Selective Production of Acetic Acid via Catalytic Fast Pyrolysis of Hexoses over Potassium Salts
Abstract
1. Introduction
2. Results and Discussion
2.1. TGA Results
2.2. Effect of Catalytic Methods
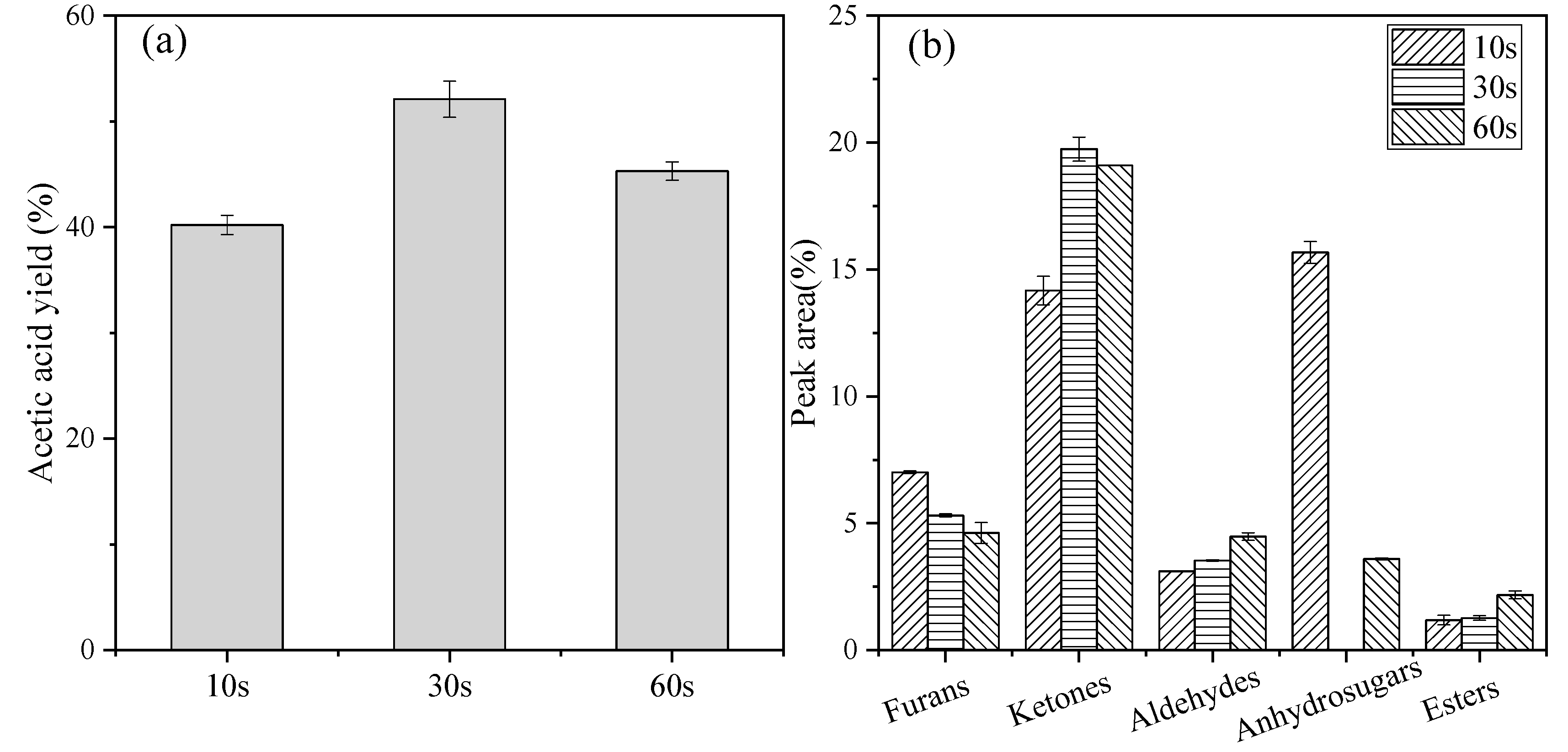
2.3. Effect of Pyrolysis Time
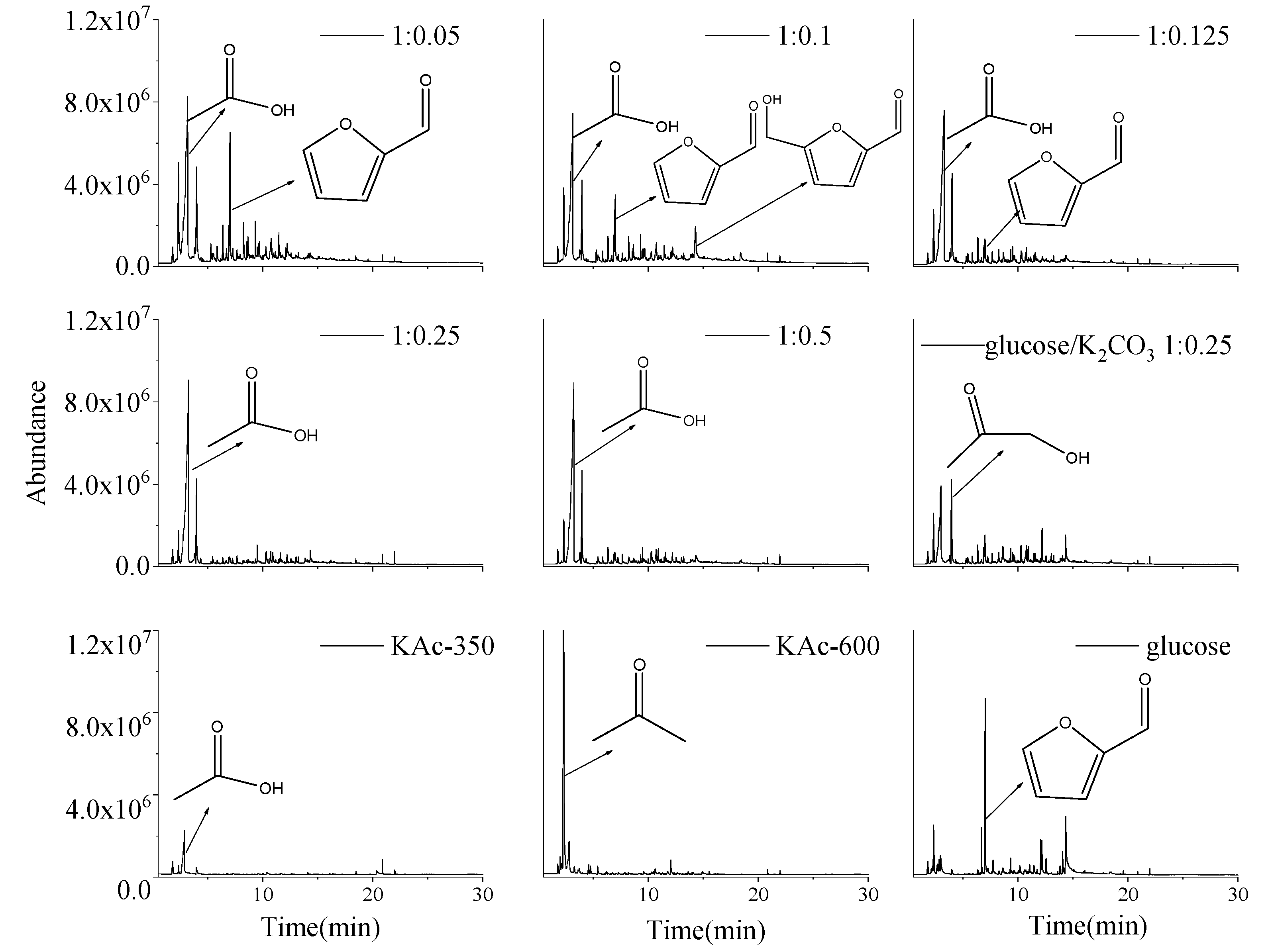
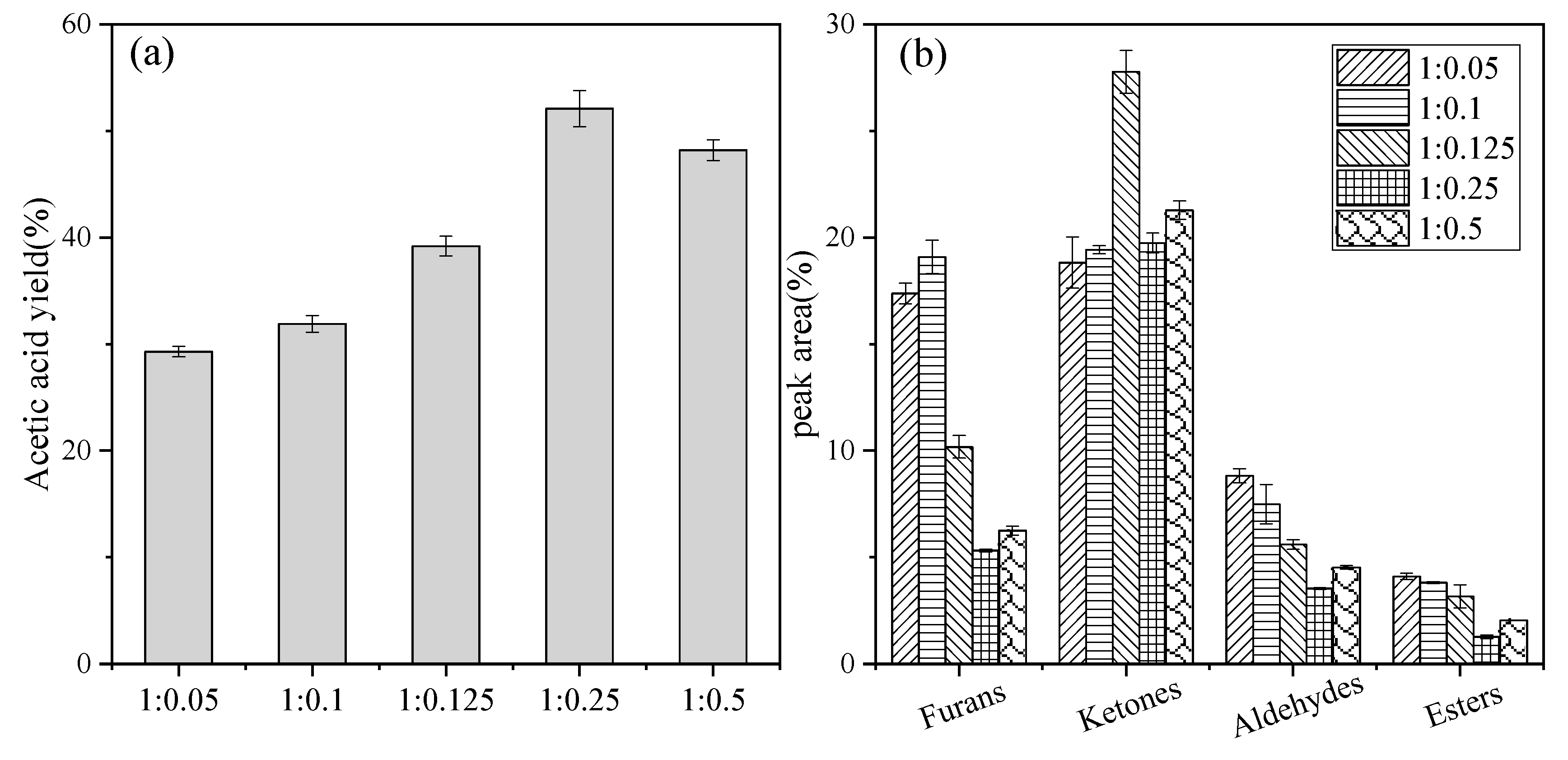
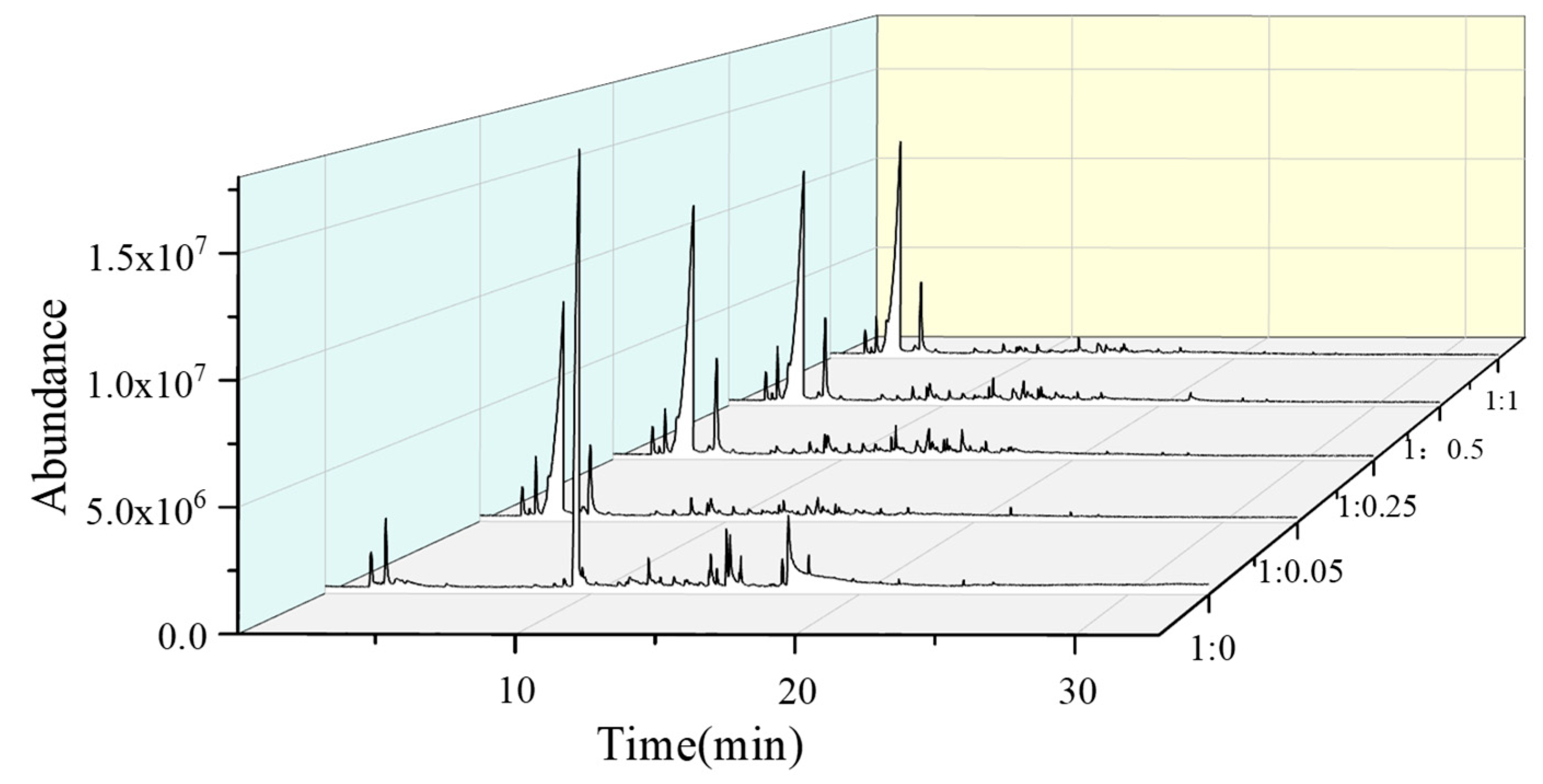
2.4. Effect of the Catalyst Dosage
2.5. Effect of Anions and Cations
2.6. Potassium Acetate Catalyzed Fructose
3. Materials and Methods
3.1. Chemicals
3.2. TGA Analysis
3.3. Py-GC/MS Analysis
3.4. Quantitative and Qualitative Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- IJmker, H.M.; Gramblicka, M.; Kersten, S.R.A.; van der Ham, A.G.J.; Schuur, B. Acetic acid extraction from aqueous solutions using fatty acids. Sep. Purif. Technol. 2014, 125, 256–263. [Google Scholar] [CrossRef]
- Feng, Y.; Yin, H.; Wang, A.; Xue, W. Selectively catalytic oxidation of 1,2-propanediol to lactic, formic, and acetic acids over Ag nanoparticles under mild reaction conditions. J. Catal. 2015, 326, 26–37. [Google Scholar] [CrossRef]
- Budiman, A.W.; Nam, J.S.; Park, J.H.; Mukti, R.I.; Chang, T.S.; Bae, J.W.; Choi, M.J. Review of Acetic Acid Synthesis from Various Feedstocks Through Different Catalytic Processes. Catal. Surv. Asia 2016, 20, 173–193. [Google Scholar] [CrossRef]
- Ganster, J.; Fink, H.P. Cellulose and cellulose acetate. In Bio-Based Plastics: Materials and Applications, 1st ed.; Kabasci, S., Ed.; Wiley: West Sussex, UK, 2014; pp. 35–62. [Google Scholar]
- Jin, F.; Kishita, A.; Moriya, T.; Enomoto, H.; Sato, N. A New Process for Producing Ca/Mg Acetate Deicer with Ca/Mg Waste and Acetic Acid Produced by Wet Oxidation of Organic Waste. Chem. Lett. 2002, 31, 88–89. [Google Scholar] [CrossRef]
- Huo, Z.; Fang, Y.; Yao, G.; Zeng, X.; Ren, D.; Jin, F. Improved two-step hydrothermal process for acetic acid production from carbohydrate biomass. J. Energy Chem. 2015, 24, 207–212. [Google Scholar] [CrossRef]
- Jin, F.; Zhang, G.; Jin, Y.; Watanabe, Y.; Kishita, A.; Enomoto, H. A new process for producing calcium acetate from vegetable wastes for use as an environmentally friendly deicer. Bioresour. Technol. 2010, 101, 7299–7306. [Google Scholar] [CrossRef]
- Yoneda, N.; Kusano, S.; Yasui, M.; Pujado, P.; Wilcher, S. Recent advances in processes and catalysts for the production of acetic acid. Appl. Catal. A Gen. 2001, 221, 253–265. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Yang, Y.-H. Microbial production of volatile fatty acids: Current status and future perspectives. Rev. Environ. Sci. Bio/Technol. 2017, 16, 327–345. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Lee, B.-R.; Sathiyanarayanan, G.; Song, H.-S.; Kim, J.; Jeon, J.-M.; Kim, J.-H.; Park, S.-H.; Yu, J.-H.; Park, K.; et al. Medium engineering for enhanced production of undecylprodigiosin antibiotic in Streptomyces coelicolor using oil palm biomass hydrolysate as a carbon source. Bioresour. Technol. 2016, 217, 141–149. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Lee, B.R.; Sathiyanarayanan, G.; Song, H.S. Biomass-derived molecules modulate the behavior ofStreptomyces coelicolorfor antibiotic production. Biotech 2016, 6, 223–231. [Google Scholar]
- Sunley, G.J.; Watson, D.J. High productivity methanol carbonylation catalysis using iridium: The Cativa™ process for the manufacture of acetic acid. Catal. Today 2000, 58, 293–307. [Google Scholar] [CrossRef]
- Kochloefl, K. Development of Industrial Solid Catalysts. Chem. Eng. Technol. 2001, 24, 229–234. [Google Scholar] [CrossRef]
- Fielder, E.; Grossmann, G.; Burkhard, K.D.; Weiss, G.; Witte, C. Methanol. Economic analysis. Int. J. Hydrogen Energy 2005, 30, 769–842. [Google Scholar]
- Mahfud, F.H.; Van Geel, F.P.; Venderbosch, R.; Heeres, H.J. Acetic Acid Recovery from Fast Pyrolysis Oil. An Exploratory Study on Liquid-Liquid Reactive Extraction using Aliphatic Tertiary Amines. Sep. Sci. Technol. 2008, 43, 3056–3074. [Google Scholar] [CrossRef]
- Chu, W.; Ooka, Y.; Kamiya, Y.; Okuhara, T. Reaction path for oxidation of ethylene to acetic acid over Pd/WO3–ZrO2 in the presence of water. Catal. Lett. 2005, 101, 225–228. [Google Scholar] [CrossRef]
- Jin, F.; Zhou, Z.; Kishita, A.; Enomoto, H.; Kishida, H.; Moriya, T. A New Hydrothermal Process for Producing Acetic Acid from Biomass Waste. Chem. Eng. Res. Des. 2007, 85, 201–206. [Google Scholar] [CrossRef]
- Jin, F.; Enomoto, H. Hydrothermal conversion of biomass wastes into resources. Prog. Nat. Sci. 2005, 15, 14–22. [Google Scholar] [CrossRef]
- Jin, F.; Zhou, Z.; Moriya, T.; Kishida, H.; Higashijima, H.; Enomoto, H. Controlling Hydrothermal Reaction Pathways to Improve Acetic Acid Production from Carbohydrate Biomass. Environ. Sci. Technol. 2005, 39, 1893–1902. [Google Scholar] [CrossRef]
- Vasiliu, M.; Guynn, K.; Dixon, D.A. Prediction of the Thermodynamic Properties of Key Products and Intermediates from Biomass. J. Phys. Chem. C 2011, 115, 15686–15702. [Google Scholar] [CrossRef]
- Dong, C.Q.; Zhang, Z.F.; Lu, Q.; Yang, Y.P. Characteristics and mechanism study of analytical fast pyrolysis of poplar wood. Energy Convers. Manag. 2012, 57, 49–59. [Google Scholar] [CrossRef]
- Garlapalli, R.K.; Wirth, B.; Reza, M.T. Pyrolysis of hydrochar from digestate: Effect of hydrothermal carbonization and pyrolysis temperatures on pyrochar formation. Bioresour. Technol. 2016, 220, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Kabir, G.; Hameed, B. Recent progress on catalytic pyrolysis of lignocellulosic biomass to high-grade bio-oil and bio-chemicals. Renew. Sustain. Energy Rev. 2017, 70, 945–967. [Google Scholar] [CrossRef]
- Mettler, M.S.; Vlachos, D.G.; Dauenhauer, P.J. Top ten fundamental challenges of biomass pyrolysis for biofuels. Energy Environ. Sci. 2012, 5, 7797–7809. [Google Scholar] [CrossRef]
- Akalın, M.K.; Karagöz, S.; Akalin, M.K. Analytical pyrolysis of biomass using gas chromatography coupled to mass spectrometry. TrAC Trends Anal. Chem. 2014, 61, 11–16. [Google Scholar] [CrossRef]
- Galletti, G.C.; Bocchini, P. Pyrolysis/gas chromatography/mass spectrometry of lignocellulose. Rapid Commun. Mass Spectrom. 1995, 9, 815–826. [Google Scholar] [CrossRef] [PubMed]
- Nowakowski, D.; Jones, J.M.; Brydson, R.; Ross, A. Potassium catalysis in the pyrolysis behaviour of short rotation willow coppice. Fuel 2007, 86, 2389–2402. [Google Scholar] [CrossRef]
- Fahmi, R.; Bridgwater, T.; Darvell, L.; Jones, J.; Yates, N.; Thain, S.; Donnison, I. The effect of alkali metals on combustion and pyrolysis of Lolium and Festuca grasses, switchgrass and willow. Fuel 2007, 86, 1560–1569. [Google Scholar] [CrossRef]
- Shimada, N.; Kawamoto, H.; Saka, S. Different action of alkali/alkaline earth metal chlorides on cellulose pyrolysis. J. Anal. Appl. Pyrolysis 2008, 81, 80–87. [Google Scholar] [CrossRef]
- Nowakowski, D.; Jones, J.M. Uncatalysed and potassium-catalysed pyrolysis of the cell-wall constituents of biomass and their model compounds. J. Anal. Appl. Pyrolysis 2008, 83, 12–25. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, M.; Chen, M.; Min, F.; Zhang, S.; Ren, Z.; Yan, Y. Catalytic effects of six inorganic compounds on pyrolysis of three kinds of biomass. Thermochim. Acta 2006, 444, 110–114. [Google Scholar] [CrossRef]
- Patwardhan, P.R.; Satrio, J.A.; Brown, R.; Shanks, B.H. Product distribution from fast pyrolysis of glucose-based carbohydrates. J. Anal. Appl. Pyrolysis 2009, 86, 323–330. [Google Scholar] [CrossRef]
- Fabbri, D.; Chiavari, G.; Prati, S.; Vassura, I.; Vangelista, M. Gas chromatography/mass spectrometric characterisation of pyrolysis/silylation products of glucose and cellulose. Rapid Commun. Mass Spectrom. 2002, 16, 2349–2355. [Google Scholar] [CrossRef]
- Carlson, T.R.; Jae, J.; Lin, Y.-C.; Tompsett, G.A.; Huber, G. Catalytic fast pyrolysis of glucose with HZSM-5: The combined homogeneous and heterogeneous reactions. J. Catal. 2010, 270, 110–124. [Google Scholar] [CrossRef]
- Lu, Q.; Yang, X.-C.; Dong, C.-Q.; Zhang, Z.-F.; Zhang, X.; Zhu, X. Influence of pyrolysis temperature and time on the cellulose fast pyrolysis products: Analytical Py-GC/MS study. J. Anal. Appl. Pyrolysis 2011, 92, 430–438. [Google Scholar] [CrossRef]
- Srinivasan, V.; Adhikari, S.; Chattanathan, S.A.; Park, S. Catalytic Pyrolysis of Torrefied Biomass for Hydrocarbons Production. Energy Fuels 2012, 26, 7347–7353. [Google Scholar] [CrossRef]
- Judd, M.D.; Plunkett, B.A.; Pope, M.I. The thermal decomposition of calcium, sodium, silver and copper(II) acetates. J. Therm. Anal. Calorim. 1974, 6, 555–563. [Google Scholar] [CrossRef]
- Afzal, M.; Butt, P.K.; Ahmad, H. Kinetics of thermal decomposition of metal acetates. J. Therm. Anal. Calorim. 1991, 37, 1015–1023. [Google Scholar] [CrossRef]
- Shuping, Z.; Yulong, W.; Mingde, Y.; Chun, L.; Junmao, T. Pyrolysis characteristics and kinetics of the marine microalgae Dunaliella tertiolecta using thermogravimetric analyzer. Bioresour. Technol. 2010, 101, 359–365. [Google Scholar] [CrossRef]
- Mattonai, M.; Ribechini, E. A comparison of fast and reactive pyrolysis with in situ derivatisation of fructose, inulin and Jerusalem artichoke (Helianthus tuberosus). Anal. Chim. Acta 2018, 1017, 66–74. [Google Scholar] [CrossRef]
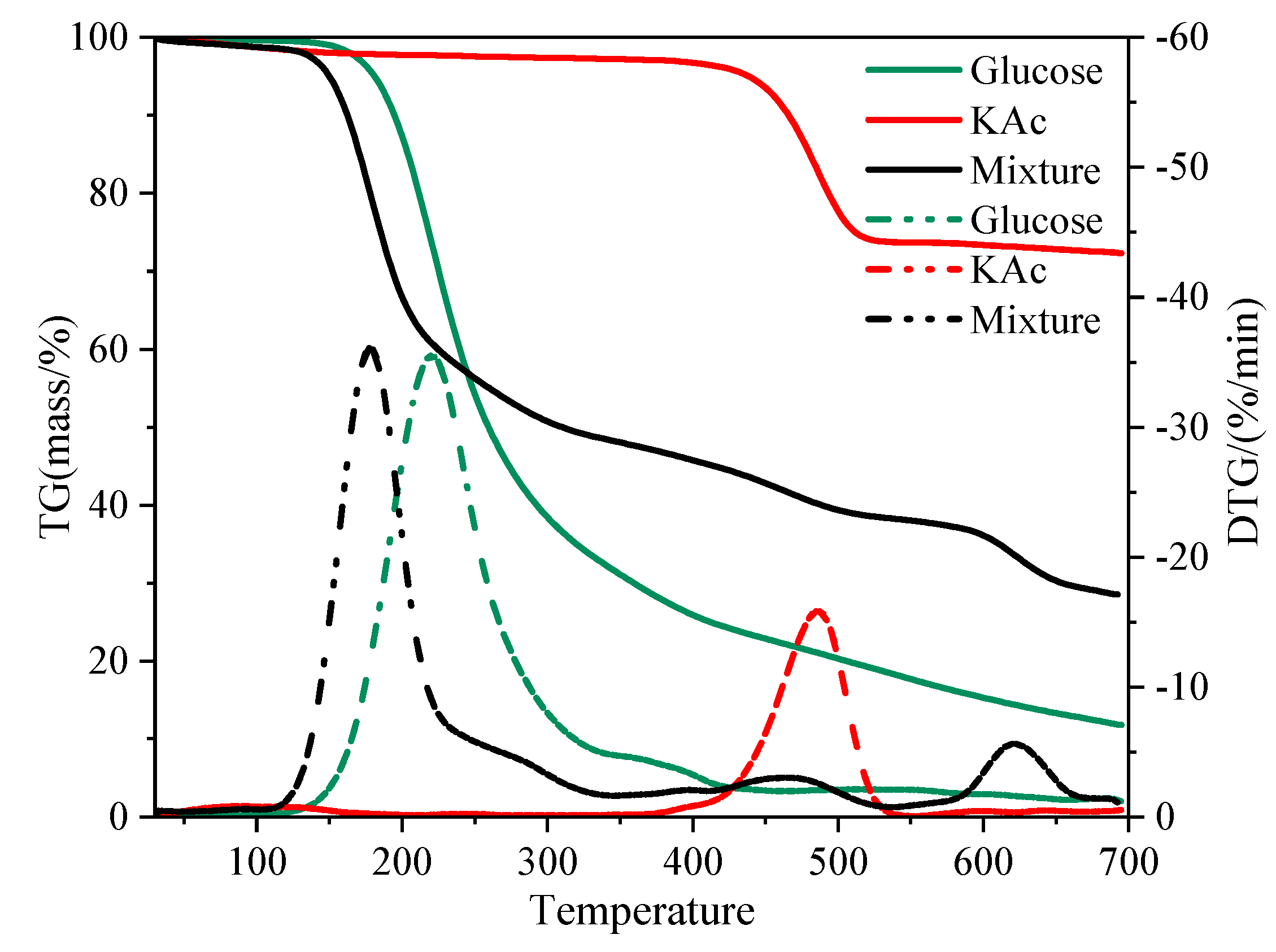

| Samples | Ti a (°C) | Tmax b (°C) | Residue c (%) |
|---|---|---|---|
| Glucose | 188 | 220 | 32 |
| GLU-KAc d | 158 | 178 | 28 |
| GLU-NaAc d | 159 | 179 | 27 |
| GLU-K2CO3 d | 152 | 171 | 30 |
| KAc | 449 | 485 | 97 |
| NaAc | 464 | 503 | 99 |
| K2CO3 | - | - | 100 |
| Products | Peak Area (%) | ||
|---|---|---|---|
| No Catalyst | In Situ | On-Line | |
| Acetic acid | 2.44 ± 0.03 | 60.83 ± 0.21 | 33.06 ± 0.02 |
| Aldehydes | |||
| Methylglyoxal | - | 3.06 ± 0.05 | 4.26 ± 0.17 |
| Hydroxyl-acetaldehyde | 5.28 ± 0.27 | - | - |
| Ketones | |||
| Acetone | 7.88 ± 0.34 | - | 4.13 ± 0.21 |
| 1-Hydroxyl-2-propanone | 0.61 ± 0.01 | 7.50 ± 0.04 | 8.55 ± 0.03 |
| 3-Methyl-1,2-cyclopentanedione | 0.71 ± 0.32 | - | 2.59 ± 0.11 |
| 2,3-Dihydro-3,5-dihydroxyl-6-methyl-4H-pyran-4-one | 0.41 ± 0.00 | 0.85 ± 0.04 | - |
| Furans | |||
| Furan | 1.38 ± 0.21 | - | 1.79 ± 0.33 |
| Furfural | 24.3 ± 0.09 | - | 12.1 ± 0.14 |
| 3-Methyl-furan | - | - | 1.39 ± 0.57 |
| 2-Furanmethanol | - | 1.09 ± 0.08 | 1.57 ± 0.19 |
| 5-Methyl-2-furancarboxaldehyde | 1.63 ± 0.02 | 0.32 ± 0.01 | 1.4 ± 0.24 |
| 2,5-Furandicarboxaldehyde | - | - | 2.26 ± 0.31 |
| 5-Hydroxymethyl-2-furancarboxaldehyde (5-HMF) | 15.93 ± 0.17 | 1.18 ± 0.29 | 0.63 ± 0.01 |
| 2,5-Dimethyl-4-hydroxyl-3(2H)-furanone | 0.63 ± 0.23 | 1.39 ± 0.15 | 1.54 ± 0.22 |
| Anhydrosugars | |||
| Levoglucosan | - | - | 1.35 ± 0.34 |
| 1,4:3,6-Dianhydro-α-d-glucopyranose | - | - | 7.3 ± 0.02 |
| Products | Time (s) | ||
|---|---|---|---|
| 10 s | 30 s | 60 s | |
| Acetic acid | 47.79 ± 019 | 60.83 ± 0.21 | 53.82 ± 0.03 |
| Methylglyoxal | 3.11 ± 0.27 | 3.06 ± 0.05 | 4.48 ± 0.24 |
| 1-Hydroxyl-2-propanone | 7.10 ± 0.31 | 7.50 ± 0.04 | 9.14 ± 0.17 |
| Furfural | 0.61 ± 0.15 | - | 2.7 ± 0.04 |
| 2-Furanmethanol | - | 1.09 ± 0.08 | - |
| 2,5-Dimethyl-4-hydroxyl-3(2H)-furanone | 0.55 ± 0.02 | 1.39 ± 0.15 | 1.44 ± 0.08 |
| 5-Hydroxymethyl-2-furancarboxaldehyde (5-HMF) | 5.40 ± 0.47 | 1.18 ± 0.29 | 0.49 ± 0.42 |
| Butyrolactone | 0.81 ± 0.33 | 1.74 ± 0.04 | - |
| 3-Ethyl-2-hydroxyl-2-cyclopenten-1-one | 0.63 ± 0.11 | 1.04 ± 0.16 | 1.29 ± 0.34 |
| 2,3-Pentanedione | 1.30 ± 0.01 | 2.23 ± 0.01 | 1.69 ± 0.17 |
| Propanoic acid, 2-oxo-, methyl ester | 1.19 ± 0.21 | 0.55 ± 0.11 | 2.18 ± 0.24 |
| Levoglucosan | 15.68 ± 0.39 | - | 3.60 ± 0.44 |
| Products | Peak Area (%) (Glucose/KAc) | ||||
|---|---|---|---|---|---|
| 1:0.05 | 1:0.1 | 1:0.125 | 1:0.25 | 1:0.5 | |
| Acetic acid | 34.22 ± 0.03 | 37.24 ± 0.29 | 45.83 ± 0.11 | 60.83 ± 0.21 | 56.25 ± 0.03 |
| Furans | |||||
| Furan | 0.27 ± 0.10 | - | - | - | - |
| Furfural | 7.79 ± 0.27 | 5.34 ± 0.32 | 2.84 ± 0.01 | - | - |
| 2-Furanmethanol | 1.41 ± 0.31 | 1.2 ± 0.19 | 0.87 ± 0.04 | 1.09 ± 0.08 | 0.52 ± 0.31 |
| 2,4-Dihydroxyl-2,5-dimethyl-3(2H)-furan-3-one | 0.82 ± 0.14 | 0.57 ± 0.13 | 0.38 ± 0.14 | 0.39 ± 0.01 | - |
| 3-Furanmethanol | 1.47 ± 0.12 | 1.3 ± 0.08 | 1.2 ± 0.33 | 0.58 ± 0.05 | 0.75 ± 0.07 |
| 5-Methyl-2-furancarboxaldehyde | 1.58 ± 0.33 | 1.34 ± 0.02 | 0.83 ± 0.04 | 0.32 ± 0.01 | 0.53 ± 0.13 |
| 2(5H)-Furanone | 0.87 ± 0.41 | 0.74 ± 0.11 | 0.61 ± 0.26 | - | 0.46 ± 0.05 |
| 2,5-Dimethyl-4-hydroxyl- 3(2H)-furanone | 2.13 ± 0.01 | 2.37 ± 0.37 | 2.00 ± 0.11 | 1.39 ± 0.15 | 1.41 ± 0.24 |
| 5-Acetyldihydro-2(3H)-furanone | 0.35 ± 0.00 | 0.57 ± 0.15 | 0.35 ± 0.17 | 0.38 ± 0.02 | 0.39 ± 0.06 |
| 5-Hydroxymethyl-2- furancarboxaldehyde | 0.52 ± 0.25 | 5.49 ± 0.29 | 0.98 ± 0.09 | 1.18 ± 0.29 | 2.19 ± 0.22 |
| Ketones | |||||
| 2-Pentanone | - | - | 5.58 ± 0.37 | - | - |
| 2,3-Pentanedione | 1.46 ± 0.22 | 2.05 ± 0.11 | 1.11 ± 0.21 | 2.23 ± 0.01 | 2.15 ± 0.11 |
| 1-Hydroxyl-2-propanone | 7.76 ± 0.13 | 7.04 ± 0.27 | 10.49 ± 0.05 | 7.5 ± 0.04 | 8.34 ± 0.24 |
| 3-Hydroxyl-2-butanone | 0.41 ± 0.21 | 0.37 ± 0.01 | 0.4 ± 0.01 | 0.57 ± 0.15 | 0.49 ± 0.21 |
| 2-Cyclopenten-1-one | 1.00 ± 0.14 | 0.67 ± 0.11 | 0.73 ± 0.08 | 0.59 ± 0.11 | 0.58 ± 0.09 |
| 1-Acetyloxyl-2-propanone | 0.7 ± 0.01 | 0.65 ± 0.09 | 0.84 ± 0.39 | 0.56 ± 0.01 | 0.74 ± 0.04 |
| 2-Cyclopentene-1,4-dione | 2.16 ± 0.28 | 1.87 ± 0.07 | 1.40 ± 0.01 | 0.27 ± 0.07 | 0.99 ± 0.01 |
| 2-Cyclohexen-1-one | 0.63 ± 0.26 | 0.49 ± 0.15 | 0.60 ± 0.03 | 0.41 ± 0.12 | 0.49 ± 0.00 |
| 1,3-Dihydroxyacetone dimer | 0.27 ± 0.38 | 0.26 ± 0.01 | 0.34 ± 0.25 | 0.25 ± 0.00 | 0.29 ± 0.01 |
| Butyrolactone | 0.77 ± 0.02 | 0.73 ± 0.25 | 1.11 ± 0.12 | 1.74 ± 0.04 | 0.94 ± 0.33 |
| 3-Methyl-1,2-cyclopentanedione | 1.84 ± 0.13 | 1.02 ± 0.24 | 1.77 ± 0.23 | 1.90 ± 0.08 | 1.88 ± 0.05 |
| 2-Hydroxyl-gamma-butyrolactone | - | 0.66 ± 0.01 | 0.69 ± 0.02 | 1.03 ± 0.06 | 1.57 ± 0.02 |
| 3-Ethyl-2-hydroxyl-2-cyclopenten-1-one | - | 0.46 ± 0.19 | 0.89 ± 0.05 | 1.04 ± 0.16 | 1.02 ± 0.14 |
| 2,3-Dihydro-3,5-dihydroxyl-6-methyl-4H-pyran-4-one | 1.4 ± 0.05 | 2.19 ± 0.23 | 0.9 ± 0.15 | 0.85 ± 0.04 | 1.07 ± 0.13 |
| Aldehydes | |||||
| Methylgloxal | 7.44 ± 0.28 | 6.27 ± 0.01 | 4.87 ± 0.27 | 3.06 ± 0.05 | 3.94 ± 0.18 |
| 2-Butenal | 0.81 ± 0.22 | 0.83 ± 0.24 | - | - | - |
| 3-Methyl-butanal | 0.57 ± 0.06 | 0.39 ± 0.16 | 0.72 ± 0.01 | 0.47 ± 0.02 | 0.59 ± 0.16 |
| Esters | |||||
| 2-Propenoic acid, 2-hydroxyethyl ester | 1.10 ± 0.07 | 0.86 ± 0.01 | 0.50 ± 0.16 | 0.19 ± 0.01 | 0.22 ± 0.01 |
| Acetic acid, methyl ester | 0.76 ± 0.01 | 0.64 ± 0.13 | 0.69 ± 0.04 | 0.33 ± 0.31 | 0.48 ± 0.02 |
| Propanoic acid, 2-oxo-, methyl ester | 1.86 ± 0.12 | 1.56 ± 0.11 | 1.76 ± 0.03 | 0.55 ± 0.11 | 1.15 ± 0.09 |
| Group | Peak area (%) | |||
|---|---|---|---|---|
| No Catalyst | KAc | NaAc | K2CO3 | |
| Acetic acid | 2.44 ± 0.03 | 60.83 ± 0.21 | 50.31 ± 0.14 | 21.85 ± 0.06 |
| Aldehydes | 6.14 ± 0.21 | 3.53 ± 0.41 | 4.19 ± 0.23 | 7.78 ± 0.03 |
| Ketones | 25.37 ± 0.34 | 19.74 ± 0.22 | 26.69 ± 0.19 | 33.7 ± 0.23 |
| Furans | 50.13 ± 0.35 | 5.31 ± 0.19 | 7.24 ± 0.32 | 16.94 ± 0.35 |
| Esters | 6.97 ± 0.19 | 1.27 ± 0.07 | 2.42 ± 0.44 | 3.45 ± 0.18 |
| Products | Peak Area (%) | ||
|---|---|---|---|
| NaAc | KAc | K2CO3 | |
| Acetic acid | 50.31 ± 0.14 | 60.83 ± 0.21 | 21.85 ± 0.06 |
| Methylglyoxal | 3.69 ± 0.01 | 3.06 ± 0.05 | 6.14 ± 0.22 |
| Acetone | - | - | 7.18 ± 0.14 |
| 1-Hydroxyl-2-propanone | 9.14 ± 0.22 | 7.5 ± 0.04 | 11.63 ± 0.03 |
| Furfural | - | - | 2.75 ± 0.02 |
| 3-Methyl-1,2-cyclopentanedione | 1.43 ± 0.01 | 1.9 ± 0.08 | 2.18 ± 0.09 |
| 5-Methyl-2-furancarboxaldehyde | 0.48 ± 0.01 | 0.32 ± 0.01 | 1.22 ± 0.33 |
| 2,3-Dihydro-3,5-dihydroxyl-6-methyl-4H-pyran-4-one | 3.02 ± 0.14 | 0.85 ± 0.04 | 2.75 ± 0.19 |
| 2-Furanmethanol | 2.29 ± 0.17 | 1.09 ± 0.08 | 1.79 ± 0.22 |
| 2,5-Dimethyl-4-hydroxyl-3(2H)-furanone | 1.61 ± 0.18 | 1.39 ± 0.15 | 2.79 ± 0.01 |
| 5-Hydroxymethyl-2-furancarboxaldehyde | 1.31 ± 0.00 | 1.18 ± 0.29 | 4.50 ± 0.25 |
| Butyrolactone | 0.93 ± 0.02 | 1.74 ± 0.04 | 0.94 ± 0.42 |
| 3-Ethyl-2-hydroxyl-2-cyclopenten-1-one | 0.46 ± 0.09 | 1.04 ± 0.16 | 0.56 ± 0.29 |
| 2,3-Pentanedione | 0.68 ± 0.11 | 2.23 ± 0.01 | 0.96 ± 0.17 |
| Products | Peak Area (%) (Fructose/KAc) | ||||
|---|---|---|---|---|---|
| 1:0 | 1:0.05 | 1:0.25 | 1:0.5 | 1:1 | |
| Isobutane | 4.41 ± 0.03 | 4.16 ± 0.24 | 2.79 ± 0.15 | 3.27 ± 0.29 | 2.82 ± 0.06 |
| Acetic acid, anhydride with formic acid | 0.52 ± 0.33 | 5.47 ± 0.32 | 4.33 ± 0.21 | 4.53 ± 0.01 | 5.47 ± 0.01 |
| Acetic acid | - | 54.99 ± 0.33 | 55.96 ± 0.13 | 57.25 ± 0.18 | 62.64 ± 0.57 |
| 2,3-Pentanedione | - | 2.01 ± 0.11 | 1.36 ± 0.13 | 0.81 ± 0.14 | 1.83 ± 0.31 |
| 1-Hydroxyl-2-propanone | - | 8.46 ± 0.21 | 9.69 ± 0.23 | 8.73 ± 0.24 | 9.32 ± 0.43 |
| Propanoic acid, 2-oxo-, methyl ester | - | 1.49 ± 0.22 | 0.88 ± 0.01 | 1.09 ± 0.07 | 0.98 ± 0.15 |
| 1,3-Cyclopentanedione | - | 0.70 ± 0.01 | 0.82 ± 0.04 | 0.68 ± 0.27 | 0.43 ± 0.04 |
| Furfural | 58.43 ± 0.12 | 2.09 ± 0.23 | 2.03 ± 0.14 | 1.91 ± 0.24 | 1.07 ± 0.22 |
| 1-Acetyloxy- 2-propanone | - | 0.71 ± 0.05 | 0.57 ± 0.21 | 0.68 ± 0.2 | 1.00 ± 0.06 |
| 2-Cyclopentene-1,4-dione | 0.97 ± 0.03 | 0.96 ± 0.21 | 0.99 ± 0.06 | 0.74 ± 0.02 | 0.44 ± 0.30 |
| Butyrolactone | - | 1.10 ± 0.21 | 1.13 ± 0.25 | 1.28 ± 0.07 | 1.57 ± 0.04 |
| 3-Methyl-1,2-cyclopentanedione, | - | 1.34 ± 0.34 | 1.33 ± 0.21 | 1.62 ± 0.09 | 1.67 ± 0.12 |
| 2,5-Dimethyl-4-hydroxyl-3(2H)-furanone | - | 1.73 ± 0.23 | 1.9 ± 0.25 | 1.59 ± 0.01 | 1.02 ± 0.01 |
| 2-Hydroxyl-γ-butyrolactone | - | 1.01 ± 0.34 | 1.06 ± 0.11 | 0.89 ± 026 | 0.48 ± 0.16 |
| Furyl hydroxymethyl ketone | 2.65 ± 0.26 | - | - | - | - |
| 2,5-Furandicarboxaldehyde | 2.56 ± 0.43 | - | - | - | - |
| 2,3-Dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one | 4.36 ± 0.14 | 0.95 ± 0.11 | 1.92 ± 0.05 | 1.03 ± 0.19 | - |
| 1,2,6-Hexanetriol | 1.77 ± 0.31 | - | - | - | - |
| 5-Acetoxymethyl-2-furaldehyde | 1.44 ± 0.24 | - | -- | - | - |
| 5-Hydroxymethyl-2-furancarboxaldehyde | 11.85 ± 0.09 | - | - | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, W.; Zhang, Z. Selective Production of Acetic Acid via Catalytic Fast Pyrolysis of Hexoses over Potassium Salts. Catalysts 2020, 10, 502. https://doi.org/10.3390/catal10050502
Kang W, Zhang Z. Selective Production of Acetic Acid via Catalytic Fast Pyrolysis of Hexoses over Potassium Salts. Catalysts. 2020; 10(5):502. https://doi.org/10.3390/catal10050502
Chicago/Turabian StyleKang, Wenyue, and Zhijun Zhang. 2020. "Selective Production of Acetic Acid via Catalytic Fast Pyrolysis of Hexoses over Potassium Salts" Catalysts 10, no. 5: 502. https://doi.org/10.3390/catal10050502
APA StyleKang, W., & Zhang, Z. (2020). Selective Production of Acetic Acid via Catalytic Fast Pyrolysis of Hexoses over Potassium Salts. Catalysts, 10(5), 502. https://doi.org/10.3390/catal10050502




