Room-Temperature Solid-State Preparation of CoFe2O4@Coal Composites and Their Catalytic Performance in Direct Coal Liquefaction
Abstract
1. Introduction
2. Results and Discussion
2.1. Structure and Morphology Characterization of Nanocatalysts
2.2. Catalytic Properties of Samples in DCL
3. Experimental Methods
3.1. Starting Materials
3.2. Preparation of CoFe2O4@coal, CoFe2O4, Fe2O3, and Co(OH)2 Nanoparticles by Solid-State Reaction
3.3. Characterization
3.4. The Catalytic Process of DCL
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Trautmann, M.; Lang, S.; Traa, Y. Direct liquefaction of lower-rank coals and biocoals with magnetically separable catalysts as a sustainable route to fuels. Fuel 2015, 151, 102–109. [Google Scholar] [CrossRef]
- Wang, L.; Chen, P. Mechanism study of iron-based catalysts in co-liquefaction of coal with waste plastics. Fuel 2002, 81, 811–815. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Shi, S.D.; Li, Y.W. Coal liquefaction technologies-development in china and challenges in chemical reaction engineering. Chem. Eng. Sci. 2010, 65, 12–17. [Google Scholar] [CrossRef]
- Hao, H.G.; Chang, T.; Cui, L.X.; Sun, R.Q.; Gao, R. Theoretical study on the mechanism of hydrogen donation and transfer for hydrogen-donor solvents during direct coal liquefaction. Catalysts 2018, 8, 648. [Google Scholar] [CrossRef]
- Trautmann, M.; Traa, Y. Efficient direct brown coal liquefaction with sulfided Co/SiO2 catalysts. Energy Fuels 2013, 27, 5589–5592. [Google Scholar] [CrossRef]
- Song, C.; Sainia, A.K.; Yoneyamaa, Y. A new process for catalytic liquefaction of coal using dispersed MoS2 catalyst generated in situ with added H2O. Fuel 2000, 79, 249–261. [Google Scholar] [CrossRef]
- Demirel, B.; Givens, E.N. Liquefaction of Wyodak coal with molybdenum-based catalysts from phosphomolybdic acid. Fuel Process. Technol. 2000, 64, 177–187. [Google Scholar] [CrossRef]
- Demirel, B.; Givens, E.N. Liquefaction of Wyodak coal with phosphomolybdic acid. Energy Fuels 1998, 12, 607–611. [Google Scholar] [CrossRef]
- Chakma, A. Simultaneous liquefaction of a subbituminous coal and upgrading of bitumen with molten ZnCl2-based catalysts. Fuel Process. Technol. 1993, 33, 101–115. [Google Scholar] [CrossRef]
- Ogino, Y.; Ozawa, S.; Ishikawa, K. Effects of molten tin catalyst on coal conversion in a hydrogen donor solvent. Fuel Process. Technol. 1986, 14, 269–277. [Google Scholar] [CrossRef]
- Li, X.; Hu, S.X.; Jin, L.J.; Hu, H.Q. Role of iron-based catalyst and hydrogen transfer in direct coal liquefaction. Energy Fuels 2008, 22, 1126–1129. [Google Scholar] [CrossRef]
- Feng, J.; Li, J.; Li, W. Influences of chemical structure and physical properties of coal macerals on coal liquefaction by quantum chemistry calculation. Fuel Process. Technol. 2013, 109, 19–26. [Google Scholar] [CrossRef]
- Barraza, J.; Coley-Silva, E.; Piñeres, J. Effect of temperature, solvent/coal ratio and beneficiation on conversion and product distribution from direct coal liquefaction. Fuel 2016, 172, 153–159. [Google Scholar] [CrossRef]
- Şimşek, E.H.; Güleç, F.; Kavuştu, H. Application of Kalman filter to determination of coal liquefaction mechanisms using discrete time models. Fuel 2017, 207, 814–820. [Google Scholar] [CrossRef]
- Zheng, Q.; Zhang, Y.; Fouquet, T.; Zeng, X.; Kanda, H.; Goto, M. Room-temperature extraction of direct coal liquefaction residue by liquefied dimethyl ether. Fuel 2020, 262, 116528. [Google Scholar] [CrossRef]
- Şimşek, E.H.; Güleç, F.; Akçadağ, F.S. Understanding the liquefaction mechanism of Beypazarı lignite in tetralin with ultraviolet irradiation using discrete time models. Fuel Process. Technol. 2020, 198, 106227. [Google Scholar] [CrossRef]
- Ali, A.; Zhao, C. Direct liquefaction techniques on lignite coal: A review. Chin. J. Catal. 2020, 41, 375–389. [Google Scholar] [CrossRef]
- Li, Y.Z.; Cao, Y.L.; Jia, D.Z. Direct coal liquefaction with Fe3O4 nanocatalysts prepared by a simple solid-state method. Energies 2017, 10, 886. [Google Scholar] [CrossRef]
- Haghighat, F.; de Klerk, A. Direct Coal Liquefaction: Low Temperature Dissolution Process. Energy Fuels 2014, 28, 1012–1019. [Google Scholar] [CrossRef]
- Hu, H.Q.; Bai, J.F.; Zhu, H.J.; Wang, Y.; Guo, S.C. Catalytic liquefaction of coal with highly dispersed Fe2S3 impregnated in-situ. Energy Fuels 2001, 15, 830–834. [Google Scholar] [CrossRef]
- Li, Y.Z.; Ma, F.Y.; Su, X.T.; Sun, C.; Liu, J.C.; Sun, Z.Q.; Hou, Y.L. Synthesis and catalysis of oleic acid-coated Fe3O4 nanocrystals for direct coal liquefaction. Catal. Commun. 2012, 26, 231–234. [Google Scholar] [CrossRef]
- Sun, Z.Q.; Ma, F.Y.; Liu, X.J.; Wu, H.H.; Niu, C.G.; Su, X.T.; Liu, J.M. Large-scale synthesis and catalysis of oleic acid-coated Fe2O3 for co-liquefaction of coal and petroleum vacuum residues. Fuel Process. Technol. 2015, 139, 173–177. [Google Scholar] [CrossRef]
- Ma, Y.B.; Gao, Z.X.; Eli, W. Hydroformylation of dicyclopentadiene over Rh catalysts supported on Fe2O3, Co3O4 and Fe2O3-Co3O4 Mixed Oxide. Prog. React. Kinet. Mech. 2017, 42, 8–13. [Google Scholar] [CrossRef]
- Wang, L.H.; Liu, H. Mesoporous Co-CeO2 catalyst prepared by colloidal solution combustion method for reverse water-gas shift reaction. Catal. Today 2018, 316, 155–161. [Google Scholar] [CrossRef]
- Ahn, C.-I.; Jeong, D.-W.; Cho, J.M.; Na, H.-S.; Jang, W.-J.; Roh, H.-S.; Choi, J.-H.; Um, S.H.; Bae, J.W. Water gas shift reaction on the Mn-modified ordered mesoporous Co3O4. Microporous Mesoporous Mater. 2016, 221, 204–211. [Google Scholar] [CrossRef]
- Hulstona, C.K.J.; Redlich, P.J.; Jacksona, W.R.; Larkinsb, F.P.; Marshall, M. Hydrogenation of a brown coal pretreated with water-soluble nicle-molybdenum and cobalt-molybdenum catalysts. Fuel 1997, 76, 1465–1469. [Google Scholar] [CrossRef]
- Song, C.S.; Parfitt, D.S.; Schobert, H.H. Bimetallic dispersed catalysts from molecular precursors containing Mo-Co-S for coal liquefaction. Energy Fuels 1994, 8, 313–319. [Google Scholar] [CrossRef]
- Priyanto, U.; Sakanishi, K.; Mochida, I. Optimized solvent amount in the liquefaction of adaro coal with binary sulfide catalyst supported on carbon nanoparticles. Energy Fuels 2000, 14, 801–805. [Google Scholar] [CrossRef]
- Zhang, D.D.; Zong, Z.M.; Liu, J.; Wang, Y.H.; Yu, L.C.; Lv, J.H.; Wang, T.M.; Wei, X.Y.; Wei, Z.H.; Li, Y. Catalytic hydroconversion of geting bituminous coal over FeNi-S/γ-Al2O3. Fuel Process. Technol. 2015, 133, 195–201. [Google Scholar] [CrossRef]
- Wang, C.H.; Kim, J.; Tang, J.; Na, J.; Kang, Y.M.; Kim, M.; Lim, H.; Bando, Y.; Li, J.; Yamauchi, Y. Large-scale synthesis of mof-derived superporous carbon aerogels with extraordinary adsorption capacity for organic solvents. Angew. Chem. Int. Ed. Engl. 2020, 59, 2066–2070. [Google Scholar] [CrossRef]
- Li, Y.Z.; Ma, F.Y.; Su, X.T.; Shi, L.J.; Pan, B.B.; Sun, Z.Q.; Hou, Y.L. Ultra-large-scale synthesis of Fe3O4 nanoparticles and their application for direct coal liquefaction. Ind. Eng. Chem. Res. 2014, 53, 6718–6722. [Google Scholar] [CrossRef]
- Shi, Z.W.; Jin, L.J.; Zhou, Y.; Li, Y.; Hu, H.Q. Effect of hydrothermal treatment on structure and liquefaction behavior of Baiyinhua coal. Fuel Process. Technol. 2017, 167, 648–654. [Google Scholar] [CrossRef]
- Qin, L.; Xu, Z.H.; Zheng, Y.L.; Li, C.; Mao, J.W.; Zhang, G.L. Confined transformation of organometal-encapsulated MOFs into spinel CoFe2O4/C nanocubes for low-temperature catalytic oxidation. Adv. Funct. Mater. 2020. [Google Scholar] [CrossRef]
- Yang, W.W.; Li, L.; Fang, Y.R.; Shan, Y.L.; Xu, J.; Shen, H.; Yu, Y.B.; Guo, Y.B.; He, H. Interfacial structure-governed SO2 resistance of Cu/TiO2 catalysts in the catalytic oxidation of CO. Catal. Sci. Technol. 2020, 10, 1661–1674. [Google Scholar] [CrossRef]
- Xu, H.; Cao, Y.L.; Xie, J.; Hu, J.D.; Li, Y.Z.; Jia, D.Z. A construction of Ag-modified raspberry-like AgCl/Ag2WO4 with excellent visible-light photocatalytic property and stability. Mater. Res. Bull. 2018, 102, 342–352. [Google Scholar] [CrossRef]
- Hu, H.Q.; Bai, J.F.; Guo, S.C.; Chen, G.H. Coal liquefaction with in situ impregnated Fe2(MoS4)3 bimetallic catalyst. Fuel 2002, 81, 1521–1524. [Google Scholar] [CrossRef]
- Chen, Z.Z.; Xie, J.; Liu, Q.Y.; Wang, H.X.; Gao, S.S.; Shi, L.; Liu, Z.Y. Characterization of direct coal liquefaction catalysts by their sulfidation behavior and tetralin dehydrogenation activity. J. Energy Inst. 2019, 92, 1213–1222. [Google Scholar] [CrossRef]
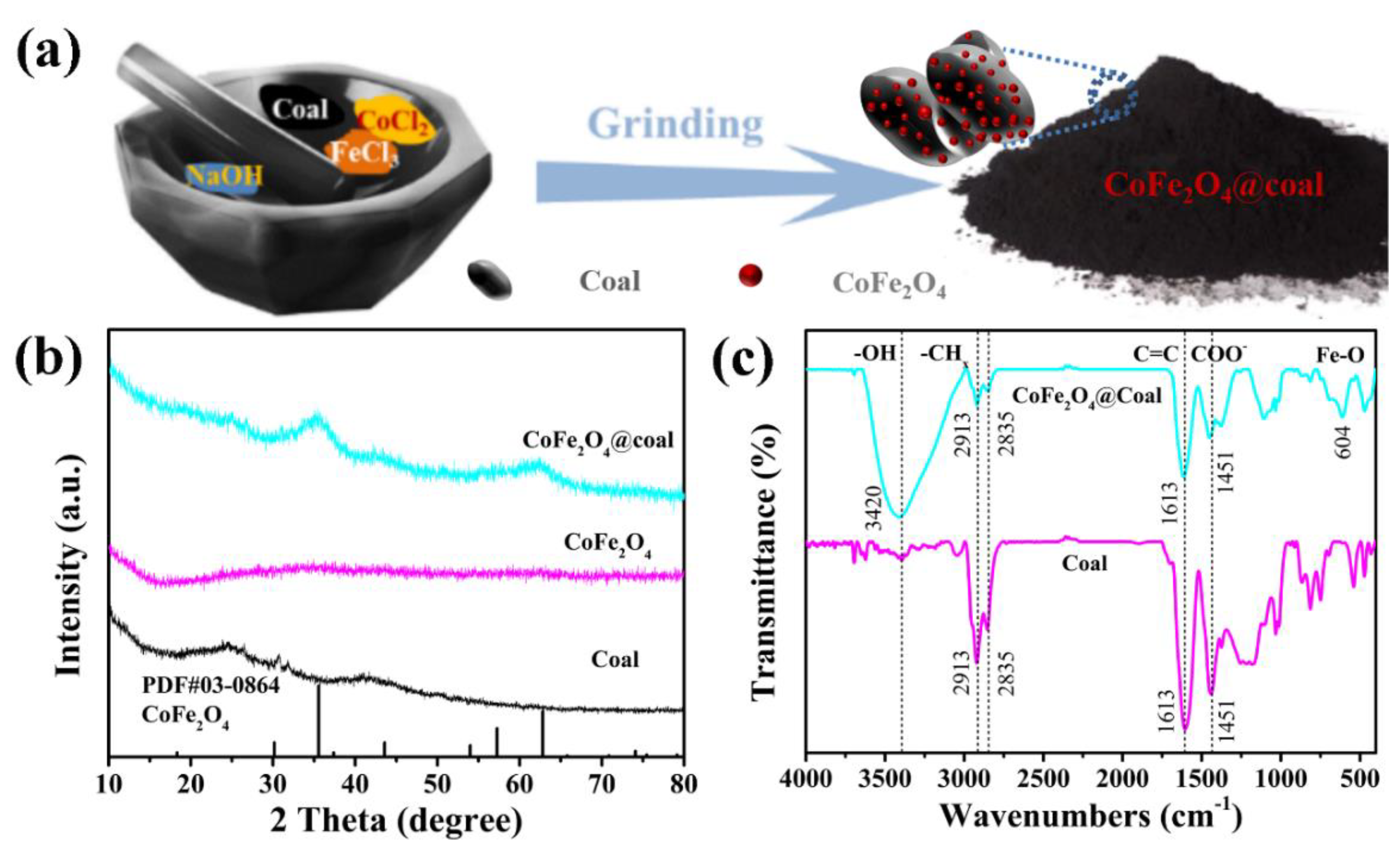
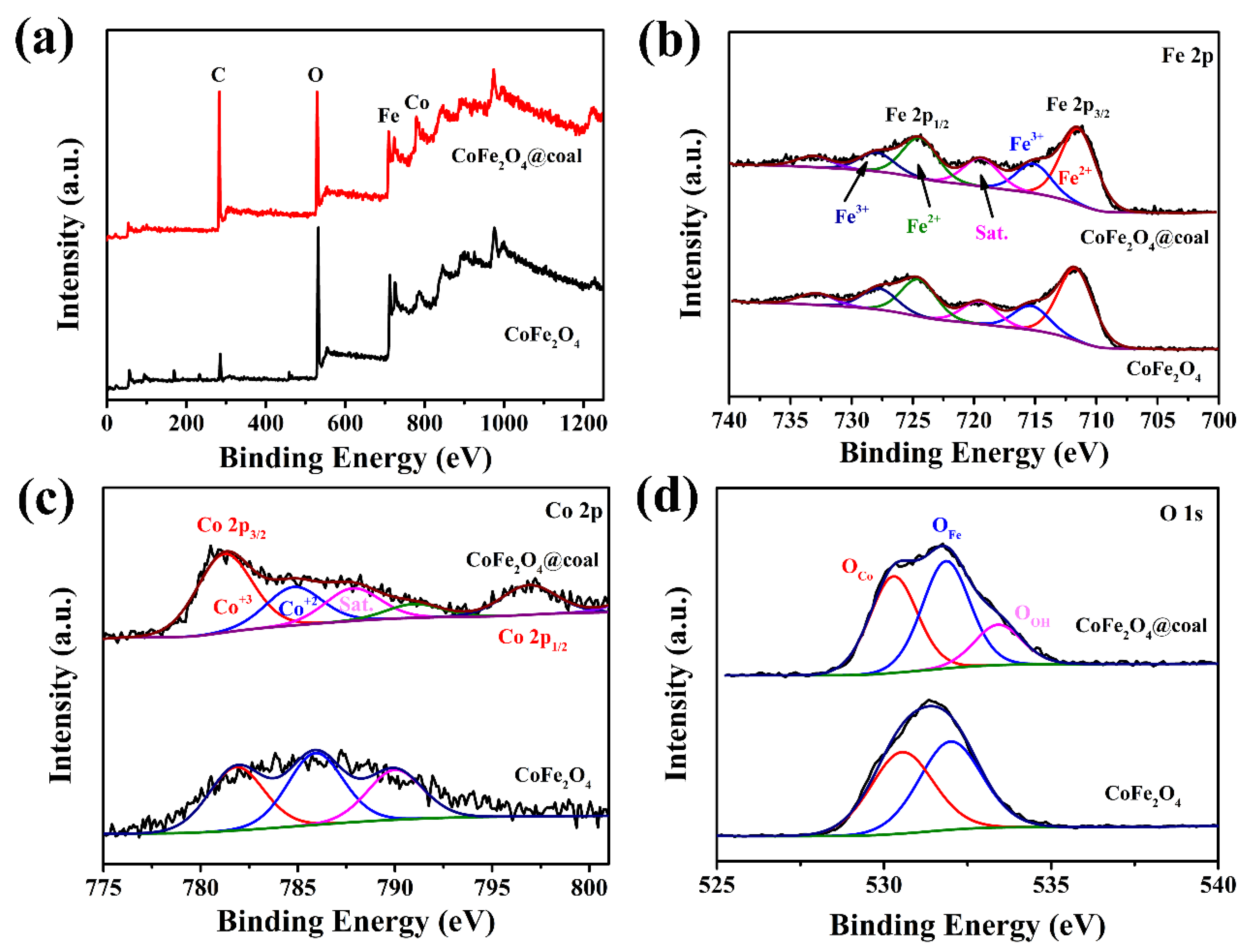

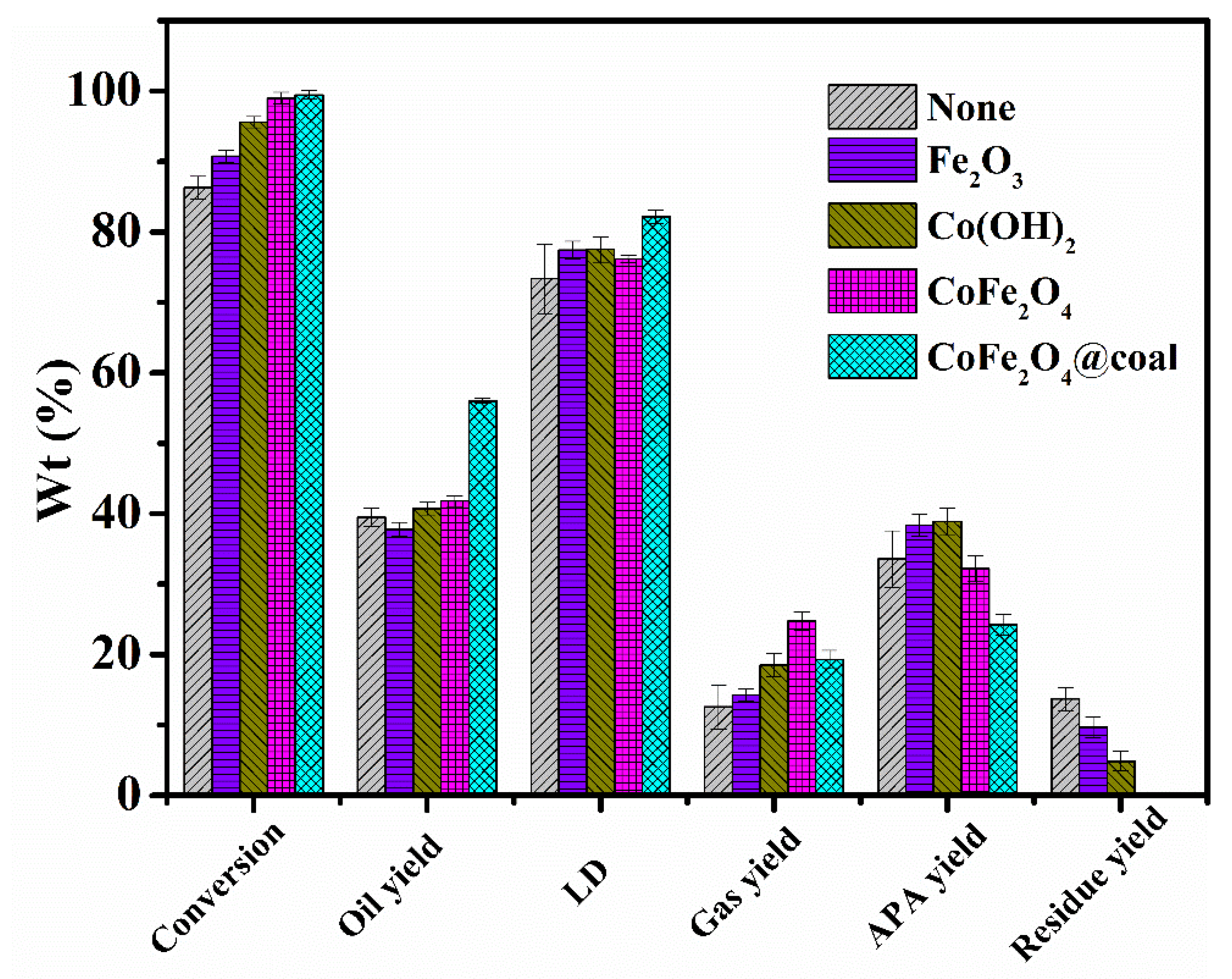
| Sample | Conversion/% | Oil Yiel/% | Liquefaction Degree/% | Gas Yield/% | APA/% | Residue Yield/% |
|---|---|---|---|---|---|---|
| / | 86.28 | 39.47 | 73.37 | 12.57 | 33.57 | 13.71 |
| Fe2O3 | 90.76 | 37.78 | 77.41 | 14.28 | 38.35 | 9.65 |
| Co(OH)2 | 95.59 | 40.71 | 77.54 | 18.49 | 38.89 | 4.84 |
| CoFe2O4 | 99.03 | 41.81 | 76.20 | 24.74 | 32.17 | 0.38 |
| CoFe2O4@coal | 99.44 | 56.01 | 82.18 | 19.30 | 24.21 | 0.11 |
| Sample | Conversion | Oil Yield | Liquefaction Degree | Gas Yield | Reference |
|---|---|---|---|---|---|
| CoFe2O4@coal | 99.44% | 56.01% | 82.18% | 19.30% | This work |
| Fe3O4 | 89.60% | 65.10% | 77.3% | - | [31] |
| Fe2(MoS4)3 | 78.20% | 70.50%(oil+gas) | - | - | [36] |
| g-FeOOH | 32.00% | - | - | - | [37] |
| FeNi-S/γ-Al2O3 | 89.40% | - | - | - | [29] |
| Oleic acid-coated Fe2O3 | 83.67% | - | - | - | [22] |
| Fe2S3 | 62.60% | 54.20% | - | - | [20] |
| Co/SiO2 | 99.00% | 55.00% | - | 44.00% | [5] |
| FeS2 | 79.00% | 47.00% | - | 32.00% | [5] |
| Fe/SiO2 | 87.00% | 44.00% | - | 43.00% | [5] |
| Oleic acid-coated Fe3O4 | 97.20% | 86.50% | 92.00% | - | [21] |
| Fe3O4 nanoparticles | 76.40% | 53.80% | - | 13.90% | [18] |
| Coal. | Proximate Analysis (wt %) 1 | Ultimate Analysis (wt %, daf 2) | H/C | O/C | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mad | Ad | Vdaf | FCd | C | H | O3 | N | S | |||
| Dahuangshan | 1.22 | 17.57 | 48.34 | 42.58 | 63.17 | 3.63 | 14.30 | 1.11 | 0.20 | 0.69 | 0.17 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Li, Y.; Wu, H.; Ma, F.; Cao, Y. Room-Temperature Solid-State Preparation of CoFe2O4@Coal Composites and Their Catalytic Performance in Direct Coal Liquefaction. Catalysts 2020, 10, 503. https://doi.org/10.3390/catal10050503
Liu B, Li Y, Wu H, Ma F, Cao Y. Room-Temperature Solid-State Preparation of CoFe2O4@Coal Composites and Their Catalytic Performance in Direct Coal Liquefaction. Catalysts. 2020; 10(5):503. https://doi.org/10.3390/catal10050503
Chicago/Turabian StyleLiu, Baolin, Yizhao Li, Hao Wu, Fengyun Ma, and Yali Cao. 2020. "Room-Temperature Solid-State Preparation of CoFe2O4@Coal Composites and Their Catalytic Performance in Direct Coal Liquefaction" Catalysts 10, no. 5: 503. https://doi.org/10.3390/catal10050503
APA StyleLiu, B., Li, Y., Wu, H., Ma, F., & Cao, Y. (2020). Room-Temperature Solid-State Preparation of CoFe2O4@Coal Composites and Their Catalytic Performance in Direct Coal Liquefaction. Catalysts, 10(5), 503. https://doi.org/10.3390/catal10050503







