Catalytic Behavior of Alkali Treated H-MOR in Selective Synthesis of Ethylenediamine via Condensation Amination of Monoethanolamine
Abstract
1. Introduction
2. Results and Discussion
2.1. Physico-Chemical Properties of the Catalyst
2.2. Acidic Properties of the Catalyst
2.3. Catalytic Performance
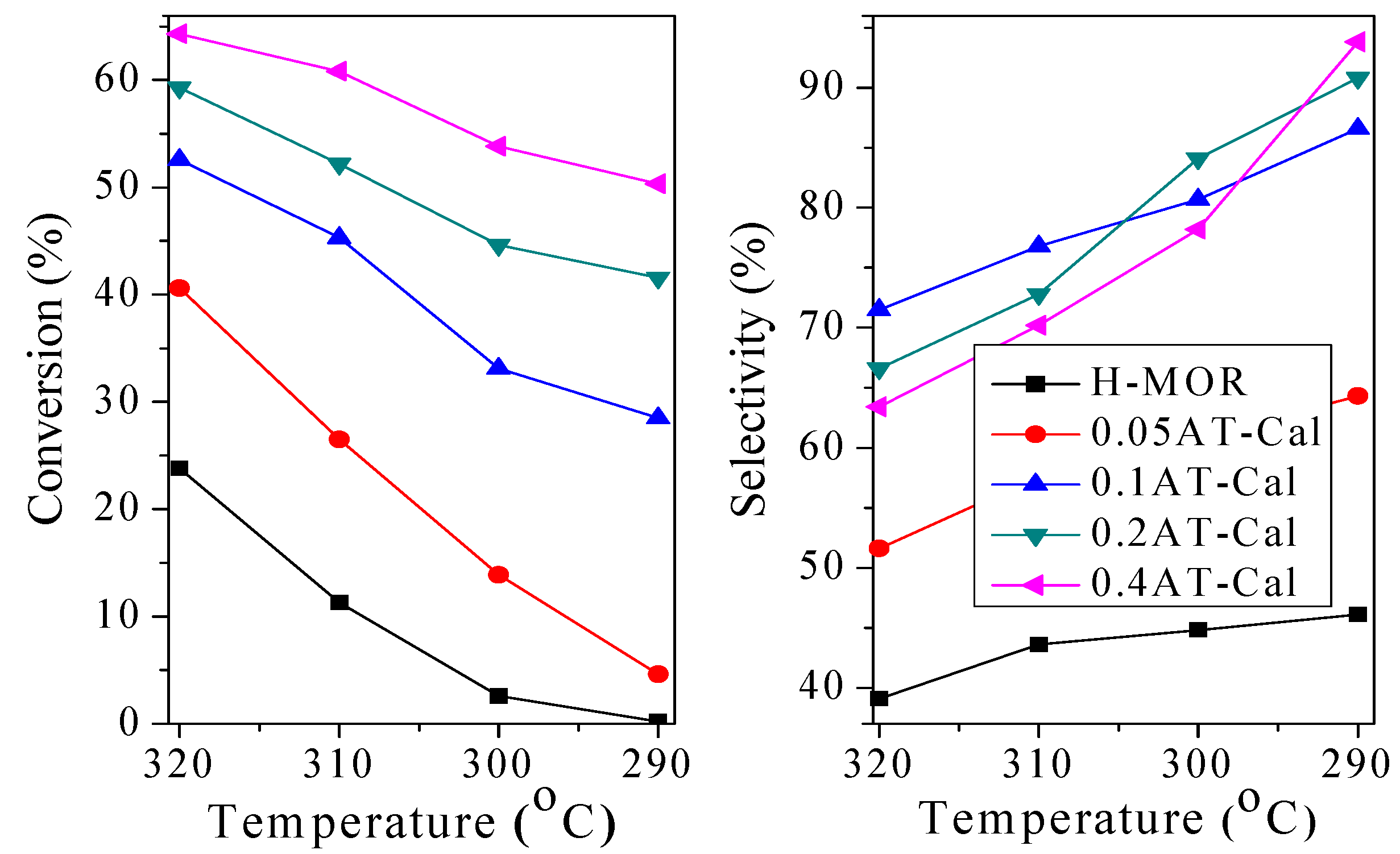
2.3.1. Effect of Alkali Treatment on Catalytic Performance
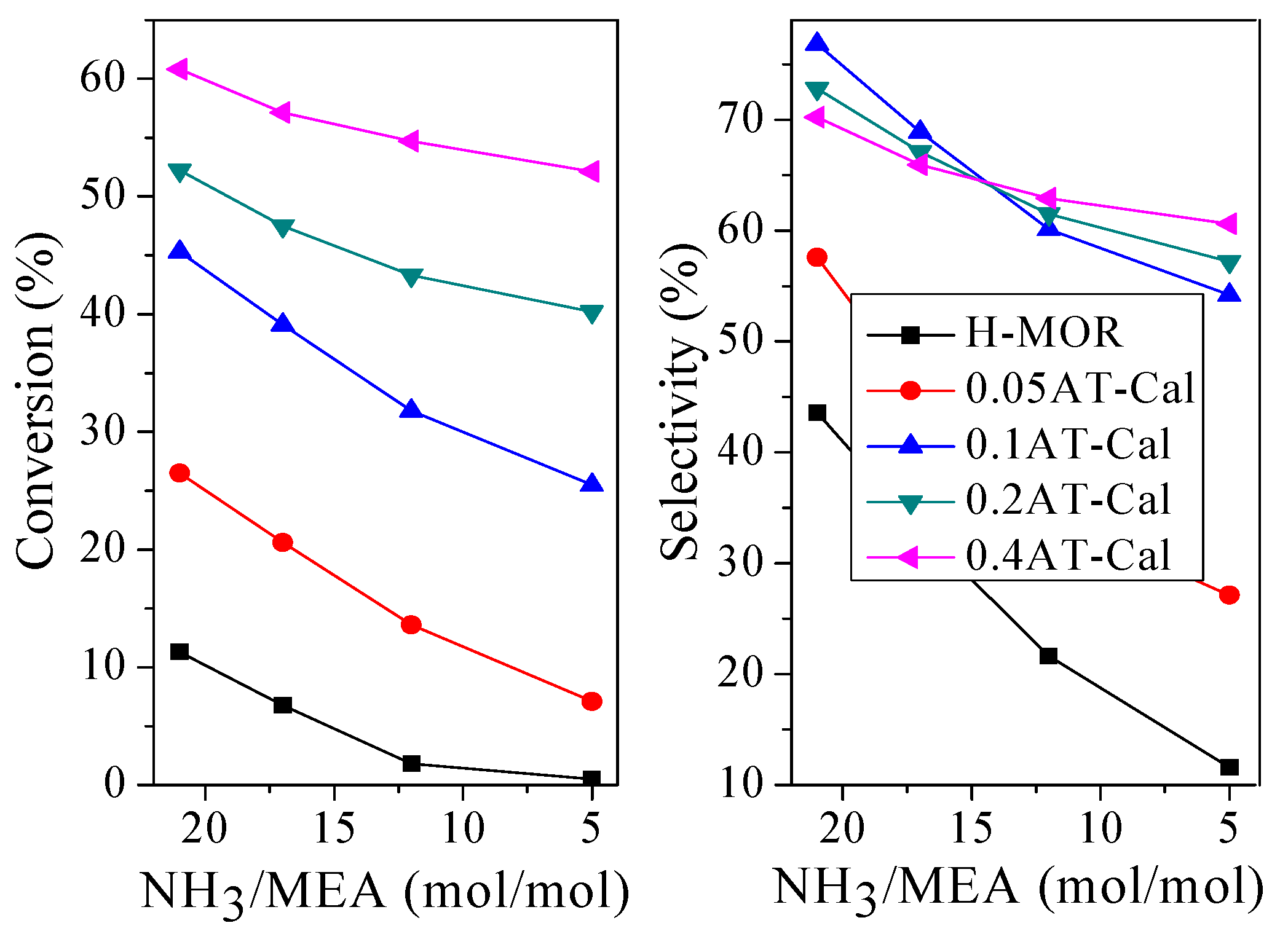
2.3.2. Effect of the Reaction Condition on the Catalytic Performance
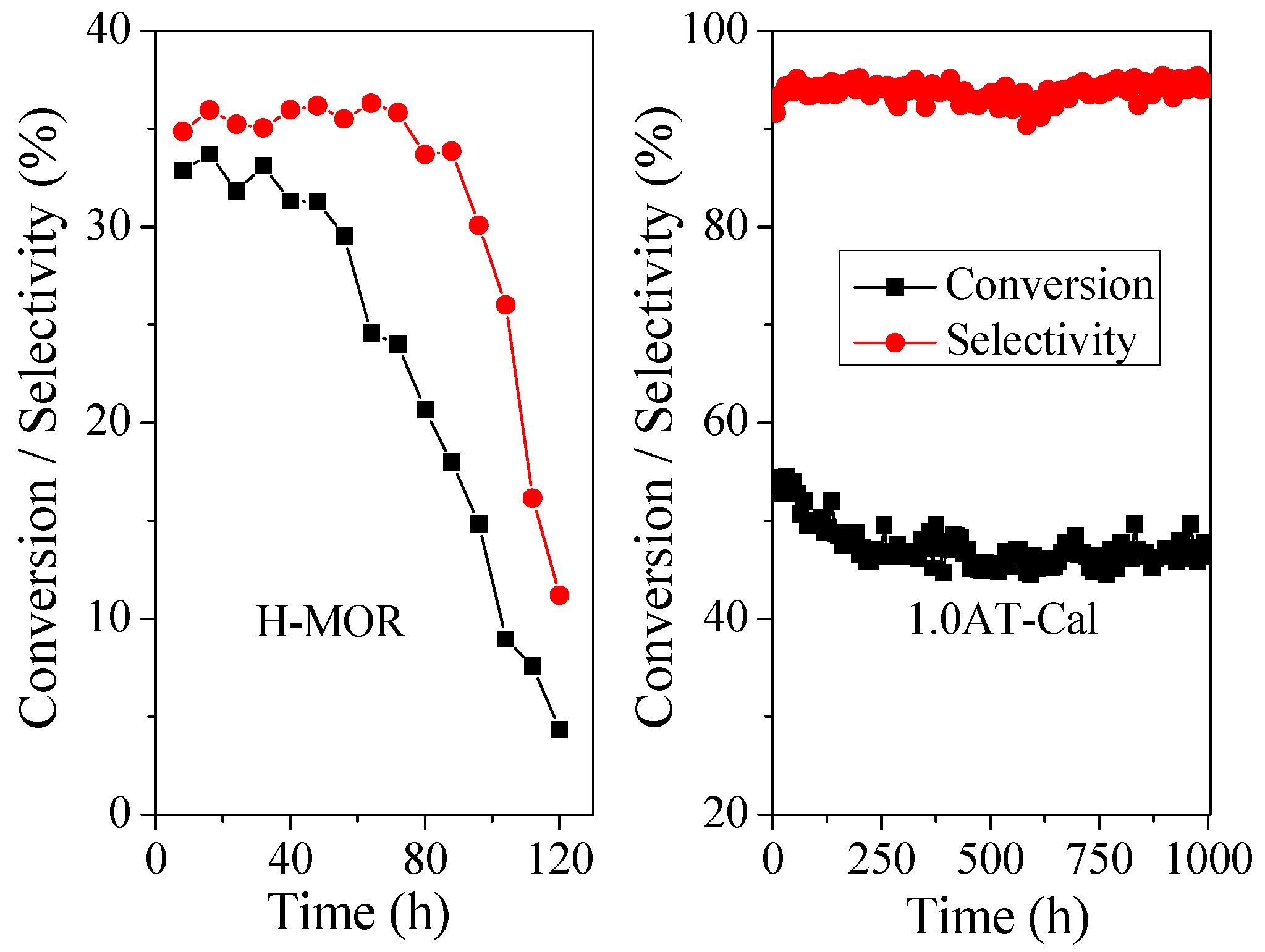
2.3.3. Catalytic Lifetime of Alkali Treated H-MOR
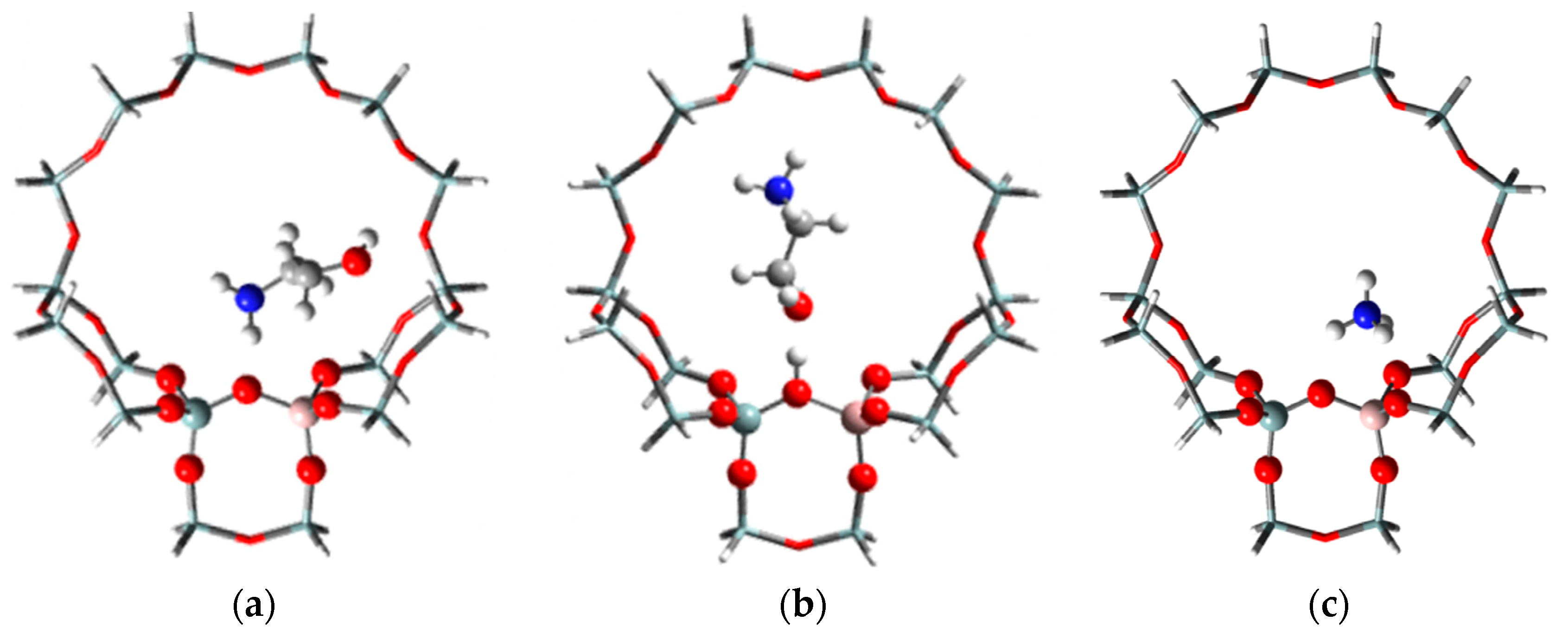
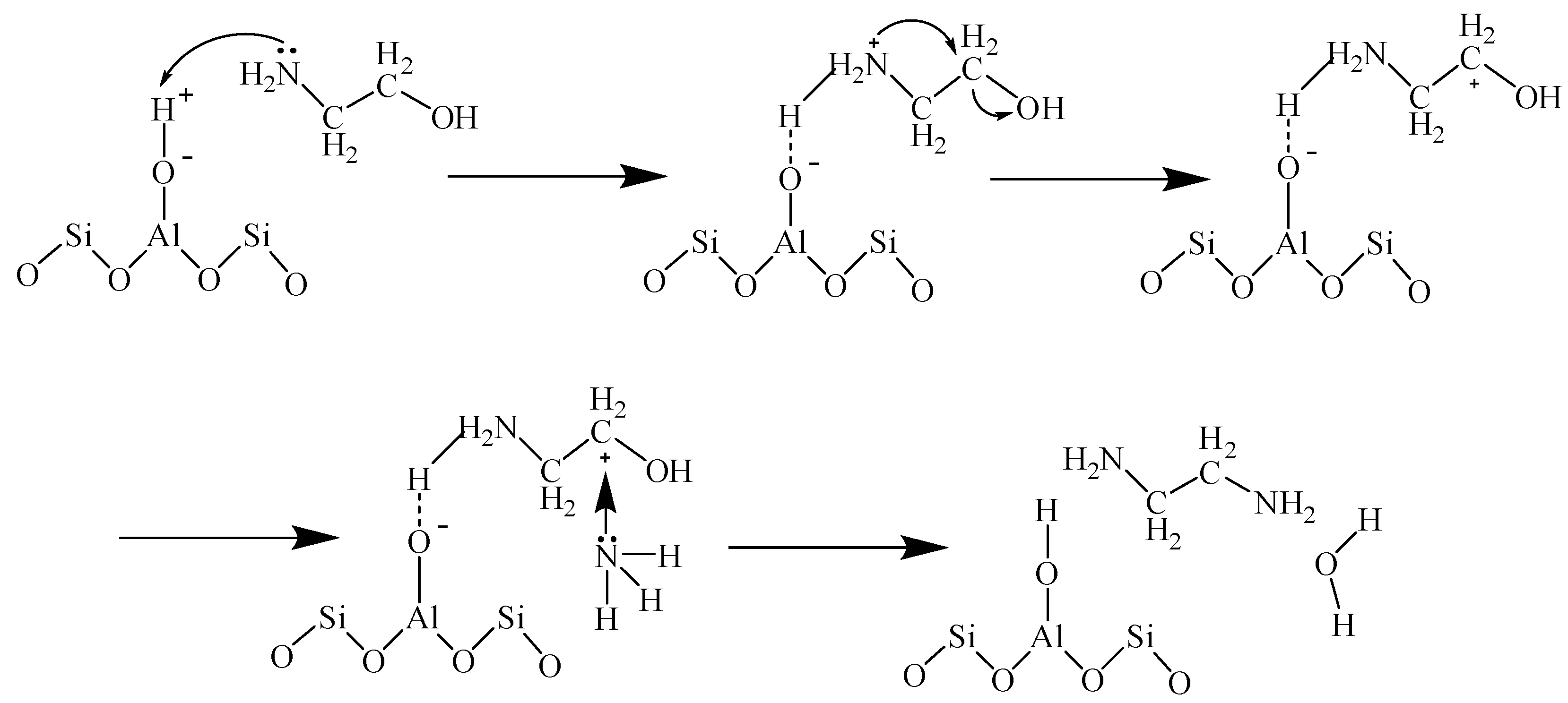
2.4. Possible Mechanism for Selective Synthesis of EDA
3. Materials and Methods
3.1. Preparation of the Catalysts
3.2. Characterization
3.3. Catalyst Evaluation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Deeba, M.; Ford, M.F.; Johnson, T.A. Shape-selective mordenite-catalyzed amination of ethanolamine to ethylenediamine. J. Mol. Catal. 1990, 60, 11–17. [Google Scholar] [CrossRef]
- Chen, X.Z.; Zhou, S.D.; Zhang, H.J. Synthesis of ethylenediamine in a tubular reactor: Experimental and theoretical kinetics. Prog. React. Kinet. Mech. 2012, 37, 411–422. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Bai, G.Y.; Yan, X.L.; Li, Y.; Zeng, T.; Wang, J.; Wang, H.F.; Xing, J.D.; Luan, D.Z.; Tang, X.Y.; et al. Amination of ethanolamine over cobalt modified H-ZSM-5 catalysts. Catal. Commun. 2007, 8, 1102–1106. [Google Scholar] [CrossRef]
- Segawa, K.; Shimura, T. Effect of dealumination of mordenite by acid leaching for selective synthesis of ethylenediamine from ethanolamine. Appl. Catal. A Gen. 2000, 194, 309–317. [Google Scholar] [CrossRef]
- Anand, R.; Jyothi, T.M.; Rao, B.S. A comparative study on the catalytic activity of ZnO modified zeolites in the synthesis of alkylpyrazines. Appl. Catal. A Gen. 2001, 208, 203–211. [Google Scholar] [CrossRef]
- Teketel, S.; Lundegaard, L.F.; Skistad, W.; Chavan, S.M.; Olsbye, U.; Lillerud, K.P.; Beato, P.; Svelle, S. Morphology-induced shape selectivity in zeolite catalysis. J. Catal. 2015, 327, 22–32. [Google Scholar] [CrossRef]
- Tijsebaert, B.; Yilmaz, B.; Müller, U.; Gies, H.; Zhang, W.; Bao, X.H.; Xiao, F.S.; Tatsumi, T.; Vos, D.D. Shape-selective synthesis of methylamines over the RRO zeolite Al-RUB-41. J. Catal. 2011, 278, 246–252. [Google Scholar] [CrossRef]
- Reule, A.A.C.; Sawada, J.S.; Semagina, N. Effect of selective 4-membered ring dealumination on mordenite-catalyzed dimethyl ether carbonylation. J. Catal. 2017, 349, 98–109. [Google Scholar] [CrossRef]
- Paixão, V.; Monteiro, R.; Andrade, M.; Fernandes, A.; Rocha, J.; Carvalho, A.P.; Martins, A. Desilication of MOR zeolite: Conventional versus microwave assisted heating. Appl. Catal. A Gen. 2011, 402, 59–68. [Google Scholar] [CrossRef]
- Paixáo, V.; Carvalho, A.P.; Rocha, J.; Fernandes, A.; Martins, A. Modification of MOR by desilication treatments: Structural, textural and acidic characterization. Microporous Mesoporous Mater. 2010, 131, 350–357. [Google Scholar] [CrossRef]
- Reddy, J.K.; Motokura, K.; Koyama, T.; Miyaji, A.; Baba, T. Effect of morphology and particle size of ZSM-5 on catalytic performance for ethylene conversion and heptane cracking. J. Catal. 2012, 289, 53–61. [Google Scholar] [CrossRef]
- Holm, M.S.; Taarning, E.; Egeblad, K.; Christensen, C.H. Catalysis with hierarchical zeolites. Catal. Today 2011, 168, 3–16. [Google Scholar] [CrossRef]
- Xu, S.L.; Zhang, M.Y.; Guo, S.J.; Li, M.R.; Huang, Q.M.; Chen, X.H. Synthesis of hierarchically porous zeolite Ti-MWW with different hard templates and their application in allyl alcohol conversion. Catal. Lett. 2020, 150, 209–221. [Google Scholar] [CrossRef]
- Zhao, S.F.; Wang, W.D.; Wang, L.Z.; Schwieger, W.; Wang, W.; Huang, J. Tuning hierarchical ZSM-5 zeolite for both gas and liquid phase biorefining. ACS Catal. 2019. [Google Scholar] [CrossRef]
- Shetti, V.N.; Kim, J.; Srivastava, R.; Choi, M.; Ryoo, R. Assessment of the mesopore wall catalytic activities of MFI zeolite with mesoporous/microporous hierarchical structures. J. Catal. 2008, 254, 296–303. [Google Scholar] [CrossRef]
- Xiao, F.S.; Wang, L.F.; Yin, C.Y.; Lin, K.F.; Di, Y.; Xu, R.; Su, D.S.; Schlögl, R.; Yokoi, T.; Tatsumi, T. Catalytic properties of hierarchical mesoporous zeolites templated with a mixture of small organic ammonium salts and mesoscale cationic polymers. Angew. Chem. 2006, 118, 3190–3193. [Google Scholar] [CrossRef]
- Yang, C.G.; Qiu, M.H.; Hu, S.W.; Chen, X.G.; Zeng, G.F.; Liu, Z.Y.; Sun, Y.H. Stable and efficient aromatic yield from methanol over alkali treated hierarchical Zn-containing HZSM-5 zeolites. Microporous Mesoporous Mater. 2016, 231, 110–116. [Google Scholar] [CrossRef]
- Monteiro, R.; Ania, C.O.; Rocha, J.; Carvalho, A.P.; Martins, A. Catalytic behavior of alkali-treated Pt/HMOR in n-hexane hydroisomerization. Appl. Catal. A Gen. 2014, 476, 148–157. [Google Scholar] [CrossRef][Green Version]
- Wang, Y.; Yokoi, T.; Namba, S.; Tatsumi, T. Effects of dealumination and desilication of Beta zeolite on catalytic performance in n-Hexane Cracking. Catalysts 2016, 6, 8. [Google Scholar] [CrossRef]
- Van Laak, A.N.C.; Sagala, S.L.; Zecevic, J.; Friederich, H.; de Jongh, P.E.; de Jong, K.P. Mesoporous mordenites obtained by sequential acid and alkaline treatments Catalysts for cumene production with enhanced accessibility. J. Catal. 2010, 276, 170–180. [Google Scholar] [CrossRef]
- Huang, S.J.; Liu, X.H.; Yu, L.L.; Miao, S.; Liu, Z.N.; Zhang, S.; Xie, S.J.; Xu, L.Y. Preparation of hierarchical mordenite zeolites by sequential steaming acid leaching alkaline treatment. Microporous Mesoporous Mater. 2014, 191, 18–26. [Google Scholar] [CrossRef]
- Cheng, X.W.; Meng, Q.Y.; Chen, J.Y.; Long, Y.C. A facile route to synthesize mesoporous ZSM-5 zeolite incorporating high ZnO loading in mesopores. Microporous Mesoporous Mater. 2012, 153, 198–203. [Google Scholar] [CrossRef]
- Groen, J.C.; Pérez-Ramíez, J. Critical appraisal of mesopore characterization by adsorption analysis. Appl. Catal. A Gen. 2004, 268, 121–125. [Google Scholar] [CrossRef]
- Qin, Z.; Shen, B.; Gao, X.; Lin, F.; Wang, B.; Xu, C. Mesoporous Y zeolite with homogeneous aluminum distribution obtained by sequential desilication-dealumination and its performance in the catalytic cracking of cumene and 1,3,5-triisopropylbenzene. J. Catal. 2011, 278, 266–275. [Google Scholar] [CrossRef]
- Wei, X.; Smirniotis, P.G. Development and characterization of mesoporosity in ZSM-12 by desilication. Microporous Mesoporous Mater. 2006, 97, 97–106. [Google Scholar] [CrossRef]
- Liu, K.F.; Xie, S.J.; Wei, H.J.; Li, X.J.; Liu, S.J.; Xu, L.Y. Alkali-treatment of template-containing MCM-22 zeolite and itsapplication in alkylation and transalkylation reactions. Appl. Catal. A Gen. 2013, 468, 288–295. [Google Scholar] [CrossRef]
- Meunier, F.C.; Verboekend, D.; Gilson, J.P.; Groen, J.C.; Pérez-Ramírez, J. Influence of crystal size and probe molecule on diffusion in hierarchical ZSM-5 zeolites prepared by desilication. Microporous Mesoporous Mater. 2012, 148, 115–121. [Google Scholar] [CrossRef]
- Jin, Y.J.; Li, Y.D.; Zhao, S.L.; Lv, Z.B.; Wang, Q.; Liu, X.L.; Wang, L. Synthesis of mesoporous MOR materials by varying temperature crystallizations and combining ternary organic templates. Microporous Mesoporous Mater. 2012, 147, 259–266. [Google Scholar] [CrossRef]
- Mei, C.S.; Wen, P.Y.; Liu, Z.C.; Liu, H.X.; Wang, Y.D.; Yang, W.M.; Xie, Z.K.; Hua, W.M.; Gao, Z. Selective production of propylene from methanol: Mesoporosity development in high silica HZSM-5. J. Catal. 2008, 258, 243–249. [Google Scholar] [CrossRef]
- Leng, K.Y.; Sun, S.N.; Wang, B.T.; Sun, L.; Xu, W.; Sun, Y.Y. Benzylation of benzene with benzyl chloride on iron-containing mesoporous mordenite. Catal. Commun. 2012, 28, 64–68. [Google Scholar] [CrossRef]
- Mochizuki, H.; Yokoi, T.; Imai, H.; Namba, S.; Kondo, J.N. Effect of desilication of H-ZSM-5 by alkali treatment on catalytic performance in hexane cracking. Appl. Catal. A Gen. 2012, 449, 188–197. [Google Scholar] [CrossRef]
- Veefkind, V.A.; Gründling, C.; Lercher, J.A. Steric aspects in methylamine and dimethylether synthesis over acidic mordenites. J. Mol. Catal. 1998, 134, 111–119. [Google Scholar] [CrossRef]
- Hao, K.; Shen, B.J.; Wang, Y.D.; Ren, J. Influence of combined alkaline treatment and Fe–Ti-loading modification on ZSM-5 zeolite and its catalytic performance in light olefin production. J. Ind. Eng. Chem. 2012, 18, 1736–1740. [Google Scholar] [CrossRef]
- Liu, Z.; Fan, W.; Ma, J. Adsorption, diffusion and catalysis of mesostructured zeolite HZSM-5. Adsorption 2012, 18, 493–501. [Google Scholar] [CrossRef]
- Choi, M.; Na, K.; Kim, J.; Sakamoto, Y.; Terasaki, O.; Ryoo, R. Stable single-unit-cell nanosheets of zeolite MFI as active and long-lived catalysts. Nature 2009, 461, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Yuan, S.P.; Wang, J.G.; Jiao, H.J.; Qin, Z.F.; Li, Y.W. A theoretical study of amines adsorption in HMOR by using ONIOM2 method. J. Mol. Catal. 2004, 220, 221–228. [Google Scholar] [CrossRef]
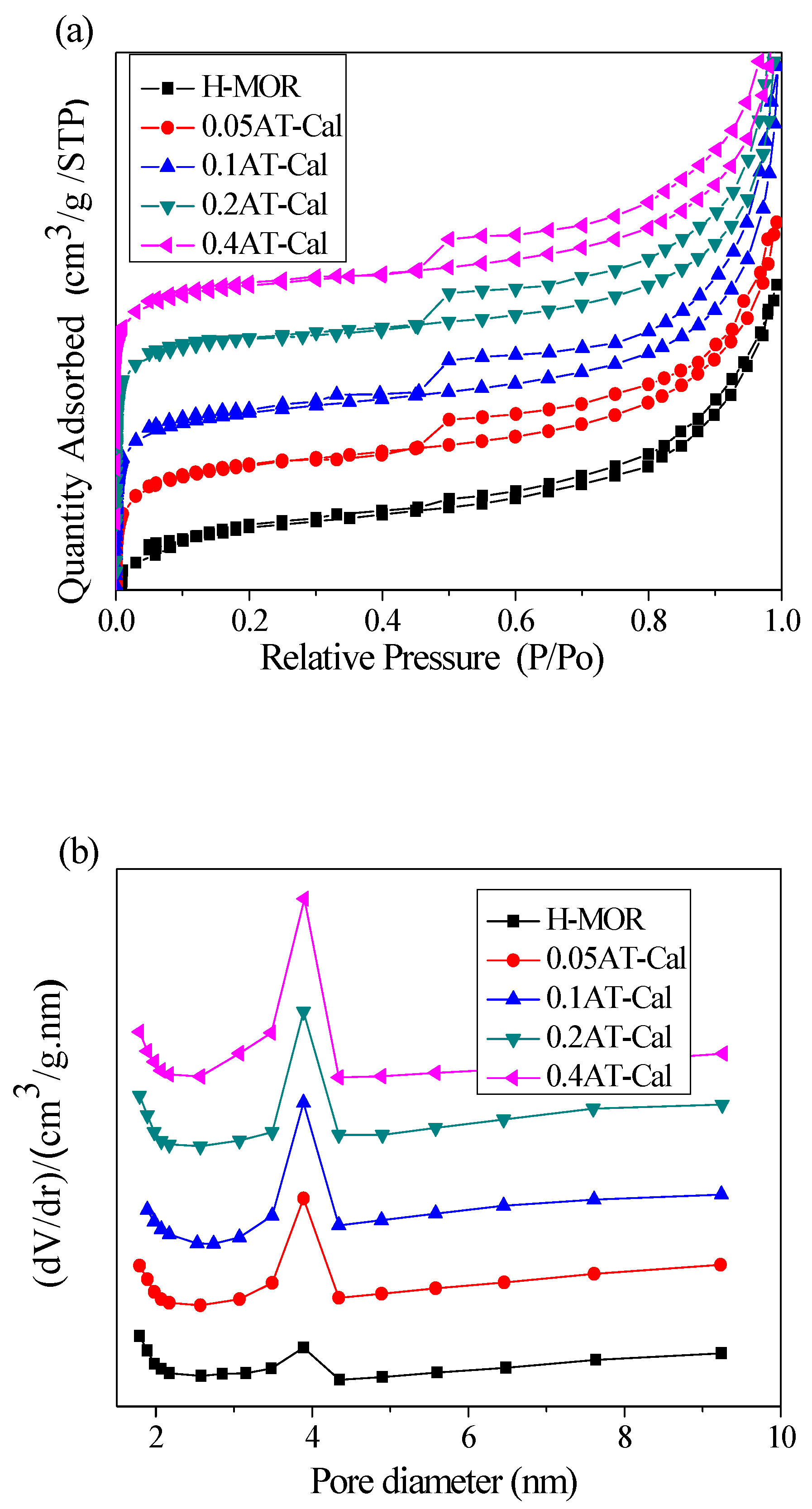

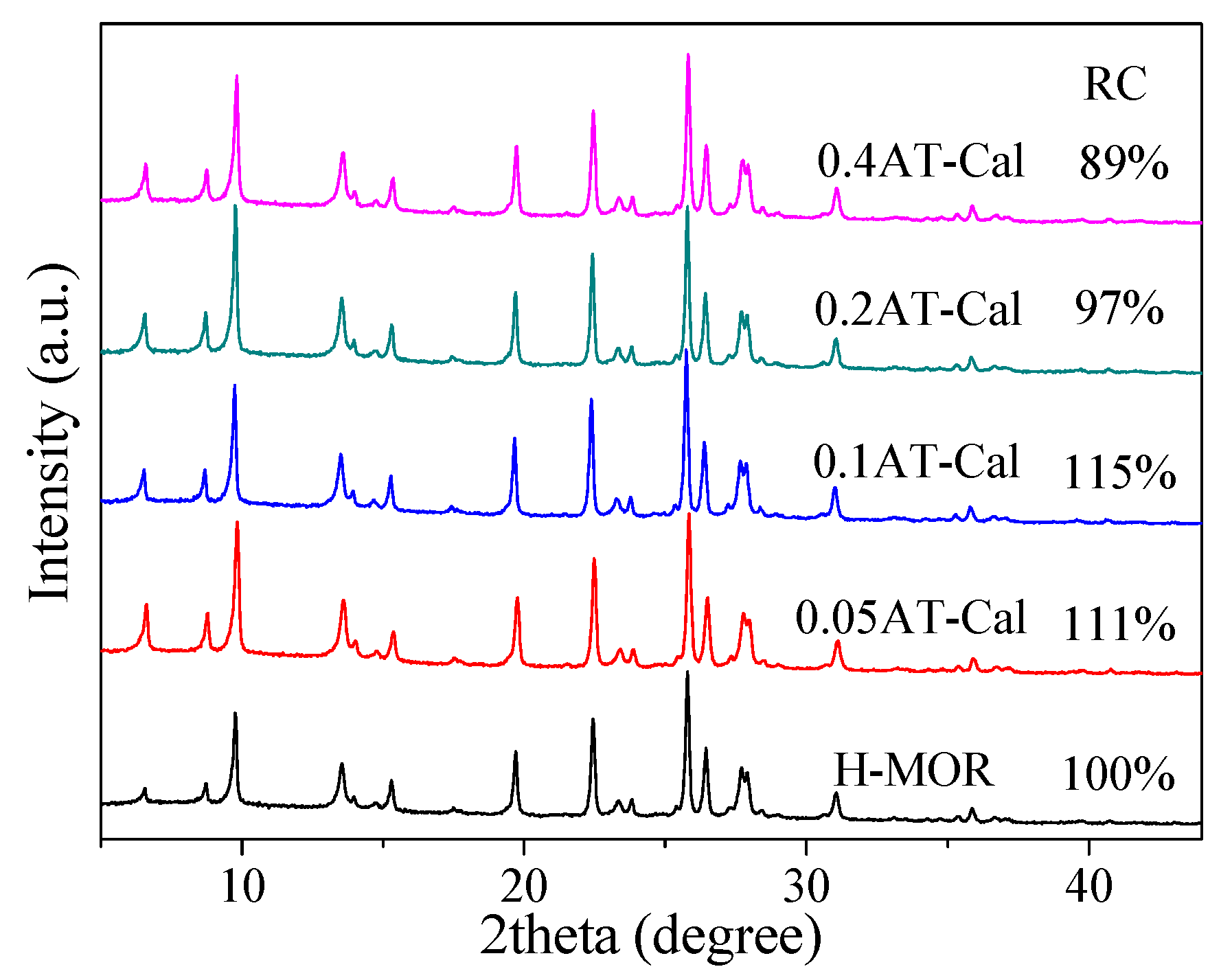
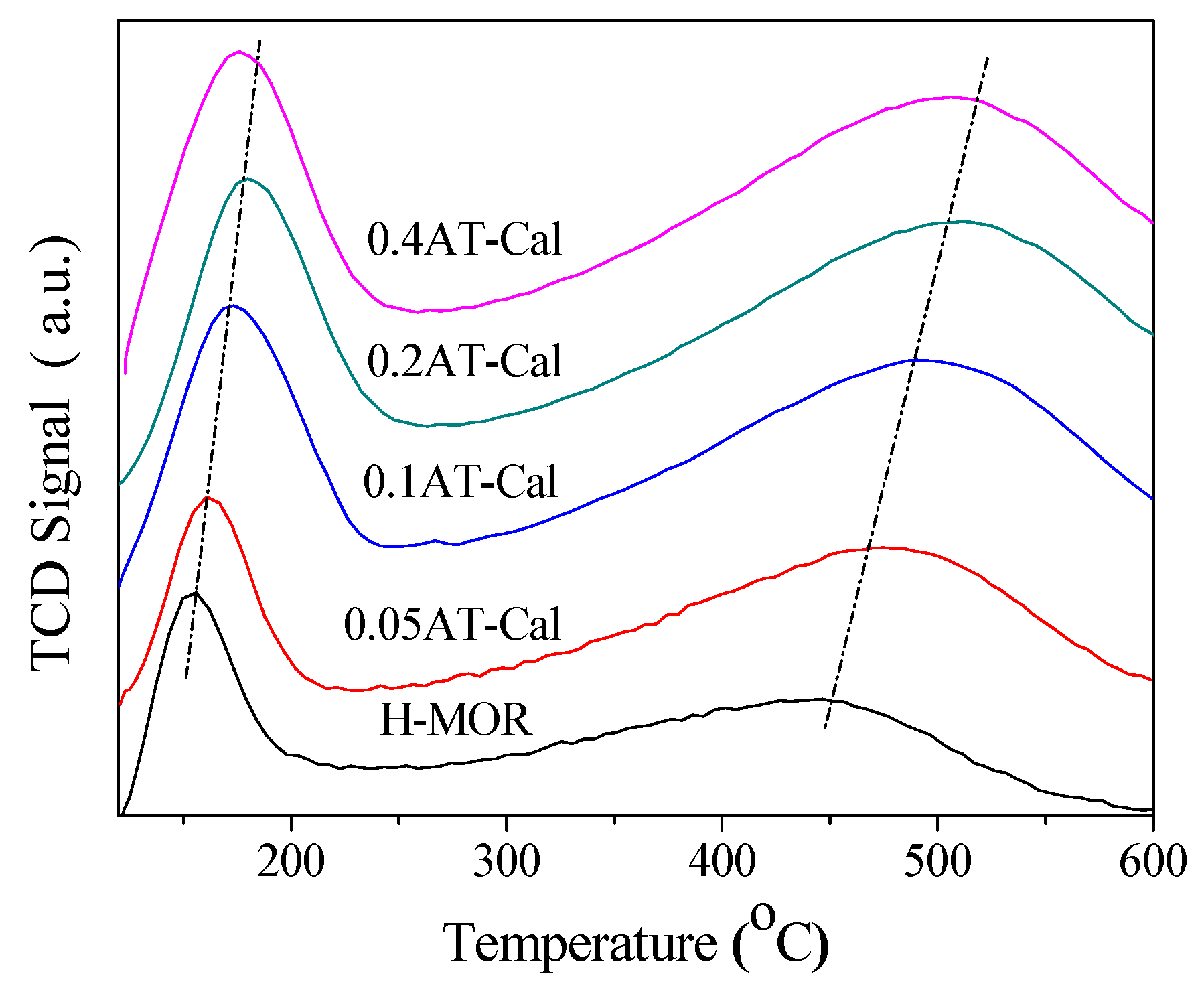

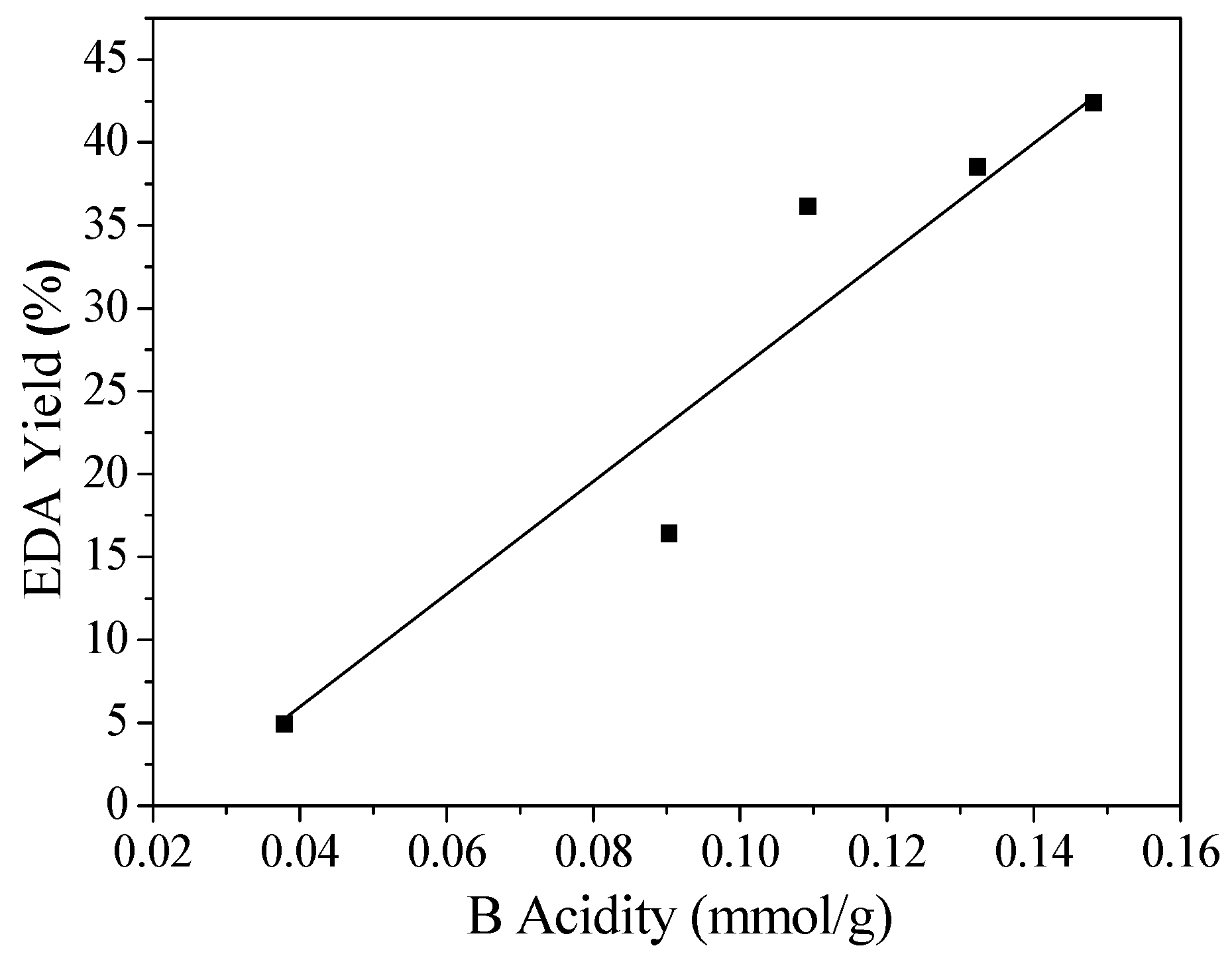
| Catalyst | SiO2/Al2O3 Molar Ratio | Relative Crystallinity (%) | Smicro (m2/g) | Sexter (m2/g) | Vmicro (cm3/g) | Vmeso (cm3/g) | Dads × 1015 (m2/s) |
|---|---|---|---|---|---|---|---|
| H-MOR | 21.6 | 100 | 336 | 35 | 0.16 | 0.11 | 5.89 |
| 0.05AT-Cal | 20.1 | 111 | 309 | 38 | 0.15 | 0.12 | 5.94 |
| 0.1AT-Cal | 19.3 | 115 | 305 | 39 | 0.14 | 0.13 | 6.18 |
| 0.2AT-Cal | 18.9 | 97 | 276 | 42 | 0.13 | 0.14 | 6.24 |
| 0.4AT-Cal | 16.4 | 89 | 205 | 42 | 0.10 | 0.15 | 7.39 |
| Catalysts | Amount of B (mmol/g) | Amount of L (mmol/g) |
|---|---|---|
| H-MOR | 0.0379 | 0.0175 |
| 0.05AT-Cal | 0.0903 | 0.0359 |
| 0.1AT-Cal | 0.1092 | 0.0373 |
| 0.2AT-Cal | 0.1323 | 0.0521 |
| 0.4AT-Cal | 0.1481 | 0.0722 |
| Catalysts | Conversion (%) | Selectivity (%) | EDA Yield (%) | ||||
|---|---|---|---|---|---|---|---|
| EDA | PIP | TEDA | AEP | Others | |||
| H-MOR | 11.3 | 43.6 | 11.3 | 3.6 | 4.0 | 37.5 | 4.9 |
| 0.05AT-Cal | 26.5 | 57.6 | 8.9 | 2.3 | 2.4 | 28.8 | 15.3 |
| 0.1AT-Cal | 45.3 | 76.8 | 6.3 | 1.6 | 2.0 | 13.3 | 34.8 |
| 0.2AT-Cal | 52.2 | 72.8 | 6.9 | 2.1 | 1.9 | 16.3 | 38.0 |
| 0.4AT-Cal | 60.8 | 70.2 | 7.5 | 2.1 | 2.3 | 17.9 | 42.7 |
| H-MOR * | 18.5 | 36.4 | 12.1 | 4.3 | 4.4 | 42.8 | 6.7 |
| H-ZSM-5 | 42.6 | 11.6 | 35.3 | 21.3 | 5.3 | 26.5 | 4.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, F.-W.; Zhang, Q.; Hui, F.; Yuan, J.; Mei, S.-N.; Yu, Q.-W.; Yang, J.-M.; Mao, W.; Liu, Z.-W.; Liu, Z.-T.; et al. Catalytic Behavior of Alkali Treated H-MOR in Selective Synthesis of Ethylenediamine via Condensation Amination of Monoethanolamine. Catalysts 2020, 10, 386. https://doi.org/10.3390/catal10040386
Zhao F-W, Zhang Q, Hui F, Yuan J, Mei S-N, Yu Q-W, Yang J-M, Mao W, Liu Z-W, Liu Z-T, et al. Catalytic Behavior of Alkali Treated H-MOR in Selective Synthesis of Ethylenediamine via Condensation Amination of Monoethanolamine. Catalysts. 2020; 10(4):386. https://doi.org/10.3390/catal10040386
Chicago/Turabian StyleZhao, Feng-Wei, Qian Zhang, Feng Hui, Jun Yuan, Su-Ning Mei, Qin-Wei Yu, Jian-Ming Yang, Wei Mao, Zhong-Wen Liu, Zhao-Tie Liu, and et al. 2020. "Catalytic Behavior of Alkali Treated H-MOR in Selective Synthesis of Ethylenediamine via Condensation Amination of Monoethanolamine" Catalysts 10, no. 4: 386. https://doi.org/10.3390/catal10040386
APA StyleZhao, F.-W., Zhang, Q., Hui, F., Yuan, J., Mei, S.-N., Yu, Q.-W., Yang, J.-M., Mao, W., Liu, Z.-W., Liu, Z.-T., & Lu, J. (2020). Catalytic Behavior of Alkali Treated H-MOR in Selective Synthesis of Ethylenediamine via Condensation Amination of Monoethanolamine. Catalysts, 10(4), 386. https://doi.org/10.3390/catal10040386





