Propane and Naphthalene Oxidation over Gold-Promoted Cobalt Catalysts Supported on Zirconia
Abstract
1. Introduction
2. Results and Discussion
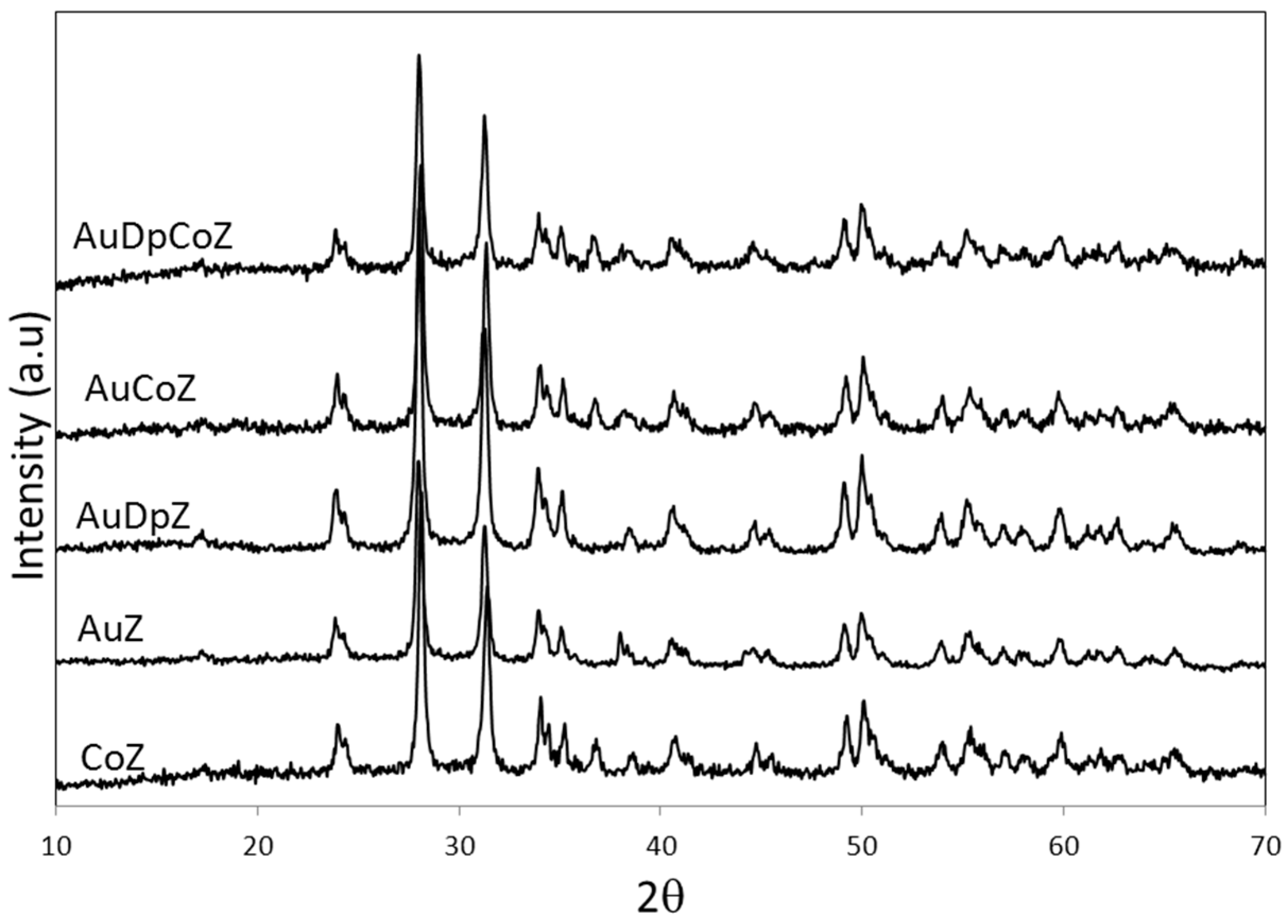
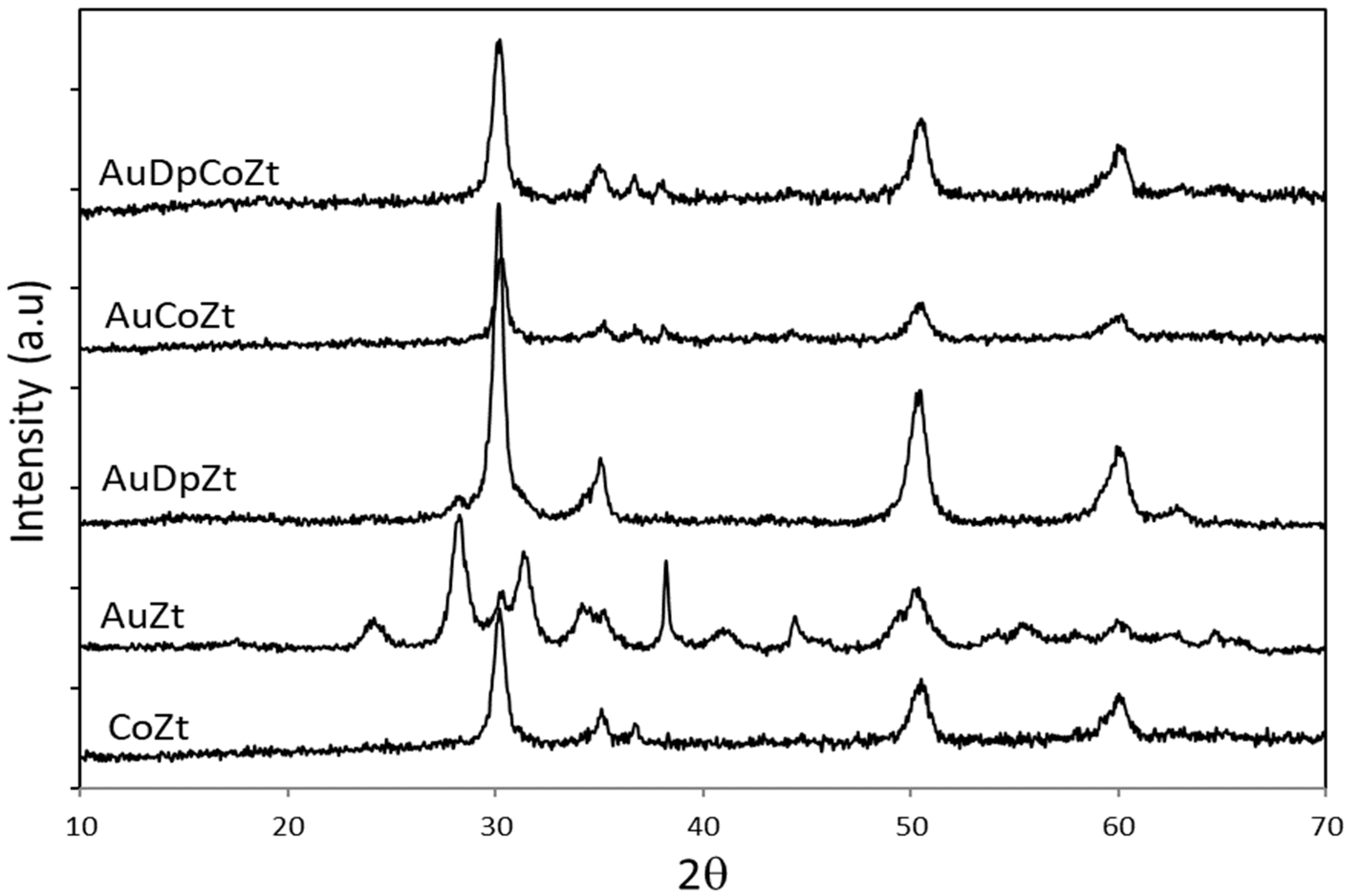
2.1. Catalyst Characterizationm
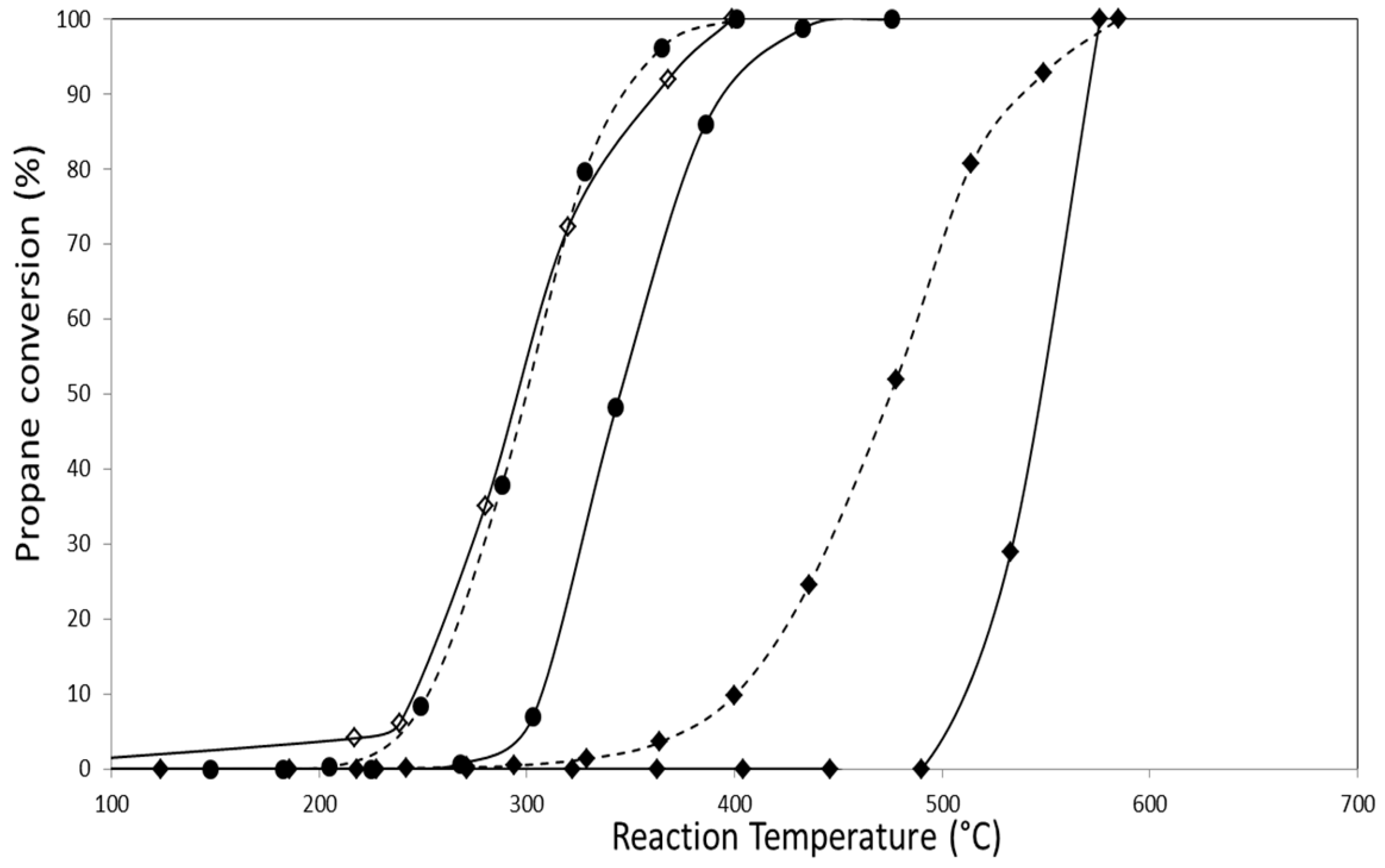
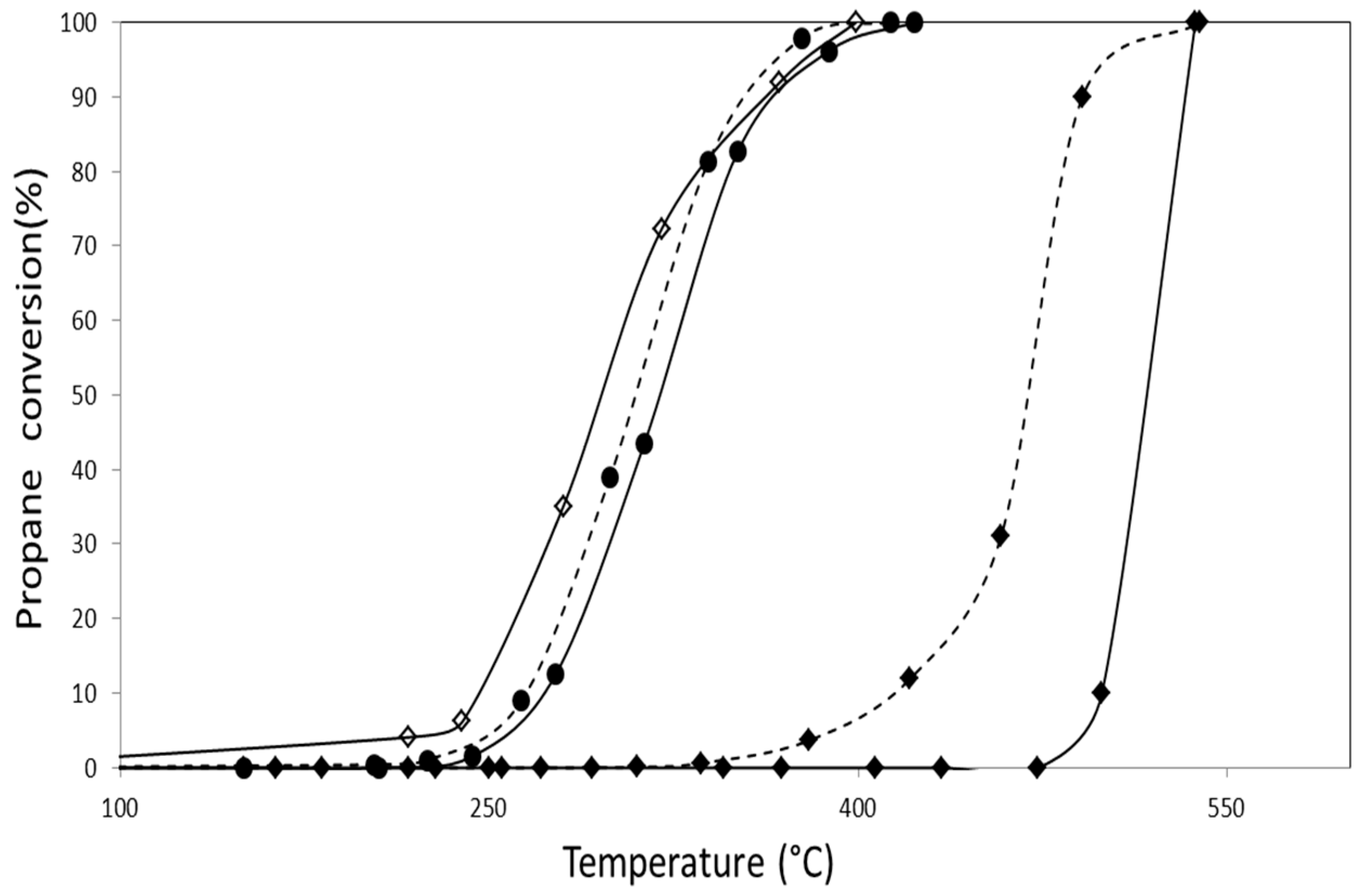
2.2. Catalytic Activity
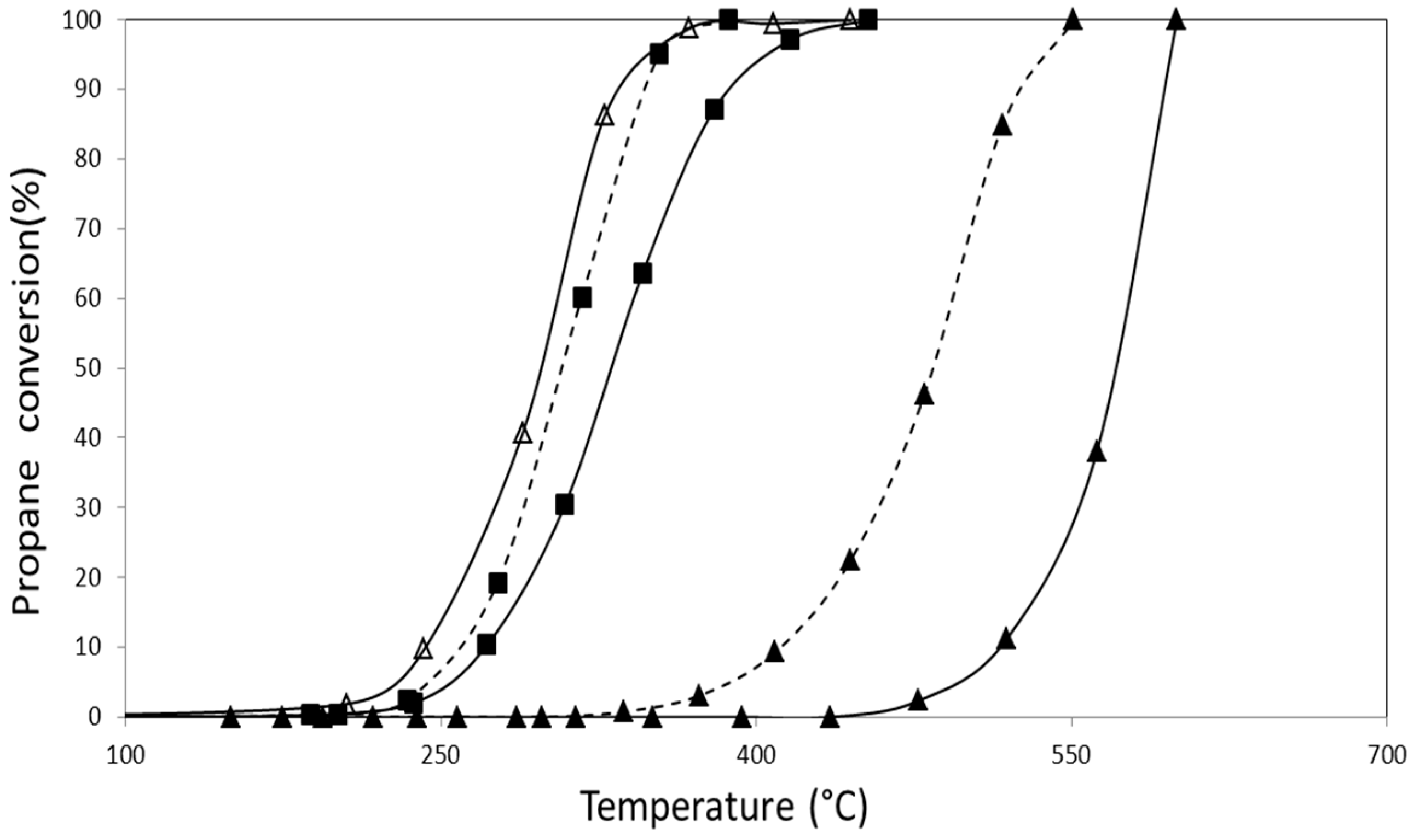
2.2.1. Catalytic Combustion of Propane in the Presence of O2/He
2.2.2. Catalytic Combustion of Propane in the Presence of O2/NO/He
2.2.3. Catalytic Combustion of Naphthalene
3. Materials and Methods
3.1. Catalyst Preparation and Characterization
3.2. Catalyst Characterization
3.3. Catalytic Activity
3.3.1. Propane Oxidation
3.3.2. Naphthalene Oxidation
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Aranda, A.; López, J.M.; Murillo, R.; Mastral, A.M.; Dejoz, A.; Vázquez, I.; Solsona, B.; Taylor, S.H.; García, A.M.D. Total oxidation of naphthalene with high selectivity using a ceria catalyst prepared by a combustion method employing ethylene glycol. J. Hazard. Mater. 2009, 171, 393–399. [Google Scholar] [CrossRef]
- Genuino, H.C.; Dharmarathna, S.; Njagi, E.C.; Mei, M.C.; Suib, S.L. Gas-Phase Total Oxidation of Benzene, Toluene, Ethylbenzene, and Xylenes Using Shape-Selective Manganese Oxide and Copper Manganese Oxide Catalysts. J. Phys. Chem. C 2012, 116, 12066–12078. [Google Scholar] [CrossRef]
- Aparicio, M.S.L.; Lick, I.D. Total oxidation of propane and naphthalene from emission sources with supported cobalt catalysts. React. Kinet. Mech. Catal. 2016, 119, 469–479. [Google Scholar] [CrossRef]
- He, C.; Cheng, J.; Zhang, X.; Douthwaite, M.; Pattisson, S.; Hao, Z. Recent Advances in the Catalytic Oxidation of Volatile Organic Compounds: A Review Based on Pollutant Sorts and Sources. Chem. Rev. 2019, 119, 4471–4568. [Google Scholar] [CrossRef]
- Haruta, M.; Kobayashi, T.; Sano, H.; Yamada, N. Novel gold catalysts for the oxidation of carbon monoxide at a temperature far below 0.DEG.C. Chem. Lett. 1987, 405–408. [Google Scholar] [CrossRef]
- Haruta, M. Gold catalysts prepared by coprecipitation for low-temperature oxidation of hydrogen and of carbon monoxide. J. Catal. 1989, 115, 301–309. [Google Scholar] [CrossRef]
- Bond, G.C.; Louis, C.; Thompson, D.T. Catalysis by Gold; Imperial College Press: London, UK, 2006; pp. 4105–4108. [Google Scholar]
- Wu, P.; Loh, K.P.; Zhao, X.S. Supported Gold Catalysts for Selective Oxidation of Organics. Sci. Adv. Mater. 2011, 3, 970–983. [Google Scholar] [CrossRef]
- De Almeida, M.P.; Martins, L.M.D.R.S.; Carabineiro, S.A.C.; Lauterbach, T.; Rominger, F.; Hashmi, A.S.K.; Pombeiro, A.J.L.; Figueiredo, J.L. Homogeneous and heterogenised new gold C-scorpionate complexes as catalysts for cyclohexane oxidation. Catal. Sci. Technol. 2013, 3, 3056–3069. [Google Scholar] [CrossRef]
- Ribeiro, A.P.C.; Martins, L.M.D.R.S.; Carabineiro, S.; Figueiredo, J.L.; Pombeiro, A.J.L. Gold nanoparticles deposited on surface modified carbon materials as reusable catalysts for hydrocarboxylation of cyclohexane. Appl. Catal. A: Gen. 2017, 547, 124–131. [Google Scholar] [CrossRef]
- Gluhoi, A.C.; Bogdanchikova, N.; Nieuwenhuys, B.E. Total oxidation of propene and propane over gold–copper oxide on alumina catalysts. Catal. Today 2006, 113, 178–181. [Google Scholar] [CrossRef]
- Haruta, M. Size- and support-dependency in the catalysis of gold. Catal. Today 1997, 36, 153–166. [Google Scholar] [CrossRef]
- Gluhoi, A.; Bogdanchikova, N.; Nieuwenhuys, B. The effect of different types of additives on the catalytic activity of Au/Al2O3 in propene total oxidation: Transition metal oxides and ceria. J. Catal. 2005, 229, 154–162. [Google Scholar] [CrossRef]
- Scirè, S.; Liotta, L.F. Supported gold catalysts for the total oxidation of volatile organic compounds. Appl. Catal. B Environ. 2012, 125, 222–246. [Google Scholar] [CrossRef]
- Trigueiro, F.; Ferreira, C.; Volta, J.-C.; Gonzalez, W.; De Oliveria, P.P. Effect of niobium addition to Co/γ-Al2O3 catalyst on methane combustion. Catal. Today 2006, 118, 425–432. [Google Scholar] [CrossRef]
- Solsona, B.; García, T.; Hutchings, G.J.; Taylor, S.H.; Makkee, M. TAP reactor study of the deep oxidation of propane using cobalt oxide and gold-containing cobalt oxide catalysts. Appl. Catal. A Gen. 2009, 365, 222–230. [Google Scholar] [CrossRef]
- Solsona, B.; Garcia, T.; Jones, C.; Taylor, S.H.; Carley, A.; Hutchings, G. Supported gold catalysts for the total oxidation of alkanes and carbon monoxide. Appl. Catal. A Gen. 2006, 312, 67–76. [Google Scholar] [CrossRef]
- Ali, A.M.; Daous, M.A.; Khamis, A.A.; Driss, H.; Burch, R.; Petrov, L. Strong synergism between gold and manganese in an Au–Mn/triple-oxide-support (TOS) oxidation catalyst. Appl. Catal. A Gen. 2015, 489, 24–31. [Google Scholar] [CrossRef]
- Solsona, B.; García, A.M.D.; Aylón, E.; Dejoz, A.M.; Vázquez, I.; Agouram, S.; Davies, T.E.; Taylor, S.H. Promoting the activity and selectivity of high surface area Ni–Ce–O mixed oxides by gold deposition for VOC catalytic combustion. Chem. Eng. J. 2011, 175, 271–278. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; Xu, B.-Q. Remarkable Nanosize Effect of Zirconia in Au/ZrO2Catalyst for CO Oxidation†. J. Phys. Chem. B 2005, 109, 9678–9683. [Google Scholar] [CrossRef]
- Campa, M.; Ferraris, G.; Gazzoli, D.; Pettiti, I.; Pietrogiacomi, D. Rhodium supported on tetragonal or monoclinic ZrO2 as catalyst for the partial oxidation of methane. Appl. Catal. B Environ. 2013, 142, 423–431. [Google Scholar] [CrossRef]
- Matsui, K.; Ohgai, M. Formation Mechanism of Hydrous Zirconia Particles Produced by the Hydrolysis of ZrOCl2 Solutions: III, Kinetics Study for the Nucleation and Crystal-Growth Processes of Primary Particles. J. Am. Ceram. Soc. 2004, 84, 2303–2312. [Google Scholar] [CrossRef]
- Aparicio, M.L.; Ocsachoque, M.A.; Gazzoli, D.; Botto, I.L.; Lick, I.D. Total Oxidation of Naphthalene with Zirconia-Supported Cobalt, Copper and Nickel Catalysts. Catalysts 2017, 7, 293. [Google Scholar] [CrossRef]
- Montaña, M.; Aparicio, M.L.; Ocsachoque, M.A.; Navas, M.B.; Barros, I.; Rodríguez-Castellón, E.; Casella, M.L.; Lick, I.D. Zirconia-Supported Silver Nanoparticles for the Catalytic Combustion of Pollutants Originating from Mobile Sources. Catalysts 2019, 9, 297. [Google Scholar] [CrossRef]
- Rhodes, M.D.; Bell, A.T. The effects of zirconia morphology on methanol synthesis from CO and H2 over Cu/ZrO2 catalysts: Part I. Steady-state studies. J. Catal. 2005, 233, 198–209. [Google Scholar] [CrossRef]
- Benito, M.; Padilla, R.M.; Rodríguez, L.; Sanz, J.; Daza, L. Zirconia supported catalysts for bioethanol steam reforming: Effect of active phase and zirconia structure. J. Power Sources 2007, 169, 167–176. [Google Scholar] [CrossRef]
- Toraya, H.; Yoshimura, M.; Somiya, S. Calibration Curve for Quantitative Analysis of the Monoclinic-Tetragonal ZrO2System by X-Ray Diffraction. J. Am. Ceram. Soc. 1984, 67, 119–121. [Google Scholar] [CrossRef]
- Jaworski, M.; Lick, I.D.; Siri, G.J.; Casella, M.L. ZrO2-modified Al2O3-supported PdCu catalysts for the water denitrification reaction. Appl. Catal. B Environ. 2014, 156, 53–61. [Google Scholar] [CrossRef]
- Wyrwalski, F.; Lamonier, J.-F.; Siffert, S.; Aboukaïs, A. Additional effects of cobalt precursor and zirconia support modifications for the design of efficient VOC oxidation catalysts. Appl. Catal. B Environ. 2007, 70, 393–399. [Google Scholar] [CrossRef]
- Labaki, M.; Siffert, S.; Lamonier, J.-F.; Zhilinskaya, E.A.; Aboukaïs, A. Total oxidation of propene and toluene in the presence of zirconia doped by copper and yttrium. Appl. Catal. B Environ. 2003, 43, 261–271. [Google Scholar] [CrossRef]
- Fierro, G. A Study of Anomalous Temperature-Programmed Reduction Profiles of Cu2O, CuO, and CuO-ZnO Catalysts. J. Catal. 1994, 148, 709–721. [Google Scholar] [CrossRef]
- Solsona, B.; Aylón, E.; Murillo, R.; Mastral, A.M.; Monzonís, A.; Agouram, S.; Davies, T.E.; Taylor, S.H.; García, A.M.D. Deep oxidation of pollutants using gold deposited on a high surface area cobalt oxide prepared by a nanocasting route. J. Hazard. Mater. 2011, 187, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Centi, G.; Cerrato, G.; D’Angelo, S.; Finardi, U.; Giamello, E.; Morterra, C.; Perathoner, S. Catalytic behavior and nature of active sites in copper-on-zirconia catalysts for the decomposition of N2O. Catal. Today 1996, 27, 265–270. [Google Scholar] [CrossRef]
- Liu, S.Y.; Yang, S.M. Complete oxidation of 2-propanol over gold-based catalysts supported on metal oxides. Appl. Catal. A Gen. 2008, 334, 92–99. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Mou, C.-Y.; Wan, B.-Z. Ultrasmall gold nanoparticles confined in zeolite Y: Preparation and activity in CO oxidation. Appl. Catal. B Environ. 2017, 218, 506–514. [Google Scholar] [CrossRef]
- Grzona, C.B.; Lick, I.D.; Rodríguez-Castellón, E.; Ponzi, M.I.; Ponzi, E.N. Cobalt and KNO3 supported on alumina catalysts for diesel soot combustion. Mater. Chem. Phys. 2010, 123, 557–562. [Google Scholar] [CrossRef]
- Yao, J.; Lu, H.; Xiao, Y.; Hou, B.; Li, D.; Jia, L. Sub-molten salt-acid treatment of LaCoO3 for a highly active catalyst towards propane combustion. Catal. Commun. 2019, 128, 105718. [Google Scholar] [CrossRef]
- Liotta, L.F.; Di Carlo, G.; Pantaleo, G.; Venezia, A.M.; Deganello, G. Co3O4/CeO2 composite oxides for methane emissions abatement: relationship between Co3O4–CeO2 interaction and catalytic activity. Appl. Catal. B Environ. 2006, 66, 217–227. [Google Scholar] [CrossRef]
- Wang, H.-T.; Liu, Y.-N.; Kang, X.-H.; Wang, Y.-F.; Yang, S.-Y.; Bian, S.-W.; Zhu, Q. Flexible hybrid yarn-shaped supercapacitors based on porous nickel cobalt sulfide nanosheet array layers on gold metalized cotton yarns. J. Colloid Interface Sci. 2018, 532, 527–535. [Google Scholar] [CrossRef]
- Solsona, B.; García, T.; Agouram, S.; Hutchings, G.J.; Taylor, S.H. The effect of gold addition on the catalytic performance of copper manganese oxide catalysts for the total oxidation of propane. Appl. Catal. B Environ. 2011, 101, 388–396. [Google Scholar] [CrossRef]
- Solsona, B.; Pérez-Cabero, M.; Vázquez, I.; Dejoz, A.; García, T.; Alvarez-Rodriguez, J.; El-Haskouri, J.; Beltrán, D.; Amorós, P.; García, A.M.D. Total oxidation of VOCs on Au nanoparticles anchored on Co doped mesoporous UVM-7 silica. Chem. Eng. J. 2012, 187, 391–400. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Zeng, X.; Zhu, T. Correlation between the physicochemical properties and catalytic performances of micro/mesoporous CoCeO mixed oxides for propane combustion. Appl. Catal. A Gen. 2019, 572, 61–70. [Google Scholar] [CrossRef]
- Solsona, B.; Sanchis, R.; Dejoz, A.M.; García, A.M.D.; Ruiz-Rodríguez, L.; Nieto, J.M.L.; Cecilia, J.A.; Rodríguez-Castellón, E. Total Oxidation of Propane Using CeO2 and CuO-CeO2 Catalysts Prepared Using Templates of Different Nature. Catalysts 2017, 7, 96. [Google Scholar] [CrossRef]
- García, A.M.D.; Sellick, D.; Varela, F.; Vázquez, I.; Dejoz, A.; Agouram, S.; Taylor, S.H.; Solsona, B. Total oxidation of naphthalene using bulk manganese oxide catalysts. Appl. Catal. A Gen. 2013, 450, 169–177. [Google Scholar] [CrossRef]
- Wu, H.; Wang, L.; Zhang, J.; Shen, Z.; Zhao, J. Catalytic oxidation of benzene, toluene and p-xylene over colloidal gold supported on zinc oxide catalyst. Catal. Commun. 2011, 12, 859–865. [Google Scholar] [CrossRef]
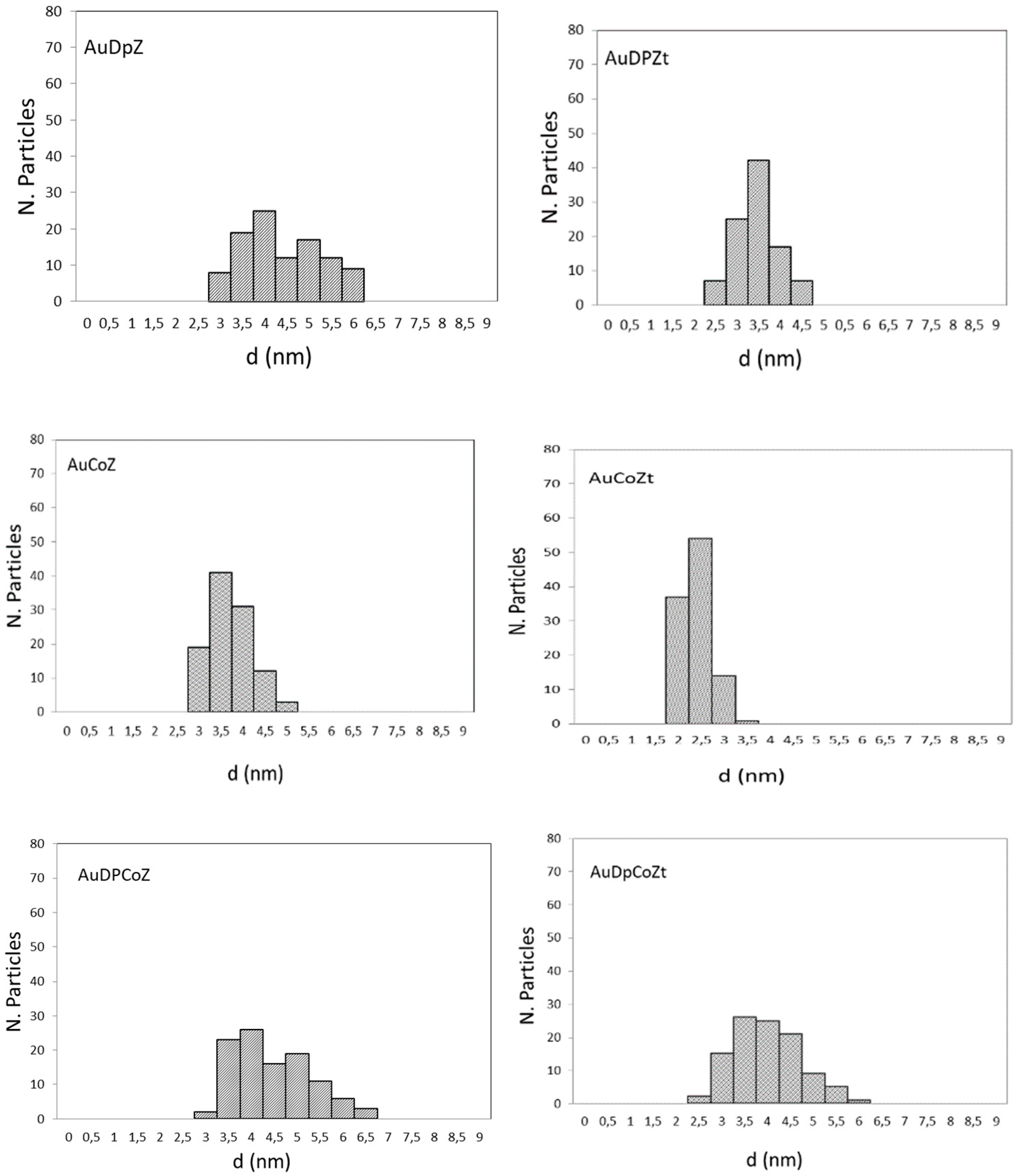
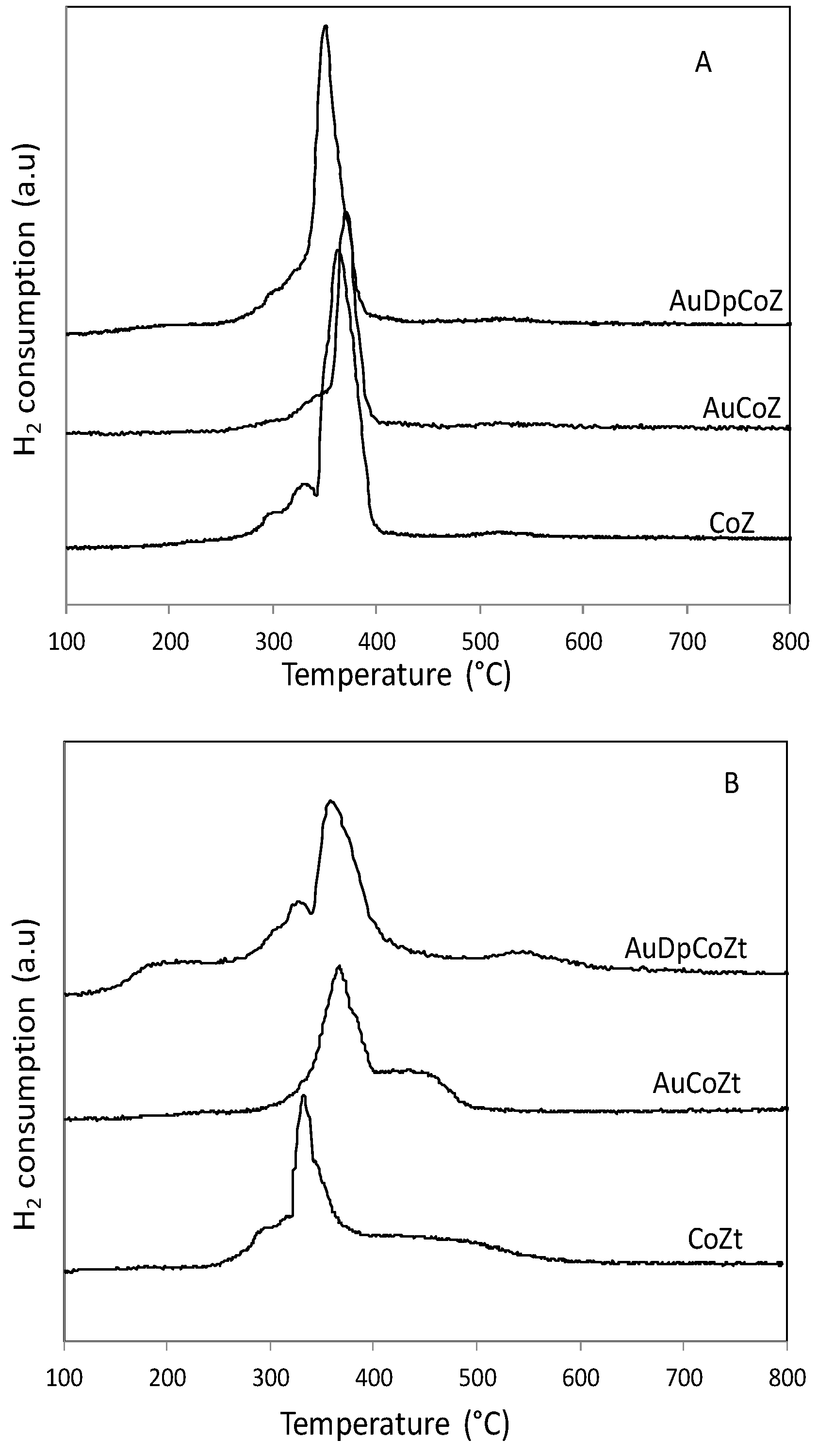
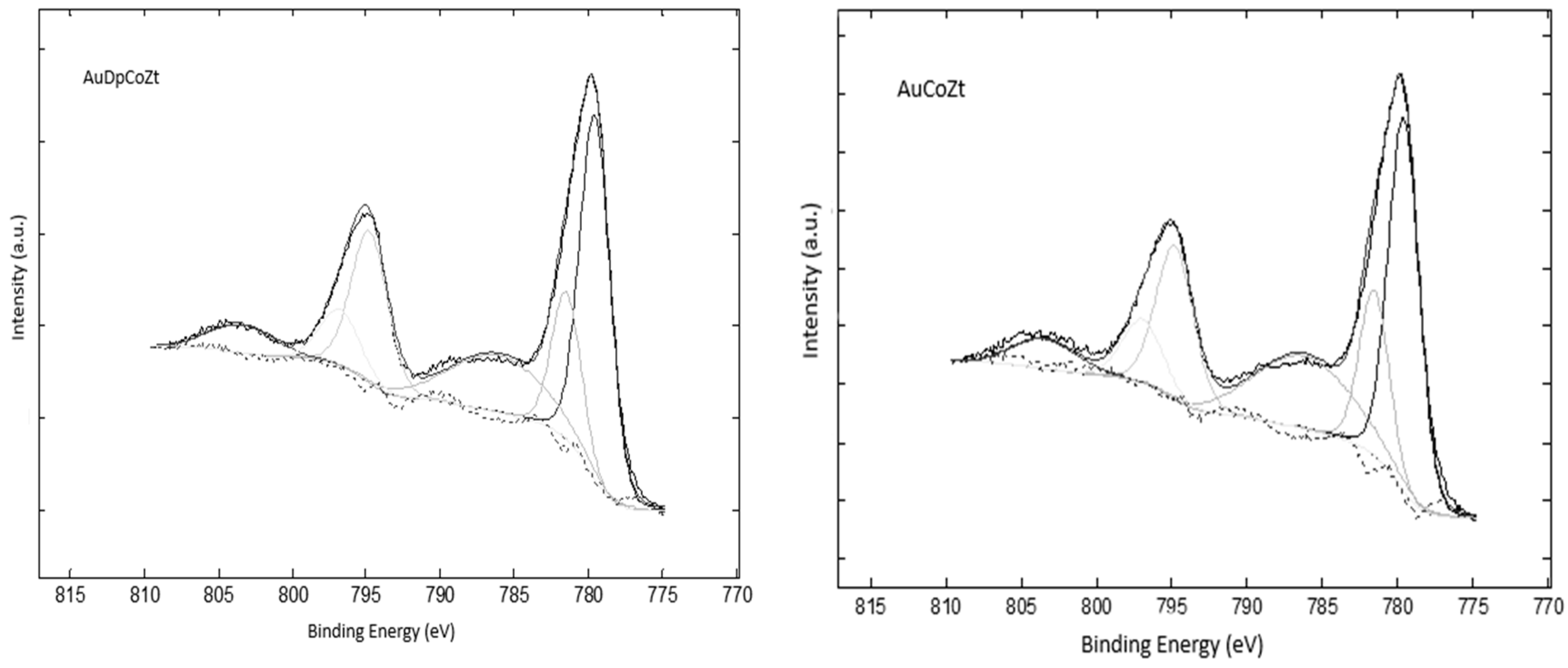

| Catalyst | SBET (m2/g) | Nominal Co/Z | EDS Co/Z | Co3O4 Crystal Size (nm) | Au Particle Size (nm) |
|---|---|---|---|---|---|
| ZrO2 (Z) | 8 | - | - | - | - |
| ZrO2.nH2O (Zt) | 340 | - | - | - | - |
| Zt 600 | 60 | - | - | - | - |
| AuZ | 8 | - | - | - | 3.6 and >30 |
| AuZt | 58 | - | - | - | 1.9 and > 20 |
| AuDpZ | 8 | - | - | - | 4.2 |
| AuDpZt | 60 | - | - | - | 3.1 |
| CoZ | 8 | 0.109 | 0.252 | 15 | - |
| CoZt | 37 | 0.109 | 0.240 | 22 | - |
| AuCoZ | 7 | - | - | 18 | 3.5 |
| AuDpCoZ | 8 | 0.109 | 0.067 | 18 | 4.3 |
| AuCoZt | 35 | - | - | 19 | 2.1 |
| AuDpCoZt | 34 | 0.109 | 0.103 | 17 | 3.2 |
| Catalyst | Co 2p3/2 | Co 2p3/2 sat | pp-sat a | Co 2p1/2 | Co 2p1/2 sat | ΔE b |
|---|---|---|---|---|---|---|
| AuCoZ | 779.7-781.6 | 786.2 | 6.9 | 794.9 | 803.6 | 15.2 |
| AuDpCoZ | 779.7-781.6 | 786.0 | 6.3 | 794.9 | 803.6 | 15.2 |
| AuCoZt | 779.5-781.5 | 786.1 | 6.5 | 794.7 | 803.4 | 15.2 |
| AuDpCoZt | 779.7-781.6 | 786.0 | 6.3 | 794.8 | 803.4 | 15.2 |
| Catalyst | Co/Z | Au/Z | Au/Co | Co2+/Co3+ |
|---|---|---|---|---|
| AuZ | --- | 0.0143 | --- | --- |
| AuZt | --- | 0.0008 | --- | --- |
| AuDpZ | --- | 0.017 | --- | --- |
| AuDpZt | --- | 0.0083 | --- | --- |
| AuCoZ | 1.4038 | 0.0247 | 0.0175 | 0.40 |
| AuDpCoZ | 2.5920 | 0.0547 | 0.0205 | 0.38 |
| AuCoZt | 0.7757 | 0.0225 | 0.0173 | 0.43 |
| AuDpCoZt | 2.0516 | 0.0613 | 0.0320 | 0.39 |
| Catalyst | Propane Combustion | |
|---|---|---|
| T50 (°C) | T100 (°C) | |
| AuZ | 550 | 576 |
| AuZt | 457 | 551 |
| AuDpZ | 478 | 585 |
| AuDpZt | 470 | 595 |
| CoZ | 300 | 400 |
| CoZt | 295 | 410 |
| AuCoZ | 342 | 442 |
| AuDpCoZ | 300 | 400 |
| AuCoZt | 320 | 410 |
| AuDpCoZt | 265 | 340 |
| Catalyst | Propane Combustion | |
|---|---|---|
| T50 (°C) in NO Absence | T50 (°C) in NO Presence | |
| AuZ | 550 | 520 |
| AuDpZ | 478 | 465 |
| CoZ | 300 | 305 |
| AuCoZ | 342 | 320 |
| AuDpCoZ | 300 | 308 |
| AuZt | 457 | 515 |
| AuDpZt | 470 | 480 |
| CoZt | 295 | 320 |
| AuCoZt | 320 | 330 |
| AuDpCoZt | 265 | 305 |
| Catalyst | Naphthalene Combustion | |
|---|---|---|
| T50 (°C) | T100 (°C) | |
| AuZ | 310 | 400 |
| AuZt | 300 | 370 |
| AuDpZ | 259 | 340 |
| AuDpZt | 230 | 300 |
| CoZ | 190 | 275 |
| CoZt | 205 | 300 |
| AuCoZ | 210 | 350 |
| AuDpCoZ | 210 | 340 |
| AuCoZt | 195 | 350 |
| AuDpCoZt | 195 | 260 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leguizamón Aparicio, M.S.; Ruiz, M.L.; Ocsachoque, M.A.; Ponzi, M.I.; Rodríguez-Castellón, E.; Lick, I.D. Propane and Naphthalene Oxidation over Gold-Promoted Cobalt Catalysts Supported on Zirconia. Catalysts 2020, 10, 387. https://doi.org/10.3390/catal10040387
Leguizamón Aparicio MS, Ruiz ML, Ocsachoque MA, Ponzi MI, Rodríguez-Castellón E, Lick ID. Propane and Naphthalene Oxidation over Gold-Promoted Cobalt Catalysts Supported on Zirconia. Catalysts. 2020; 10(4):387. https://doi.org/10.3390/catal10040387
Chicago/Turabian StyleLeguizamón Aparicio, María Silvia, María Lucia Ruiz, Marco Antonio Ocsachoque, Marta Isabel Ponzi, Enrique Rodríguez-Castellón, and Ileana Daniela Lick. 2020. "Propane and Naphthalene Oxidation over Gold-Promoted Cobalt Catalysts Supported on Zirconia" Catalysts 10, no. 4: 387. https://doi.org/10.3390/catal10040387
APA StyleLeguizamón Aparicio, M. S., Ruiz, M. L., Ocsachoque, M. A., Ponzi, M. I., Rodríguez-Castellón, E., & Lick, I. D. (2020). Propane and Naphthalene Oxidation over Gold-Promoted Cobalt Catalysts Supported on Zirconia. Catalysts, 10(4), 387. https://doi.org/10.3390/catal10040387







