Synthesis of Magnetic Fe3O4/ZnWO4 and Fe3O4/ZnWO4/CeVO4 Nanoparticles: The Photocatalytic Effects on Organic Pollutants upon Irradiation with UV-Vis Light
Abstract
1. Introduction
2. Results
2.1. Physico–Chemical Characterization of Fe3O4/ZnWO4 and Fe3O4/ZnWO4/CeVO4 Nanoparticles
2.2. Photocatalytic Reactions Using Fe3O4/ZnWO4/CeVO4
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Fe3O4 Nanoparticles
3.3. Synthesis of Fe3O4/ZnWO4 Nanoparticles
3.4. Synthesis of Fe3O4/ZnWO4/CeVO4 Nanoparticles
3.5. Evaluation of Photocatalytic Activities
3.6. Photodegradation Mechanism
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Franz, S.; Falletta, E.; Arab, H.; Murgolo, S.; Bestetti, M.; Mascolo, G. Degradation of Carbamazepine by Photo(electro)catalysis on Nanostructured TiO2 Meshes: Transformation Products and Reaction Pathways. Catalysts 2020, 10, 169. [Google Scholar] [CrossRef]
- Li, S.; Xue, B.; Chen, J.; Jiang, W.; Liu, Y. BiOCOOH Microflowers Decorated with Ag/Ag2CrO4 Nanoparticles as Highly Efficient Photocatalyst for the Treatment of Toxic Wastewater. Catalysts 2020, 10, 93. [Google Scholar] [CrossRef]
- Feijoo, S.; González-Rodríguez, J.; Fernández, L.; Vázquez-Vázquez, C.; Feijoo, G.; Moreira, M.T. Fenton and Photo-Fenton Nanocatalysts Revisited from the Perspective of Life Cycle Assessment. Catalysts 2019, 10, 23. [Google Scholar] [CrossRef]
- Włodarczyk, P.P.; Włodarczyk, B. Preparation and Analysis of Ni–Co Catalyst Use for Electricity Production and COD Reduction in Microbial Fuel Cells. Catalysts 2019, 9, 1042. [Google Scholar] [CrossRef]
- Khalid, S.; Shahid, M.; Natasha Bibi, I.; Sarwar, T.; Shah, A.; Niazi, N. A Review of Environmental Contamination and Health Risk Assessment of Wastewater Use for Crop Irrigation with a Focus on Low and High-Income Countries. Int. J. Environ. Res. Public Health 2018, 15, 895. [Google Scholar] [CrossRef]
- Schaider, L.A.; Rodgers, K.M.; Rudel, R.A. Review of Organic Wastewater Compound Concentrations and Removal in Onsite Wastewater Treatment Systems. Environ. Sci. Technol. 2017, 51, 7304–7317. [Google Scholar] [CrossRef]
- Bartolomeu, M.; Neves, M.G.P.M.S.; Faustino, M.A.F.; Almeida, A. Wastewater chemical contaminants: Remediation by advanced oxidation processes. Photochem. Photobiol. Sci. 2018, 17, 1573–1598. [Google Scholar] [CrossRef]
- Holkar, C.R.; Jadhav, A.J.; Pinjari, D.V.; Mahamuni, N.M.; Pandit, A.B. A critical review on textile wastewater treatments: Possible approaches. J. Environ. Manag. 2016, 182, 351–366. [Google Scholar] [CrossRef]
- Kweinor Tetteh, E.; Rathilal, S.; Chetty, M.; Kwaku Armah, E.; Asante-Sackey, D. Treatment of Water and Wastewater for Reuse and Energy Generation-Emerging Technologies. In Water and Wastewater Treatment; Eyvaz, M., Ed.; IntechOpen: London, UK, 2019; ISBN 978-1-78923-929-4. [Google Scholar]
- Singh, N.B.; Nagpal, G.; Agrawal, S. Rachna Water purification by using Adsorbents: A Review. Environ. Technol. Innov. 2018, 11, 187–240. [Google Scholar] [CrossRef]
- Eghbali-Arani, M.; Sobhani-Nasab, A.; Rahimi-Nasrabadi, M.; Pourmasoud, S. Green Synthesis and Characterization of SmVO4 Nanoparticles in the Presence of Carbohydrates as Capping Agents with Investigation of Visible-Light Photocatalytic Properties. J. Electron. Mater. 2018, 47, 3757–3769. [Google Scholar] [CrossRef]
- Zinatloo-Ajabshir, S.; Mortazavi-Derazkola, S.; Salavati-Niasari, M. Preparation, characterization and photocatalytic degradation of methyl violet pollutant of holmium oxide nanostructures prepared through a facile precipitation method. J. Mol. Liq. 2017, 231, 306–313. [Google Scholar] [CrossRef]
- Shon, J.-H.; Teets, T.S. Photocatalysis with Transition Metal Based Photosensitizers. Comments Inorg. Chem. 2020, 40, 53–85. [Google Scholar] [CrossRef]
- Xu, C.; Ravi Anusuyadevi, P.; Aymonier, C.; Luque, R.; Marre, S. Nanostructured materials for photocatalysis. Chem. Soc. Rev. 2019, 48, 3868–3902. [Google Scholar] [CrossRef]
- Luciani, G.; Imparato, C.; Vitiello, G. Photosensitive Hybrid Nanostructured Materials: The Big Challenges for Sunlight Capture. Catalysts 2020, 10, 103. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Yu, J.; Jaroniec, M. Graphene-based semiconductor photocatalysts. Chem. Soc. Rev. 2012, 41, 782–796. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, L.; Chen, Z.; Hu, J.; Li, S.; Wang, Z.; Liu, J.; Wang, X. Semiconductor heterojunction photocatalysts: Design, construction, and photocatalytic performances. Chem. Soc. Rev. 2014, 43, 5234. [Google Scholar] [CrossRef]
- Chen, X.; Shen, S.; Guo, L.; Mao, S.S. Semiconductor-based Photocatalytic Hydrogen Generation. Chem. Rev. 2010, 110, 6503–6570. [Google Scholar] [CrossRef]
- Hisatomi, T.; Kubota, J.; Domen, K. Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting. Chem. Soc. Rev. 2014, 43, 7520–7535. [Google Scholar] [CrossRef]
- Chen, Y.; Bai, X. A Review on Quantum Dots Modified g-C3N4-Based Photocatalysts with Improved Photocatalytic Activity. Catalysts 2020, 10, 142. [Google Scholar] [CrossRef]
- Zhang, L.; Ran, J.; Qiao, S.-Z.; Jaroniec, M. Characterization of semiconductor photocatalysts. Chem. Soc. Rev. 2019, 48, 5184–5206. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Castilla, C.; López-Ramón, M.V.; Fontecha-Cámara, M.Á.; Álvarez, M.A.; Mateus, L. Removal of Phenolic Compounds from Water Using Copper Ferrite Nanosphere Composites as Fenton Catalysts. Nanomaterials 2019, 9, 901. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Dai, K.; Zhang, J.; Lu, L.; Liang, C.; Geng, L.; Wang, Z.; Yuan, G.; Zhu, G. In situ controllable synthesis of novel surface plasmon resonance-enhanced Ag2WO4/Ag/Bi2MoO6 composite for enhanced and stable visible light photocatalyst. Appl. Surf. Sci. 2017, 391, 507–515. [Google Scholar] [CrossRef]
- Sobhani-Nasab, A.; Pourmasoud, S.; Ahmadi, F.; Wysokowski, M.; Jesionowski, T.; Ehrlich, H.; Rahimi-Nasrabadi, M. Synthesis and characterization of MnWO4/TmVO4 ternary nano-hybrids by an ultrasonic method for enhanced photocatalytic activity in the degradation of organic dyes. Mater. Lett. 2019, 238, 159–162. [Google Scholar] [CrossRef]
- Peymani-Motlagh, S.M.; Moeinian, N.; Rostami, M.; Fasihi-Ramandi, M.; Sobhani-Nasab, A.; Rahimi-Nasrabadi, M.; Eghbali-Arani, M.; Ganjali, M.R.; Jesionowski, T.; Ehrlich, H.; et al. Effect of Gd3+-, Pr3+- or Sm3+-substituted cobalt–zinc ferrite on photodegradation of methyl orange and cytotoxicity tests. J. Rare Earths 2019, 37, 1288–1295. [Google Scholar] [CrossRef]
- Gandomi, F.; Peymani-Motlagh, S.M.; Rostami, M.; Sobhani-Nasab, A.; Fasihi-Ramandi, M.; Eghbali-Arani, M.; Ahmadian, R.; Gholipour, N.; Rahimi-Nasrabadi, M.; Ganjali, M.R. Simple synthesis and characterization of Li0.5Fe2.5O4, LiMg0.5Fe2O4 and LiNi0.5Fe2O4, and investigation of their photocatalytic and anticancer properties on hela cells line. J. Mater. Sci. Mater. Electron. 2019, 30, 19691–19702. [Google Scholar] [CrossRef]
- Chiu, Y.-H.; Chang, T.-F.M.; Chen, C.-Y.; Sone, M.; Hsu, Y.-J. Mechanistic Insights into Photodegradation of Organic Dyes Using Heterostructure Photocatalysts. Catalysts 2019, 9, 430. [Google Scholar] [CrossRef]
- Wang, Q.; Nakabayashi, M.; Hisatomi, T.; Sun, S.; Akiyama, S.; Wang, Z.; Pan, Z.; Xiao, X.; Watanabe, T.; Yamada, T.; et al. Oxysulfide photocatalyst for visible-light-driven overall water splitting. Nat. Mater. 2019, 18, 827–832. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, X. Oxysulfide Semiconductors for Photocatalytic Overall Water Splitting with Visible Light. Angew. Chem. Int. Ed. 2019, 58, 15580–15582. [Google Scholar] [CrossRef]
- Hao, H.; Lang, X. Metal Sulfide Photocatalysis: Visible-Light-Induced Organic Transformations. ChemCatChem 2019, 11, 1378–1393. [Google Scholar] [CrossRef]
- Ahmed, M.; Xinxin, G. A review of metal oxynitrides for photocatalysis. Inorg. Chem. Front. 2016, 3, 578–590. [Google Scholar] [CrossRef]
- Polo, A.M.S.; Lopez-Peñalver, J.J.; Sánchez-Polo, M.; Rivera-Utrilla, J.; López-Ramón, M.V.; Rozalén, M. Halide removal from water using silver doped magnetic-microparticles. J. Environ. Manag. 2020, 253, 109731. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Janczarek, M.; Wei, Z.; Raja-Mogan, T.; Endo-Kimura, M.; Khedr, T.M.; Ohtani, B.; Kowalska, E. Morphology- and Crystalline Composition-Governed Activity of Titania-Based Photocatalysts: Overview and Perspective. Catalysts 2019, 9, 1054. [Google Scholar] [CrossRef]
- Melinte, V.; Stroea, L.; Chibac-Scutaru, A.L. Polymer Nanocomposites for Photocatalytic Applications. Catalysts 2019, 9, 986. [Google Scholar] [CrossRef]
- Kar, A.; Sain, S.; Kundu, S.; Bhattacharyya, A.; Kumar Pradhan, S.; Patra, A. Influence of Size and Shape on the Photocatalytic Properties of SnO2 Nanocrystals. ChemPhysChem 2015, 16, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.F.S.; Gouveia, A.F.; Assis, M.; de Oliveira, R.C.; Pinatti, I.M.; Penha, M.; Gonçalves, R.F.; Gracia, L.; Andrés, J.; Longo, E. ZnWO4 nanocrystals: Synthesis, morphology, photoluminescence and photocatalytic properties. Phys. Chem. Chem. Phys. 2018, 20, 1923–1937. [Google Scholar] [CrossRef]
- Zhang, W. Preparation, morphology, size quantization effect and photocatalytic properties of CdS Q-nanocrystals. Sci. China Ser. B 2003, 46, 196–206. [Google Scholar] [CrossRef]
- Li, Y.-F.; Liu, Z.-P. Particle Size, Shape and Activity for Photocatalysis on Titania Anatase Nanoparticles in Aqueous Surroundings. J. Am. Chem. Soc. 2011, 133, 15743–15752. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Tian, X.; Nie, Y.; Zhou, Z.; Yang, C.; Li, Y.; Lu, L. Oxygen Vacancy Promoted Heterogeneous Fenton-like Degradation of Ofloxacin at pH 3.2–9.0 by Cu Substituted Magnetic Fe3O4@FeOOH Nanocomposite. Environ. Sci. Technol. 2017, 51, 12699–12706. [Google Scholar] [CrossRef]
- Lu, Z.; Zhao, X.; Zhu, Z.; Yan, Y.; Shi, W.; Dong, H.; Ma, Z.; Gao, N.; Wang, Y.; Huang, H. Enhanced Recyclability, Stability, and Selectivity of CdS/C@Fe3O4 Nanoreactors for Orientation Photodegradation of Ciprofloxacin. Chem. Eur. J. 2015, 21, 18528–18533. [Google Scholar] [CrossRef]
- Hou, X.; Wang, X.; Mi, W. Progress in Fe3O4-based multiferroic heterostructures. J. Alloys Compd. 2018, 765, 1127–1138. [Google Scholar] [CrossRef]
- Mu, Q.; Sun, Y.; Guo, A.; Yu, X.; Xu, X.; Cai, A.; Wang, X. Bio-templated synthesis of Fe3O4–TiO2 composites derived from Chlorella pyrenoidosa with enhanced visible-light photocatalytic performance. Mater. Res. Express 2019, 6, 0950c3. [Google Scholar] [CrossRef]
- Mishra, P.; Patnaik, S.; Parida, K. An overview of recent progress on noble metal modified magnetic Fe3O4 for photocatalytic pollutant degradation and H2 evolution. Catal. Sci. Technol. 2019, 9, 916–941. [Google Scholar] [CrossRef]
- Amin Marsooli, M.; Rahimi Nasrabadi, M.; Fasihi-Ramandi, M.; Adib, K.; Eghbali, M.; Pourmasoud, S.; Ahmadi, F.; Sohouli, E.; Sobhani Nasab, A.; Ali Mirhosseini, S.; et al. Preparation of Fe3O4/SiO2/TiO2/PrVO4 nanocomposite in various molar ratios: Investigation on photocatalytic performance on organic contaminate and bacterial environments, and anti-cancer properties. Polyhedron 2020, 176, 114239. [Google Scholar] [CrossRef]
- Ke, J.; Adnan Younis, M.; Kong, Y.; Zhou, H.; Liu, J.; Lei, L.; Hou, Y. Nanostructured Ternary Metal Tungstate-Based Photocatalysts for Environmental Purification and Solar Water Splitting: A Review. Nano-Micro Lett. 2018, 10, 69. [Google Scholar] [CrossRef]
- García-Pérez, U.M.; de la Cruz, A.M.; Peral, J. Transition metal tungstates synthesized by co-precipitation method: Basic photocatalytic properties. Electrochim. Acta 2012, 81, 227–232. [Google Scholar] [CrossRef]
- Peng, W.; Li, Y.; Zhang, F.; Zhang, G.; Fan, X. Roles of Two-Dimensional Transition Metal Dichalcogenides as Cocatalysts in Photocatalytic Hydrogen Evolution and Environmental Remediation. Ind. Eng. Chem. Res. 2017, 56, 4611–4626. [Google Scholar] [CrossRef]
- Zawawi, S.M.M.; Yahya, R.; Hassan, A.; Mahmud, H.E.; Daud, M.N. Structural and optical characterization of metal tungstates (MWO4; M=Ni, Ba, Bi) synthesized by a sucrose-templated method. Chem. Cent. J. 2013, 7, 80. [Google Scholar] [CrossRef]
- Kuzmin, A.; Kalendarev, R.; Kursitis, A.; Purans, J. Confocal spectromicroscopy of amorphous and nanocrystalline tungsten oxide films. J. Non-Cryst. Solids 2007, 353, 1840–1843. [Google Scholar] [CrossRef]
- Hosseinpour-Mashkani, S.M.; Maddahfar, M.; Sobhani-Nasab, A. Precipitation Synthesis, Characterization, Morphological Control, and Photocatalyst Application of ZnWO4 Nanoparticles. J. Electron. Mater. 2016, 45, 3612–3620. [Google Scholar] [CrossRef]
- Hosseinpour-Mashkani, S.S.; Sobhani-Nasab, A. Investigation the effect of temperature and polymeric capping agents on the size and photocatalytic properties of NdVO4 nanoparticles. J. Mater. Sci. Mater. Electron. 2017, 28, 16459–16466. [Google Scholar] [CrossRef]
- Tedstone, A.A.; Lewis, D.J.; O’Brien, P. Synthesis, Properties, and Applications of Transition Metal-Doped Layered Transition Metal Dichalcogenides. Chem. Mater. 2016, 28, 1965–1974. [Google Scholar] [CrossRef]
- Lv, R.; Robinson, J.A.; Schaak, R.E.; Sun, D.; Sun, Y.; Mallouk, T.E.; Terrones, M. Transition Metal Dichalcogenides and Beyond: Synthesis, Properties, and Applications of Single- and Few-Layer Nanosheets. Acc. Chem. Res. 2015, 48, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Pastora, J.; Dominguez, S.; Bringas, E.; Rivero, M.J.; Ortiz, I.; Dionysiou, D.D. Review and perspectives on the use of magnetic nanophotocatalysts (MNPCs) in water treatment. Chem. Eng. J. 2017, 310, 407–427. [Google Scholar] [CrossRef]
- Lopez Maldonado, K.L.; de la Presa, P.; de la Rubia, M.A.; Crespo, P.; de Frutos, J.; Hernando, A.; Matutes Aquino, J.A.; Elizalde Galindo, J.T. Effects of grain boundary width and crystallite size on conductivity and magnetic properties of magnetite nanoparticles. J. Nanoparticle Res. 2014, 16, 2482. [Google Scholar] [CrossRef]
- Smilgies, D.-M. Scherrer grain-size analysis adapted to grazing-incidence scattering with area detectors. J. Appl. Crystallogr. 2009, 42, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- Zinatloo-Ajabshir, S.; Salavati-Niasari, M. Preparation and characterization of nanocrystalline praseodymium oxide via a simple precipitation approach. J. Mater. Sci. Mater. Electron. 2015, 26, 5812–5821. [Google Scholar] [CrossRef]
- Monshi, A.; Foroughi, M.R.; Monshi, M.R. Modified Scherrer Equation to Estimate More Accurately Nano-Crystallite Size Using XRD. World J. Nano Sci. Eng. 2012, 2, 154–160. [Google Scholar] [CrossRef]
- Umbrello, D.; Rotella, G.; Matsumura, T.; Musha, Y. Evaluation of microstructural changes by X-ray diffraction peak profile and focused ion beam/scanning ion microscope analysis. Int. J. Adv. Manuf. Technol. 2015, 77, 1465–1474. [Google Scholar] [CrossRef]
- Zou, Z.; Xuan, A.G.; Yan, Z.G.; Wu, Y.X.; Li, N. Preparation of Fe3O4 particles from copper/iron ore cinder and their microwave absorption properties. Chem. Eng. Sci. 2010, 65, 160–164. [Google Scholar] [CrossRef]
- Marsooli, M.A.; Fasihi-Ramandi, M.; Adib, K.; Pourmasoud, S.; Ahmadi, F.; Ganjali, M.R.; Sobhani Nasab, A.; Rahimi Nasrabadi, M.; Plonska-Brzezinska, M.E. Preparation and Characterization of Magnetic Fe3O4/CdWO4 and Fe3O4/CdWO4/PrVO4 Nanoparticles and Investigation of Their Photocatalytic and Anticancer Properties on PANC1 Cells. Materials 2019, 12, 3274. [Google Scholar] [CrossRef] [PubMed]
- Kalai Selvan, R.; Gedanken, A.; Anilkumar, P.; Manikandan, G.; Karunakaran, C. Synthesis and Characterization of Rare Earth Orthovanadate (RVO4; R = La, Ce, Nd, Sm, Eu & Gd) Nanorods/Nanocrystals/Nanospindles by a Facile Sonochemical Method and Their Catalytic Properties. J. Clust. Sci. 2009, 20, 291–305. [Google Scholar]
- Ekthammathat, N.; Thongtem, T.; Phuruangrat, A.; Thongtem, S. Synthesis and Characterization of CeVO4 by Microwave Radiation Method and Its Photocatalytic Activity. J. Nanomater. 2013, 2013, 1–7. [Google Scholar]
- Adib, K.; Rezvani, Z.; Rahimi-Nasrabadi, M.; Pourmortazavi, S.M. Statistically optimized synthesis of cadmium tungstate nanoplates for use as a photocatalyst. J. Mater. Sci. Mater. Electron. 2018, 29, 6377–6387. [Google Scholar] [CrossRef]
- Beshkar, F.; Zinatloo-Ajabshir, S.; Salavati-Niasari, M. Preparation and characterization of the CuCr2O4 nanostructures via a new simple route. J. Mater. Sci. Mater. Electron. 2015, 26, 5043–5051. [Google Scholar] [CrossRef]
- Deotale, A.J.; Nandedkar, R.V. Correlation between Particle Size, Strain and Band Gap of Iron Oxide Nanoparticles. Mater. Today Proc. 2016, 3, 2069–2076. [Google Scholar] [CrossRef]
- Xi, G.; Yue, B.; Cao, J.; Ye, J. Fe3O4/WO3 Hierarchical Core-Shell Structure: High-Performance and Recyclable Visible-Light Photocatalysis. Chem. Eur. J. 2011, 17, 5145–5154. [Google Scholar] [CrossRef]
- Yang, S.; Zeng, T.; Li, Y.; Liu, J.; Chen, Q.; Zhou, J.; Ye, Y.; Tang, B. Preparation of Graphene Oxide Decorated Fe3O4@SiO2 Nanocomposites with Superior Adsorption Capacity and SERS Detection for Organic Dyes. J. Nanomater. 2015, 2015, 1–8. [Google Scholar]
- Rahimi-Nasrabadi, M.; Pourmortazavi, S.M.; Ganjali, M.R.; Hajimirsadeghi, S.S.; Zahedi, M.M. Electrosynthesis and characterization of zinc tungstate nanoparticles. J. Mol. Struct. 2013, 1047, 31–36. [Google Scholar] [CrossRef]
- Sadiq Mohamed, M.J.; Bhat Denthaje, K. Novel RGO-ZnWO4-Fe3O4 Nanocomposite as an Efficient Catalyst for Rapid Reduction of 4-Nitrophenol to 4-Aminophenol. Ind. Eng. Chem. Res. 2016, 55, 7267–7272. [Google Scholar] [CrossRef]
- Rawtani, D.; Khatri, N.; Tyagi, S.; Pandey, G. Nanotechnology-based recent approaches for sensing and remediation of pesticides. J. Environ. Manag. 2018, 206, 749–762. [Google Scholar] [CrossRef] [PubMed]
- Topalov, A.; Molnár-Gábor, D.; Abramović, B.; Korom, S.; Peričin, D. Photocatalytic removal of the insecticide fenitrothion from water sensitized with TiO2. J. Photochem. Photobiol. Chem. 2003, 160, 195–201. [Google Scholar] [CrossRef]
- Vela, N.; Calín, M.; Yáñez-Gascón, M.J.; Garrido, I.; Pérez-Lucas, G.; Fenoll, J.; Navarro, S. Photocatalytic oxidation of six pesticides listed as endocrine disruptor chemicals from wastewater using two different TiO2 samples at pilot plant scale under sunlight irradiation. J. Photochem. Photobiol. Chem. 2018, 353, 271–278. [Google Scholar] [CrossRef]
- Ahmed, S.; Rasul, M.G.; Brown, R.; Hashib, M.A. Influence of parameters on the heterogeneous photocatalytic degradation of pesticides and phenolic contaminants in wastewater: A short review. J. Environ. Manag. 2011, 92, 311–330. [Google Scholar] [CrossRef]
- Shifu, C.; Lei, J.; Wenming, T.; Xianliang, F. Fabrication, characterization and mechanism of a novel Z-scheme photocatalyst NaNbO3/WO3 with enhanced photocatalytic activity. Dalton Trans. 2013, 42, 10759. [Google Scholar] [CrossRef]
- Ishibashi, K.; Fujishima, A.; Watanabe, T.; Hashimoto, K. Detection of active oxidative species in TiO2 photocatalysis using the fluorescence technique. Electrochem. Commun. 2000, 2, 207–210. [Google Scholar] [CrossRef]
- Ibrahim, A.M.A.; Al-Ashqar, S.M.A. Spectroscopic and kinetic studies on the degradation of methylene blue using the supramolecular coordination polymer [(Ph3Sn)4Fe(CN)6] as catalyst. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2012, 92, 238–244. [Google Scholar] [CrossRef]
- Choi, K.-H.; Oh, S.-L.; Kim, D.-Y.; Jung, J.-S. Size Dependent Photocatalytic Activity of Photofunctional Magnetic Core–Shell (Fe3O4@ TiO2) Particles. J. Nanosci. Nanotechnol. 2013, 13, 7134–7137. [Google Scholar] [CrossRef]
- Zhou, Y.X.; Tong, L.; Chen, X.B.; Zeng, X.H. Fe3O4-ZnWO4 hybrid microspheres: Facile synthesis and magnetically recyclable photocatalytic performance. Micro-Nano Lett. 2013, 8, 32–34. [Google Scholar] [CrossRef]
- Rajamohan, S.; Kumaravel, V.; Muthuramalingam, R.; Ayyadurai, S.; Abdel-Wahab, A.; Sub Kwak, B.; Kang, M.; Sreekantan, S. Fe3O4-Ag2WO4: Facile synthesis, characterization and visible light assisted photocatalytic activity. New J. Chem. 2017, 41, 11722–11730. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, F.; Gao, Y.; Liu, Y.; Fang, P.; Wang, S. A novel synthetic route for magnetically retrievable Bi2WO6 hierarchical microspheres with enhanced visible photocatalytic performance. J. Mater. Chem. A 2013, 1, 7027. [Google Scholar] [CrossRef]
- Zhou, Y.-X.; Tong, L.; Zeng, X.-H.; Chen, X.-B. Fe3O4@Bi2WO6 Core–Shell Structured Microspheres: Facile Construction and Magnetically Recyclable Photocatalytic Activity Under Visible-Light. J. Nanosci. Nanotechnol. 2015, 15, 9868–9873. [Google Scholar] [CrossRef] [PubMed]
- Karunakaran, C.; Vinayagamoorthy, P.; Jayabharathi, J. Nonquenching of Charge Carriers by Fe3O4 Core in Fe3O4/ZnO Nanosheet Photocatalyst. Langmuir 2014, 30, 15031–15039. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, W.-N.; Zhao, M.-L.; Zhang, C.-Y.; Huang, C.-X.; Cheng, S.; Xu, H.-M.; Qian, H.-S. Magnetically Recyclable Fe3O4@ZnxCd1–xS Core–Shell Microspheres for Visible Light-Mediated Photocatalysis. Langmuir 2018, 34, 9264–9271. [Google Scholar] [CrossRef] [PubMed]
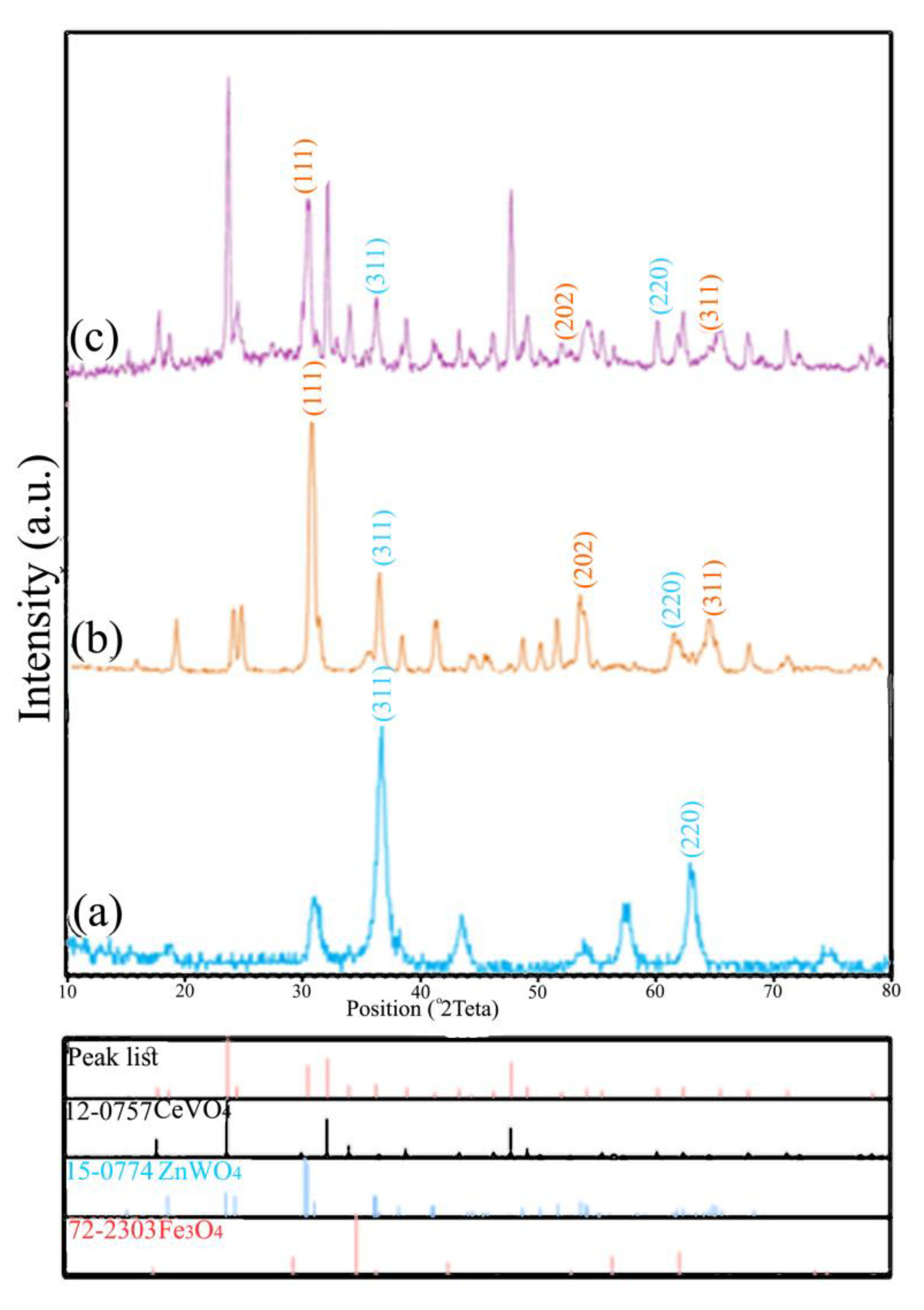

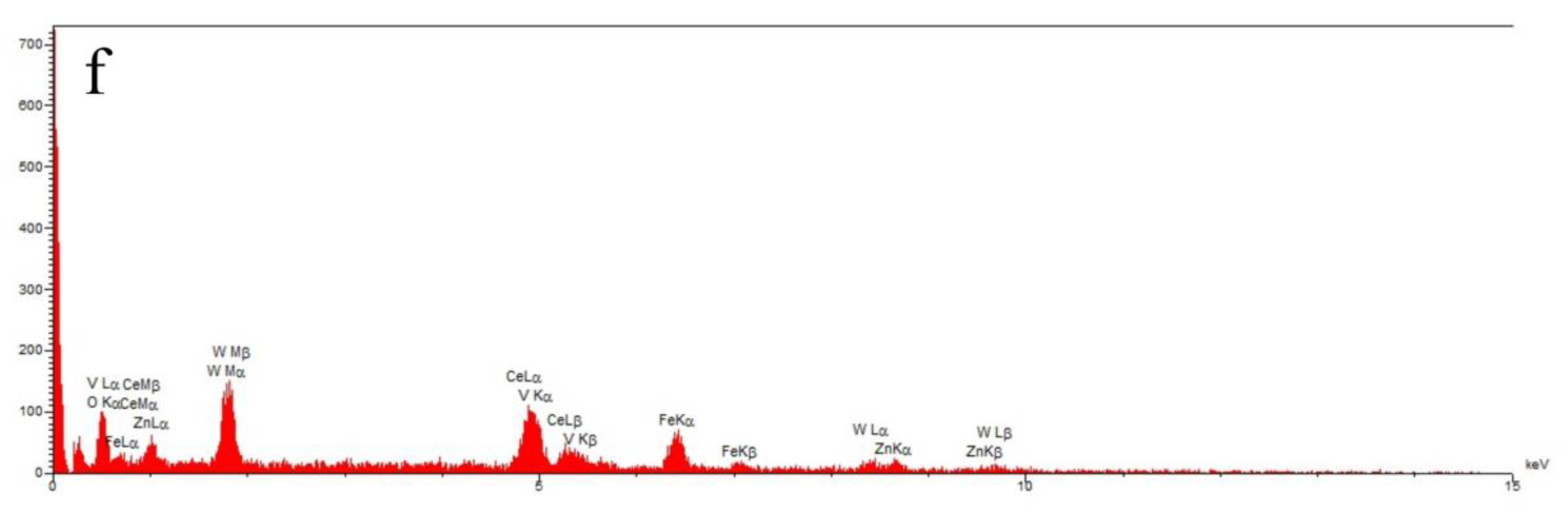
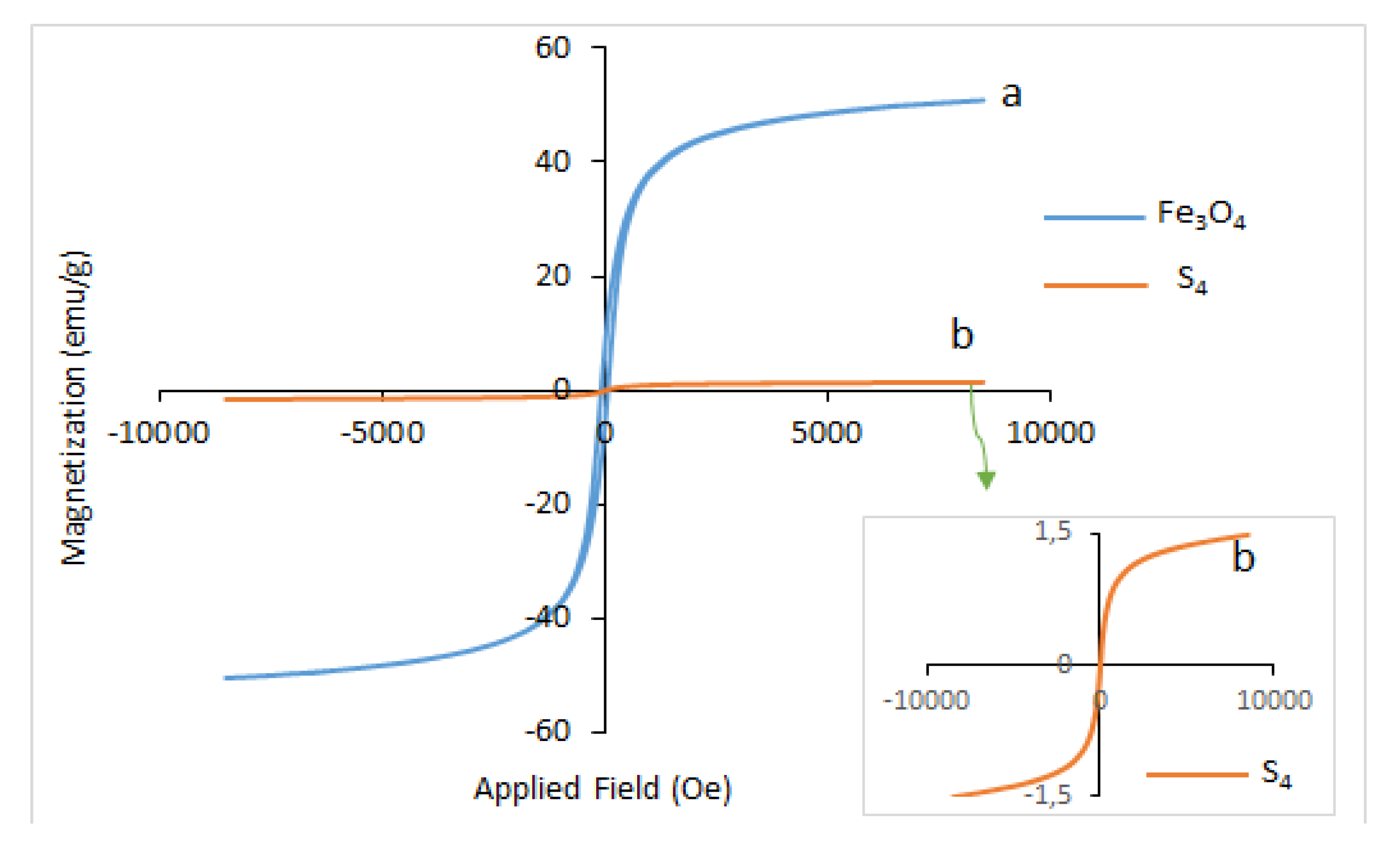
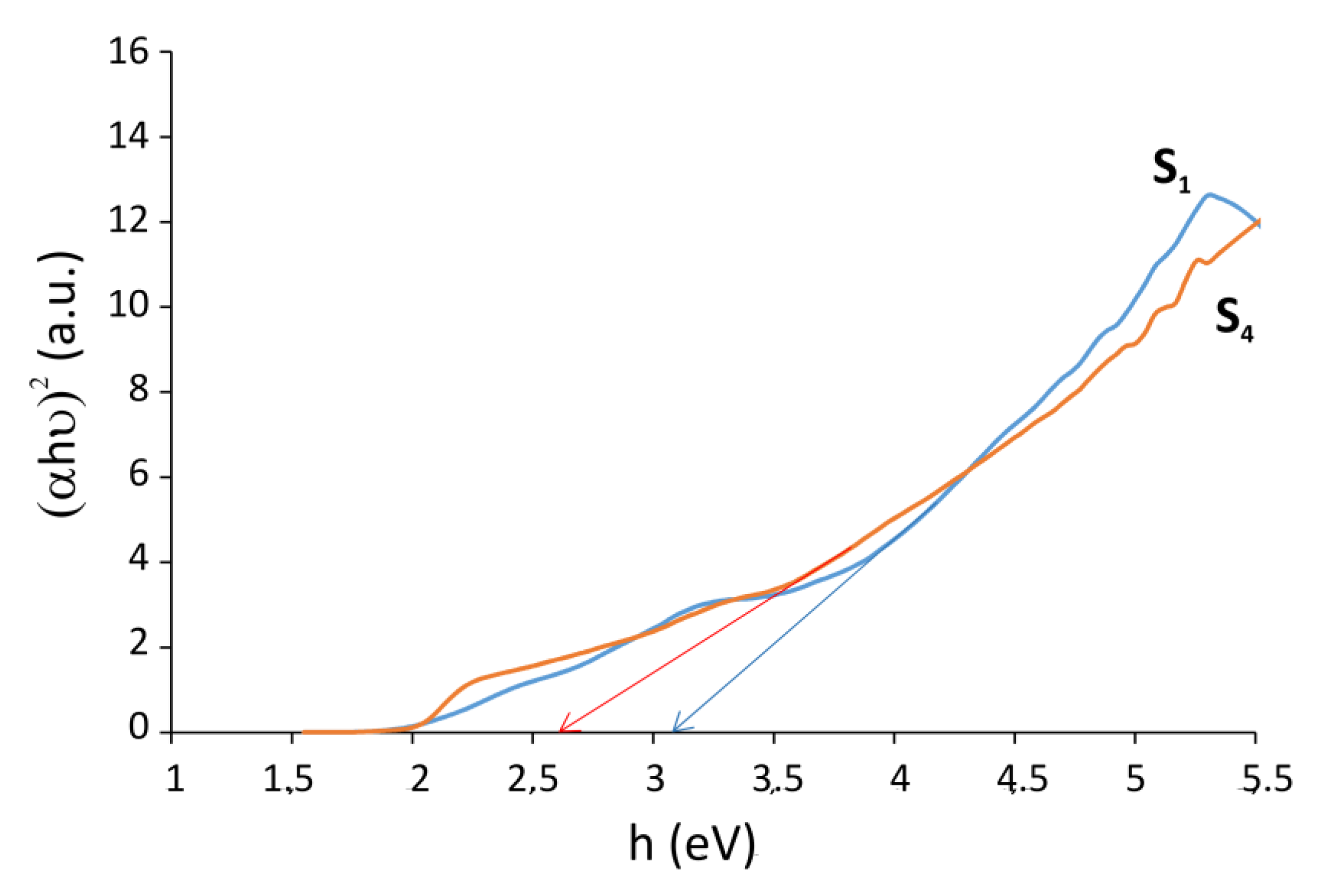
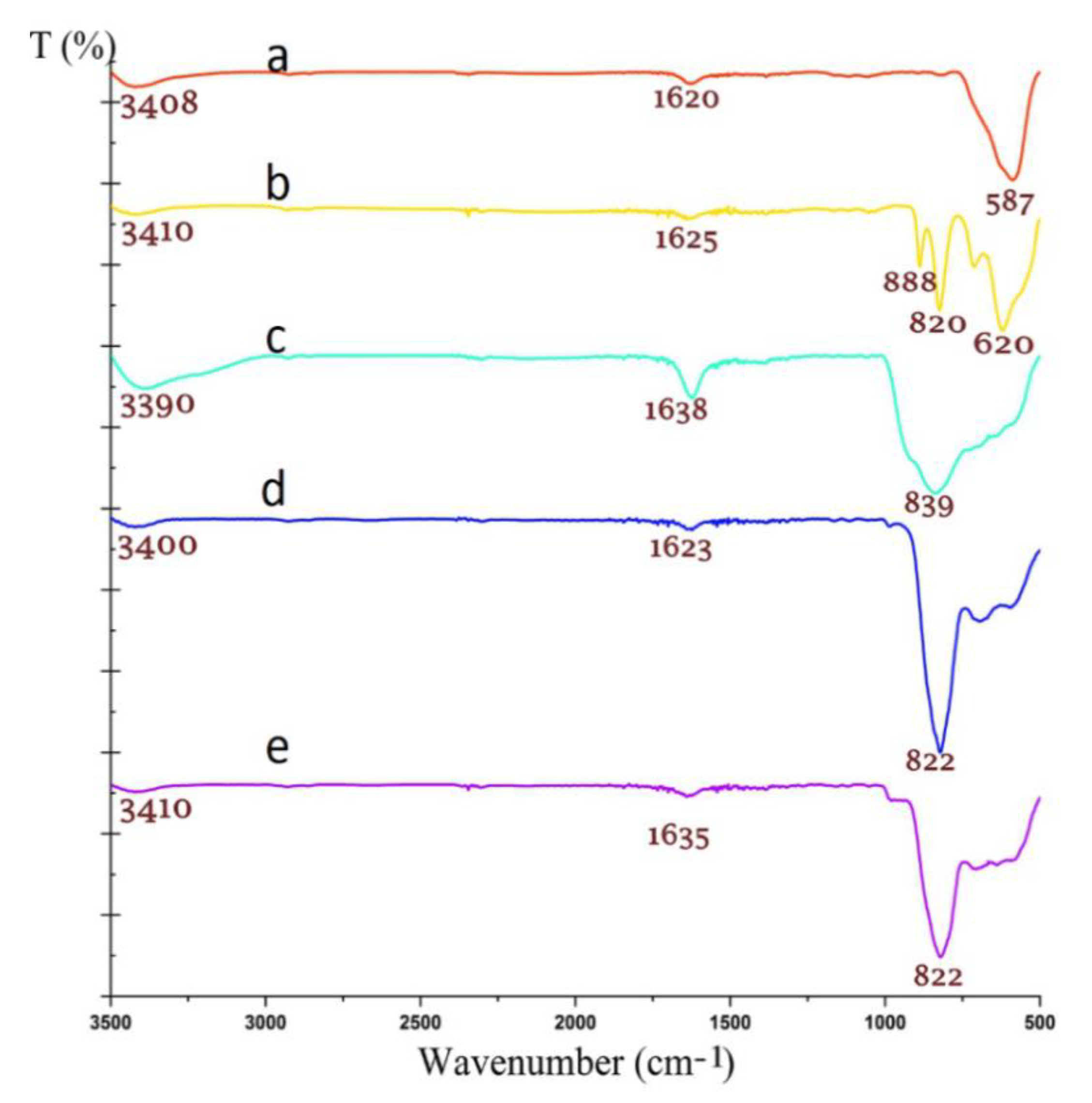
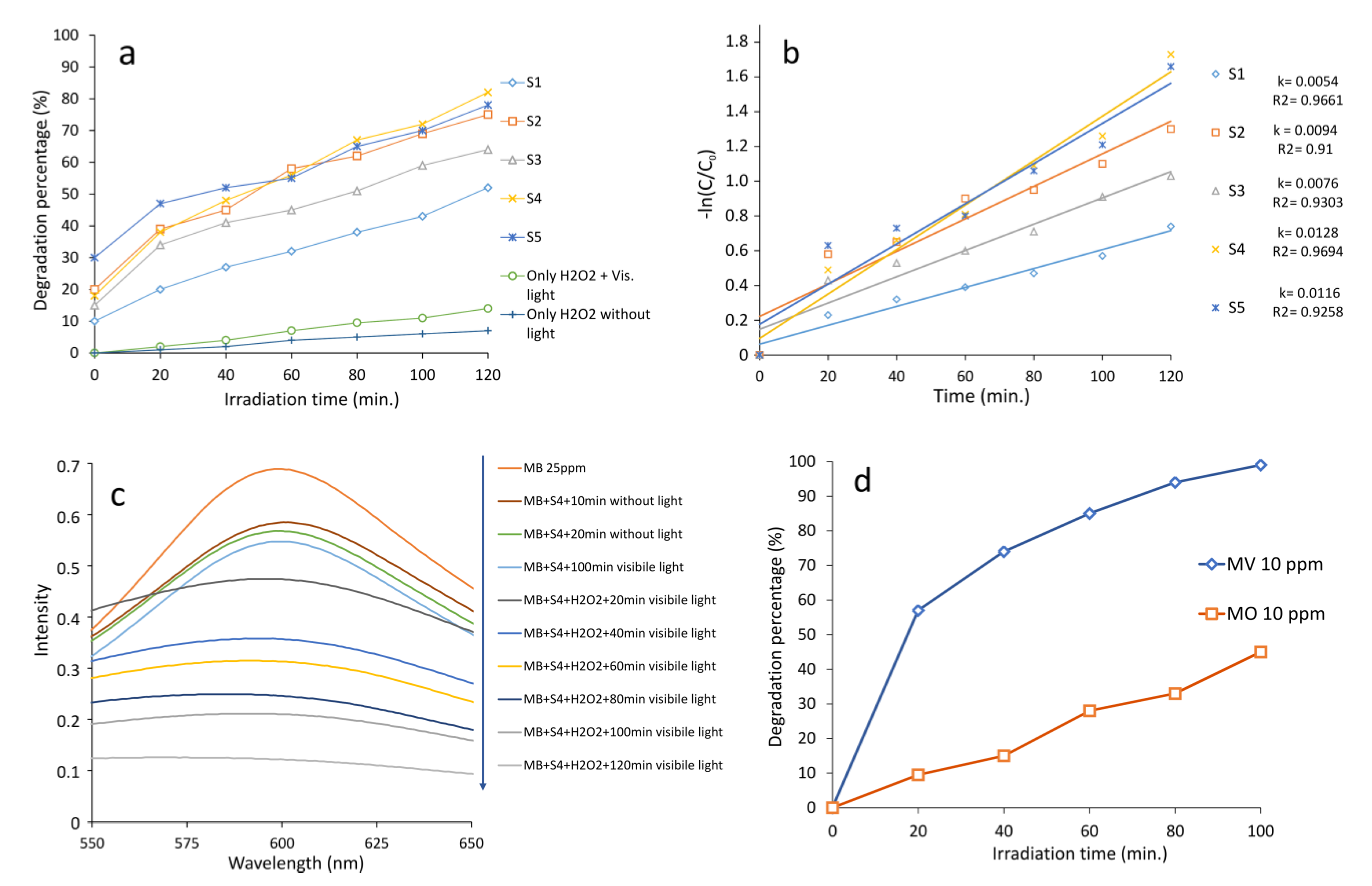

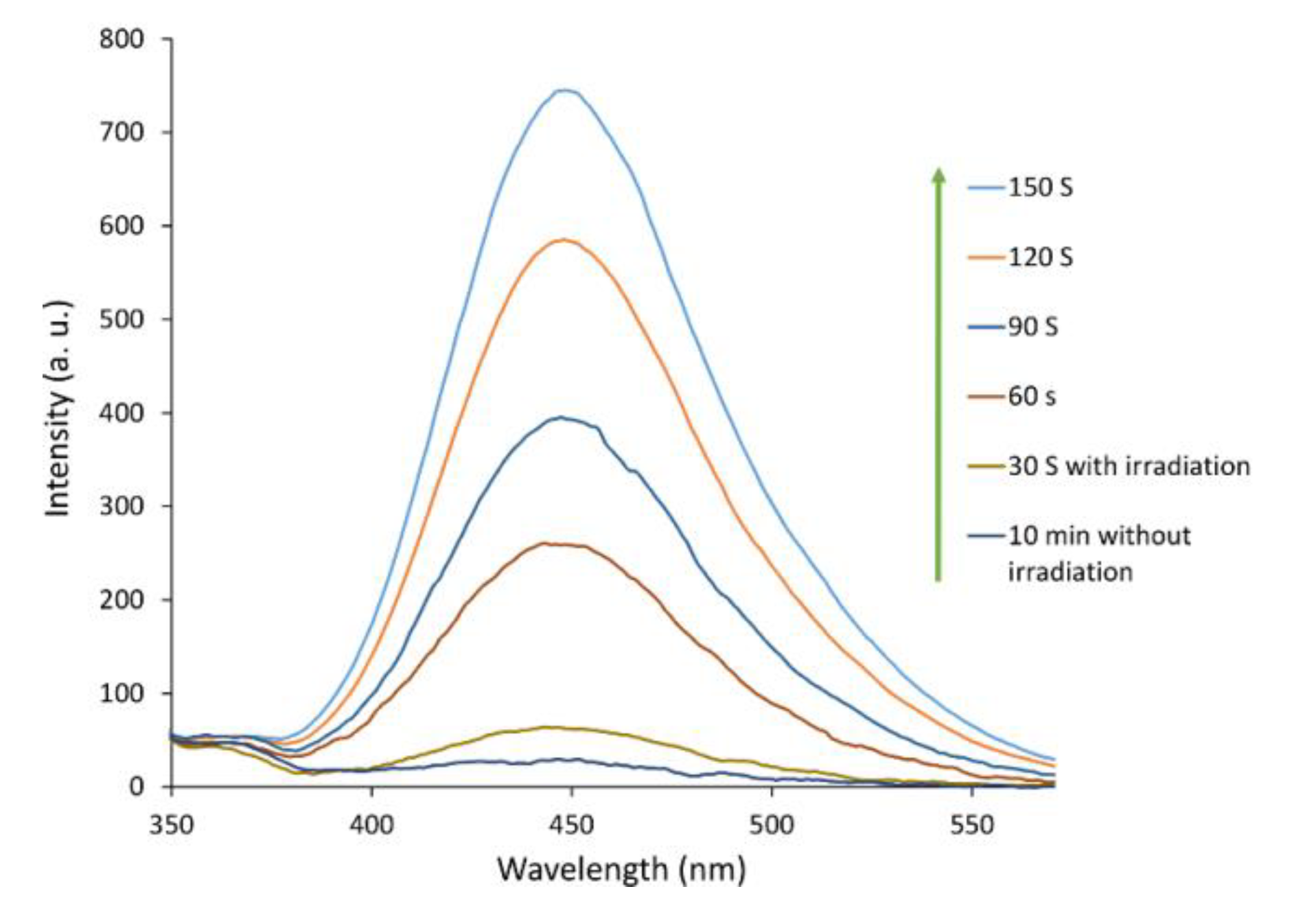

| Sample | 2 Theta (Degrees) | hkl | Crystallite Sizes Dhkl (nm) | Average Crystallite Size (nm) | A (Å) | B (Å) | C (Å) |
|---|---|---|---|---|---|---|---|
| Fe3O4 | 35.6 | (311) | 15.8 | 0.15 | 8.3740 | 8.3740 | 8.3740 |
| 63.1 | (220) | 14.3 | |||||
| Fe3O4/ZnWO4 | 35.6 | (311) | 16.33 | 36.3 | 4.6910 | 5.7200 | 4.9250 |
| 63.1 | (220) | 15.40 | |||||
| 30.47 | (111) | 12.60 | |||||
| 53.63 | (202) | 13.62 | |||||
| 64.78 | (311) | 14.79 | |||||
| Fe3O4/ZnWO4/CeVO4 | 35.6 | (311) | 16.68 | 58.7 | 7.3990 | 7.3990 | 6.4960 |
| 63.1 | (220) | 16.01 | |||||
| 30.47 | (111) | 14.02 | |||||
| 53.63 | (202) | 15.32 | |||||
| 64.78 | (311) | 16.14 | |||||
| 24.03 | (200) | 14.63 | |||||
| 32.40 | (112) | 12.67 | |||||
| 47.86 | (322) | 11.96 |
| Samples | Light Source | Pollutant | Degradation (%) | The Amount of Catalyst Used (g/100 mL) | Time (min) | Ref. |
|---|---|---|---|---|---|---|
| Fe3O4/TiO2 (120 nm) | UV | RhB | 5.4 | 0.1 | 50 | [79] |
| Fe3O4/TiO2 (420 nm) | UV | RhB | 25.8 | 0.1 | 50 | [79] |
| Fe3O4/ZnWO4 | UV | RhB | 100 | 0.1 | 300 | [80] |
| Fe3O4/Ag2WO4 | Vis. | Fast green | 81 | - | 120 | [81] |
| Fe3O4/CdWO4 | Vis. | MB | 32 | 0.02 | 120 | [62] |
| Fe3O4/CdWO4/PrVO4 | Vis. | MB | 68 | 0.02 | 120 | [62] |
| Bi2WO6 | Vis. | RhB | 62.5 | - | 180 | [82] |
| Fe3O4/Bi2WO6 | Vis. | RhB | 95.8 | - | 180 | [82] |
| Fe3O4/Bi2WO6 | Vis. | RhB | 66 | 0.1 | 180 | [83] |
| Fe3O4/SiO2/Bi2WO6 | Vis. | RhB | 100 | - | 100 | [82] |
| ZnO | UV | MB | 80 | 0.01 | 60 | [84] |
| Fe3O4/ZnO | UV | MB | 94 | 0.01 | 60 | [84] |
| Fe3O4/ZnxCd1-cS | Vis. | MB | 92 | 0.01 | 35 | [85] |
| Fe3O4/ZnWO4/CeVO4 | Vis | MB | 84 | 0.02 | 120 | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amin Marsooli, M.; Rahimi Nasrabadi, M.; Fasihi-Ramandi, M.; Adib, K.; Pourmasoud, S.; Ahmadi, F.; Eghbali, M.; Sobhani Nasab, A.; Tomczykowa, M.; Plonska-Brzezinska, M.E. Synthesis of Magnetic Fe3O4/ZnWO4 and Fe3O4/ZnWO4/CeVO4 Nanoparticles: The Photocatalytic Effects on Organic Pollutants upon Irradiation with UV-Vis Light. Catalysts 2020, 10, 494. https://doi.org/10.3390/catal10050494
Amin Marsooli M, Rahimi Nasrabadi M, Fasihi-Ramandi M, Adib K, Pourmasoud S, Ahmadi F, Eghbali M, Sobhani Nasab A, Tomczykowa M, Plonska-Brzezinska ME. Synthesis of Magnetic Fe3O4/ZnWO4 and Fe3O4/ZnWO4/CeVO4 Nanoparticles: The Photocatalytic Effects on Organic Pollutants upon Irradiation with UV-Vis Light. Catalysts. 2020; 10(5):494. https://doi.org/10.3390/catal10050494
Chicago/Turabian StyleAmin Marsooli, Mohammad, Mehdi Rahimi Nasrabadi, Mahdi Fasihi-Ramandi, Kourosh Adib, Saeid Pourmasoud, Farhad Ahmadi, Mohammad Eghbali, Ali Sobhani Nasab, Monika Tomczykowa, and Marta E. Plonska-Brzezinska. 2020. "Synthesis of Magnetic Fe3O4/ZnWO4 and Fe3O4/ZnWO4/CeVO4 Nanoparticles: The Photocatalytic Effects on Organic Pollutants upon Irradiation with UV-Vis Light" Catalysts 10, no. 5: 494. https://doi.org/10.3390/catal10050494
APA StyleAmin Marsooli, M., Rahimi Nasrabadi, M., Fasihi-Ramandi, M., Adib, K., Pourmasoud, S., Ahmadi, F., Eghbali, M., Sobhani Nasab, A., Tomczykowa, M., & Plonska-Brzezinska, M. E. (2020). Synthesis of Magnetic Fe3O4/ZnWO4 and Fe3O4/ZnWO4/CeVO4 Nanoparticles: The Photocatalytic Effects on Organic Pollutants upon Irradiation with UV-Vis Light. Catalysts, 10(5), 494. https://doi.org/10.3390/catal10050494






