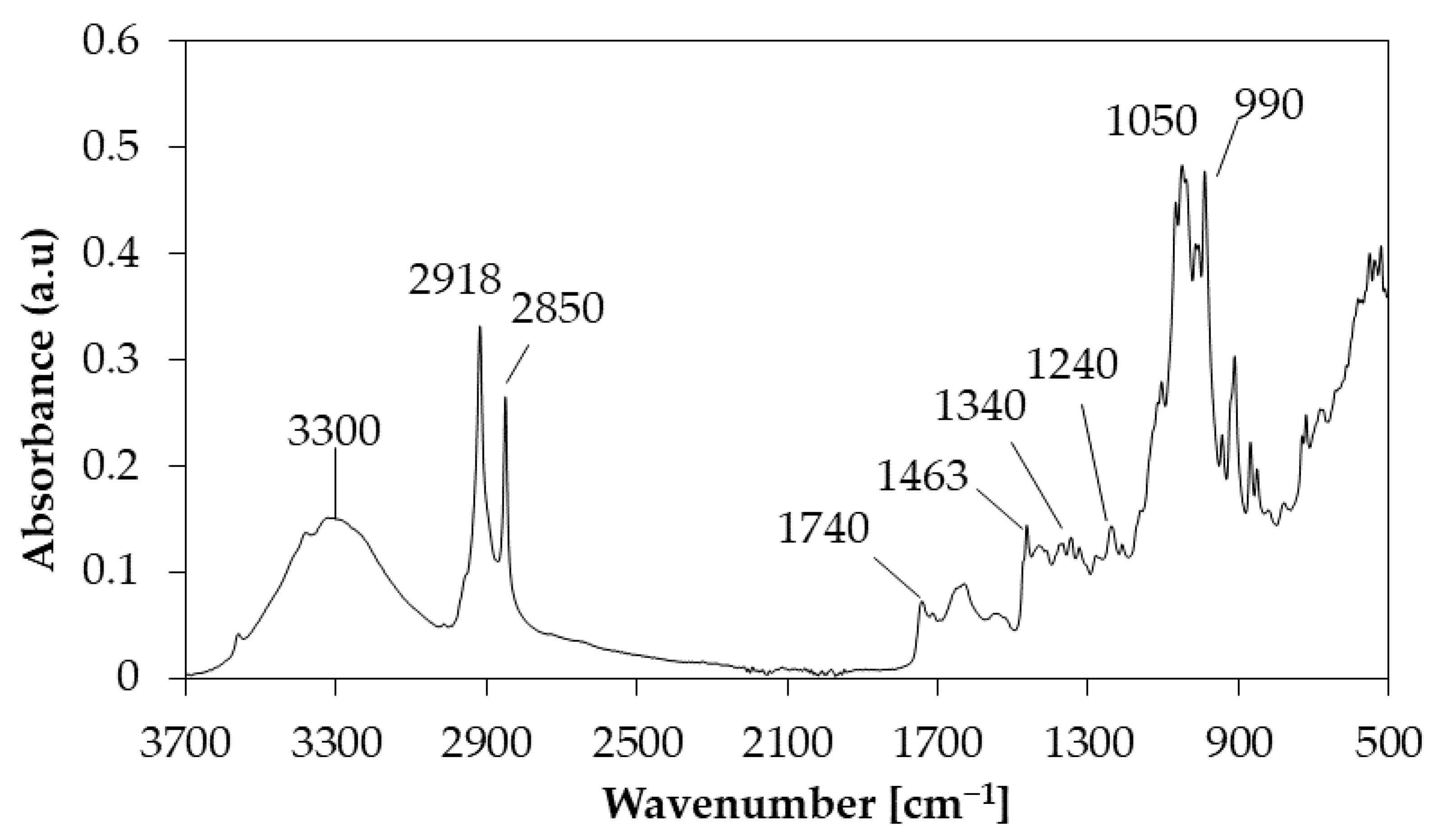
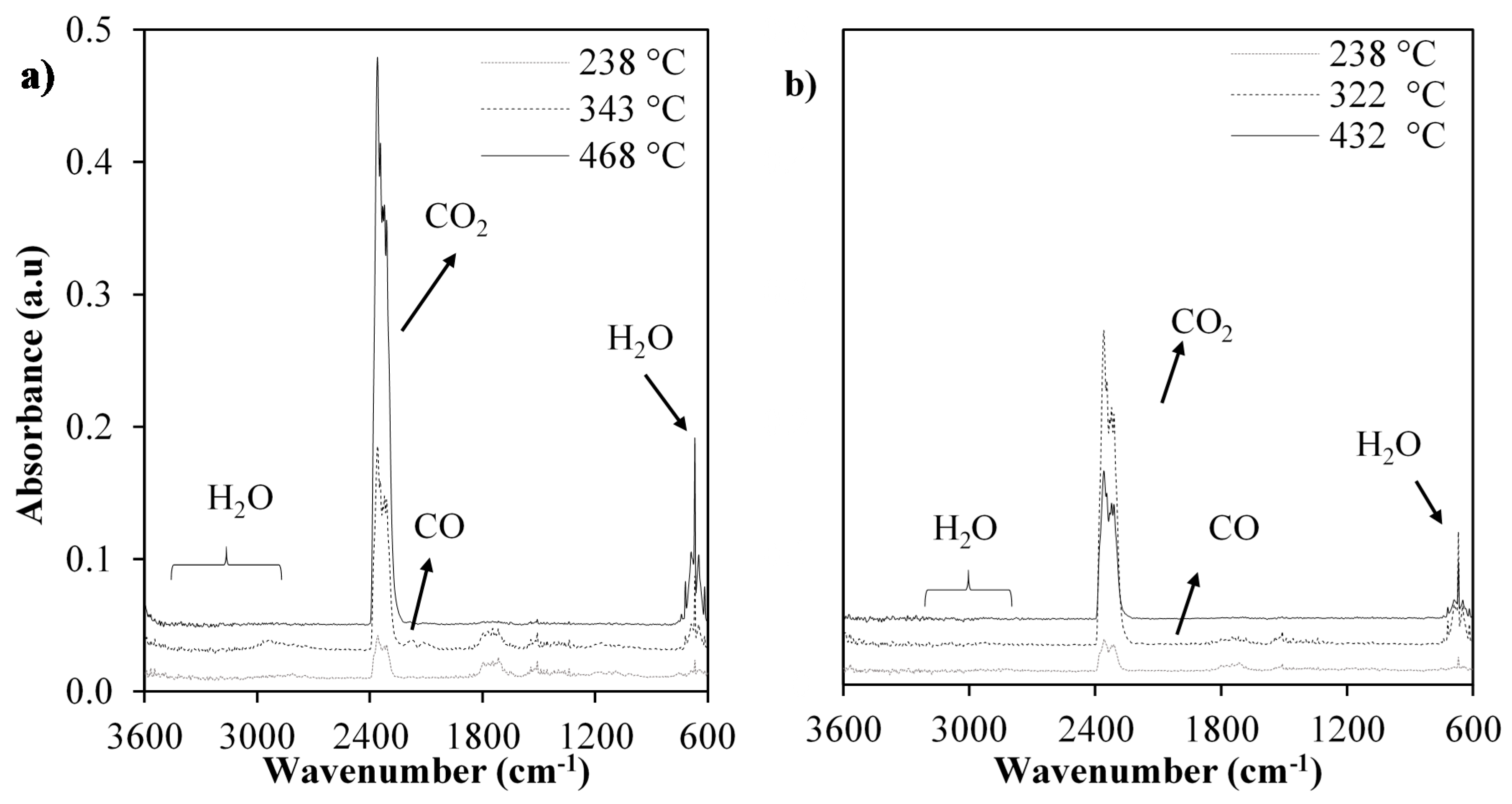
2.3. TG-FTIR Analysis
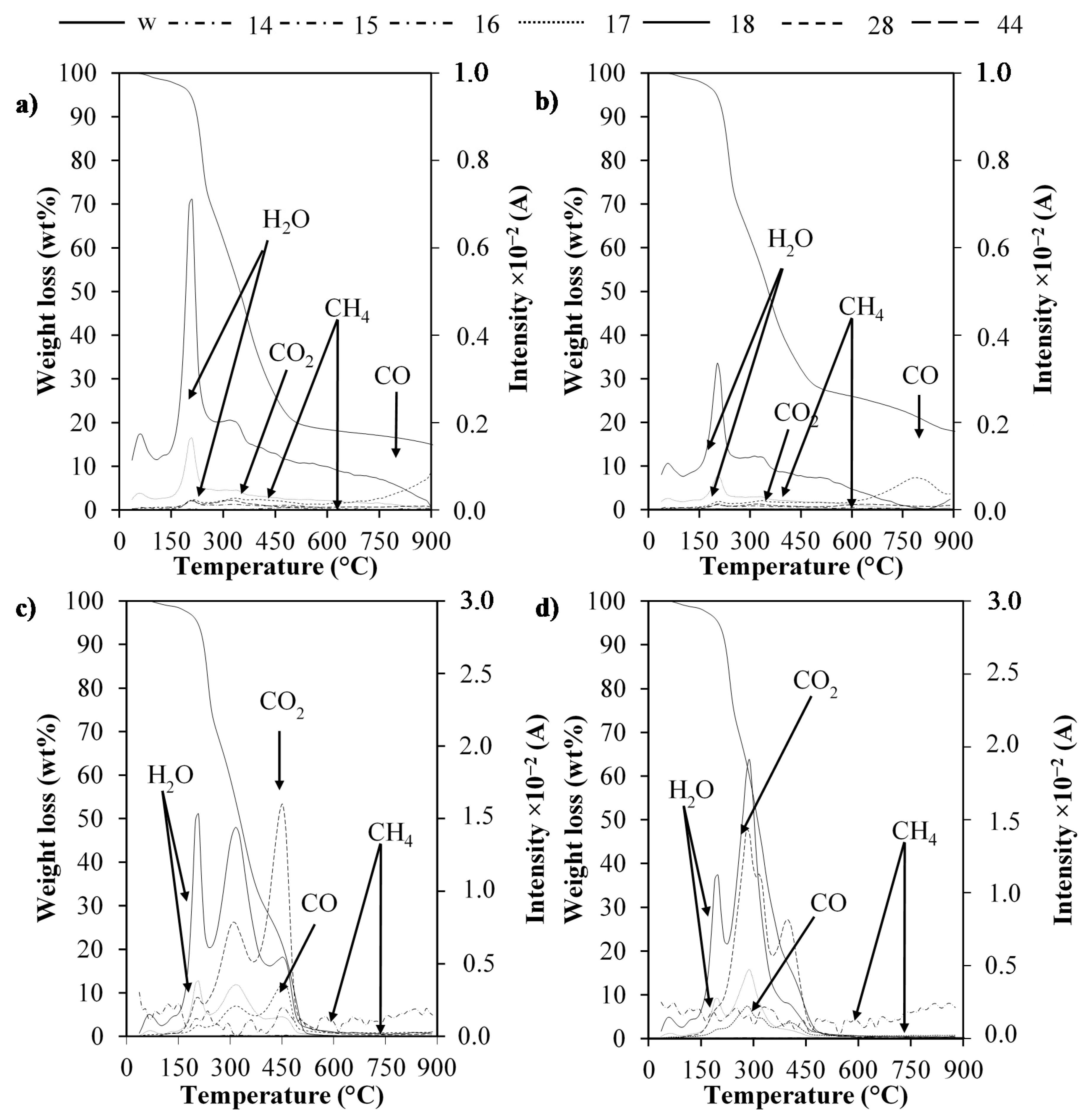
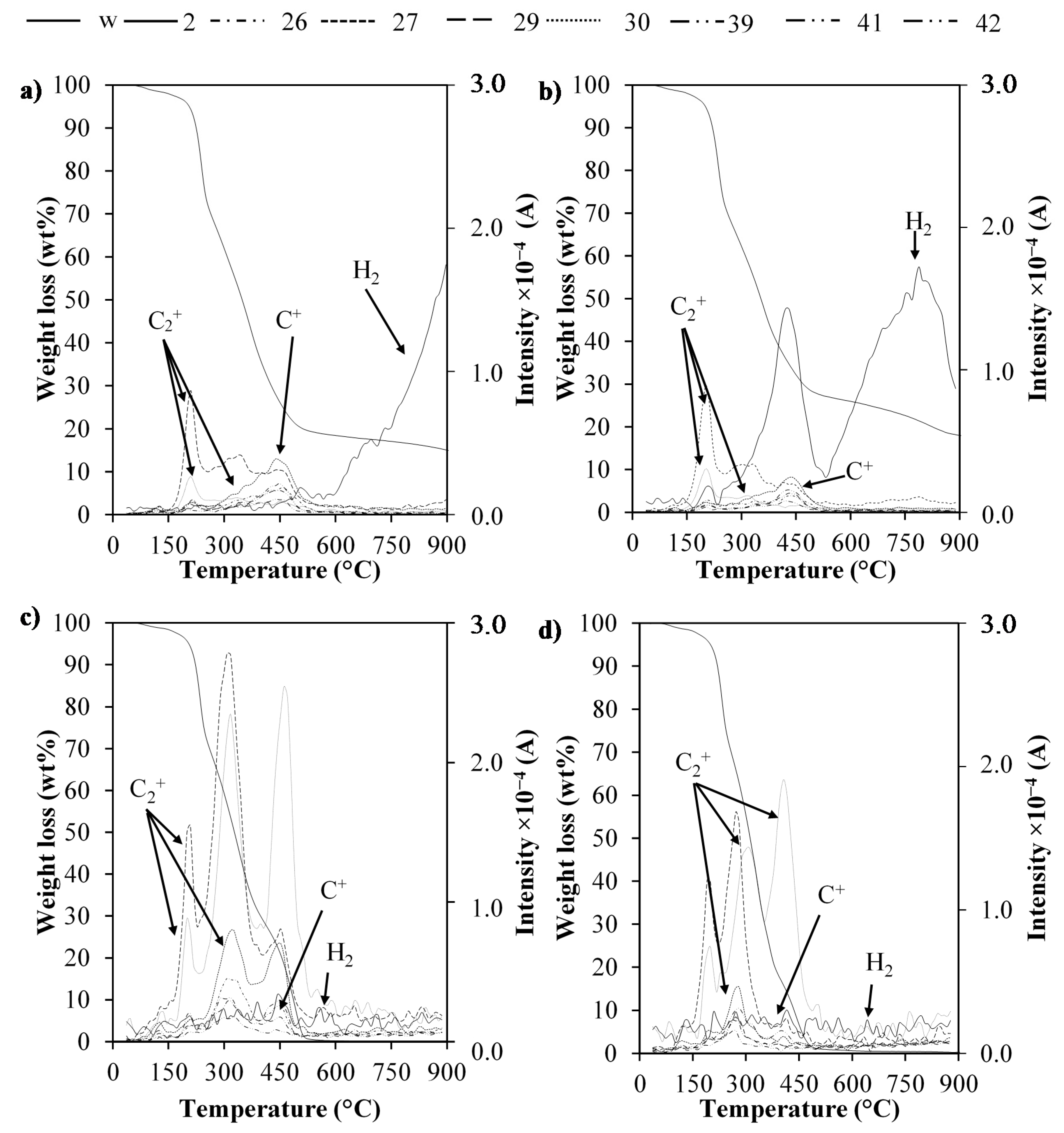
2.4. TG-MS Analysis
2.5. Kinetic Model
3. Materials and Methods
3.1. Biomass Recolection, Pretreatment and Characerization
3.2. Catalyst Synthesis
3.3. TG Analysis
3.4. TG-FTIR Analysis
3.5. TG-MS Analysis
3.6. Kinetic Model
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mishra, R.K.; Mohanty, K. Pyrolysis kinetics and thermal behavior of waste sawdust biomass using thermogravimetric analysis. Bioresour. Technol. 2018, 251, 63–74. [Google Scholar] [CrossRef]
- Organización de las Naciones Unidas (ONU) Combatir el Cambio Climático—Desarrollo Sostenible. Available online: http://www.un.org/sustainabledevelopment/es/combatir-el-cambio-climatico/ (accessed on 11 April 2017).
- Ansari, K.B.; Gaikar, V.G. Pressmud as an alternate resource for hydrocarbons and chemicals by thermal pyrolysis. Ind. Eng. Chem. Res. 2014, 53, 1878–1889. [Google Scholar] [CrossRef]
- Xiang, Z.; Liang, J.; Morgan, H.M., Jr.; Liu, Y.; Mao, H.; Bu, Q. Thermal behavior and kinetic study for co-pyrolysis of lignocellulosic biomass with polyethylene over Cobalt modified ZSM-5 catalyst by thermogravimetric analysis. Bioresour. Technol. 2018, 247, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Loy, A.C.M.; Gan, D.K.W.; Yusup, S.; Chin, B.L.F.; Lam, M.K.; Shahbaz, M.; Unrean, P.; Acda, M.N.; Rianawati, E. Thermogravimetric kinetic modelling of in-situ catalytic pyrolytic conversion of rice husk to bioenergy using rice hull ash catalyst. Bioresour. Technol. 2018, 261, 213–222. [Google Scholar] [CrossRef]
- Zhao, M.; Memon, M.Z.; Ji, G.; Yang, X.; Vuppaladadiyam, A.K.; Song, Y.; Raheem, A.; Li, J.; Wang, W.; Zhou, H. Alkali metal bifunctional catalyst-sorbents enabled biomass pyrolysis for enhanced hydrogen production. Renew. Energy 2020, 148, 168–175. [Google Scholar] [CrossRef]
- Wu, Y.; Liao, Y.; Liu, G.; Ma, X. Syngas production by chemical looping gasification of biomass with steam and CaO additive. Int. J. Hydrog. Energy 2018, 43, 19375–19383. [Google Scholar] [CrossRef]
- Gunasee, S.D.; Carrier, M.; Gorgens, J.F.; Mohee, R. Pyrolysis and combustion of municipal solid wastes: Evaluation of synergistic effects using TGA-MS. J. Anal. Appl. Pyrolysis 2016, 121, 50–61. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, G.; Zhan, J.; Lee, J.-Y.; Wang, H.; Wang, C. Combustion behaviors and kinetics analysis of coal, biomass and plastic. Thermochim. Acta 2018, 669, 140–148. [Google Scholar] [CrossRef]
- Sharma, H.B.; Sarmah, A.K.; Dubey, B. Hydrothermal carbonization of renewable waste biomass for solid biofuel production: A discussion on process mechanism, the influence of process parameters, environmental performance and fuel properties of hydrochar. Renew. Sustain. Energy Rev. 2020, 123, 109761. [Google Scholar] [CrossRef]
- Jaswal, R.; Shende, A.; Nan, W.; Amar, V.; Shende, R. Hydrothermal liquefaction and photocatalytic reforming of pinewood (pinus ponderosa)-derived acid hydrolysis residue for hydrogen and bio-oil production. Energy Fuels 2019, 33, 6454–6462. [Google Scholar] [CrossRef]
- Duman, G.; Watanabe, T.; Uddin, M.A.; Yanik, J. Steam gasification of safflower seed cake and catalytic tar decomposition over ceria modified iron oxide catalysts. Fuel Process. Technol. 2014, 126, 276–283. [Google Scholar] [CrossRef]
- Guo, F.; Li, X.; Liu, Y.; Peng, K.; Guo, C.; Rao, Z. Catalytic cracking of biomass pyrolysis tar over char-supported catalysts. Energy Convers. Manag. 2018, 167, 81–90. [Google Scholar] [CrossRef]
- Lucia, U. Overview on fuel cells. Renew. Sustain. Energy Rev. 2014, 30, 164–169. [Google Scholar] [CrossRef]
- Portafolio “Colombia tiene potencial para producir energía con biomasa” | Infraestructura | Economía | Portafolio. Available online: https://www.portafolio.co/economia/infraestructura/colombia-tiene-potencial-para-producir-energia-con-biomasa-505377 (accessed on 18 February 2019).
- Escalante, H.; Orduz, J.; Zapata, H.; Cardona, M.C.; Duarte, M. Atlas del Potencial Energético de la Biomasa Residual en Colombia; Universidad Industrial de Santander: Bucaramanga, Colombia, 2011. [Google Scholar]
- García-Torres, R.; Rios-Leal, E.; Martínez-Toledo, Á.; Ramos-Morales, F.; Cruz-Sánchez, S.; Cuevas-Díaz, M.D.C. Uso de cachaza y bagazo de caña de azúcar en la remoción de hidrocarburos en el suelo contaminado. Rev. Int. Contam. Ambient. 2011, 27, 31–39. [Google Scholar]
- Procaña Asociación Colombiana de Productores y Proveedores de caña de azucar. Available online: http://www.procana.org/new/quienes-somos/subproductos-y-derivados-de-la-caña.html (accessed on 13 March 2017).
- Sanchez, N.; Ruiz, R.Y.; Cifuentes, B.; Cobo, M. Dataset for controlling sugarcane press-mud fermentation to increase bioethanol steam reforming for hydrogen production. Waste Manag. 2019, 98, 1–13. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, J.; Ge, X.; Chen, M. By-products recycling for syngas cleanup in biomass pyrolysis—An overview. Renew. Sustain. Energy Rev. 2016, 59, 1246–1268. [Google Scholar] [CrossRef]
- Domínguez, A.; Menéndez, J.A.; Pis, J.J. Hydrogen rich fuel gas production from the pyrolysis of wet sewage sludge at high temperature. J. Anal. Appl. Pyrolysis 2006, 77, 127–132. [Google Scholar] [CrossRef]
- Yang, H.; Ji, G.; Clough, P.T.; Xu, X.; Zhao, M. Kinetics of catalytic biomass pyrolysis using Ni-based functional materials. Fuel Process. Technol. 2019, 195, 106145. [Google Scholar] [CrossRef]
- Gupta, N.; Tripathi, S.; Balomajumder, C. Characterization of pressmud: A sugar industry waste. Fuel 2011, 90, 389–394. [Google Scholar] [CrossRef]
- Gangavati, P.B.; Safi, M.J.; Singh, A.; Prasad, B.; Mishra, I.M. Pyrolysis and thermal oxidation kinetics of sugar mill press mud. Thermochim. Acta 2005, 428, 63–70. [Google Scholar] [CrossRef]
- Yu, H.; Liu, Y.; Liu, J.; Chen, D. High catalytic performance of an innovative Ni/magnesium slag catalyst for the syngas production and tar removal from biomass pyrolysis. Fuel 2019, 254, 115622. [Google Scholar] [CrossRef]
- Garba, M.U.; Inalegwu, A.; Musa, U.; Aboje, A.A.; Kovo, A.S.; Adeniyi, D.O. Thermogravimetric characteristic and kinetic of catalytic co-pyrolysis of biomass with low- and high-density polyethylenes. Biomass Convers. Biorefin. 2018, 8, 143–150. [Google Scholar] [CrossRef]
- Akubo, K.; Nahil, M.A.; Williams, P.T. Pyrolysis-catalytic steam reforming of agricultural biomass wastes and biomass components for production of hydrogen/syngas. J. Energy Inst. 2019, 92, 1987–1996. [Google Scholar] [CrossRef]
- Albertazzi, S.; Basile, F.; Brandin, J.; Einvall, J.; Fornasari, G.; Hulteberg, C.; Sanati, M.; Trifirò, F.; Vaccari, A. Pt-Rh/MgAl(O) catalyst for the upgrading of biomass-generated synthesis gases. Energy Fuels 2009, 23, 573–579. [Google Scholar] [CrossRef]
- Santamaria, L.; Arregi, A.; Alvarez, J.; Artetxe, M.; Amutio, M.; Lopez, G.; Bilbao, J.; Olazar, M. Performance of a Ni/ZrO2 catalyst in the steam reforming of the volatiles derived from biomass pyrolysis. J. Anal. Appl. Pyrolysis 2018, 136, 222–231. [Google Scholar] [CrossRef]
- Ammendola, P.; Lisi, L.; Piriou, B.; Ruoppolo, G. Rh-perovskite catalysts for conversion of tar from biomass pyrolysis. Chem. Eng. J. 2009, 154, 361–368. [Google Scholar] [CrossRef]
- Lu, Q.; Li, W.; Zhang, X.; Liu, Z.; Cao, Q.; Xie, X.; Yuan, S. Experimental study on catalytic pyrolysis of biomass over a Ni/Ca-promoted Fe catalyst. Fuel 2020, 263, 116690. [Google Scholar] [CrossRef]
- Wang, C.; Li, L.; Zeng, Z.; Xu, X.; Ma, X.; Chen, R.; Su, C. Catalytic performance of potassium in lignocellulosic biomass pyrolysis based on an optimized three-parallel distributed activation energy model. Bioresour. Technol. 2019, 281, 412–420. [Google Scholar] [CrossRef]
- Balasundram, V.; Ibrahim, N.; Kasmani, R.M.; Hamid, M.K.A.; Isha, R.; Hasbullah, H.; Ali, R.R. Thermogravimetric catalytic pyrolysis and kinetic studies of coconut copra and rice husk for possible maximum production of pyrolysis oil. J. Clean. Prod. 2017, 167, 218–228. [Google Scholar] [CrossRef]
- Federici, J.A.; Vlachos, D.G. Experimental studies on syngas catalytic combustion on Pt/Al2O3 in a microreactor. Combust. Flame 2011, 158, 2540–2543. [Google Scholar] [CrossRef]
- Li, S.; Zheng, H.; Zheng, Y.; Tian, J.; Jing, T.; Chang, J.S.; Ho, S.H. Recent advances in hydrogen production by thermo-catalytic conversion of biomass. Int. J. Hydrog. Energy 2019, 44, 14266–14278. [Google Scholar] [CrossRef]
- Rioche, C.; Kulkarni, S.; Meunier, F.C.; Breen, J.P.; Burch, R. Steam reforming of model compounds and fast pyrolysis bio-oil on supported noble metal catalysts. Appl. Catal. B Environ. 2005, 61, 130–139. [Google Scholar] [CrossRef]
- Tomishige, K.; Asadullah, M.; Kunimori, K. Syngas production by biomass gasification using Rh/CeO2/SiO2 catalysts and fluidized bed reactor. Catal. Today 2004, 89, 389–403. [Google Scholar] [CrossRef]
- Cifuentes, B.; Valero, M.F.; Conesa, J.A.J.; Cobo, M. Hydrogen Production by Steam Reforming of Ethanol on Rh-Pt Catalysts: Influence of CeO2, ZrO2, and La2O3 as Supports. Catalysts 2015, 5, 1872–1896. [Google Scholar] [CrossRef]
- Cifuentes, B.; Hernández, M.; Monsalve, S.; Cobo, M. Hydrogen production by steam reforming of ethanol on a RhPt/CeO2/SiO2 catalyst: Synergistic effect of the Si:Ce ratio on the catalyst performance. Appl. Catal. A Gen. 2016, 523, 283–293. [Google Scholar] [CrossRef]
- Yuan, R.; Yu, S.; Shen, Y. Pyrolysis and combustion kinetics of lignocellulosic biomass pellets with calcium-rich wastes from agro-forestry residues. Waste Manag. 2019, 87, 86–96. [Google Scholar] [CrossRef]
- Garrido, M.A.; Font, R.; Conesa, J.A. Kinetic study and thermal decomposition behavior of viscoelastic memory foam. Energy Convers. Manag. 2016, 119, 327–337. [Google Scholar] [CrossRef]
- Safar, M.; Lin, B.J.; Chen, W.H.; Langauer, D.; Chang, J.S.; Raclavska, H.; Pétrissans, A.; Rousset, P.; Pétrissans, M. Catalytic effects of potassium on biomass pyrolysis, combustion and torrefaction. Appl. Energy 2019, 235, 346–355. [Google Scholar] [CrossRef]
- Conesa, J.A.; Soler, A. Decomposition kinetics of materials combining biomass and electronic waste. J. Therm. Anal. Calorim. 2017, 128, 225–233. [Google Scholar] [CrossRef]
- Ding, Y.; Ezekoye, O.A.; Zhang, J.; Wang, C.; Lu, S. The effect of chemical reaction kinetic parameters on the bench-scale pyrolysis of lignocellulosic biomass. Fuel 2018, 232, 147–153. [Google Scholar] [CrossRef]
- Zhang, X.; Deng, H.; Hou, X.; Qiu, R.; Chen, Z. Pyrolytic behavior and kinetic of wood sawdust at isothermal and non-isothermal conditions. Renew. Energy 2019, 142, 284–294. [Google Scholar] [CrossRef]
- Dhyani, V.; Bhaskar, T. A comprehensive review on the pyrolysis of lignocellulosic biomass. Renew. Energy 2018, 129, 695–716. [Google Scholar] [CrossRef]
- Gu, X.; Ma, X.; Li, L.; Liu, C.; Cheng, K.; Li, Z. Pyrolysis of poplar wood sawdust by TG-FTIR and Py–GC/MS. J. Anal. Appl. Pyrolysis 2013, 102, 16–23. [Google Scholar] [CrossRef]
- Gogoi, M.; Konwar, K.; Bhuyan, N.; Borah, R.C.; Kalita, A.C.; Nath, H.P.; Saikia, N. Assessments of pyrolysis kinetics and mechanisms of biomass residues using thermogravimetry. Bioresour. Technol. Rep. 2018, 4, 40–49. [Google Scholar] [CrossRef]
- Caballero, A.; Conesa, J.A. Mathematical considerations for nonisothermal kinetics in thermal decomposition. J. Anal. Appl. Pyrolysis 2005, 73, 85–100. [Google Scholar] [CrossRef]
- Arenas, C.N.; Navarro, M.V.; Martínez, J.D. Pyrolysis kinetics of biomass wastes using isoconversional methods and the distributed activation energy model. Bioresour. Technol. 2019, 288, 121485. [Google Scholar] [CrossRef]
- Anca-Couce, A.; Berger, A.; Zobel, N. How to determine consistent biomass pyrolysis kinetics in a parallel reaction scheme. Fuel 2014, 123, 230–240. [Google Scholar] [CrossRef]
- Yang, J.; Chen, H.; Zhao, W.; Zhou, J. TG-FTIR-MS study of pyrolysis products evolving from peat. J. Anal. Appl. Pyrolysis 2016, 117, 296–309. [Google Scholar] [CrossRef]
- Naik, S.; Goud, V.V.; Rout, P.K.; Jacobson, K.; Dalai, A.K. Characterization of Canadian biomass for alternative renewable biofuel. Renew. Energy 2010, 35, 1624–1631. [Google Scholar] [CrossRef]
- Ministerio de Minas y Energía de Colombia. El Carbón Colombiano: Fuente de Energía para el Mundo; Ministerio de Minas y Energía de Colombia: Bogotá, Colombia, 2016. [Google Scholar]
- Demirbaş, A. Gaseous products from biomass by pyrolysis and gasification: Effects of catalyst on hydrogen yield. Energy Convers. Manag. 2002, 43, 897–909. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, J.; Shanks, B.H.; Brown, R.C. The deleterious effect of inorganic salts on hydrocarbon yields from catalytic pyrolysis of lignocellulosic biomass and its mitigation. Appl. Energy 2015, 148, 115–120. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Carpio, R.B.; Zhang, Y.; Kuo, C.T.; Chen, W.T.; Schideman, L.C.; de Leon, R.L.; Charles, L.; Leon, R.L. De Characterization and thermal decomposition of demineralized wastewater algae biomass. Algal Res. 2019, 38, 101399. [Google Scholar] [CrossRef]
- Sebestyén, Z.; Barta-Rajnai, E.; Bozi, J.; Blazsó, M.; Jakab, E.; Miskolczi, N.; Czégény, Z. Thermo-catalytic Pyrolysis of biomass and plastic mixtures using HZSM-5. Appl. Energy 2017, 207, 114–122. [Google Scholar] [CrossRef]
- Fong, M.J.B.; Loy, A.C.M.; Chin, B.L.F.; Lam, M.K.; Yusup, S.; Jawad, Z.A. Catalytic pyrolysis of Chlorella vulgaris: Kinetic and thermodynamic analysis. Bioresour. Technol. 2019, 289, 121689. [Google Scholar] [CrossRef] [PubMed]
- Minh Loy, A.C.; Yusup, S.; Fui Chin, B.L.; Wai Gan, D.K.; Shahbaz, M.; Acda, M.N.; Unrean, P.; Rianawati, E. Comparative study of in-situ catalytic pyrolysis of rice husk for syngas production: Kinetics modelling and product gas analysis. J. Clean. Prod. 2018, 197, 1231–1243. [Google Scholar] [CrossRef]
- Long, Y.; Ruan, L.; Lv, X.; Lv, Y.; Su, J.; Wen, Y. TG-FTIR analysis of pyrolusite reduction by major biomass components. Chin. J. Chem. Eng. 2015, 23, 1691–1697. [Google Scholar] [CrossRef]
- Mehmood, M.A.; Ahmad, M.S.; Liu, Q.; Liu, C.G.; Tahir, M.H.; Aloqbi, A.A.; Tarbiah, N.I.; Alsufiani, H.M.; Gull, M. Helianthus tuberosus as a promising feedstock for bioenergy and chemicals appraised through pyrolysis, kinetics, and TG-FTIR-MS based study. Energy Convers. Manag. 2019, 194, 37–45. [Google Scholar] [CrossRef]
- Conesa, J.A.; Domene, A. Biomasses pyrolysis and combustion kinetics through n-th order parallel reactions. Thermochim. Acta 2011, 523, 176–181. [Google Scholar] [CrossRef][Green Version]
- García, R.; Pizarro, C.; Lavín, A.G.; Bueno, J.L. Biomass proximate analysis using thermogravimetry. Bioresour. Technol. 2013, 139, 1–4. [Google Scholar] [CrossRef]
- Quiroga, E.; Moltó, J.; Conesa, J.A.; Valero, M.F.; Cobo, M. Data of Pyrolysis and Combustion of Sugarcane Residual Biomass over Rh-Pt/CeO2 -SiO2 Catalyst by tg, TG-FTIR and TG-MS. Mendeley Dataset. Available online: https://data.mendeley.com/datasets/gkysct9wjz/1 (accessed on 17 October 2019).

| Moisture (as Received) [wt%] | 2 |
|---|---|
| Elemental Analysis [wt%] | |
| C | 50.0 |
| H | 7.2 |
| N | 0.9 |
| S | N.D |
| O (by difference) * | 33.7 |
| Proximate Analysis [wt%] | |
| Ashes 1 | 8.1 |
| Volatile matter 1 | 82.8 |
| Fixed carbon 1 | 9.1 |
| Calorific Value [MJ kg−1] | |
| HHVdb | 22.9 |
| LHVdb | 21.3 |
| ICP-MS [mg g−1] | |
| Al | 3.30 |
| K | 2.58 |
| Fe | 2.52 |
| Si | 1.07 |
| Mg | 1.04 |
| Species | Functional Group | Wavenumber (cm−1) |
| CH4 | C–H | 2924, 1440 |
| CO2 | C=O | 2361 |
| CO | C–O | 2115 |
| Aldehydes, ketones and acids | C=O | 1900–1600 |
| Carboxylic acids | C=O | 1173 |
| Aromatics | C=C | 1640 |
| Overtones of CO2 | C=O | 726–586 |
| Hydroxyl group | O–H | 3900–3600 |
| H2O | O–H | 1509, 1757 |
| Hydroxyl group of phenolic compounds | O–H | 1336, 1450 |
| Phenols | C–O–H | 1223 |
| Species | Key Ion Fragment | m/z | Non-Catalytic Pyrolysis | Catalytic Pyrolysis | Non-Catalytic Combustion | Catalytic Combustion |
|---|---|---|---|---|---|---|
| H2 | H2+ | 2 | 1.0 | 2.4 | 0.5 | 0.6 |
| Methane | CH3+ | 15 | 1.0 | 0.6 | 1.8 | 1.5 |
| CH4+ | 16 | 1.0 | 0.8 | 16.6 | 21.2 | |
| Water | OH+ | 17 | 1.0 | 0.6 | 2.6 | 2.2 |
| H2O+ | 18 | 1.0 | 0.5 | 2.3 | 1.9 | |
| CO | CO+ | 28 | 1.0 | 1.2 | 3.6 | 2.3 |
| C2+ Hydrocarbons | C2H2+ | 26 | 1.0 | 0.7 | 2.2 | 1.3 |
| C2H3+ | 27 | 1.0 | 0.6 | 2.1 | 1.0 | |
| C3+Hydrocarbons | C3H5+ | 41 | 1.0 | 0.8 | 2.0 | 1.5 |
| C3H6+ | 42 | 1.0 | 0.8 | 3.1 | 2.8 | |
| C3H7+ | 43 | 1.0 | 0.8 | 7.3 | 5.9 | |
| Aldehydes | CHO+ | 29 | 1.0 | 0.9 | 3.4 | 1.7 |
| Formaldehyde | CH2O+ | 30 | 1.0 | 0.9 | 11.1 | 8.7 |
| CO2 | CO2+ | 44 | 1.0 | 0.9 | 28.5 | 26.6 |
| Alcohols | CH2OH+ | 31 | 1.0 | 0.8 | 56.5 | 52.4 |
| C2H5OH+ | 46 | 1.0 | 1.0 | 14.1 | 13.1 |
| Component | Kinetic Parameter | Non-catalytic Pyrolysis | Catalytic Pyrolysis | Non-catalytic Combustion | Catalytic Combustion |
|---|---|---|---|---|---|
| 1 | Tmax | 243 | 243 | 238 | 238 |
| ki0 [s−1] | 2 × 1012 | 1 × 109 | 3 × 1013 | 5 × 1011 | |
| Ei [kJ·mol−1] | 133.6 | 104.2 | 144.2 | 127.5 | |
| ni | 1.0 | 0.8 | 1.3 | 1.3 | |
| ci0 | 0.18 | 0.17 | 0.24 | 0.26 | |
| 2 | Tmax | 338 | 327 | 343 | 322 |
| ki0 [s−1] | 4 × 108 | 7 × 104 | 7 × 107 | 1 × 1010 | |
| Ei [kJ·mol−1] | 108.9 | 75.1 | 110.0 | 130.3 | |
| ni | 4.3 | 4.6 | 2.9 | 3.0 | |
| ci0 | 0.28 | 0.48 | 0.49 | 0.53 | |
| 3 | Tmax | 375 | 369 | 468 | 432 |
| ki0 [s−1] | 5 × 107 | 4 × 109 | 2 × 1013 | 5 × 109 | |
| Ei [kJ·mol−1] | 120.2 | 141.7 | 210.5 | 156.6 | |
| ni | 3.0 | 2.6 | 1.2 | 0.9 | |
| ci0 | 0.36 | 0.18 | 0.21 | 0.13 | |
| Cinert0 | 0.18 | 0.18 | 0.07 | 0.07 | |
| VC | 3.4% | 1.1% | 2.7% | 1.3% | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quiroga, E.; Moltó, J.; Conesa, J.A.; Valero, M.F.; Cobo, M. Kinetics of the Catalytic Thermal Degradation of Sugarcane Residual Biomass Over Rh-Pt/CeO2-SiO2 for Syngas Production. Catalysts 2020, 10, 508. https://doi.org/10.3390/catal10050508
Quiroga E, Moltó J, Conesa JA, Valero MF, Cobo M. Kinetics of the Catalytic Thermal Degradation of Sugarcane Residual Biomass Over Rh-Pt/CeO2-SiO2 for Syngas Production. Catalysts. 2020; 10(5):508. https://doi.org/10.3390/catal10050508
Chicago/Turabian StyleQuiroga, Eliana, Julia Moltó, Juan A. Conesa, Manuel F. Valero, and Martha Cobo. 2020. "Kinetics of the Catalytic Thermal Degradation of Sugarcane Residual Biomass Over Rh-Pt/CeO2-SiO2 for Syngas Production" Catalysts 10, no. 5: 508. https://doi.org/10.3390/catal10050508
APA StyleQuiroga, E., Moltó, J., Conesa, J. A., Valero, M. F., & Cobo, M. (2020). Kinetics of the Catalytic Thermal Degradation of Sugarcane Residual Biomass Over Rh-Pt/CeO2-SiO2 for Syngas Production. Catalysts, 10(5), 508. https://doi.org/10.3390/catal10050508







