Overview of Recent Advances in Immobilisation Techniques for Phenol Oxidases in Solution
Abstract
1. Introduction
2. Recent Advances in Laccase and Tyrosinase Immobilisation and Insolubilisation Techniques
2.1. Adsorption/Noncovalent Bonding
2.1.1. Physical Adsorption
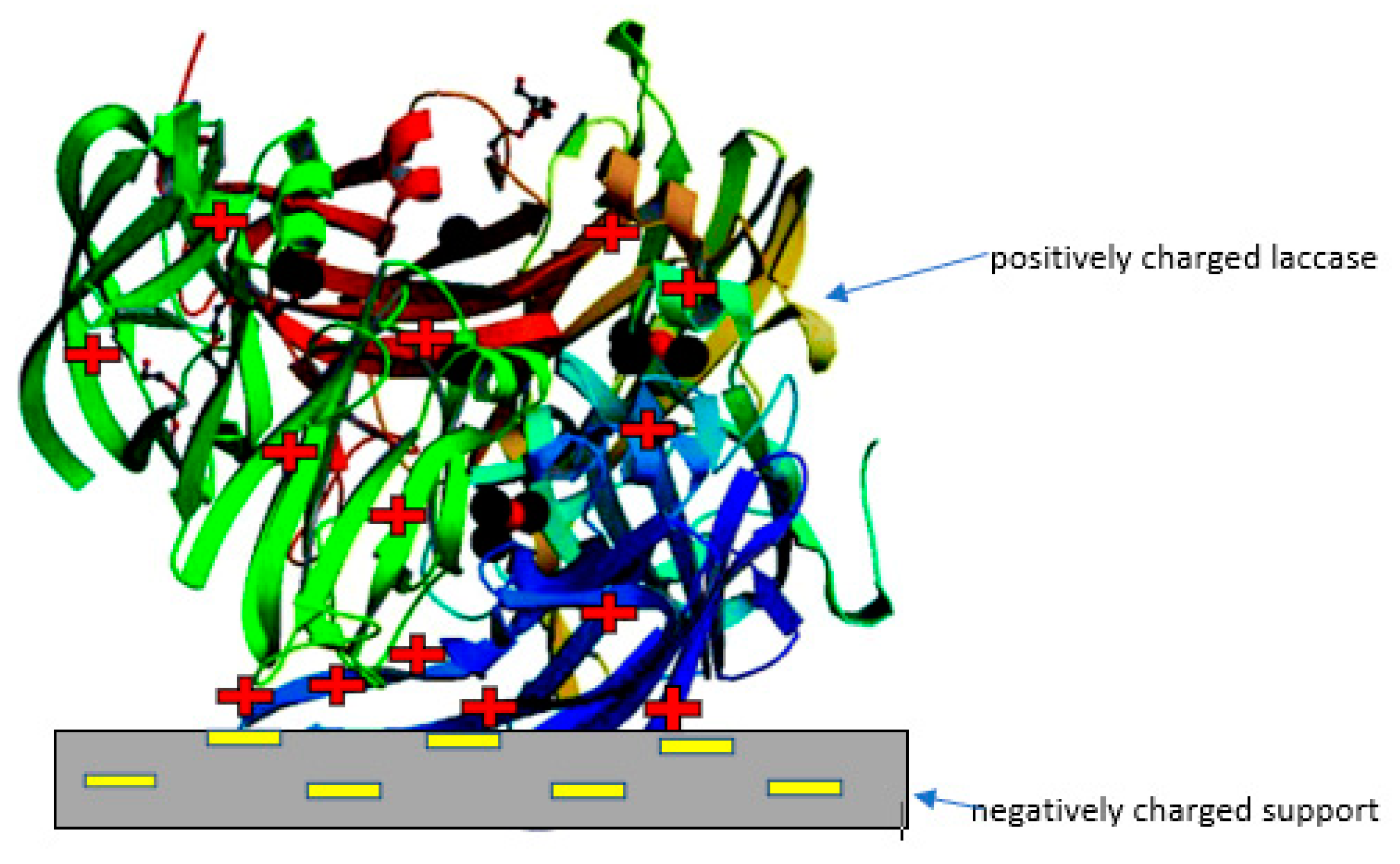
2.1.2. Electrostatic Interaction/Ionic Binding
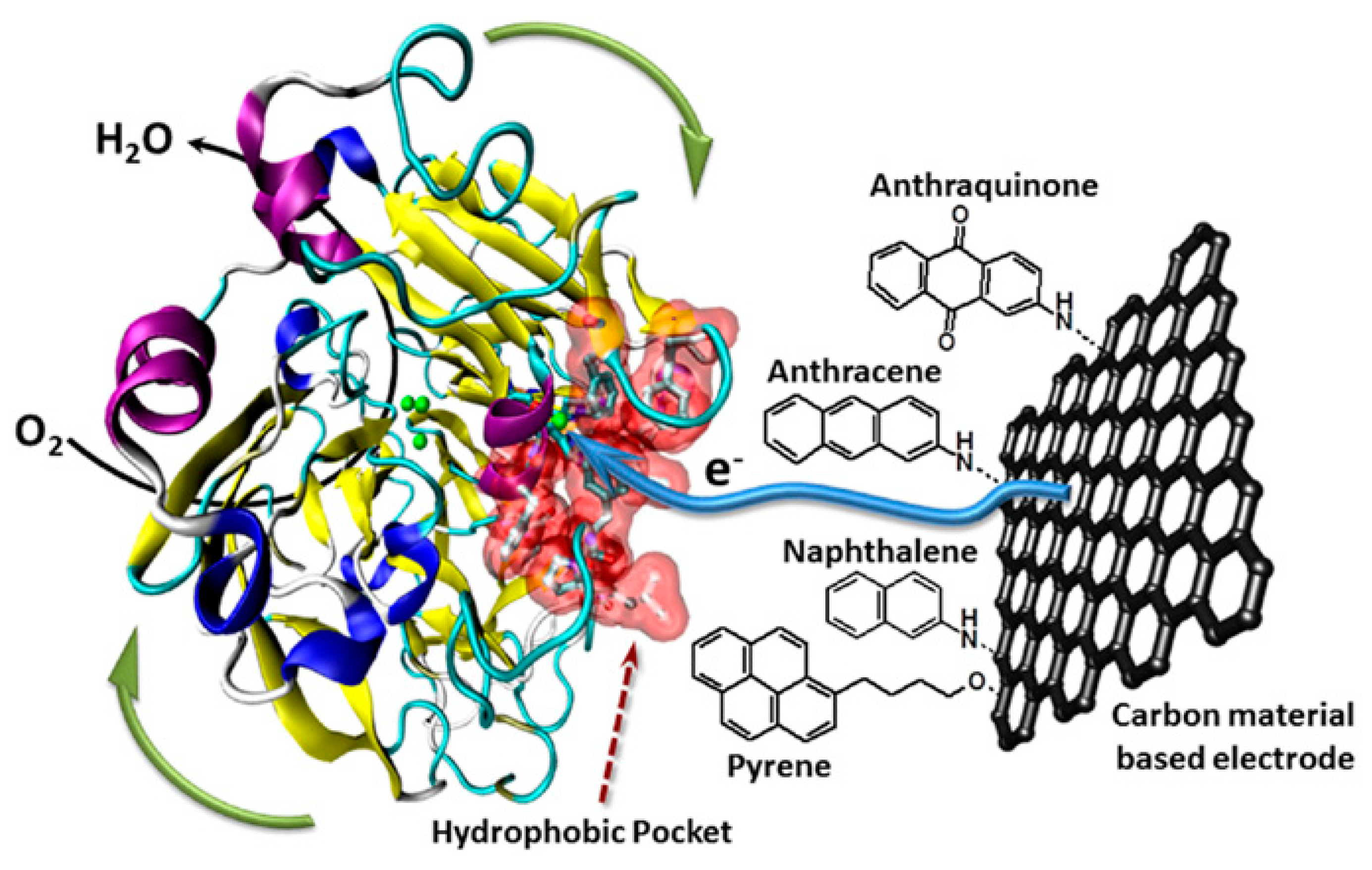
2.1.3. Hydrophobic Adsorption
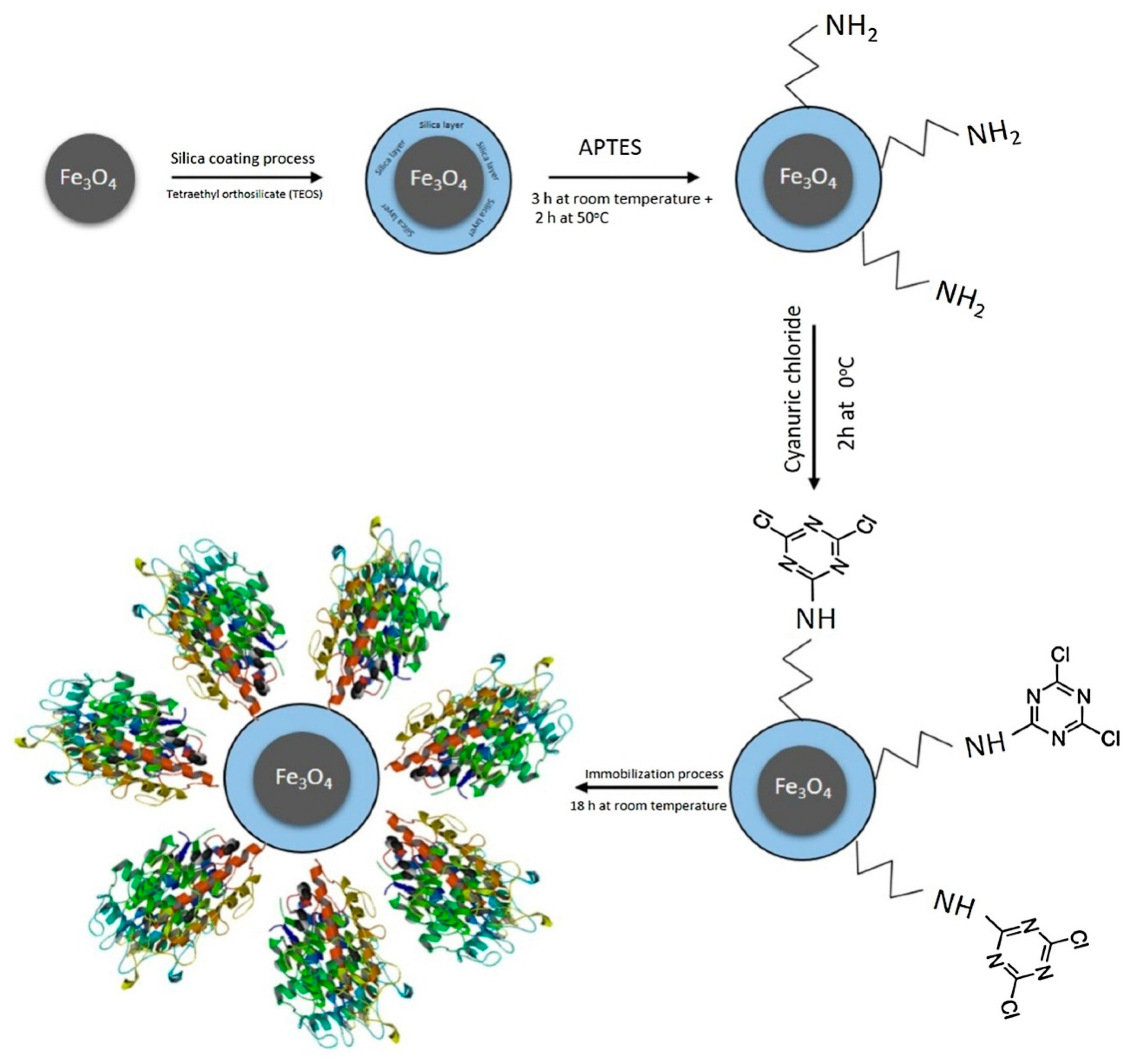
2.2. Covalent Bonding
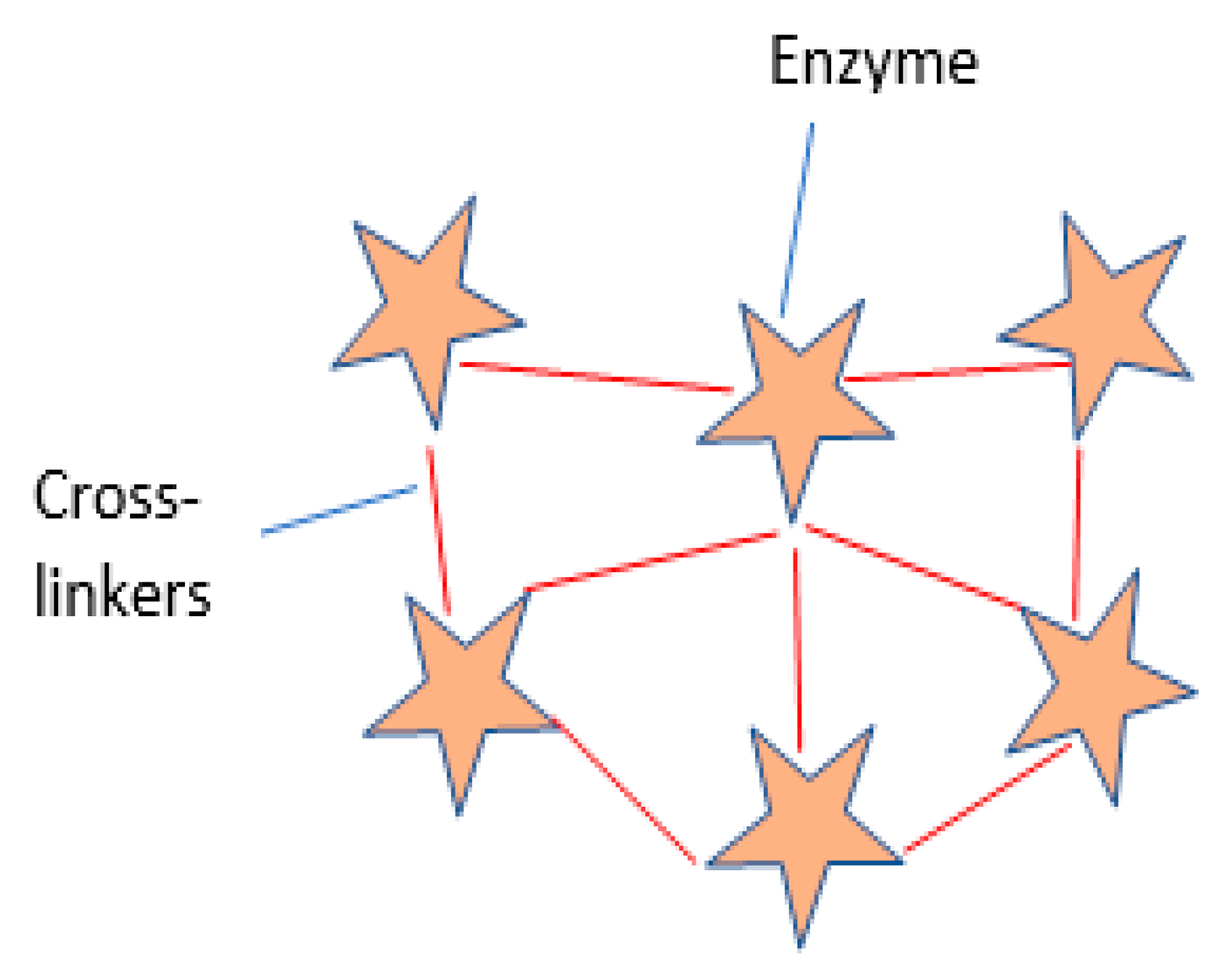
2.3. Cross-Linking
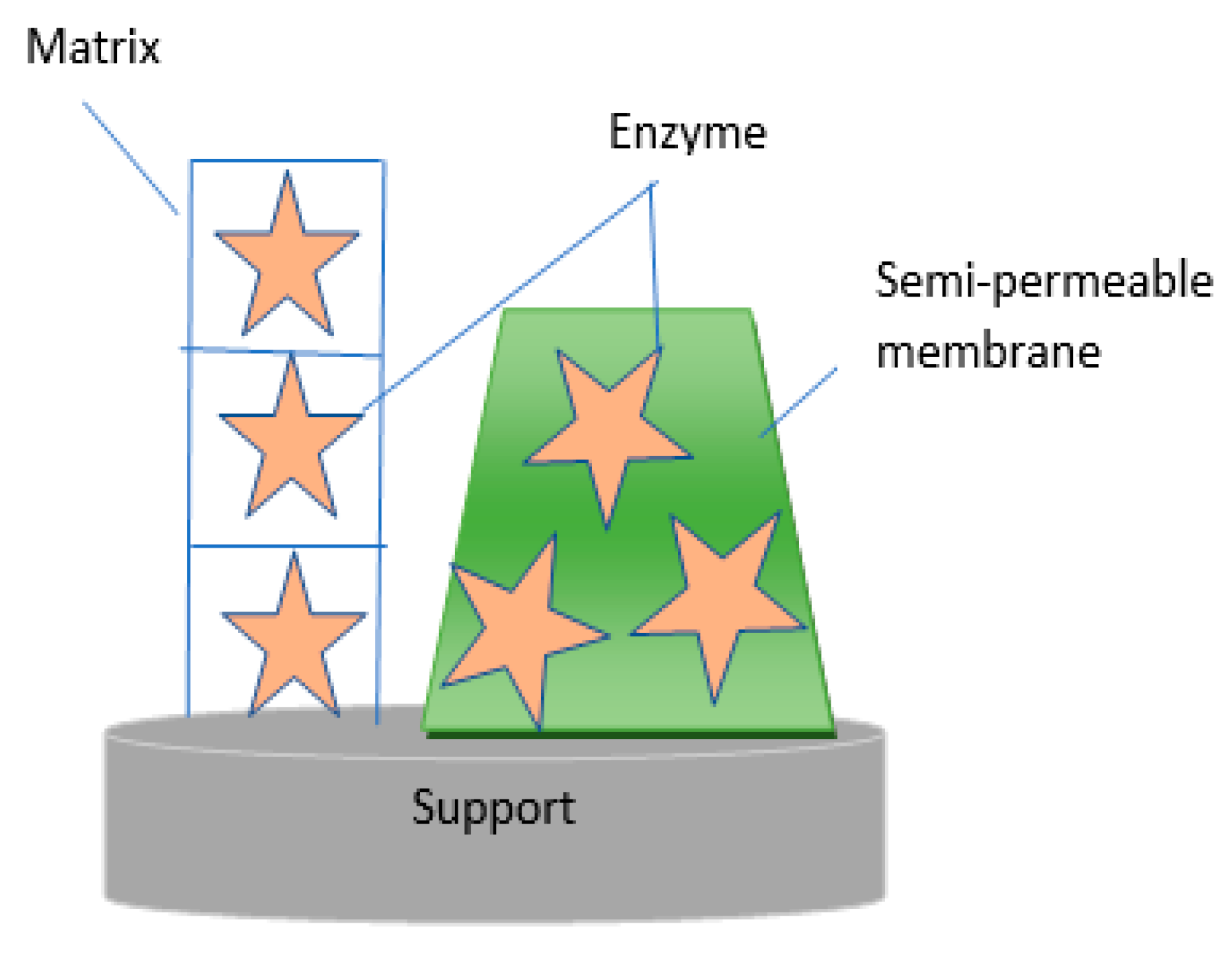
2.4. Entrapment/Encapsulation
2.5. Support Matrices for Immobilisation of Laccases and Tyrosinases
2.5.1. CNTs as Support for Enzymes
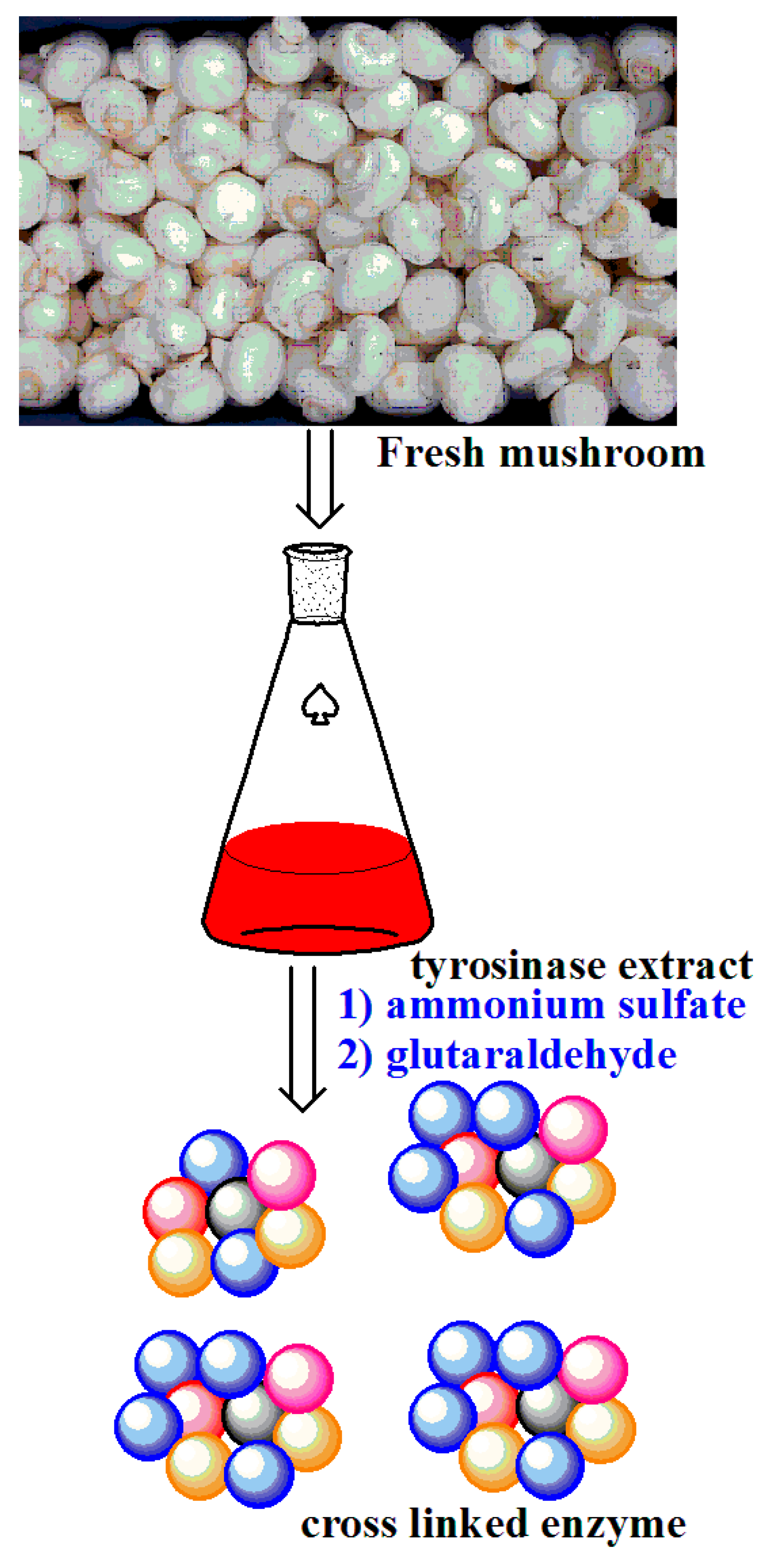
2.5.2. Cross-Linked Enzyme Aggregates as Supports
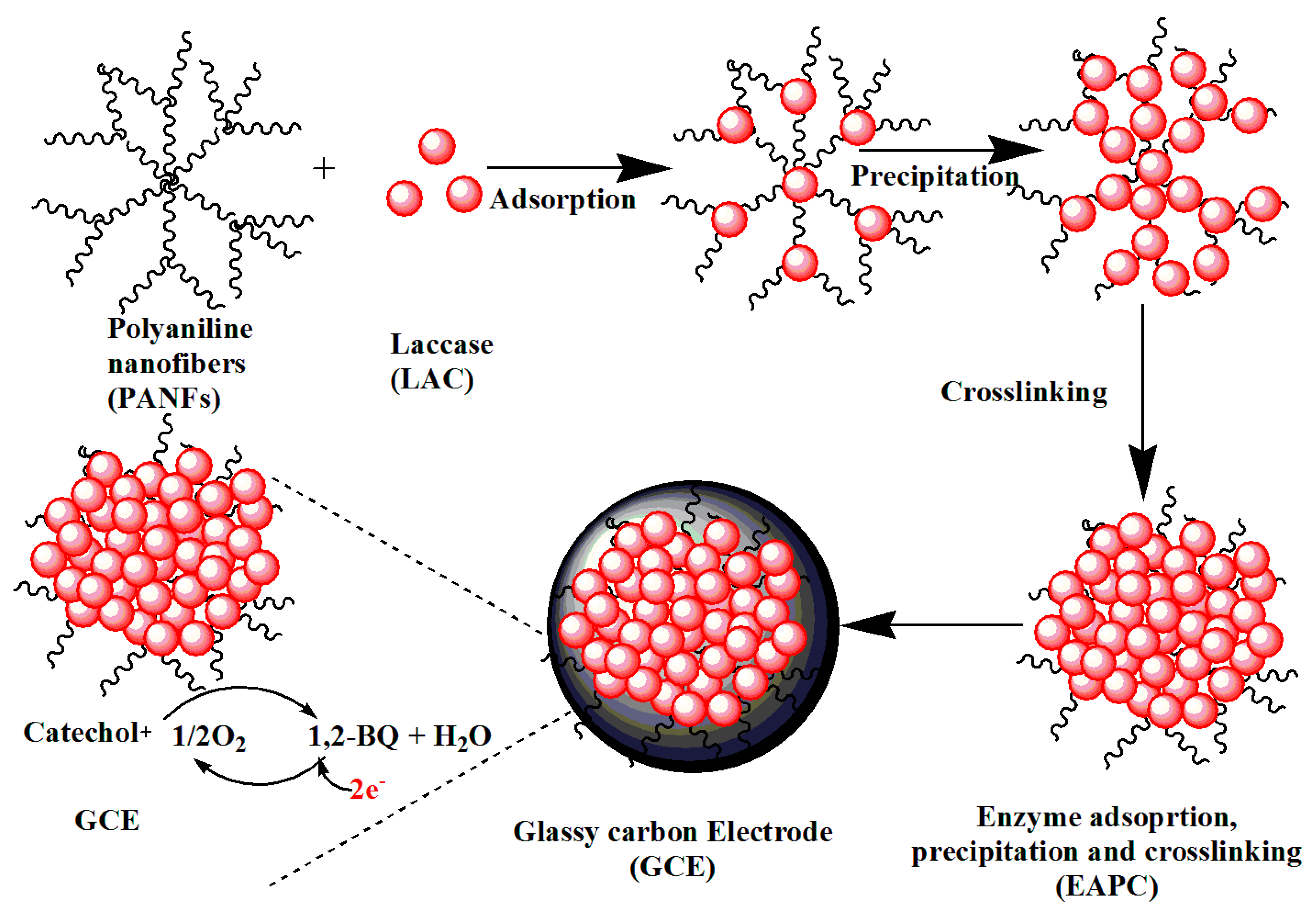
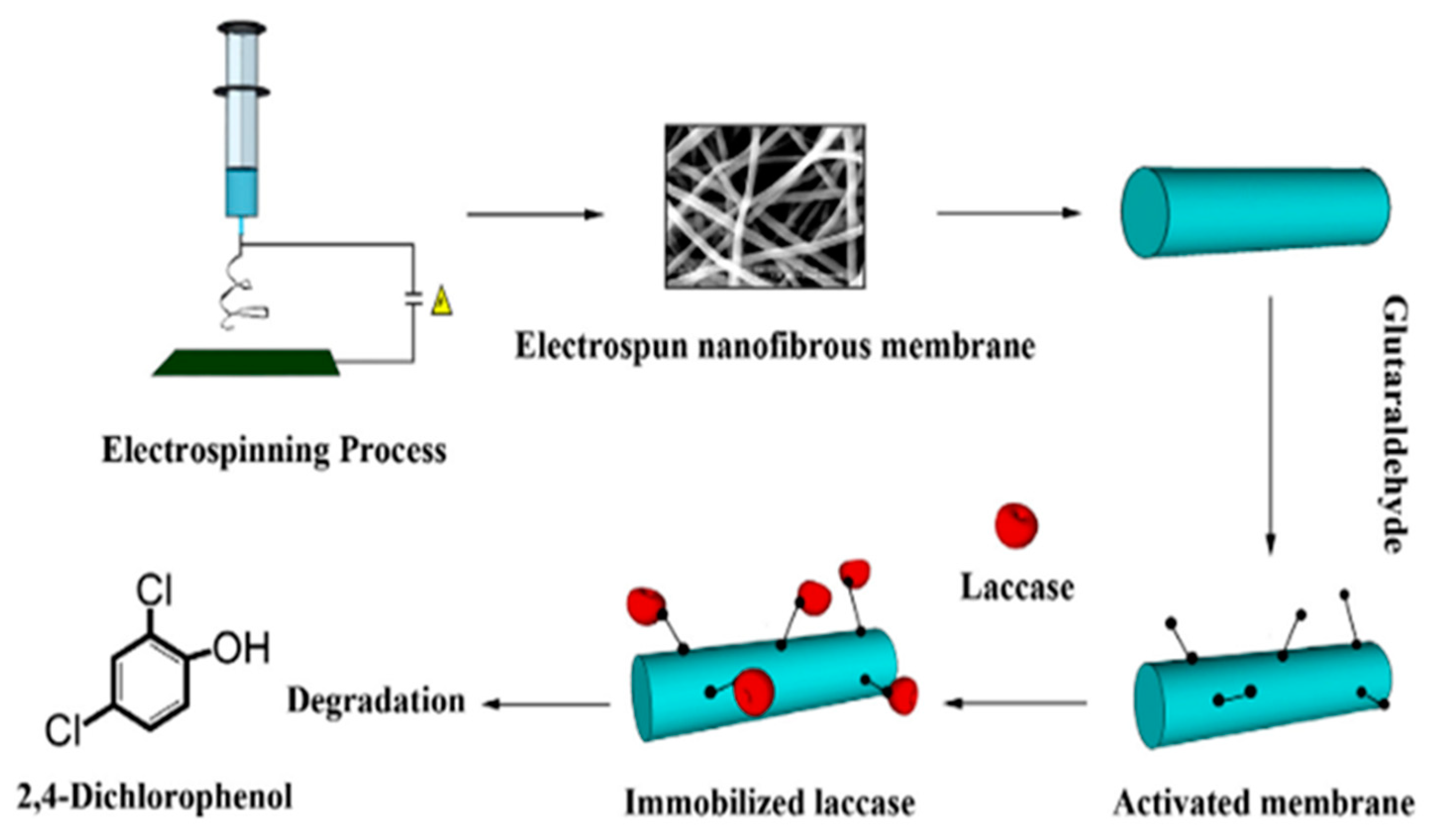
2.5.3. Enzyme Nanofibre Supports
2.5.4. Laccase Supported on PVDF Membranes by Electron Beam Irradiation
2.5.5. Insolubilisation of Laccases and Tyrosinases
Laccase Supported on Cross-Linked Chitosan Beads for Decolourisation of Dyes
Laccase and Tyrosinases Supported on Chitosan for Removal of Acetaminophen
3. Performance of Immobilised/Insolubilised Laccases and Tyrosinases
3.1. Operational Stability
3.2. Storage Stability
3.3. pH Stability
3.4. Thermal Stability
3.5. Effect of Inhibitors on Enzyme Activity
3.6. Kinetic Behaviour
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hongyan, L.; Zexiong, Z.; Shiwei, X.; He, X.; Yinian, Z. Study on transformation and degradation of bisphenol A by Trametes versicolor laccase and simulation of molecular docking. Chemosphere 2019, 224, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Kotik, M.; Markov, E. Biodegradation of phenolic compounds by Basidiomycota and its phenol oxidases: A review. Chemosphere 2016, 149, 373–382. [Google Scholar]
- Dinçer, A.; Becerik, S.; Aydemir, T. Immobilization of tyrosinase on chitosan-clay composite beads. Int. J. Biol. Macromol. 2012, 50, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Giroud, F.; Minteer, S.D. Anthracene-modified pyrenes immobilized on carbon nanotubes for direct electroreduction of O2 by laccase. Electrochem. Commun. 2013, 34, 157–160. [Google Scholar] [CrossRef]
- Xu, H.; Sun, X.; Wang, S.; Song, C.; Wang, S. Development of laccase/graphene oxide membrane for enhanced synthetic dyes separation and degradation. Sep. Purif. Technol. 2018, 204, 255–260. [Google Scholar] [CrossRef]
- Beck, S.; Berry, E.; Duke, S.; Miliken, A.; Patterson, H.; Prewett, D.L.; Rae, T.C.; Sridgar, V.; Wendland, N.; Gregory, B.W.; et al. Characterization of Trametes versicolor laccase-catalyzed degradation of estrogenic pollutants: Substrate limitation and product identification. Int. Biodeterior. Biodegrad. 2018, 127, 146–159. [Google Scholar] [CrossRef]
- Bilal, M.; Adeel, M.; Rasheed, T.; Zhao, Y.; Iqbal, M.N. Emerging contaminants of high concern and their enzyme-assisted biodegradation—A review. Environ. Int. 2019, 124, 336–353. [Google Scholar] [CrossRef]
- Pang, R.; Li, M.; Zhang, C. Degradation of phenolic compounds by laccase immobilized on carbon nanomaterials: Diffusional limitation investigation. Talanta 2015, 131, 38–45. [Google Scholar] [CrossRef]
- Sun, K.; Li, S.; Yu, J.; Gong, R.; Si, Y.; Liu, X.; Chu, G. Cu2+ þ -assisted laccase from Trametes versicolor enhanced self-polyreaction of triclosan. Chemosphere 2019, 225, 745–754. [Google Scholar] [CrossRef]
- Songulashvili, G.; Jimenéz-Tobón, G.A.J.; Jaspers, C.; Penninckx, M.J. Immobilized laccase of Cerrena unicolor for elimination of endocrine disruptor micropollutants. Fungal Biol. 2012, 6, 883–889. [Google Scholar] [CrossRef]
- Li, F.; Yu, Y.; Wang, Q.; Yuan, J.; Wang, P.; Fan, X. Polymerization of dopamine catalyzed by laccase: Comparison of enzymatic and conventional methods. Enzym. Microb. Technol. 2018, 119, 58–64. [Google Scholar] [CrossRef]
- Bilal, M.; Rasheed, T.; Nabeel, F.; Iqbal, M.N.; Zhao, Y. Hazardous contaminants in the environment and their laccase-assisted degradation—A review. J. Environ. Manag. 2019, 234, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Fortes, C.C.S.; Daniel-da-Silva, A.D.; Xavier, A.M.R.B.; Tavares, A.P.M. Opmtimization of enzyme immobilization on functionalized magnetic nanoparticles for laccase biocatalytic reactions. Chem. Eng. Process. 2017, 117, 1–8. [Google Scholar] [CrossRef]
- Ghosh, B.; Saha, R.; Bhattacharya, D.; Mukhopadhyay, M. Laccase and its source of sustainability in an enzymatic biofuel cell. Bioresour. Technol. Rep. 2019, 6, 268–278. [Google Scholar] [CrossRef]
- Ba, S.; Kumar, V.V. Recent developments in the use of tyrosinase and laccase in environmental applications. Crit. Rev. Biotechnol. 2017, 37, 819–832. [Google Scholar] [CrossRef]
- Brugnari, T.; Pereira, M.G.; Bubna, G.A.; de Freitas, E.N.; Contato, A.G.; Correa, R.C.G.; Castoldi, R.; de Souza, C.G.M.; Polizeli, M.T.; Bracht, A.; et al. A highly reusable MANAE-agarose-immobilized Pleurotusostreatus laccase for degradation of bisphenol A. Sci. Total Environ. 2018, 634, 1346–1351. [Google Scholar] [CrossRef]
- Lassouane, F.; Aït-Amar, H.; Amrani, S.; Rodriguez-Couto, S. A promising laccase immobilization approach for Bisphenol A removal from aqueous solutions. Bioresour. Technol. 2019, 271, 360–367. [Google Scholar] [CrossRef]
- Wen, X.; Zeng, Z.; Du, C.; Huang, D. Immobilized laccase on bentonite-derived mesoporous materials for removal of tetracycline. Chemosphere 2019, 222, 865–871. [Google Scholar] [CrossRef]
- Costa, J.B.; Lima, M.J.; Sampaio, M.J.; Neves, M.C.; Faria, J.L.; Torres, S.M.; Tavares, A.P.M.; Silva, C.G. Enhanced biocatalytic sustainability of laccase by immobilization on functionalized carbon nanotubes/polysulfone membranes. Chem. Eng. J. 2019, 355, 974–985. [Google Scholar] [CrossRef]
- Fathali, Z.; Rezaei, S.; Ali, M.; Habibi-Rezaei, M. Catalytic phenol removal using entrapped cross-linked laccase aggregates. Int. J. Biol. Macromol. 2019, 122, 359–366. [Google Scholar] [CrossRef]
- Bayramoglu, G.; Akbulut, A.; Arica, M.Y. Immobilization of tyrosinase on modified diatom biosilica: Enzymatic removal of phenolic compounds from aqueous solution. J. Hazard. Mater. 2013, 244, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Liu, L.; Chen, C.; Kang, X.; Xie, Q. Effective immobilization of tyrosinase via enzyme catalytic polymerization of L-DOPA for highly sensitive phenol and atrazine sensing. Talanta 2016, 160, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Kampmann, M.; Boll, S.; Kossuch, J.; Bielechi, J.; Uhl, S.; Kleiner, B.; Wichmann, R. Efficient immobilization of mushroom tyrosinase utilizing whole cells from Agaricusbisporus and its application for degradation of bisphenol A. Water Res. 2014, 57, 295–303. [Google Scholar] [CrossRef]
- Carmango, J.R.; Bacarrin, M.; Pereira, P.A.; Campos, A.M.; Oliveira, G.G.; Fhilo, O.F.; Oliveira, O.N.; Janegitz, B.C. Electrochemical biosensor made with tyrosinase immobilized in a matrix of nanodiamonds and potato starch for detecting phenolic compounds. Anal. Chim. Acta 2018, 1034, 137–143. [Google Scholar]
- Lasmi, K.; Derder, H.; Kernad, A.; Sam, S.; Abib, H.; Belhousse, S.; Tighilt, F.Z.; Hamdani, K.; Gabouze, N. Tyrosinase immobilization on functionalized porous silicon surface for optical monitoring of pyrocatechol. Appl. Surf. Sci. 2018, 446, 3–9. [Google Scholar] [CrossRef]
- Antecka, K.; Zdarta, J.; Siwińska-Stefańska, K.; Sztuk, G.; Jankowsaka, E.; Oleskowicz-Popiel, P.; Jesionowski, T. Synergistic degradation of dye wastewaters using binary or ternary oxide systems with immobilized laccase. Catalysts 2018, 8, 402. [Google Scholar] [CrossRef]
- Bilal, M.; Asgher, M.; Iqbal, H.M.N.; Hu, H. Bio-based degradation of emerging endocrine-disrupting and dye-based pollutants using cross-linked enzyme aggregates. Environ. Sci. Pollut. Res. 2017, 24, 7035–7041. [Google Scholar] [CrossRef]
- Carlsson, N.; Gustafsson, H.; Thörn, C.; Olsson, L.; Holmberg, K.; Åkerman, B. Enzymes immobilized in mesoporous silica: A physical—Chemical perspective. Adv. Colloid Interface Sci. 2014, 205, 339–360. [Google Scholar] [CrossRef]
- Jun, L.Y.; Yon, L.S.; Mubarak, N.M.; Bing, C.H.; Pan, S.; Danquah, M.K.; Abudullah, E.C.; Khalid, M. An overview of immobilized enzyme technologies for dye and phenolic removal from wastewater. J. Environ. Chem. Eng. 2019, 7, 102961–102975. [Google Scholar] [CrossRef]
- An, N.; Hui, C.; Yu, X.; Shen, D.; Hua, W. Immobilization of enzymes on clay minerals for biocatalysts and biosensors. Appl. Clay Sci. 2015, 114, 283–296. [Google Scholar] [CrossRef]
- Amaly, N.; Si, Y.; Chen, Y.; Moghazzy, A.Y.; Zhao, C.; Zhang, R.; Sun, G. Reusable anionic sulfonate functionalized nanofibrous membranes for cellulase enzyme adsorption and separation. Colloids Surf. B Biointerfaces 2018, 170, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Polyák, P.; Urbán, E.; Nagy, G.N.; Vértessy, B.G.; Pukánszky, B. The role of enzyme adsorption in the enzymatic degradation of an aliphatic polyester. Enzym. Microb. Technol. 2019, 120, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.A.; Wahab, W.A.A.; Abdel-Hameed, S.M. Comparative study in kinetics and thermodynamic characteristics of immobilized caseinase on novel support from basalt by physical adsorption and covalent binding. Biocatal. Agric. Biotechnol. 2019, 18, 101028–101039. [Google Scholar] [CrossRef]
- Arica, M.Y.; Salih, B.; Celikbicak, O.; Bayramoglu, G. Immobilization of laccase on the fibrous polymer-grafted film and study of textile dye degradation by MALDI—ToF-MS. Chem. Eng. Res. Des. 2017, 128, 107–119. [Google Scholar] [CrossRef]
- Lonappan, L.; Liu, Y.; Rouissi, T.; Kaur, S.; Verma, M.; Surampalli, R.Y. Adsorptive immobilization of agro-industrially produced crude laccase on various micro-biochars and degradation of diclofenac. Sci. Total Environ. 2018, 640, 1251–1258. [Google Scholar] [CrossRef]
- Hyun, J.; Hong, S.; Jin, H.; Ha, S.; Kim, J. Precipitated and chemically-crosslinked laccase over polyaniline nanofiber for high performance phenol sensing. Chemosphere 2016, 143, 142–147. [Google Scholar]
- Li, D.; Luo, L.; Pang, Z.; Ding, L.; Wang, Q.; Ke, H.; Huang, F.; Wei, Q. Novel phenolic biosensor based on a magnetic polydopamine-laccase-nickel nanoparticle loaded carbon nanofiber composite. Appl. Mater. Interfaces 2014, 6, 5144–5151. [Google Scholar] [CrossRef]
- Allertz, P.J.; Berger, S.; Sellenk, G.; Dittmer, C.; Dietze, M.; Stahmann, K.P.; Salchert, K. Approaching immobilization of enzymes onto open porous Basotect. Catalysts 2017, 7, 359. [Google Scholar] [CrossRef]
- Özen, F.; Günel, A.; Baran, A. DNA-binding, enzyme inhibition, and photochemical properties of chalcone-containing metallophthalocyanine compounds. Bioorg. Chem. 2018, 81, 71–78. [Google Scholar] [CrossRef]
- Holmberg, K. Interactions between surfactants and hydrolytic enzymes. Colloids Surf. B Biointerfaces 2018, 168, 169–177. [Google Scholar] [CrossRef]
- Ichi-Ribault, S.E.; Zebda, A.; Tingry, S.; Petit, M.; Suherman, A.L.; Boualam, A.; Cinquin, P.; Martin, D.K. Performance and stability of chitosan-MWCNTs-laccase biocathode: Effect of MWCNTs surface charges and ionic strength. J. Electroanal. Chem. 2017, 799, 26–33. [Google Scholar] [CrossRef]
- Gascon, V.; Blanco, R.M. Efficient retention of laccase by non-covalent immobilization on amino-functionalized ordered mesoporous silica. Appl. Catal. A Gen. 2014, 482, 116–126. [Google Scholar] [CrossRef]
- Xia, T.; Liu, C.; Hu, J.; Guo, C. Improved performance of immobilized laccase on amine-functioned magnetic Fe3O2 nanoparticles modified with polyethylenimine. Chem. Eng. J. 2016, 295, 201–206. [Google Scholar] [CrossRef]
- Abreu Silveira, E.; Perez, S.M.; Basso, A.; Serban, S.; Mamede, R.P.; Tardioli, P.W.; Farinas, C.S.; Casterjon, N.; Lorenete, G.; Martin, J.; et al. Biocatalyst engineering of Thermomyces lanuginosus lipase adsorbed on hydrophobic supports: Modulation of enzyme properties for ethanolysis of oil in solvent-free systems. J. Biotechnol. 2019, 289, 126–134. [Google Scholar] [CrossRef]
- Nalder, T.D.; Kurtovic, I.; Barrow, C.J.; Marshall, S.N. A simplified method for active-site titration of lipases immobilised on hydrophobic supports. Enzym. Microb. Technol. 2018, 113, 18–23. [Google Scholar] [CrossRef]
- Urena, Y.R.C.; Lisboa-filho, P.N.; Szardenings, M.; Gatjen, L.; Noeske, P.M.; Rischka, K. Formation and composition of adsorbates on hydrophobic carbon surfaces from aqueous laccase-maltodextrin mixture suspension. Appl. Surf. Sci. 2016, 385, 216–224. [Google Scholar] [CrossRef]
- Tavares, A.P.M.; Silva, C.G.; Draz, G.; Faria, J.L. Laccase immobilization over multi-walled carbon nanotubes: Kinetic, thermodynamic and stability studies. J. Colloid Interface Sci. 2015, 454, 52–60. [Google Scholar] [CrossRef]
- Han, Z.; Zhao, L.; Yu, P.; Chen, J.; Wu, F.; Mao, L. Comparative investigation of small laccase immobilized on carbon nanomaterials for direct bioelectrocatalysis of oxygen reduction. Electrochem. Commun. 2019, 101, 82–87. [Google Scholar] [CrossRef]
- Cosnier, S.; Holzinger, M.; Goff, A.L. Recent advances in carbon nanotube-based enzymatic fuel cells. Front. Bioeng. Biotechnol. 2014, 2, 1–6. [Google Scholar] [CrossRef]
- Cieh, N.L.; Sulaiman, S.; Mokhtar, M.N.; Naim, M.N. Bleached kenaf microfiber as a support matrix for cyclodextrin glucanotransferase immobilization via covalent binding by different coupling agents. Process Biochem. 2017, 56, 81–89. [Google Scholar] [CrossRef]
- Sun, H.; Yang, H.; Huang, W.; Zhang, S. Immobilization of laccase in a sponge-like hydrogel for enhanced durability in enzymatic degradation of dye pollutants. J. Colloid Interface Sci. 2015, 450, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Asgher, M.; Noreen, S.; Bilal, M. Enhancing catalytic functionality of Trametes versicolor IBL-04 laccase by immobilization on chitosan microspheres. Chem. Eng. Res. Des. 2017, 119, 1–11. [Google Scholar] [CrossRef]
- Shari, M.; Robatjazi, S.; Sadri, M.; Mosaabadi, J.M. Immobilization of organophosphorus hydrolase enzyme by covalent attachment on modified cellulose micro fibers using different chemical activation strategies: Characterization and stability studies. Chin. J. Chem. Eng. 2019, 27, 191–199. [Google Scholar] [CrossRef]
- Abdollahi, K.; Yazdani, F.; Panahi, R. Covalent immobilization of tyrosinase onto cyanuric chloride crosslinked amine-functionalized superparamagnetic nanoparticles: Synthesis and characterization of the recyclable nanobiocatalyst. Int. J. Biol. Macromol. 2017, 94, 396–405. [Google Scholar] [CrossRef]
- Koloti, L.E.; Gule, N.P.; Arotiba, O.A.; Malinga, S.P. Laccase-immobilized dendritic nanofibrous membranes as a novel approach towards the removal of bisphenol A. Environ. Technol. 2018, 39, 392–404. [Google Scholar] [CrossRef]
- Harir, M.; Bellahcene, M.; Baratto, M.C.; Pollini, S.; Rossolini, G.M.; Trabalzini, L.; Fatarella, E.; Pogni, R. Isolation and characterization of a novel tyrosinase produced by Sahara soil actinobacteria and immobilization on nylon nanofiber membranes. J. Biotechnol. 2018, 265, 54–64. [Google Scholar] [CrossRef]
- Xu, R.; Chi, C.; Li, F.; Zhang, B. Laccase—Polyacrylonitrile nanofibrous membrane: Highly immobilized, stable, reusable, and efficacious for 2,4,6-trichlorophenol removal. Appl. Mater. Interfaces 2013, 5, 12554–12560. [Google Scholar] [CrossRef]
- Soumano, L.; Bellenger, J.; Jones, J.P.; Cabana, H. A hybrid bioreactor based on insolubilized tyrosinase and laccase catalysis and micro filtration membrane remove pharmaceuticals from wastewater. Chemosphere 2018, 201, 749–755. [Google Scholar]
- Tirunagari, H.; Basetty, S.; Rode, H.B.; Fadnavis, N.W. Crosslinked enzyme aggregates (CLEA) of phytase with soymilk proteins. J. Biotechnol. 2018, 282, 67–69. [Google Scholar] [CrossRef]
- Jabbari, S.; Dabirmanesh, B.; Khajeh, K. Specificity enhancement towards phenolic substrate by immobilization of laccase on surface plasmon resonance sensor chip. J. Mol. Catal. B Enzym. 2015, 121, 32–36. [Google Scholar] [CrossRef]
- Kumar, V.V.; Sivanesan, S.; Cabana, H. Magnetic cross-linked laccase aggregates—Bioremediation tool for decolorization of distinct classes of recalcitrant dyes. Sci. Total Environ. 2014, 487, 830–839. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Xu, X.; Yang, X.; Ye, X.; Lin, J. Cross-linked enzyme aggregates of Cerrena laccase: Preparation, enhanced NaCl tolerance and decolorization of Remazol Brilliant Blue Reactive. J. Taiwan Inst. Chem. Eng. 2016, 65, 1–7. [Google Scholar] [CrossRef]
- Pavinatto, A.; Mercante, L.A.; Facure, M.H.M.; Pena, R.B. Ultrasensitive biosensor based on polyvinylpyrrolidone/chitosan/reduced graphene oxide electrospun nanofibers for 17 α—Ethinylestradiol electrochemical detection. Appl. Surf. Sci. 2018, 458, 431–437. [Google Scholar] [CrossRef]
- Pinto, S.C.; Rodrigues, A.R.; Saraiva, J.M.A.; Lopes-da-Silva, J.A. Catalytic activity of trypsin entrapped in electrospunpoly(ϵ-caprolactone) nanofibers. Enzym. Microb. Technol. 2015, 79, 8–18. [Google Scholar] [CrossRef]
- Chimphango, A.F.A.; van Zyl, W.H.; Görgens, J.F. In situ enzymatic aided formation of xylan hydrogels and encapsulation of horse radish peroxidase for slow release. Carbohydr. Polym. 2012, 88, 1109–1117. [Google Scholar] [CrossRef]
- Taheran, M.; Naghdi, M.; Brar, S.K.; Knystautas, E.J.; Verma, M.; Surampalli, R.Y. Degradation of chlortetracycline using immobilized laccase on polyacrylonitrile-biochar composite nanofibrous membrane. Sci. Total Environ. 2017, 605, 315–321. [Google Scholar] [CrossRef]
- Mansor, A.F.; Mohidem, N.A.; Zawawi, W.N.I.W.M.; Othman, N.S.; Endud, S.; Mat, H. The optimization of synthesis conditions for laccase entrapment in mesoporous silica microparticles by response surface methodology. Microporous Mesoporous Mater. 2016, 220, 308–314. [Google Scholar] [CrossRef]
- Canbolat, M.F.; Basri, H.; Gultekin, F. Enzymatic behavior of laccase following interaction with γ-CD and immobilization into PCL nanofibers. Anal. Biochem. 2017, 528, 13–18. [Google Scholar] [CrossRef]
- Dai, Y.; Yao, J.; Song, Y.; Liu, X.; Wang, S.; Yuan, Y. Enhanced performance of immobilized laccase in electrospun fibrous membranes by carbon nanotubes modification and its application for bisphenol A removal from water. J. Hazard. Mater. 2016, 317, 485–493. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, Z.; Chen, G.; Wang, Z. An improved method to encapsulate laccase from Trametes vesrsicolor with enhanced stability and catalytic activity. Catalysts 2018, 286, 1–11. [Google Scholar]
- Gill, J.; Orsat, V.; Kermasha, S. Optimization of encapsulation of a microbial laccase enzymatic extract using selected matrices. Process Biochem. 2018, 65, 55–61. [Google Scholar] [CrossRef]
- Oliveira, S.F.; da Luz, J.M.R.; Kasuya, M.C.M.; Ladeira, L.O.; Junior, A.C. Enzymatic extract containing lignin peroxidase immobilized on carbon nanotubes: Potential biocatalyst in dye decolourization. Saudi J. Biol. Sci. 2018, 25, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Tang, R.; Zhou, Q.; Li, F.; Zhang, B. Enhancement of catalytic activity of immobilized laccase for diclofenac biodegradation by carbon nanotubes. Chem. Eng. J. 2015, 262, 88–95. [Google Scholar] [CrossRef]
- Ji, C.; Hou, J.; Chen, V. Cross-linked carbon nanotubes-based biocatalytic membranes for micro-pollutants degradation: Performance, stability, and regeneration. J. Membr. Sci. 2016, 520, 869–880. [Google Scholar] [CrossRef]
- Xu, D.; Yang, Z. Cross-linked tyrosinase aggregates for elimination of phenolic compounds from wastewater. Chemosphere 2013, 92, 391–398. [Google Scholar] [CrossRef]
- Xu, R.; Zhou, Q.; Li, F.; Zhang, B. Laccase immobilization on chitosan/poly(vinyl alcohol) composite nanofibrous membranes for 2,4-dichlorophenol removal. Chem. Eng. J. 2013, 222, 321–329. [Google Scholar] [CrossRef]
- Palvannan, T.; Saravanakumar, T.; Unnithan, A.R.; Chung, N.; Kim, D.; Park, S. Efficient transformation of phenyl urea herbicide chloroxuron by laccase immobilized on zein polyurethane nanofiber. J. Mol. Catal. B Enzym. 2014, 99, 156–162. [Google Scholar] [CrossRef]
- Xu, R.; Si, Y.; Wu, X.; Li, F.; Zhang, B. Triclosan removal by laccase immobilized on mesoporous nanofibers: Strong adsorption and efficient degradation. Chem. Eng. J. 2014, 255, 63–70. [Google Scholar] [CrossRef]
- Jahangiri, E.; Thomas, I.; Schulze, A.; Seiwert, B.; Cabana, H.; Schlosser, D. Characterisation of electron beam irradiation-immobilised laccase for application in wastewater treatment. Sci. Total Environ. 2018, 624, 309–322. [Google Scholar] [CrossRef]
- Ma, H.; Meng, G.; Cui, B.; Si, J.; Dai, Y. Chitosan crosslinked with genipin as supporting matrix for biodegradation of synthetic dyes: Laccase immobilization and characterization. Chem. Eng. Res. Des. 2018, 132, 664–676. [Google Scholar] [CrossRef]
- Ba, S.; Haroune, L.; Cruz-Morato, C.; Jacquet, C.; Touahar, I.E.; Philippe-Bellenger, J.; Legault, C.Y.; Jones, J.P.; Cabana, H. Synthesis and characterization of combined cross-linked laccase and tyrosinase aggregates transforming acetaminophen as a model phenolic compound in wastewaters. Sci. Total Environ. 2014, 487, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Valerio, S.G.; Alves, J.S.; Klein, M.P.; Rodrigues, R.C.; Hertz, P.F. High operational stability of invertase from Saccharomyces cerevisiae immobilized on chitosan nanoparticles. Carbohydr. Polym. 2013, 92, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Ma, F.; Han, Y.; Zhang, X.; Yu, H. Removal of sulfonamide antibiotics by oriented immobilized laccase on Fe3O4 nanoparticles with natural mediators. J. Hazard. Mater. 2014, 279, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Yaohua, G.; Ping, X.; Feng, J.; Keren, S. Co-immobilization of laccase and ABTS onto novel dual-functionalized cellulose beads for highly improved biodegradation of indole. J. Hazard. Mater. 2019, 365, 118–124. [Google Scholar] [CrossRef]
- Fatarella, E.; Spinelli, D.; Ruzzante, M.; Pogni, R. Nylon 6 film and nanofiber carriers: Preparation and laccase immobilization performance. J. Mol. Catal. B Enzym. 2018, 351, 985–994. [Google Scholar] [CrossRef]
- Lonappan, L.; Liu, Y.; Rouissi, T.; Pourcel, F.; Brar, S.K.; Verma, M.; Surampalli, R.Y. Covalent immobilization of laccase on citric acid functionalized micro-biochars derived from different feedstock and removal of diclofenac. Chem. Eng. J. 2018, 351, 985–994. [Google Scholar] [CrossRef]
- Wan, D.; Tian, L.; Li, X.; Li, B.; Zhang, Q. A versatile strategy for enzyme immobilization: Fabricating lipase/inorganic hybrid nanostructures on macroporous resins with enhanced catalytic properties. Biochem. Eng. J. 2018, 139, 101–108. [Google Scholar] [CrossRef]
- Shao, B.; Liu, Z.; Zheng, G.; Liu, Y.; Yang, X.; Zhou, C.; Chen, M.; Liu, Y.; Jiang, Y.; Yan, M. Immobilization of laccase on hollow mesoporous carbon nanospheres: Noteworthy immobilization, excellent stability and efficacious for antibiotic contaminants removal. J. Hazard. Mater. 2019, 362, 318–326. [Google Scholar] [CrossRef]
- Maryšková, M.; Ardao, I.; García-González, C.A.; Martinová, L.; Rotková, J.; Ševců, A. Polyamide 6/chitosan nanofibers as support for the immobilization of Trametes versicolor laccase for the elimination of endocrine disrupting chemicals. Enzym. Microb. Technol. 2016, 89, 31–38. [Google Scholar] [CrossRef]
- Wang, Q.; Cui, J.; Li, G.; Zhang, J.; Li, D.; Huang, F.; Wei, Q. Laccase immobilized on a PAN/adsorbents composite nanofibrous membrane for catechol treatment by a biocatalysis/adsorption process. Molecules 2014, 19, 3376–3388. [Google Scholar] [CrossRef]
- Misra, N.; Kumar, V.; Goel, N.K.; Varshney, L. Laccase immobilization on radiation synthesized epoxy functionalized polyethersulfone beads and their application for degradation of acid dye. Polymer 2014, 55, 6017–6024. [Google Scholar] [CrossRef]
- Machado, A.; Tavares, A.P.M.; Rocha, C.M.R.; Cristóvão, R.O.; Teixeira, J.A.; Macedo, E.A. Immobilization of commercial laccase on spent grain. Process Biochem. 2012, 47, 1095–1101. [Google Scholar]
- Taheran, M.; Naghdi, M.; Brar, S.K.; Knystautas, E.J.; Verma, M.; Surampalli, R.Y. Covalent immobilization of laccase onto nanofibrous membrane for degradation of pharmaceutical residues in water. ACS Sustain. Chem. Eng. 2017, 5, 10430–10438. [Google Scholar] [CrossRef]
- Sathishkumar, P.; Kamala-Kannan, S.; Cho, M.; Kim, J.S.; Hadibarata, T.; Salim, M.R.; Oh, B.T. Laccase immobilization on cellulose nanofiber: The catalytic efficiency and recyclic application for simulated dye effluent treatment. J. Mol. Catal. B Enzym. 2014, 100, 111–120. [Google Scholar] [CrossRef]
- El Aty, A.A.A.; Mostafa, F.A.; Hassan, M.E.; Hamed, E.R.; Esawy, M.A. Covalent immobilization of Alternariatenuissima KM651985 laccase and some applied aspects. Biocatal. Agric. Biotechnol. 2017, 9, 74–81. [Google Scholar] [CrossRef]
- Li, G.; Nandgaonkar, A.G.; Lu, K.; Krause, W.E.; Lucia, L.A.; Wei, Q. Laccase immobilized on PAN/O-MMT composite a de novo adsorption and biocatalytic synergy. RSC Adv. 2016, 6, 41420–41427. [Google Scholar] [CrossRef]
- Olajuyigbe, F.M.; Adetuyi, Y.; Fatokun, C.O. Characterization of free and immobilized laccase from Cyberlindnerafabianii and application in degradation of bisphenol A. Int. J. Biol. Macromol. 2019, 125, 856–864. [Google Scholar] [CrossRef]
- Jaiswal, N.; Pandey, V.P.; Dwivedi, U.N. Purification of a thermostable laccase from Leucaenaleucocephala using a copper alginate entrapment approach and the application of the laccase in dye decolorization. Process Biochem. 2014, 49, 1196–1204. [Google Scholar] [CrossRef]
- Jiang, X.; Yu, Y.; Li, X.; Kong, X.Z. High yield preparation of uniform polyurea microspheres through precipitation polymerization and their application as laccase immobilization support. Chem. Eng. J. 2017, 328, 1043–1050. [Google Scholar] [CrossRef]
- Wen, X.; Du, C.; Wan, J.; Zheng, G.; Huang, D.; Yin, L.; Deng, R.; Tan, S.; Zhang, J. Immobilizing laccase on kaolinite and its application in treatment of malachite green effluent with the coexistence of Cd (П). Chemosphere 2019, 217, 843–850. [Google Scholar] [CrossRef]
- Yuan, H.; Chen, L.; Hong, F.F.; Zhu, M. Evaluation of nanocellulose carriers produced by four different bacterial strains for laccase immobilization. Carbohydr. Polym. 2018, 196, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Samak, N.A.; Tan, Y.; Sui, K.; Xia, T.; Wang, K.; Guo, C.; Liu, C. CotA laccase immobilized on functionalized magnetic graphene oxide nano-sheets for efficient biocatalysis. Mol. Catal. 2018, 445, 269–278. [Google Scholar] [CrossRef]
- Saoudi, O.; Ghaouar, N. Biocatalytic characterization of free and immobilized laccase from Trametes versicolor in its activation zone. Int. J. Biol. Macromol. 2019, 128, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Lu, Y.; Lin, J.; Hu, C.; Tsai, H. Quick screening of true tyrosinase inhibitors from natural products using tyrosinase-immobilized magnetic nanoparticles and a magnetic microplate. J. Chin. Chem. Soc. 2018, 65, 1075–1081. [Google Scholar] [CrossRef]
- Stapelberg, J.; Nqephe, M.; Lambrechts, I.; Crampton, B.; Lall, N. Selected South African plants with tyrosinase enzyme inhibition and their effect on gene expression. S. Afr. J. Bot. 2018, 10, 6–11. [Google Scholar] [CrossRef]
- Jaiswal, N.; Pandey, V.P.; Dwivedi, U.N. Immobilization of papaya laccase in chitosan led to improved multipronged stability and dye discoloration. Int. J. Biol. Macromol. 2016, 86, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Drozd, R.; Rakoczy, R.; Wasak, A.; Junka, A.; Fijałkowski, K. The application of magnetically modified bacterial cellulose for immobilization of laccase. Int. J. Biol. Macromol. 2018, 108, 462–470. [Google Scholar] [CrossRef]
- Liu, C.; Saeki, D.; Cheng, L.; Luo, J.; Matsuyama, H. Polyketone-based membrane support improves the organic solvent resistance of laccase catalysis. J. Colloid Interface Sci. 2019, 544, 230–240. [Google Scholar] [CrossRef]
- Touahar, I.E.; Haroune, L.; Ba, S.; Bellenger, J.; Cabana, H. Characterization of combined cross-linked enzyme aggregates from laccase, versatile peroxidase and glucose oxidase, and their utilization for the elimination of pharmaceuticals. Sci. Total Environ. 2014, 481, 90–99. [Google Scholar] [CrossRef]
- Hassani, T.; Ba, S.; Cabana, H. Formation of enzyme polymer engineered structure for laccase and cross-linked laccase aggregates stabilization. Bioresour. Technol. 2013, 128, 640–645. [Google Scholar] [CrossRef]
- Yildiz, H.B.; Caliskan, S.; Kamaci, M.; Caliskan, A.; Yilmaz, H. L-Dopa synthesis catalyzed by tyrosinase immobilized in poly(ethyleneoxide) conducting polymers. Int. J. Biol. Macromol. 2013, 56, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Kashefi, S.; Borghei, S.M.; Mahmoodi, N.M. Covalently immobilized laccase onto graphene oxide nanosheets: Preparation, characterization, and biodegradation of azo dyes in colored wastewater. J. Mol. Liq. 2019, 276, 153–162. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ndlovu, T.; Ba, S.; Malinga, S.P. Overview of Recent Advances in Immobilisation Techniques for Phenol Oxidases in Solution. Catalysts 2020, 10, 467. https://doi.org/10.3390/catal10050467
Ndlovu T, Ba S, Malinga SP. Overview of Recent Advances in Immobilisation Techniques for Phenol Oxidases in Solution. Catalysts. 2020; 10(5):467. https://doi.org/10.3390/catal10050467
Chicago/Turabian StyleNdlovu, Thandanani, Sidy Ba, and Soraya P Malinga. 2020. "Overview of Recent Advances in Immobilisation Techniques for Phenol Oxidases in Solution" Catalysts 10, no. 5: 467. https://doi.org/10.3390/catal10050467
APA StyleNdlovu, T., Ba, S., & Malinga, S. P. (2020). Overview of Recent Advances in Immobilisation Techniques for Phenol Oxidases in Solution. Catalysts, 10(5), 467. https://doi.org/10.3390/catal10050467



