Abstract
Wastewater from the textile industry has a substantial impact on water quality. Synthetic dyes used in the textile production process are often discharged into water bodies as residues. Highly colored wastewater causes various of problems for the aquatic environment such as: reducing light penetration, inhibiting photosynthesis and being toxic to certain organisms. Since most dyes are resistant to biodegradation and are not completely removed by conventional methods (adsorption, coagulation-flocculation, activated sludge, membrane filtration) they persist in the environment. Advanced oxidation processes (AOPs) based on hydrogen peroxide (H2O2) have been proven to decolorize only some of the dyes from wastewater by photocatalysis. In this article, we compared two very different photocatalytic systems (UV/peroxydisulfate and UV/H2O2). Photocatalyzed activation of peroxydisulfate (PDS) generated sulfate radicals (SO4•−), which reacted with the selected anthraquinone dye of concern, Acid Blue 129 (AB129). Various conditions, such as pH and concentration of PDS were applied, in order to obtain an effective decolorization effect, which was significantly better than in the case of hydroxyl radicals. The kinetics of the reaction followed a pseudo-first order model. The main reaction pathway was also proposed based on quantum chemical analysis. Moreover, the toxicity of the solution after treatment was evaluated using Daphnia magna and Lemna minor, and was found to be significantly lower compared to the toxicity of the initial dye.
1. Introduction
A source of clean water is important for various industrial, social and economic development sectors; therefore, it has to be constantly monitored for impurities. Increased human activity has introduced a wide range of toxic chemicals including inorganic (e.g., chromium, mercury, lead) and organic (e.g., pesticides, surfactants, pharmaceuticals) pollutants into the aqueous environment [1,2]. A significant source of such polluting compounds is wastewater from the textile industry, which is classified as the most polluting of all industrial sectors in terms of effluent volume and its chemical content [3]. The chemical loads of textile effluents originate from the residues of textile production processes, such as printing, scouring, bleaching and dyeing [4]. During the batch dyeing process, which is a common method for dying textiles, approximately 10% to 15% of the synthetic dyes used are lost, due to the inefficiency of the operation [5]. The residues are discharged into the effluent, from which they cannot be effectively removed by conventional wastewater treatment processes [6].
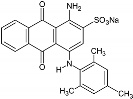
Dyes may be classified by their application or chemical structure into direct dyes (polyazo compounds, stilbenes, oxazines), basic dyes (diazahemicyanine, hemicyanine, cyanine, thiazine, acridine) or solvent dyes (azo, anthraquinone) [7]. Polyazo dyes have three or more N=N bonds in the molecule, and the number of azo groups attached to its center determines the color index of the dye (CI, systematic classification of colors by their saturation, brightness and hue) [8]. Designed to be highly stable towards light, temperature, water and detergents, dyes persist in the environment [9]. The presence of one or more benzene rings in their structure makes them more recalcitrant to biodegradation [10]. Moreover, dyes discharged into water even at a low concentration (even below 1 mg/L) are not only highly visible, which affects the aesthetic quality and transparency of water bodies (lakes, rivers) [11], but they also disturb the aquatic life by reducing light penetration and inhibiting photosynthesis, which causes oxygen deficiency [12]. Azo and anthraquinone dyes represent around 90% of all organic colorants [13]. They pose a threat to aquatic organisms (bacteria, algae, fish) by being toxic (lethal effect, genotoxic, mutagenic, carcinogenic) [14,15]. In particular, Acid Blue 129 (AB129), which is an acidic dye with an anthraquinone structure, being extensively used in the dyeing of silk, wool, cotton, paper, leather and nylon [16], was found to be associated with an ecotoxic hazard and danger of bioaccumulation [17]. The properties and structure of Acid blue 129 (AB129) are described in Table 1.

Table 1.
Acid blue 129 (AB129) properties and structure
The conventional treatments of textile effluents involve, among others things: adsorption, coagulation-flocculation, membrane filtration and activated sludge [18,19]. However, these methods are not completely efficient and have several shortcomings. The adsorption process usually involves the use of activated carbon, which is expensive and incurs additional to regeneration and disposal costs [20]. Several dyes can inhibit bacteria development in activated sludge or cause membrane fouling using the filtration method [21,22]. Coagulation–flocculation is a pH-dependent process, which generates an extensive amount of concentrated sludge and is not suitable for all dyes [23].
Considering the obstacles in conventional textile wastewater treatment, alternative methods were developed. One of which is the advanced oxidation process (AOP), which utilizes highly reactive oxidizing intermediates like hydroxyl radicals (•OH) [24]. These radicals are often catalytically generated from hydrogen peroxide (H2O2) or ozone. For example, the Fenton reaction uses iron as a catalyst for producing •OH. AOPs can also utilize ozone (O3) to produce •OH, which is used for decolorization of the azo dyes C.I. Reactive black 5 [25]. Ultraviolet (UV) radiation can catalyze the generation of •OH by photolysis of H2O2. This process was reported to be effective in the degradation of some dyes [26,27]. UV is also extensively used in combination with O3 [28]. In one study, besides acting as a catalyst, UV radiation also contributed to the enhanced removal of total organic carbon (TOC) and chemical oxygen demand (COD), in a decolorization experiment of Reactive Blue 19 [29]. While the oxidation-reduction potential (ORP) of •OH/H2O is 2.8 V (at an acidic pH) and •OH/OH- is 1.89 V (at an alkaline pH) [30], the use of H2O2 to generate radicals is not cost-effective. For example, Argun and Karatas [31] reported that 4 g/L of H2O2 and 0.2 g/L of iron salt were used to decolorize 200 mg/L of synthetic dye. Similarly, Meric et al. [32] used 0.4 g/L of H2O2 and 0.1 g/L of iron salt to decolorize 100 mg/L of dye. Therefore, high consumption of H2O2 provides an economical challenge and increases the need to find cheaper and more effective substitutes, e.g., permanganate, ozone, persulfate anions or sulfate radicals [33]. Sulfate radicals (SO4•−) and •OH- based oxidation processes have comparable reaction rates for the removal of some pharmaceuticals [34], but sulfate radicals usually have a longer half-life (30–40 μs) than •OH (10−3 μs) [35,36]. Both radicals differ also in their reaction behavior, whereby SO4•− favors electron transfer and •OH reacts more by addition and H-abstraction [37,38]. This is reflected in the different types of dyes treated. For example, Tang and Huren [39] reported that •OH is ineffective for the oxidation of anthraquinone dyes, while degradation by SO4•− is effective [40]. SO4•− may be generated by catalytic activation of peroxydisulfate (PDS) by: heat, UV radiation, transition metals, electrolysis, transition metals or radiolysis [41,42]. While PDS in the form of sodium persulfate is cheaper (0.74 USD/kg) [43] and safer to handle than liquid H2O2 (1.5 USD/kg), it is more expensive if calculated per mol (0.18 USD/mol PDS, 0.05 USD/mol H2O2), and hence the amount of radicals generated. Despite the many advantages of persulfate treatment, its disadvantages also have to be taken into consideration, such as post-treatment toxicity. Post-contamination with sulfate salts may be thought a small problem in comparison to the toxic by-products formed in a SO4•− system, including transformation products of target contaminants (e.g., polynitrophenol compounds formed from nitrophenols [44]) and the by-products generated from effluent organic matter. SO4•− is known to be more prone to form such post-contamination; therefore, toxicity studies after persulfate treatment are recommended [41].
In this study, photocatalyzed decolorization experiments of anthraquinone dye AB129 were conducted under various conditions. The work was performed to evaluate the role of sulfate and hydroxyl radicals in the dye oxidation. Pseudo-first order rate kinetics were also evaluated, and a simple pathway was proposed. Finally, the post-treatment toxicity of by-products was measured. To the best of our knowledge, this is the first time that the UV application of PDS has been used for the catalyzed oxidation of anthraquinone dye. Table 2 shows the various methods used to degrade anthraquinone dyes.

Table 2.
Methods used to degrade anthraquinone dyes.
2. Results and Discussion
Several experiments were performed, including the influence of •OH and SO4•− on the decolorization efficiency, effect of PDS concentration, pH, possible reaction pathways, and the ecotoxicity of by-products.
2.1. Influence of •OH and SO4•− on AB129 Decolorization
To determine the decolorization efficiency of both radical species on AB129, we performed experiments with PDS (source of SO4•−) and H2O2 (•OH) catalyzed by UV. It is known that from 1 mole of oxidant, 2 moles of radicals may be generated, according to Equations (1) and (2):
Figure 1 shows that UV irradiation alone [similarly to PDS (Figure 2) and H2O2 (data not shown) w/o UV activation] was not able to degrade the dye. The dye seems to be resistant to direct UV photolysis, as the energy of the photons with a wavelength ranging from 313 to 578 nm is too low to degrade the molecule of the dye. Also, decolorization by •OH is relatively slow and reached only 12% after one hour of the experiment. Only the UV-catalyzed SO4•− oxidation process was found to be effective in the decolorization of AB129, whereby the effect was about 90% of the initial dye concentration (25 mg/L). Homolysis of the peroxide bond of PDS occurs when catalyzed by UV, which results in the generation of SO4•− [52]. In proposed UV/PDS system (in near neutral or acidic pH) the only type of free radicals formed could be SO4•−. Water molecules could be oxidized to produce •OH but this process is very slow (k = 6.6 × 102 s−1) [53] and therefore, not significant in the timeframe of the experiment.
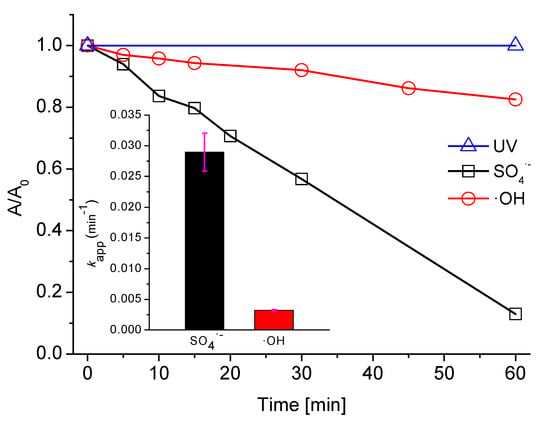
Figure 1.
Decolorization (absorbance at 595 nm) of AB129 with SO4•−, •OH and ultraviolet (UV) radiation alone (conditions: 25 mg/L AB129, 2.5 mM PDS, 10 mM H2O2, UV 150 W). The inset shows decolorization kinetics of AB129 by SO4•− and •OH, (the fuchsia error bars represent the slope error).
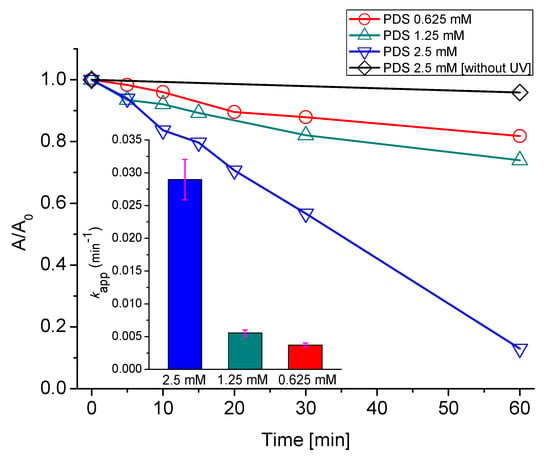
Figure 2.
Decolorization (absorbance at 595 nm) of AB129 by UV/peroxydisulfate (PDS) system (25 mg/L AB129, UV 150 W). The fuchsia error bars represent the slope error
Despite the H2O2 concentrations being four times higher than in the case of PDS (10 mM vs. 2.5 mM), the generated •OH radicals did not react with the AB129 as effectively due to the following possible reasons. Although H2O2 and PDS molecules have a similar bond length of 1.453 Å and 1.497 Å [54], H2O2 peroxide bond energy (51.0 kcal/mol) is significantly higher than PDS (33.5 kcal/mol) and, therefore, it is more difficult to be cleaved by UV irradiation [55]. Furthermore, •OH has an almost ten times faster recombination rate (k = 5.2 × 109 M−1 s−1) [56] than SO4•− (k = 5.0 × 108 M−1 s−1) [57] and, therefore, a smaller amount of generated radicals is available to react with the contaminant, compared to SO4•−. Further differences in the decolorization of AB129 may be due to intrinsic differences in the reaction mechanisms. While SO4•− works more by electron abstraction because of a higher electron affinity (2.43 eV) than •OH (1.83 eV), •OH acts more through hydrogen abstraction or addition [58]. This makes SO4•− more selective and highly reactive towards organic contaminants containing non-bonding electron pairs of atoms such as O, N and S [59]. The first steps of the reaction between AB129 and sulfate radicals are described in more detail in the subsection “Formation of by-products”.
The apparent first order (kapp) rate constants, shown in the inset of Figure 1 and calculated based on Equation (9), are one order of magnitude different higher for SO4•− than for •OH (0.029 min−1 and 0.0032 min−1 for SO4•− and •OH, respectively). Both radicals are susceptible to electron transfer; however, SO4•− shows a much lower energy barrier for this reaction, which results in markedly higher kapp. Therefore, it was decided to focus solely on PDS for a better understanding of its reaction mechanism with the AB129 dye.
2.2. Effect of PDS Concentration
To determine the optimal decolorization conditions, the concentration of PDS was changed from 0.625 to 2.5 mM, as depicted in Figure 2, where the inset shows the decolorization kinetics of AB129 by different PDS concentrations.
The concentrations of 0.625 mM and 1.25 mM achieved only 18% and 26% decolorization, respectively, and are almost comparable with the blank experiment w/o UV light. A further increase to 2.5 mM caused a significant improvement. The kinetic of the dye removal is significantly faster, the decrease is linear, and after 60 min the dye decolorization reached 87%. The apparent first-order rate constants calculated were 0.0037 min−1, 0.0056 min−1 and 0.029 min−1 for 0.625 mM, 1.25 mM and 2.5 mM PDS, respectively. Therefore, for the four-fold increase in the PDS concentration, the rate constant increased roughly eight times. This may be because the low concentrations of oxidant did not produce enough SO4•- to degrade the dye effectively [60]. Finally, 2.5 mM was chosen as the optimal concentration in the experiment in terms of efficiency and economy, because higher PDS concentrations are not economically feasible.
2.3. Effect of the Initial pH
The other parameter that significantly influences the decolorization efficiency is the initial pH of the solution. The initial solution pH was varied in the interval between 3 and 11, as shown in Figure 3.
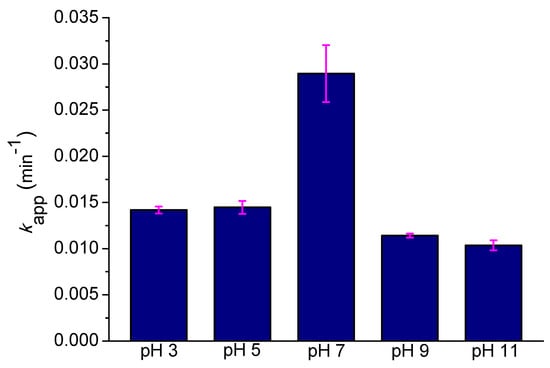
Figure 3.
Effect of the initial pH on AB129 decolorization rate constant (conditions: 25 mg/L AB129, absorbance measured at 595 nm, UV 150 W). The fuchsia error bars represent the slope error.
It can be inferred that the pH conditions had a significant influence on the UV catalyzed PDS oxidation system. The apparent first-order rate constant increased noticeably from the pH range of 3 to 5 (0.0141 min−1 and 0.0145 min−1) to pH 7 (0.029 min−1) and decreased back to half-values for a higher pH (0.0114 min−1 and 0.0107 min−1 for pH 9 and 11, respectively). The results show that the neutral conditions are the most optimal for the decolorization reaction. Under an alkaline pH, the hydroxides (OH−) in the solution undergo reactions with SO4•− to generate •OH (Equation (3)), which is a significantly less effective radical species in this respect.
Furthermore, the generated •OH further reacts with SO4•− (Equation (4)), decreasing the number of available radicals.
Under an acidic pH, the further breakdown of PDS to SO4•− may be catalyzed by acid activation (Equation (5) and (6)).
However, the generation of SO4•- catalyzed by acid conditions and UV together would yield high concentrations of those radicals. In excess, SO4•− may favor reactions like scavenging (Equation (7)) [61] or recombination (Equation (8)) over reactions with the dye.
This may explain kapp being lower in an acidic pH compared to a neutral pH, which was also observed by Liang et al. [62] in a PDS oxidation system. Overall, the most favorable condition for oxidation of AB129 is at pH of 7. Alkaline and acidic pH conditions caused inhibition of the reaction by the possible reasons explained. After evaluating the effect of pH on the decolorization process, the study focused on the identification of post-treatment intermediates.
2.4. Formation of by-Products
To determine the possible formation of by-products, the absorbance spectra during the decolorization of AB129 were recorded, as depicted in Figure 4.
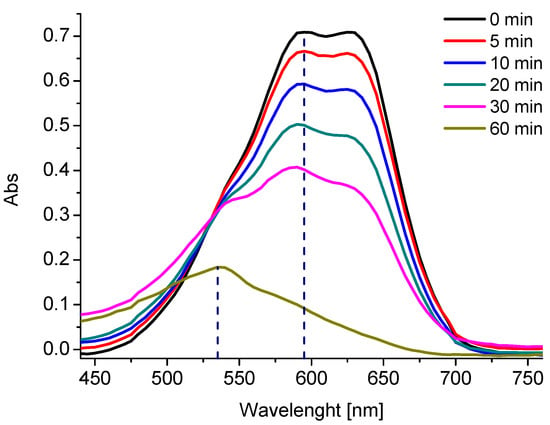
Figure 4.
Absorbance spectra of AB129 during decolorization tests (conditions: 25 mg/L AB129, 2.5 mM PDS).
At the beginning, a double peak at 595 and 630 nm was recorded. During the experiment, the peak at 630 nm slowly disappeared, followed by the peak at 595 nm. At 20 min, a new peak at 535 nm was formed, which dominated at the end of the experiment (60 min). This peak may represent the formed by-products.
Quantum chemical calculations were performed to determine the most probable pathway of the reaction between the sulfate radical and AB129. Firstly, the geometry of AB129 was optimized, as shown in Figure 5.
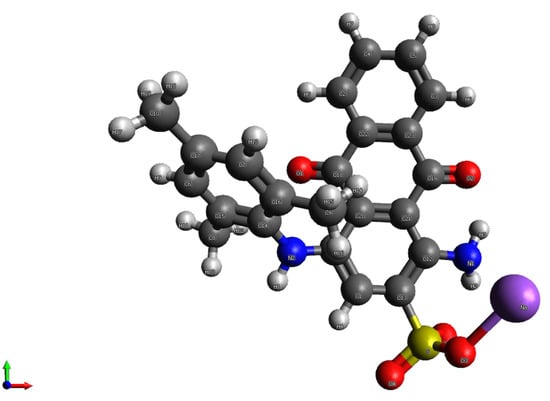
Figure 5.
Optimized AB129 molecule obtained using B3LYP/6-31G**.
Then the CPCM approach was applied in order to model the solvent (water) effect on the calculated transition energies of the species with the def2-TZVP basis set. The λmax of the visible spectrum was computed to be 594 nm, almost the same as the wavelength, which was used for determination of AB129 (595 nm), and corresponded to the HOMO → LUMO and HOMO-1 → LUMO (overall E = 2.087 eV) transitions of AB129. Moreover, the values of HOMO and LUMO were found to be −5.413 and −2.863 eV, respectively, whereas the difference in the energy (HOMO-LUMO energy gap) was 2.55 eV. Similar values were recently computed and reported for Acid Blue 113 by Asghar et al. [63]. Figure 6 shows the HOMO and LUMO of AB129 obtained at B3LYP/6-31G** and a map of the electron density of the AB129 molecule.
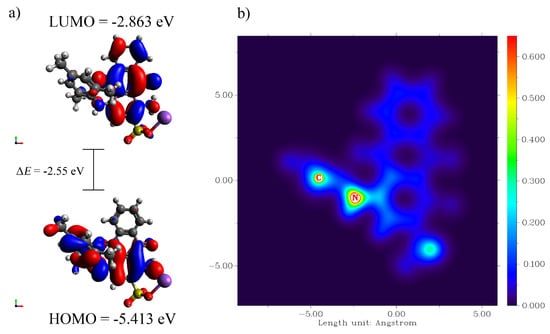
Figure 6.
(a) HOMO and LUMO of AB129 obtained at B3LYP/6-31G** and (b) map of the electron density (X, Y plane) of AB129 molecule (the sodium atom was removed for simplicity).
According to the frontier orbital theory, chemical reactions preferentially occur at the position of the molecule wherein their frontier orbital intensely overlap [64]. Moreover, the most probable reaction pathway for sulfate radicals that have a very strong electrophilic character is a direct attack on one of the atoms of the contaminant molecule, usually the one with the highest electron density in the HOMO of the aromatic molecule [65]. Figure 6b shows that one of the positions with the highest electron densities (in the HOMO of AB129) is the region near to nitrogen atom from the secondary amine. From this, it is possible to conclude that there is a higher preference for the –NH- group, and that the main product forming in this system is a derivative of hydroxylated anthraquinone.
Furthermore, according to Liu et al. [66], a Hirshfeld charge may be successfully employed to determine the reactive sites of the electrophilic reactions. Apart from the oxygen atoms, which are probably not involved in the reactions reported in this study, and a high O/C ratio is often correlated with a slow reaction between the molecules and the oxidants [67], the N atom of AB129 may be characterized by the smallest Hirshfeld charge (−0.424), which is even smaller than the second nitrogen (N1: −0.398) from the primary amine located on the anthraquinone. This may provide further confirmation that the first and crucial reaction of the sulfate radical with AB129 is an electron transfer from the –NH- moiety, which splits the AB129 molecule and creates the anthraquinone derivative.
This conclusion may be supported in several other ways. Primarily, the intermediate that was formed absorbs photons of higher energy (Vis peak shifted to the left side of the spectrum i.e., 535 nm), which is typical for the anthraquinone derivatives with a much lower molecular weight [39]. A similar observation was made by Tang and An, who observed radical driven splitting of Acid Blue 40 with the formation of a yellow intermediate absorbing light in a similar region to that reported in this study [39].
Considering the possible formation of by-products, their toxicity on model plant fronds and freshwater crustaceans was evaluated and is discussed below.
2.5. Ecotoxicity
In addition to a determination of decolorization efficiency, it is important to consider the toxicity of the system for living organisms, since post-treatment by-products may sometimes be more toxic than the initial contaminant [41]. Tests using plant fronds Daphnia magna and freshwater crustaceans Lemna minor are often performed in toxicological studies, because they are simple, fast and cost-effective. Moreover, they represent both plants and animals, which may tell us more about the impact on the ecosystem, and selected microorganisms are very informative in terms of the potential toxicity of wastewater [68,69,70]. For example, Sackey et al. found that Daphnia magna and Lemna minor are effective for testing the toxicity of leachates [71]. Moreover, Castro et al. [72] investigated the potential toxicity of effluents from the textile industry before and after treatment, and concluded that the raw textile effluent was very toxic. Therefore, toxicity tests were performed using the same conditions mentioned in the methodology of the decolorization test, except different concentrations of PDS and AB129 were used, as shown in Table 3 and Table 4.

Table 3.
Toxicity of AB129 by-products on Daphnia magna (* Time 0 min = samples without PDS addition).

Table 4.
Toxicity of AB129 by-products on Lemna minor (* Time 0 min = samples without PDS addition).
According the guidelines for the interpretation of the obtained toxicity results given by Kudlek [73], samples characterized by a toxic effect of <25% are nontoxic. Only the lowest concentration of PDS/AB129 (0.2/0.2 mM) was nontoxic for the Lemna minor test organisms, whereas Daphnia magna organisms were more sensitive to the action of PDS/AB129, and classified the post-processed samples subjected to both 0.2/0.2 mM and 0.5/0.5 mM as low toxic (a toxicity effect of between 25% and 50%). A toxicity effect higher than 50% classified the samples as being toxic. Such results were noted in the samples between 5 and 20 min of the experiment tested on Daphnia magna, where the concentration of PDS/AB129 was equal to 1/1 mM and 2/2 mM.
Nonetheless, both tests indicated that the toxicity of the initial solutions increased along with the PDS/AB129 concentration. In both tests, up to approximately 10 min of the experiment, the toxicity increased due to the effect of the addition of PDS. However, in both cases, after this time, a decreasing trend in toxicity was detected. After 45 min of the experiment, the toxicity for Daphnia roughly halved for all of the concentrations analyzed and for Lemna it decreased even more. This may indicate that by-products of AB129 after treatment are less toxic than the original dye. Moreover, PDS toxicity is only temporary, because it is quickly decomposed and exhibits lower toxicity to the analyzed organisms.
3. Materials and Methods
3.1. Chemicals
Sodium persulfate (Na2S2O8, purity ≥98%), hydrogen peroxide (H2O2, 30% w/w in water), sodium hydroxide (NaOH, 97% powder), sulfuric acid (H2SO4, 95%–98%), and Acid blue (AB129, C23H19N2NaO5S, 25% dye content) were purchased from Sigma Aldrich (Prague, Czech Republic). Hydrochloric acid (HCl, >35%) was purchased from Avantor Performance Materials Poland (Gliwice, Poland). Deionized water (18.2 MΩ·cm) obtained from ELGA purelab flex system (ELGA, Veolia Water, Marlow, UK) was used in all of the experiments.
3.2. Analytical
A pH meter TMultiLine® Multi 3430 IDS from WTW (Weilheim, Germany) equipped with SenTix pH electrodes was used to measure the acidity of the reaction mixture. The visible spectrum of the samples was measured by a UV-Vis spectrophotometer DR 3900 from Hach (Vancouver, WA, USA) within the 440–760 nm wavelength range, recorded every 5 nm.
3.3. Decolorization Test
Decolorization experiments of AB129 were performed based on a modified method of Neamtu et al. [74]. Firstly, a solution of AB129 (25 mg/L) and PDS (various concentrations of 0.625 mM, 1.25 mM and 2.5 mM) or H2O2 (10 mM) was prepared in a 100 mL reactor. Then, pH conditions were adjusted by adding a minimal amount of concentrated NaOH or H2SO4 solution, and the prepared reactor was exposed to UV radiation under constant magnetic stirring. The UV light source was provided by a model TQ 150 medium-pressure mercury UV lamp (Heraeus, Hanau, Germany) placed in a quartz glass (DURAN 50) cooling jacket fed by recirculating tap water. This step maintained a constant temperature of the mixture of 21 ± 1 °C. According to the data provided by the manufacturer, the TQ 150 lamp operated in the cooling jacket emanates radiation with a wavelength λexc of 313, 365, 405, 436, 546, 578 nm, and radiation flux equal to 2.5, 5.8, 2.9, 3.6, 4.6, 4.2 W, respectively. The absorbance spectra were measured in 1 mL quartz cuvettes by a UV-Vis spectrometer at the wavelength of 595 nm according to Palencia et al. [75]. The analyses were performed several times and averages and standard deviations were calculated by Origin 9 software [OriginLab].
3.4. Kinetic Test and AB129 Structure Modelling
A pseudo-first order kinetic model was used to describe the decolorization of AB129 by SO4•− and •OH (Equation (9)) [76].
where C0 and Ct are the initial (t = 0) and time-dependent concentrations (at time t), proportional to the measured absorbance A, respectively, and kapp is an apparent rate constant [77].
3.5. Quantum Chemical Analysis
The initial coordinates of the AB129 structure were obtained with the Avogadro program [78]. The structure of the AB129 was further optimized using the Orca program package [79], and the results were validated with Gaussian 16 software [63], both in the gaseous and liquid phase at the B3LYP/6-31G** level of study, as suggested in a recent work on the Acid blue 113 oxidation [63]. Time-dependent density functional theory TD-DFT was used to predict the excited state properties of AB129. The outputs were later visualized with the Avogadro program, whereas the electron densities and Hirshfeld charges were visualized and computed by Multiwfn software [80,81].
3.6. Ecotoxicity Test
Two different bio-tests were used to determine the toxicity of the post-treatment products: the Lemna sp. growth inhibition test (GIT) and the Daphtoxkit F bioassay. In the GIT, plant fronds of freshwater vascular plants Lemna minor from our own breeding were used. The test is based on calculating the number of plant fronds growing for 7 days in a tested and blank sample, prepared according to the OECD Guideline 221. The test was performed at a temperature of 25 ± 1 °C by a constant exposure to light with an illuminance of 6000 lux.
The Daphtoxkit F bioassay from Tigret (Warszawa, Poland) uses freshwater crustaceans Daphnia magna to measure their immobility or mortality after 24 h exposure to tested post-process samples, in comparison to standard freshwater (ISO medium prepared according ISO 6341). The test was performed on 1-day-old test organisms, according to the OECD Guideline 202. NaOH (0.1 mol/L) and HCl (0.1 mol/L) solutions were used for pH corrections during the toxicity tests. The toxicity of both tests was calculated using the following equation 10 [73]:
where E is the toxicity effect (%), NC is the number of living organisms (plant fronds or freshwater crustaceans) in the control sample, and NT is the number of living organisms (plant fronds or freshwater crustaceans) in the test sample. Interpretation of the results obtained from both of the toxicity tests was performed based on the toxicity classification presented in Table 5, and according to guidelines proposed by Mahugo Santana et al. [82].

Table 5.
Interpretation of the toxicity results.
4. Conclusions
In this work, we focused on sulfate and hydroxyl radical-based oxidation processes catalyzed by UV for the treatment of the model dye Acid Blue 129. SO4•− at a concentration of 2.5 mM successfully decolorized 25 mg/L of the dye up to 87% within 60 min, whereas •OH at a concentration of 10 mM was significantly less effective. The pseudo-first-order kinetic rate constant of the optimal reaction conditions, including neutral pH, was found to be 0.029 min−1. The probable reaction pathway of AB129 with SO4•− was determined using quantum chemical calculations, indicating electron transfer from the –NH- moiety, which splits the AB129 molecule creating the anthraquinone derivative. Ecotoxicity tests of the by-products showed a lower toxicity than the toxicity of the initial dye and only a temporary effect of PDS.
Author Contributions
Conceptualization, S.W. and K.K.; methodology, K.K. and S.W.; software, S.W.; validation, S.W., E.K. and M.Č.; investigation, T.K. and K.K.; writing—original draft preparation, K.K. and E.K.; writing—review and editing, K.G., S.W., D.S., V.V.T.P., and M.Č.; supervision, S.W. and M.Č. All authors have read and agreed to the published version of the manuscript
Funding
This research was supported by the Ministry of Education, Youth and Sports in the Czech Republic under the “Inter Excellence – Action programme“ within the framework of the project ‘‘Exploring the role of ferrates and modified nano zero-valent iron in the activation process of persulfates“ (registration number LTAUSA18078) and the Research Infrastructures NanoEnviCz (Project No. LM2018124). This work was also supported by the Ministry of Education, Youth and Sports of the Czech Republic and the European Union - European Structural and Investment Funds in the frames of Operational Programme Research, Development and Education - project Hybrid Materials for Hierarchical Structures (HyHi, Reg. No. CZ.02.1.01/0.0/0.0/16_019/0000843). Finally, the work was supported by the Ministry of Science and Higher Education Republic of Poland within statutory funds No. 08/040/BK_19/0119, and by the National Centre for Research and Development in Poland (POIR.04.01.02-00-0062/16).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pal, A.; He, Y.; Jekel, M.; Reinhard, M.; Gin, K.Y.H. Emerging contaminants of public health significance as water quality indicator compounds in the urban water cycle. Environ. Int. 2014, 71, 46–62. [Google Scholar] [CrossRef]
- Tsiampalis, A.; Frontistis, Z.; Binas, V.; Kiriakidis, G.; Mantzavinos, D. Degradation of sulfamethoxazole using iron-doped titania and simulated solar radiation. Catalysts 2019, 9, 612. [Google Scholar] [CrossRef]
- Şen, S.; Demirer, G.N. Anaerobic treatment of real textile wastewater with a fluidized bed reactor. Water Res. 2003, 37, 1868–1878. [Google Scholar] [CrossRef]
- Ozturk, E.; Yetis, U.; Dilek, F.B.; Demirer, G.N. A chemical substitution study for a wet processing textile mill in Turkey. J. Clean. Prod. 2009, 17, 239–247. [Google Scholar] [CrossRef]
- Inoue, M.; Okada, F.; Sakurai, A.; Sakakibara, M. A new development of dyestuffs degradation system using ultrasound. Ultrason. Sonochem. 2006, 13, 313–320. [Google Scholar] [CrossRef]
- Eren, Z. Ultrasound as a basic and auxiliary process for dye remediation: A review. J. Environ. Manag. 2012, 104, 127–141. [Google Scholar] [CrossRef]
- Julkapli, N.M.; Bagheri, S.; Hamid, S.B.A. Recent advances in heterogeneous photocatalytic decolorization of synthetic dyes. Sci. World J. 2014, 2014, 692307. [Google Scholar] [CrossRef]
- Ghaly, A.E.; Ananthashankar, R.; Alhattab, M.; Ramakrishnan, V.V. Production, Characterization and Treatment of Textile Effluents: A Critical Review. J. Chem. Eng. Process. Technol. 2014, 5, 1000182. [Google Scholar]
- Chequer, F.D.; de Oliveira, G.A.R.; Ferraz, E.R.A.; Carvalho, J.; Zanoni, M.B.; de Oliveir, D.P. Textile Dyes: Dyeing Process and Environmental Impact. Eco-Friendly Text. Dye. Finish. 2013, 6, 151–176. [Google Scholar]
- Wang, J.; Guo, B.; Zhang, X.; Zhang, Z.; Han, J.; Wu, J. Sonocatalytic degradation of methyl orange in the presence of TiO2 catalysts and catalytic activity comparison of rutile and anatase. Ultrason. Sonochem. 2005, 12, 331–337. [Google Scholar] [CrossRef]
- Wijetunga, S.; Li, X.F.; Jian, C. Effect of organic load on decolourization of textile wastewater containing acid dyes in upflow anaerobic sludge blanket reactor. J. Hazard. Mater. 2010, 177, 792–798. [Google Scholar] [CrossRef]
- Entezari, M.H.; Al-Hoseini, Z.S.; Ashraf, N. Fast and efficient removal of Reactive Black 5 from aqueous solution by a combined method of ultrasound and sorption process. Ultrason. Sonochem. 2008, 15, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Crini, G.; Badot, P.-M. Sorption Processes and Pollution: Conventional and Non-Conventional Sorbents for Pollutant Removal from Wastewaters; Presses universitaires de Franche-Comté: Besançon, France, 2010; ISBN 2848673044. [Google Scholar]
- Puvaneswari, N.; Muthukrishnan, J.; Gunasekaran, P. Toxicity assessment and microbial degradation of azo dyes. Indian J. Exp. Biol. 2006, 44, 618–626. [Google Scholar]
- Oh, S.W.; Kang, M.N.; Cho, C.W.; Lee, M.W. Detection of carcinogenic amines from dyestuffs or dyed substrates. Dye. Pigment. 1997, 33, 119–135. [Google Scholar] [CrossRef]
- Fat’hi, M.R.; Asfaram, A.; Hadipour, A.; Roosta, M. Kinetics and thermodynamic studies for removal of Acid Blue 129 from aqueous solution by almond shell. J. Environ. Health Sci. Eng. 2014, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Acid Blue 129. Available online: http://datasheets.scbt.com/sc-214468.pdf (accessed on 22 April 2020).
- Pala, A.; Tokat, E. Color removal from cotton textile industry wastewater in an activated sludge system with various additives. Water Res. 2002, 36, 2920–2925. [Google Scholar] [CrossRef]
- Harrelkas, F.; Azizi, A.; Yaacoubi, A.; Benhammou, A.; Pons, M.N. Treatment of textile dye effluents using coagulation-flocculation coupled with membrane processes or adsorption on powdered activated carbon. Desalination 2009, 235, 330–339. [Google Scholar] [CrossRef]
- Cooper, P. Removing colour from dyehouse waste waters—A critical review of technology available. J. Soc. Dye. Colour. 1993, 109, 97–100. [Google Scholar] [CrossRef]
- Fane, A.G.; Fell, C.J.D. A review of fouling and fouling control in ultrafiltration. Desalination 1987, 62, 117–136. [Google Scholar] [CrossRef]
- Georgiou, D.; Melidis, P.; Aivasidis, A. Use of a microbial sensor: Inhibition effect of azo-reactive dyes on activated sludge. Bioprocess Biosyst. Eng. 2002, 25, 79–83. [Google Scholar]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of various recent wastewater dye removal methods: A review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Li, Q.; Wang, L.; Fang, X.; Zhang, L.; Li, J.; Xie, H. Synergistic effect of photocatalytic degradation of hexabromocyclododecane in water by UV/TiO2/persulfate. Catalysts 2019, 9, 189. [Google Scholar] [CrossRef]
- Tehrani, A.R.; Mahmood, N.M.; Arami, M. Study of the efficiency of effective parameters on decolorization of CI. Reactive black 5 wastewater by ozonation. J. Color Sci. Technol. 2008, 2, 67–75. [Google Scholar]
- Muruganandham, M.; Swaminathan, M. Photochemical oxidation of reactive azo dye with UV-H2O2 process. Dye. Pigment. 2004, 62, 269–275. [Google Scholar] [CrossRef]
- Mohey El-Dein, A.; Libra, J.A.; Wiesmann, U. Mechanism and kinetic model for the decolorization of the azo dye Reactive Black 5 by hydrogen peroxide and UV radiation. Chemosphere 2003, 52, 1069–1077. [Google Scholar] [CrossRef]
- Lovato, M.E.; Gilliard, M.B.; Cassano, A.E.; Martín, A.M. Kinetics of the degradation of n-butyl benzyl phthalate using O3/UV, direct photolysis, direct ozonation and UV effects. Environ. Sci. Pollut. Res. 2015, 22, 909–917. [Google Scholar] [CrossRef]
- Tehrani-Bagha, A.R.; Amini, F.L. Decolorization of a Reactive Dye by UV-Enhanced Ozonation. Prog. Col. Color. Coat. 2010, 3, 1–8. [Google Scholar]
- Ghernaout, D. Advanced oxidation phenomena in electrocoagulation process: A myth or a reality? Desalin. Water Treat. 2013, 51, 7536–7554. [Google Scholar] [CrossRef]
- Argun, M.E.; Karatas, M. Application of Fenton process for decolorization of reactive black 5 from synthetic wastewater: Kinetics and thermodynamics. Environ. Prog. Sustain. Energy 2011, 30, 540–548. [Google Scholar] [CrossRef]
- Meriç, S.; Kaptan, D.; Ölmez, T. Color and COD removal from wastewater containing Reactive Black 5 using Fenton’s oxidation process. Chemosphere 2004, 54, 435–441. [Google Scholar] [CrossRef]
- Li, J.; Li, R.; Zou, L.; Liu, X. Efficient degradation of norfloxacin and simultaneous electricity generation in a persulfate-photocatalytic fuel cell system. Catalysts 2019, 9, 835. [Google Scholar] [CrossRef]
- Rickman, K.A.; Mezyk, S.P. Kinetics and mechanisms of sulfate radical oxidation of β-lactam antibiotics in water. Chemosphere 2010, 81, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Olmez-Hanci, T.; Arslan-Alaton, I. Comparison of sulfate and hydroxyl radical based advanced oxidation of phenol. Chem. Eng. J. 2013, 224, 10–16. [Google Scholar] [CrossRef]
- Zheng, J.; Li, J.; Bai, J.; Xiaohantan, X.; Zeng, Q.; Li, L.; Zhou, B. Efficient degradation of refractory organics using sulfate radicals generated directly from WO3 photoelectrode and the catalytic reaction of sulfate. Catalysts 2017, 7, 346. [Google Scholar] [CrossRef]
- Neta, P.; Madhavan, V.; Zemel, H.; Fessenden, R.W. Rate Constants and Mechanism of Reaction of SO4 with Aromatic Compounds. J. Am. Chem. Soc. 1977, 99, 163–164. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical Review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (⋅OH/⋅O− in Aqueous Solution. J. Phys. Chem. Ref. Data 1988, 17, 513. [Google Scholar] [CrossRef]
- Tang, W.Z. Huren an Photocatalytic degradation kinetics and mechanism of acid blue 40 by TiO2/UV in aqueous solution. Chemosphere 1995, 31, 4171–4183. [Google Scholar] [CrossRef]
- Li, X.; Tang, S.; Yuan, D.; Tang, J.; Zhang, C.; Li, N.; Rao, Y. Improved degradation of anthraquinone dye by electrochemical activation of PDS. Ecotoxicol. Environ. Saf. 2019, 177, 77–85. [Google Scholar] [CrossRef]
- Wacławek, S.; Lutze, H.V.; Grübel, K.; Padil, V.V.T.; Černík, M.; Dionysiou, D.D. Chemistry of persulfates in water and wastewater treatment: A review. Chem. Eng. J. 2017, 330, 44–62. [Google Scholar] [CrossRef]
- Yang, J.; Zeng, Z.; Huang, Z.; Cui, Y. Acceleration of persulfate activation by MIL-101(Fe) with vacuum thermal activation: Effect of FeII/FeIII mixed-valence center. Catalysts 2019, 9, 906. [Google Scholar] [CrossRef]
- Duan, X.; Sun, H.; Kang, J.; Wang, Y.; Indrawirawan, S.; Wang, S. Insights into heterogeneous catalysis of persulfate activation on dimensional-structured nanocarbons. ACS Catal. 2015, 5, 4629–4636. [Google Scholar] [CrossRef]
- Ji, Y.; Shi, Y.; Yang, Y.; Yang, P.; Wang, L.; Lu, J.; Li, J.; Zhou, L.; Ferronato, C.; Chovelon, J.M. Rethinking sulfate radical-based oxidation of nitrophenols: Formation of toxic polynitrophenols, nitrated biphenyls and diphenyl ethers. J. Hazard. Mater. 2019, 361, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Gözmen, B.; Kayan, B.; Gizir, A.M.; Hesenov, A. Oxidative degradations of reactive blue 4 dye by different advanced oxidation methods. J. Hazard. Mater. 2009, 168, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Lizama, C.; Freer, J.; Baeza, J.; Mansilla, H.D. Optimized photodegradation of reactive blue 19 on TiO2 and ZnO suspensions. Catal. Today 2002, 76, 235–246. [Google Scholar] [CrossRef]
- Tehrani-Bagha, A.R.; Mahmoodi, N.M.; Menger, F.M. Degradation of a persistent organic dye from colored textile wastewater by ozonation. Desalination 2010, 260, 34–38. [Google Scholar] [CrossRef]
- Bilal, M.; Rasheed, T.; Iqbal, H.M.N.; Li, C.; Wang, H.; Hu, H.; Wang, W.; Zhang, X. Photocatalytic degradation, toxicological assessment and degradation pathway of C.I. Reactive Blue 19 dye. Chem. Eng. Res. Des. 2018, 129, 384–390. [Google Scholar] [CrossRef]
- Becelic-Tomin, M.; Dalmacija, B.; Rajic, L.; Tomasevic, D.; Kerkez, D.; Watson, M.; Prica, M. Degradation of anthraquinone dye reactive blue 4 in pyrite ash catalyzed fenton reaction. Sci. World J. 2014, 2014, 234654. [Google Scholar] [CrossRef]
- Lovato, M.E.; Fiasconaro, M.L.; Martín, C.A. Degradation and toxicity depletion of RB19 anthraquinone dye in water by ozone-based technologies. Water Sci. Technol. 2017, 75, 813–822. [Google Scholar] [CrossRef]
- Radovic, M.; Mitrovic, J.; Kostic, M.; Bojic, D.; Petrovic, M.; Najdanovic, S.; Bojic, A. Comparison of ultraviolet radiation/hydrogen peroxide, Fenton and photo-Fenton processes for the decolorization of reactive dyes. Hem. Ind. 2015, 69, 657–665. [Google Scholar] [CrossRef]
- Sharma, S.; Patel, S.; Ruparelia, J. Feasibility study on degradation of RR120 dye from water by O3, O3/UV and O3/UV/Persulfate. In In Multi-disciplinary Sustainable Engineering: Current and Future Trends, Proceedings of the 5th Nirma University International Conference on Engineering, Ahmedabad, India, 26–28 November 2015; CRC Press: Boca Raton, FL, USA, 2016; p. 233. [Google Scholar]
- Herrmann, H.; Reese, A.; Zellner, R. Time-resolved UV/VIS diode array absorption spectroscopy of SOx− (x=3, 4, 5) radical anions in aqueous solution. J. Mol. Struct. 1995, 348, 183–186. [Google Scholar] [CrossRef]
- Tan, C.; Gao, N.; Deng, Y.; Zhang, Y.; Sui, M.; Deng, J.; Zhou, S. Degradation of antipyrine by UV, UV/H2O2 and UV/PS. J. Hazard. Mater. 2013, 260, 1008–1016. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.S.; He, X.; Khan, H.M.; Khan, J.A.; O’Shea, K.E.; Boccelli, D.L.; Dionysiou, D.D. Efficient removal of endosulfan from aqueous solution by UV-C/peroxides: A comparative study. J. Hazard. Mater. 2013, 263, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Neta, P.; Huie, R.E.; Ross, A.B. Rate Constants for Reactions of Inorganic Radicals in Aqueous Solution. J. Phys. Chem. Ref. Data 1988, 17, 1027–1284. [Google Scholar] [CrossRef]
- De Laat, J.; Le, T.G. Kinetics and modeling of the Fe(III)/H2O2 system in the presence of sulfate in acidic aqueous solutions. Environ. Sci. Technol. 2005, 39, 1811–1818. [Google Scholar] [CrossRef]
- Yang, Z.; Su, R.; Luo, S.; Spinney, R.; Cai, M.; Xiao, R.; Wei, Z. Comparison of the reactivity of ibuprofen with sulfate and hydroxyl radicals: An experimental and theoretical study. Sci. Total Environ. 2017, 590, 751–760. [Google Scholar] [CrossRef]
- Ghauch, A.; Baalbaki, A.; Amasha, M.; El Asmar, R.; Tantawi, O. Contribution of persulfate in UV-254 nm activated systems for complete degradation of chloramphenicol antibiotic in water. Chem. Eng. J. 2017, 317, 1012–1025. [Google Scholar] [CrossRef]
- Silveira, J.E.; Garcia-Costa, A.L.; Cardoso, T.O.; Zazo, J.A.; Casas, J.A. Indirect decolorization of azo dye Disperse Blue 3 by electro-activated persulfate. Electrochim. Acta 2017, 258, 927–932. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, H.; Hou, L. Degradation of C. I. Acid Orange 7 in aqueous solution by a novel electro/Fe3O4/PDS process. J. Hazard. Mater. 2014, 276, 182–191. [Google Scholar] [CrossRef]
- Liang, C.; Wang, Z.S.; Bruell, C.J. Influence of pH on persulfate oxidation of TCE at ambient temperatures. Chemosphere 2007, 66, 106–113. [Google Scholar] [CrossRef]
- Asghar, A.; Bello, M.M.; Raman, A.A.A.; Daud, W.M.A.W.; Ramalingam, A.; Zain, S.B.M. Predicting the degradation potential of Acid blue 113 by different oxidants using quantum chemical analysis. Heliyon 2019, 5, e02396. [Google Scholar] [CrossRef]
- Lang, A.R. Dyes and Pigments: New Research; Nova Science Publishers: Hauppauge, NY, USA, 2009; ISBN 978-1-60876-195-1. [Google Scholar]
- Cinar, Z. The Role of Molecular Modeling in TiO2 Photocatalysis. Molecules 2017, 22, 556. [Google Scholar] [CrossRef]
- Liu, S.; Rong, C.; Lu, T. Information conservation principle determines electrophilicity, nucleophilicity, and regioselectivity. J. Phys. Chem. A 2014, 118, 3698–3704. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Ye, T.; Wei, Z.; Luo, S.; Yang, Z.; Spinney, R. Quantitative Structure–Activity Relationship (QSAR) for the Oxidation of Trace Organic Contaminants by Sulfate Radical. Environ. Sci. Technol. 2015, 49, 13394–13402. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, P.; Sree, K.S.; Appenroth, K.J. Duckweeds for water remediation and toxicity testing. Toxicol. Environ. Chem. 2016, 98, 1127–1154. [Google Scholar] [CrossRef]
- Žaltauskaitė, J.; Sujetovienė, G.; Čypaitė, A.; Aužbikavičiūtė, A. Lemna Minor as a Tool for Wastewater Toxicity Assessment and Pollutants Removal Agent. In Proceedings of the 9th International Conference on Environmental Engineering, Vilnius, Lithuania, 22–23 May 2014. [Google Scholar]
- Mkandawire, M.; Teixeira Da Silva, J.A.; Dudel, E.G. The lemna bioassay: Contemporary issues as the most standardized plant bioassay for aquatic ecotoxicology. Crit. Rev. Environ. Sci. Technol. 2014, 44, 154–197. [Google Scholar] [CrossRef]
- Sackey, L.N.A.; Kočí, V.; van Gestel, C.A.M. Ecotoxicological effects on Lemna minor and Daphnia magna of leachates from differently aged landfills of Ghana. Sci. Total Environ. 2020, 698, 134295. [Google Scholar] [CrossRef]
- Castro, A.M.; Nogueira, V.; Lopes, I.; Rocha-Santos, T.; Pereira, R. Evaluation of the potential toxicity of effluents from the textile industry before and after treatment. Appl. Sci. 2019, 9, 3804. [Google Scholar] [CrossRef]
- Kudlek, E. Identification of Degradation By-Products of Selected Pesticides during Oxidation and Chlorination Processes. Ecol. Chem. Eng. S 2019, 26, 571–581. [Google Scholar] [CrossRef]
- Neamtu, M.; Siminiceanu, I.; Yediler, A.; Kettrup, A. Kinetics of decolorization and mineralization of reactive azo dyes in aqueous solution by the UV/H2O2 oxidation. Dye. Pigment. 2002, 53, 93–99. [Google Scholar] [CrossRef]
- Palencia, M.; Martínez, J.M.; Arrieta, Á. Removal of Acid Blue 129 dye by Polymer-Enhanced Ultrafiltration (PEUF). J. Sci. Technol. Appl. 2017, 2, 65–74. [Google Scholar] [CrossRef]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters, 3rd ed.; Wiley: Hoboken, NJ, USA, 2012; ISBN 1118591488. [Google Scholar]
- Silvestri, D.; Wacławek, S.; Venkateshaiah, A.; Krawczyk, K.; Sobel, B.; Padil, V.V.T.; Černík, M.; Varma, R.S. Synthesis of Ag nanoparticles by a chitosan-poly(3-hydroxybutyrate) polymer conjugate and their superb catalytic activity. Carbohydr. Polym. 2020, 232, 115806. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef]
- Neese, F. The ORCA program system. WIRES Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Fu, R.; Lu, T.; Chen, F.W. Comparing methods for predicting the reactive site of electrophilic substitution. Acta Phys.-Chim. Sin. 2014, 30, 628–639. [Google Scholar]
- Santana, C.M.; Ferrera, Z.S.; Padrón, M.E.T.; Rodríguez, J.J.S. Methodologies for the extraction of phenolic compounds from environmental samples: New approaches. Molecules 2009, 14, 298–320. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).