Synergistic Effect in Au-Cu Bimetallic Catalysts for the Valorization of Lignin-Derived Compounds
Abstract
1. Introduction
2. Results and Discussion
2.1. Catalyst Characterization
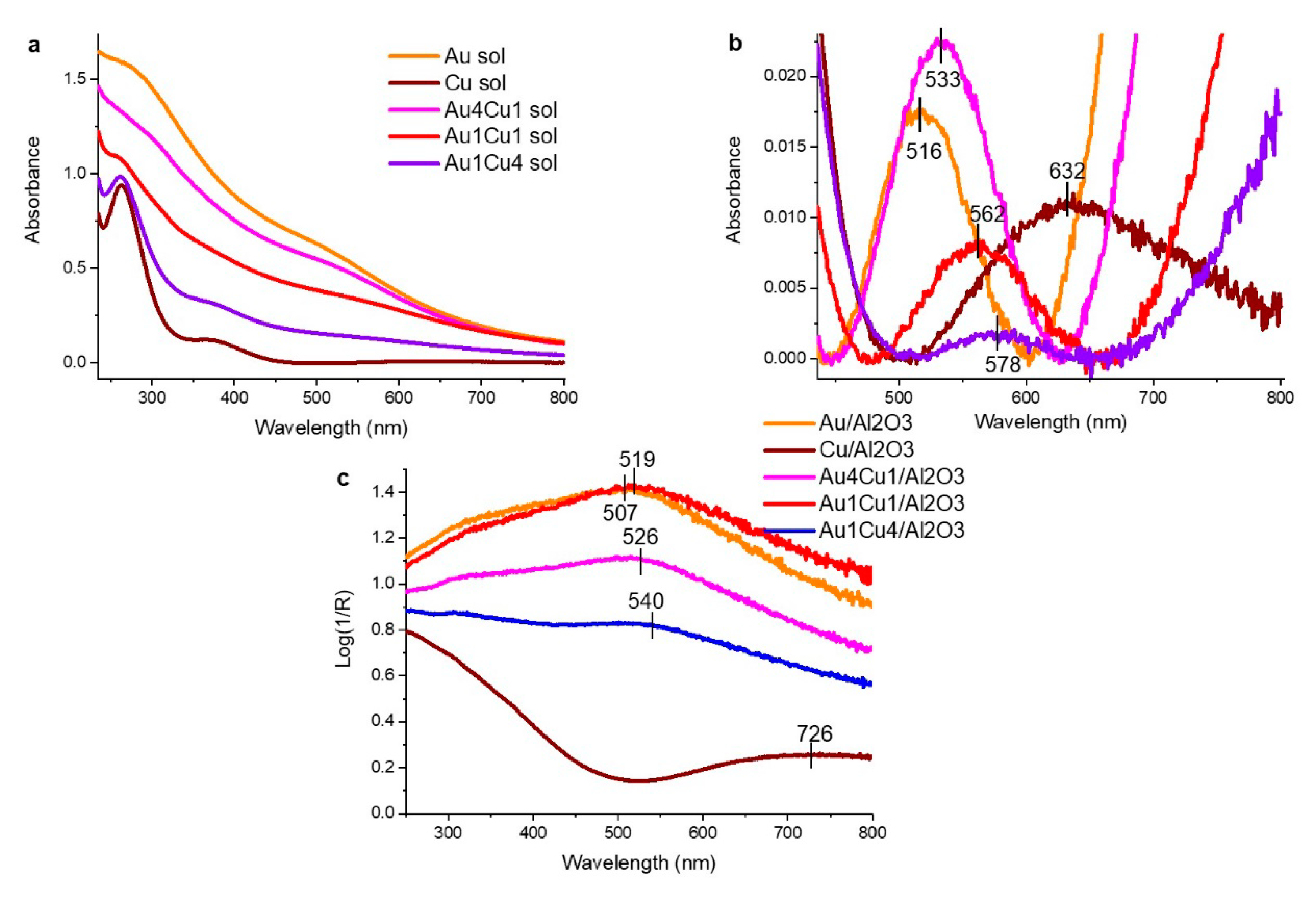
2.1.1. Energy Dispersive X-Ray (EDX) and UV-vis Spectra of the Parent Sols
2.1.2. ICP-OES (Inductively Coupled Plasma Optical Emission Spectroscopy) Measurements
2.1.3. XPS Analyses
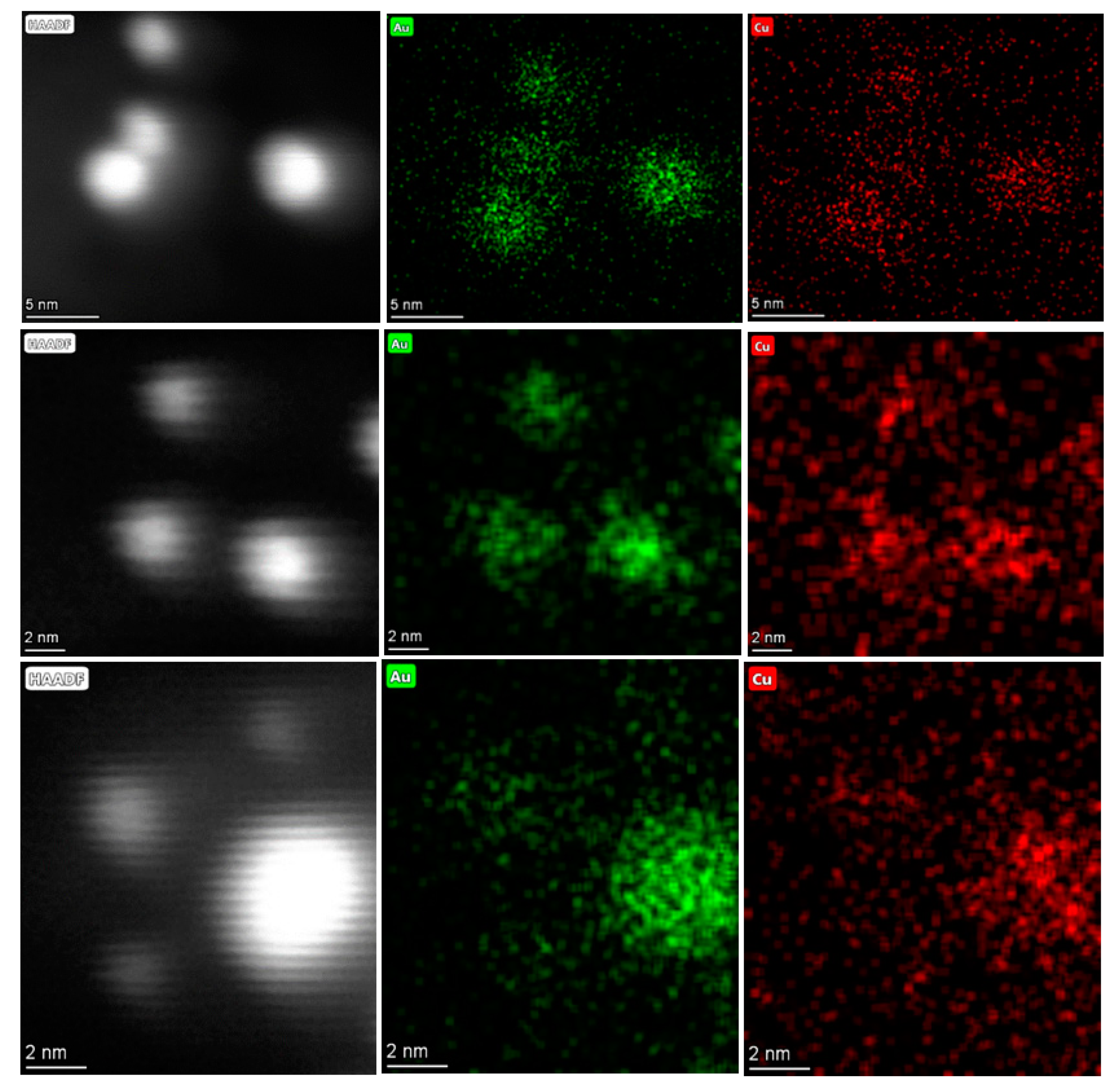
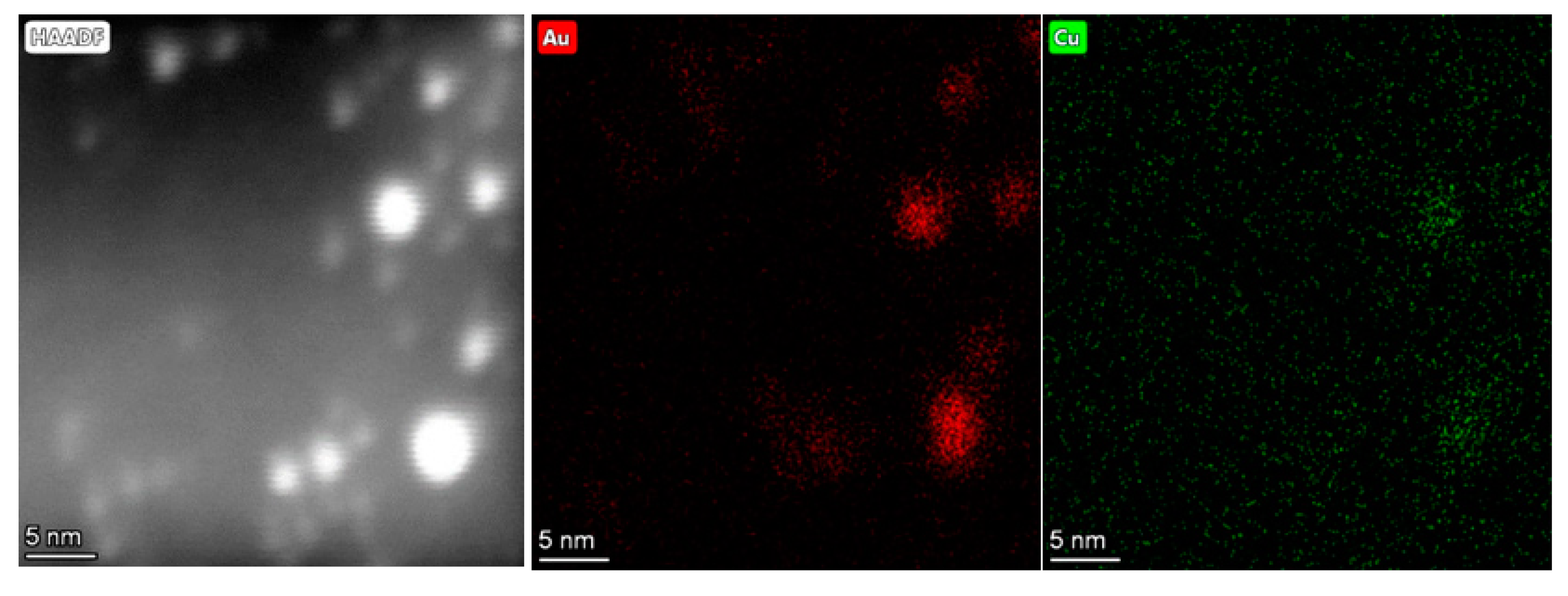
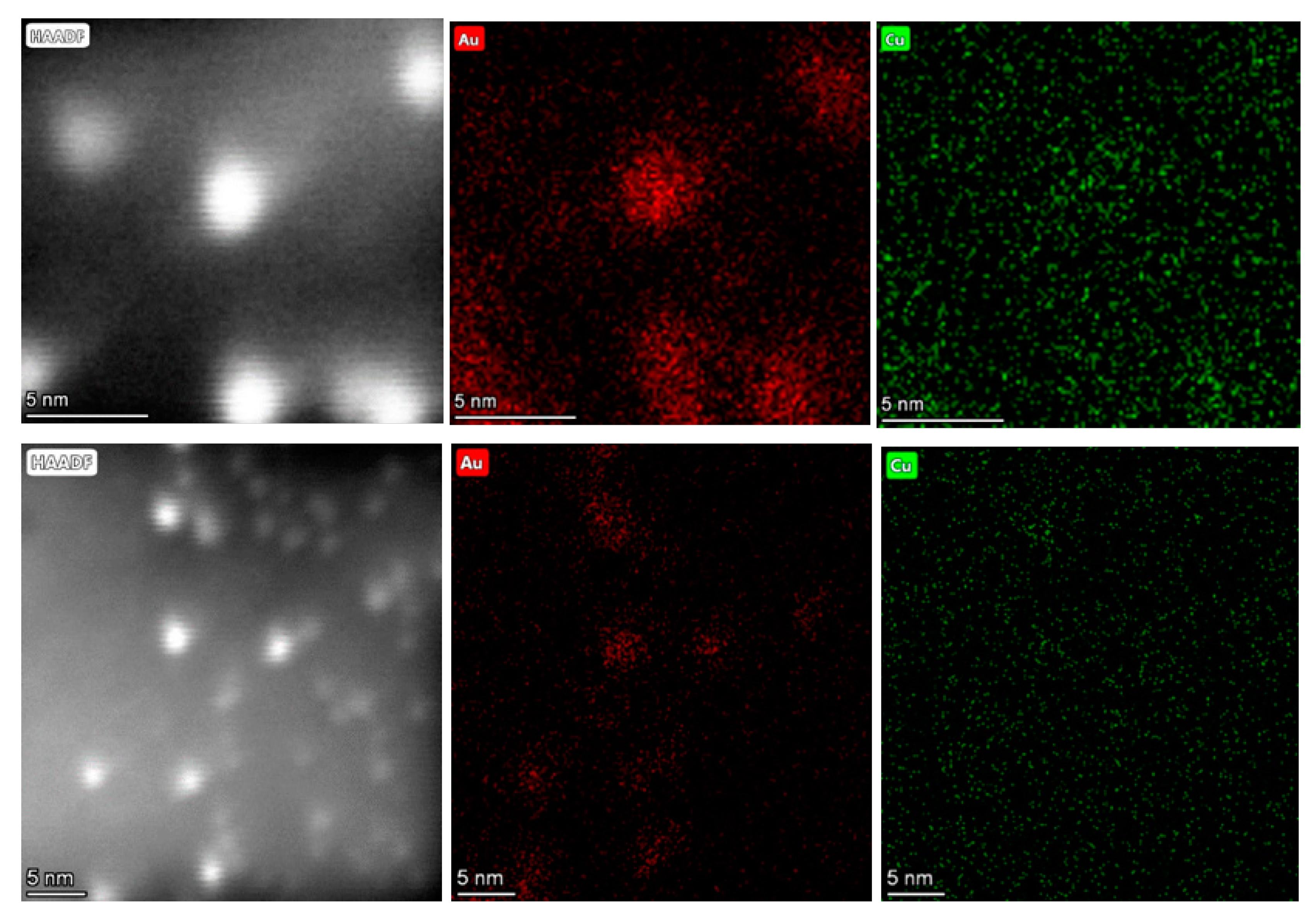
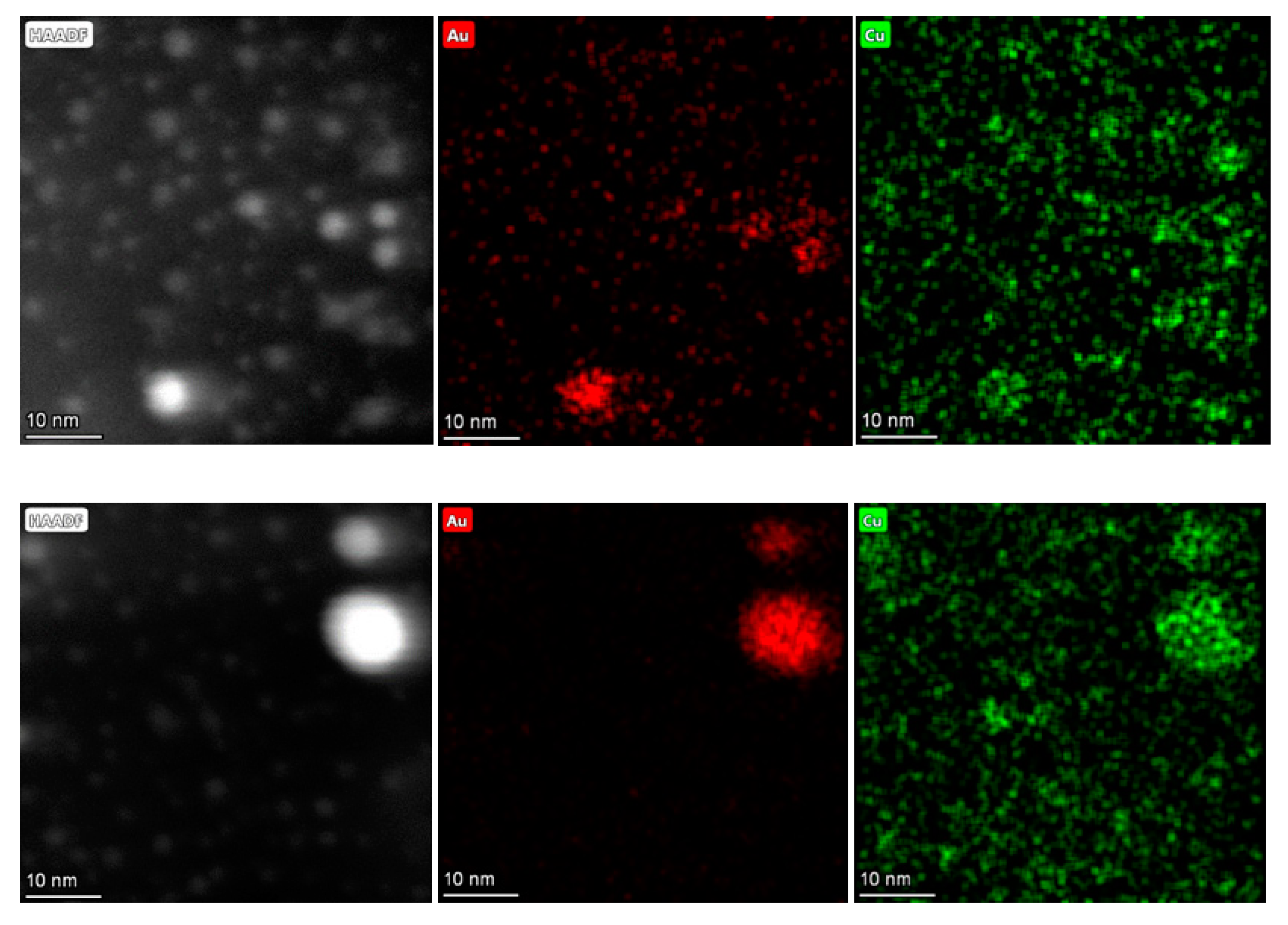
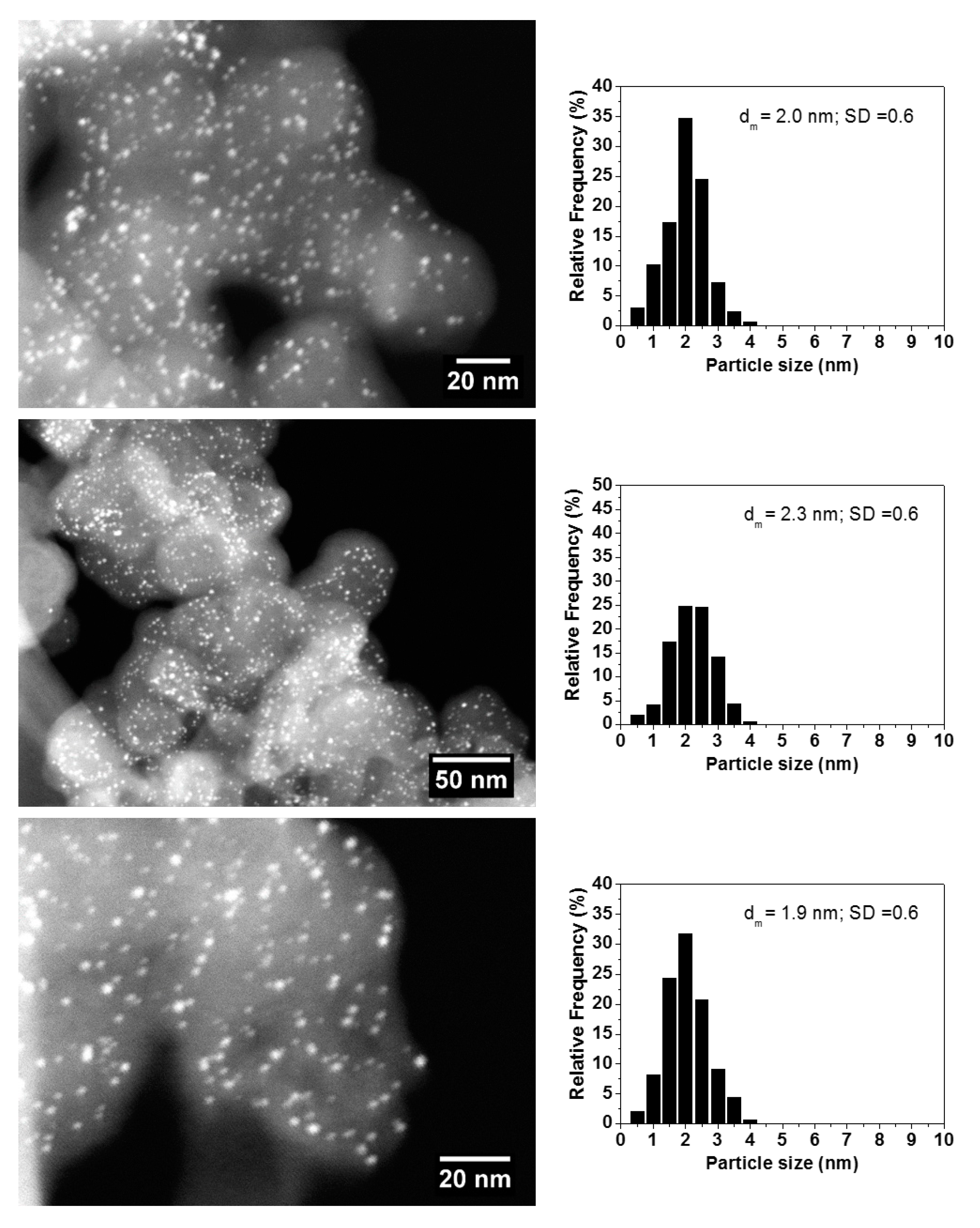
2.1.4. High Angular Annular Dark Field Scanning Transmission Electron Microscopy (HAADF-STEM)
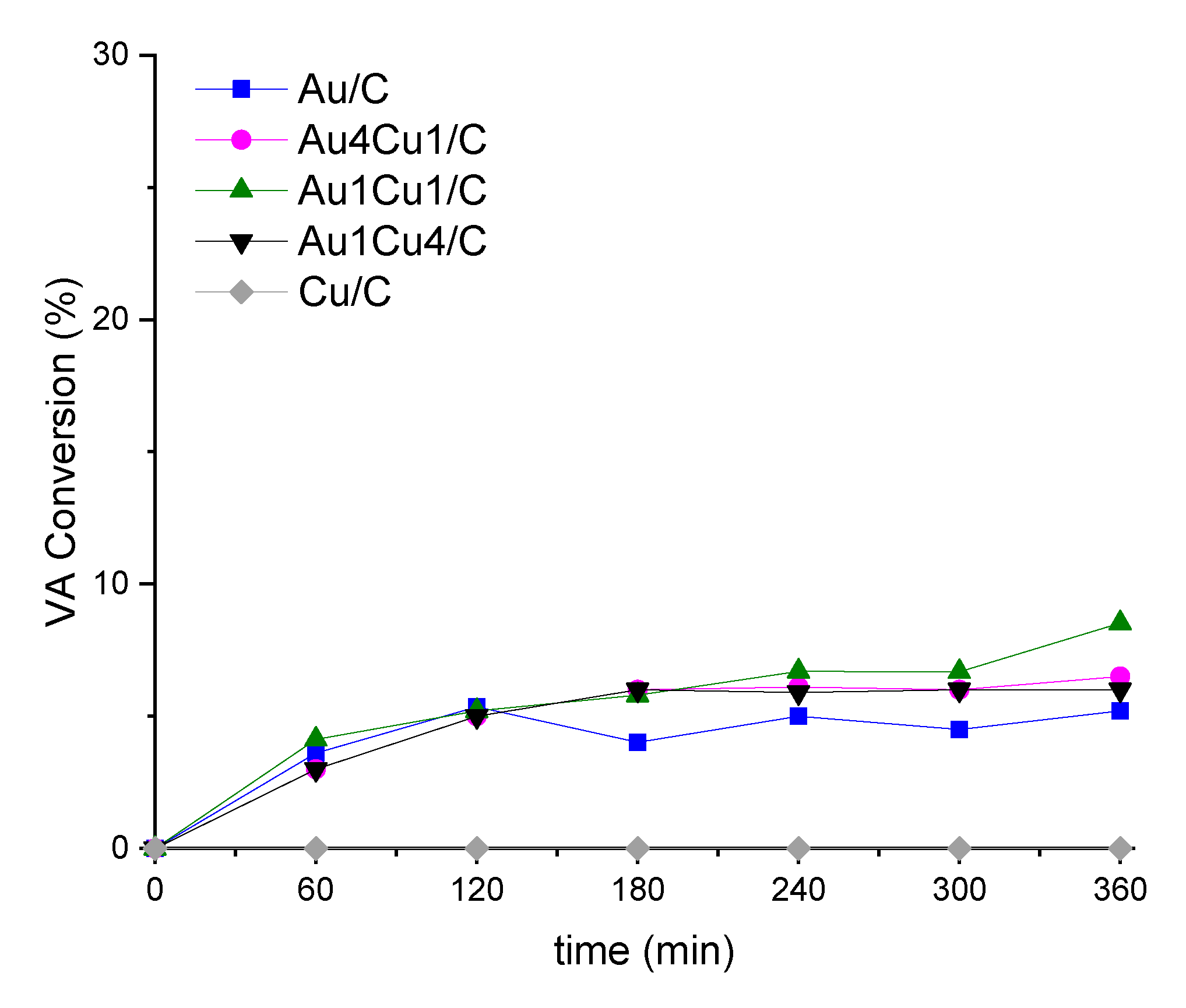
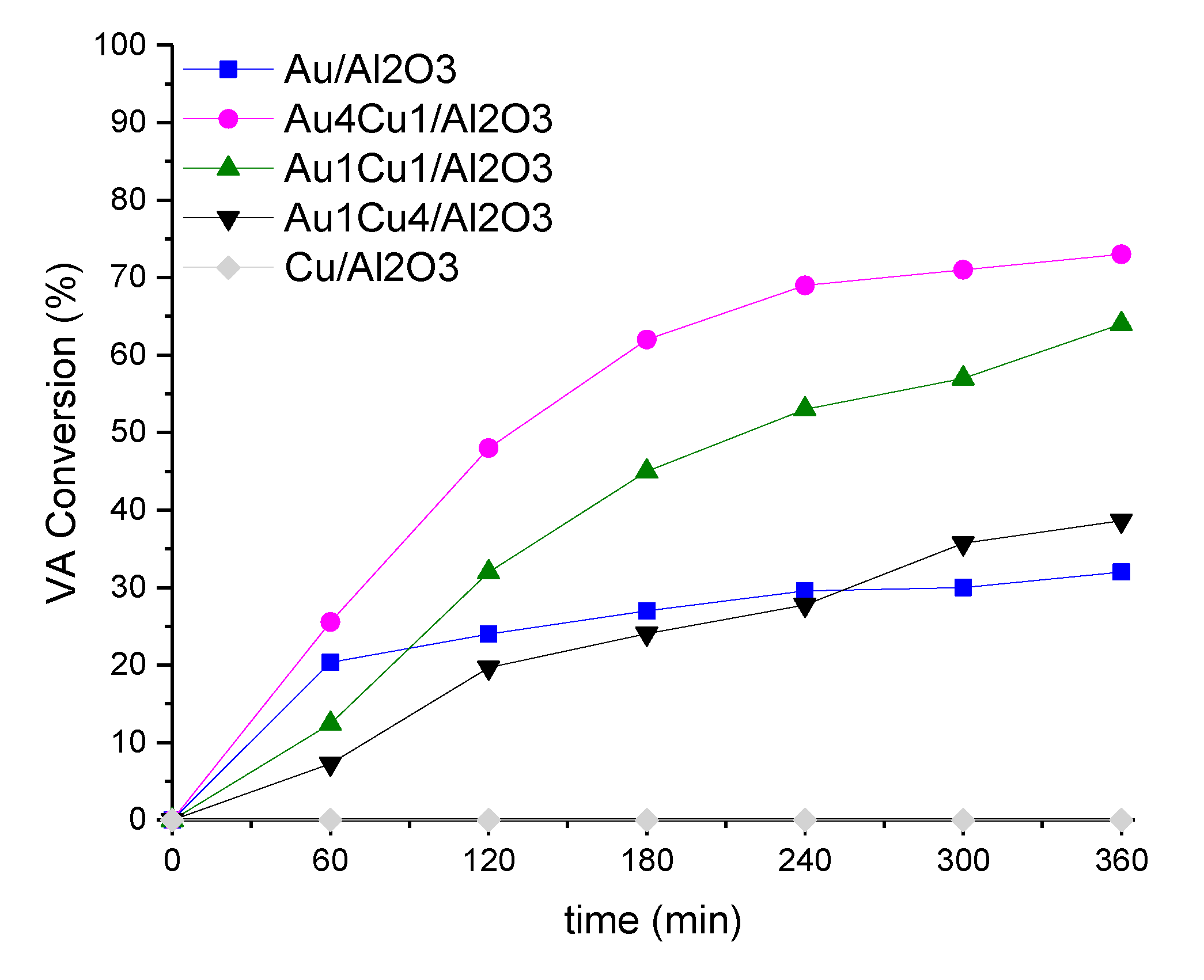
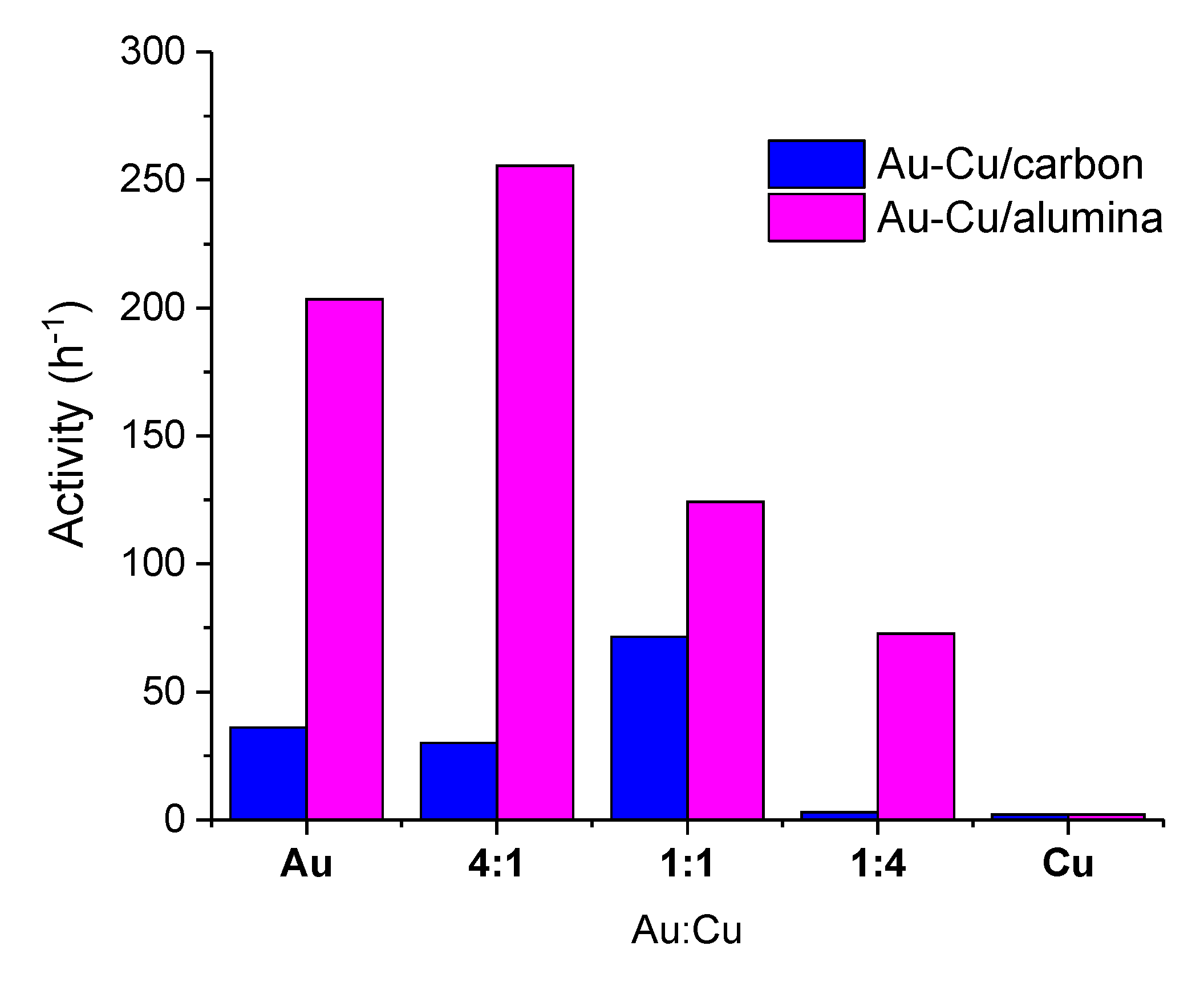
2.2. Catalytic Behaviour
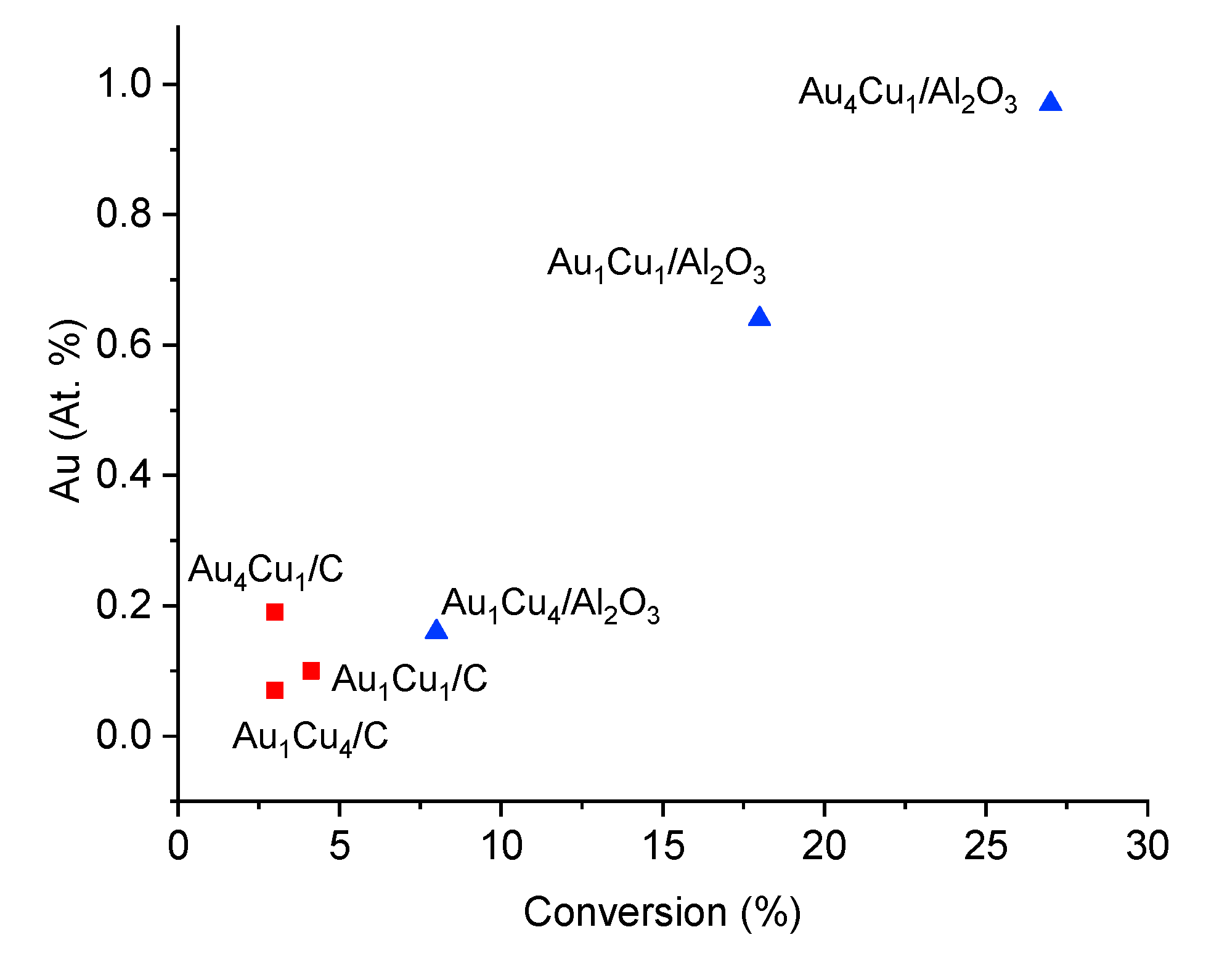
2.2.1. Correlation of Catalytic Results and Characterization
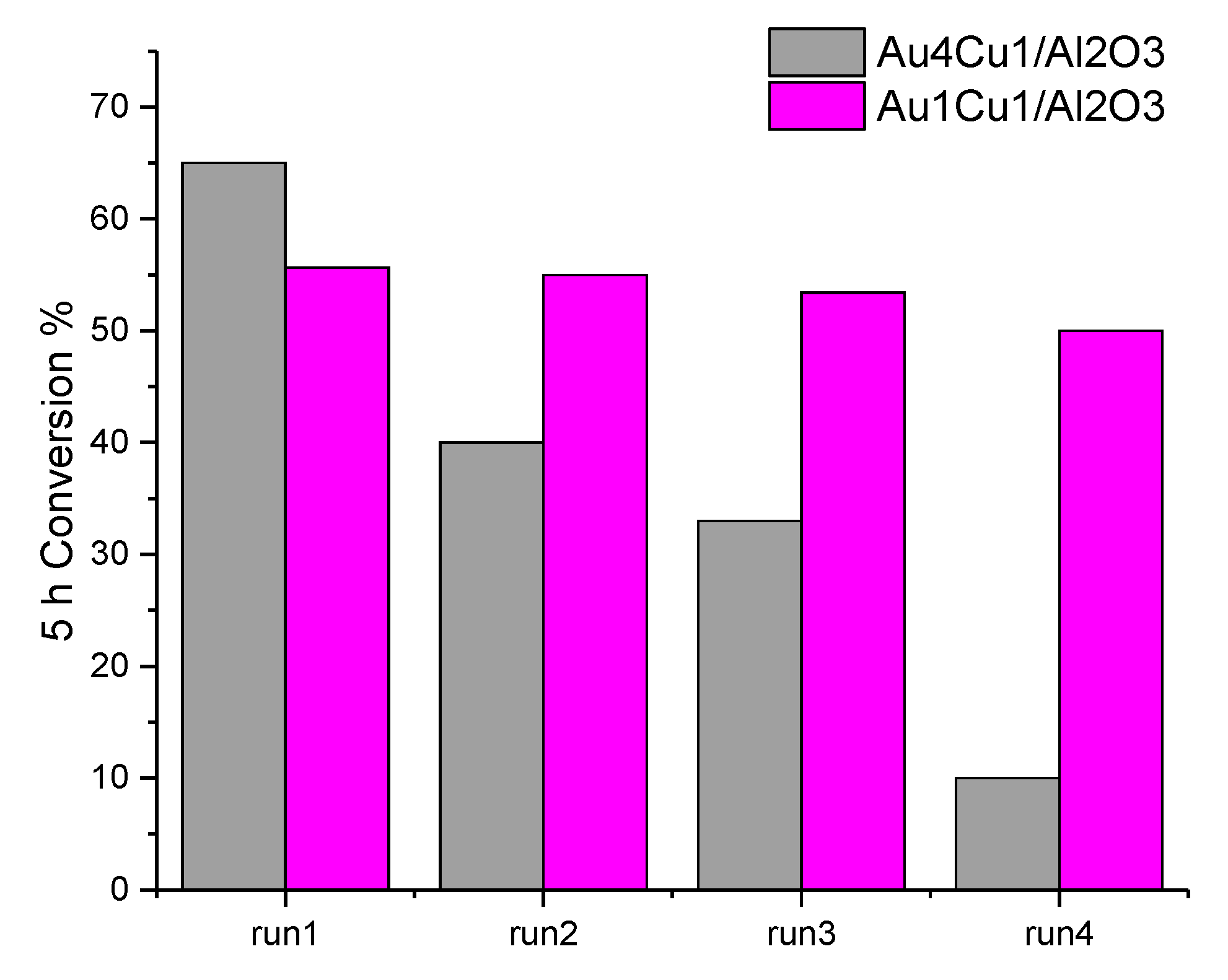
2.2.2. Stability Tests
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bruijnincx, P.C.A.; Weckhuysen, B.M. Lignin up for break-down. Nat. Chem. 2014, 6, 1035–1036. [Google Scholar] [CrossRef]
- Heitner, C.; Schmidt, J.A.; Group, F. Lignin and Lignans; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Zakzeski, J.; Bruijnincx, P.C.A.; Jongerius, A.L.; Weckhuysen, B.M. The catalytic valorization of lignin for the production of renewable chemicals. Chem. Rev. 2010, 110, 3552–3599. [Google Scholar] [CrossRef]
- Wang, Z. Green Chemistry: Recent Advances in Developing Catalytic Processes in Environmentally-Benign Solvent Systems. Available online: http://ccc.chem.pitt.edu/wipf/frontiers/zhiyong.pdf (accessed on 1 February 2020).
- Zakzeski, J.; Jongerius, A.L.; Weckhuysen, B.M. Transition metal catalyzed oxidation of Alcell lignin, soda lignin, and lignin model compounds in ionic liquids. Green Chem. 2010, 12, 1225–1236. [Google Scholar] [CrossRef]
- Wu, X.; Guo, S.; Zhang, J. Selective oxidation of veratryl alcohol with composites of Au nanoparticles and graphene quantum dots as catalysts. Chem. Commun. 2015, 51, 6318–6321. [Google Scholar] [CrossRef]
- Díaz-González, M.; Vidal, T.; Tzanov, T. Phenolic compounds as enhancers in enzymatic and electrochemical oxidation of veratryl alcohol and lignins. Appl. Microbiol. Biotechnol. 2011, 89, 1693–1700. [Google Scholar] [CrossRef] [PubMed]
- Gutman, A.L.; Shkolnik, E.; Tishin, B.; Nisnevich, G. Process and Intermediates for Production of Donepezil and Related Compounds. U.S. Patent 6492522B1, 10 December 2002. [Google Scholar]
- Lahtinen, P.; Korpi, H.; Haavisto, E.; Leskelä, M.; Repo, T. Parallel screening of homogeneous copper catalysts for the oxidation of benzylic alcohols with molecular oxygen in aqueous solutions. J. Comb. Chem. 2004, 6, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Mate, V.R.; Shirai, M.; Rode, C.V. Heterogeneous Co3O4 catalyst for selective oxidation of aqueous veratryl alcohol using molecular oxygen. Catal. Commun. 2013, 33, 66–69. [Google Scholar] [CrossRef]
- Melián-Rodríguez, M.; Saravanamurugan, S.; Kegnæs, S.; Riisager, A. Aerobic Oxidation of Veratryl Alcohol to Veratraldehyde with Heterogeneous Ruthenium Catalysts. Top. Catal. 2015, 58, 1036–1042. [Google Scholar] [CrossRef]
- Stucchi, M.; Cattaneo, S.; Cappella, A.; Wang, W.; Wang, D.; Villa, A.; Prati, L. Catalytic Oxidation of Methoxy Substituted Benzyl Alcohols as Model for Lignin Valorisation. Catal. Today 2019. [Google Scholar] [CrossRef]
- Olmos, C.M.; Chinchilla, L.E.; Cappella, A.M.; Villa, A.; Delgado, J.J.; Hungría, A.B.; Blanco, G.; Calvino, J.J.; Prati, L.; Chen, X. Selective oxidation of veratryl alcohol over Au-Pd/Ce0.62Zr0.38O2 catalysts synthesized by sol-immobilization: Effect of Au:Pd molar ratio. Nanomaterials 2018, 8, 669. [Google Scholar] [CrossRef]
- Haruta, M.; Kobayashi, T.; Sano, H.; Yamada, N. Novel Gold Catalysts for the Oxidation of Carbon Monoxide at a Temperature far Below 0 °C. Chem. Lett. 1987, 16, 405–408. [Google Scholar] [CrossRef]
- Hutchings, G.J. Vapor phase hydrochlorination of acetylene: Correlation of catalytic activity of supported metal chloride catalysts. J. Catal. 1985, 96, 292–295. [Google Scholar] [CrossRef]
- Silva, T.A.G.; Teixeira-Neto, E.; López, N.; Rossi, L.M. Volcano-like behavior of Au-Pd core-shell nanoparticles in the selective oxidation of alcohols. Sci. Rep. 2014, 4, 5766. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, S.; Stucchi, M.; Villa, A.; Prati, L. Gold Catalysts for the Selective Oxidation of Biomass-Derived Products. ChemCatChem 2019, 11, 309–323. [Google Scholar] [CrossRef]
- Schwank, J. Gold in bimetallic catalysts. Gold Bull. 1985, 18, 2–10. [Google Scholar] [CrossRef]
- Markó, I.E.; Giles, P.R.; Tsukazaki, M.; Brown, S.M.; Urch, C.J. Copper-catalyzed oxidation of alcohols to aldehydes and ketones: An efficient, aerobic alternative. Science 1996, 274, 2044–2046. [Google Scholar] [CrossRef]
- Albadi, J.; Alihoseinzadeh, A.; Razeghi, A. Novel metal oxide nanocomposite of Au/CuO–ZnO for recyclable catalytic aerobic oxidation of alcohols in water. Catal. Commun. 2014, 49, 1–5. [Google Scholar] [CrossRef]
- Zhan, W.; Wang, J.; Wang, H.; Zhang, J.; Liu, X.; Zhang, P.; Chi, M.; Guo, Y.; Guo, Y.; Lu, G.; et al. Crystal Structural Effect of AuCu Alloy Nanoparticles on Catalytic CO Oxidation. J. Am. Chem. Soc. 2017, 139, 8846–8854. [Google Scholar] [CrossRef]
- Destro, P.; Marras, S.; Manna, L.; Colombo, M.; Zanchet, D. AuCu alloy nanoparticles supported on SiO2: Impact of redox pretreatments in the catalyst performance in CO oxidation. Catal. Today 2017, 282, 105–110. [Google Scholar] [CrossRef]
- Della Pina, C.; Falletta, E.; Rossi, M. Highly selective oxidation of benzyl alcohol to benzaldehyde catalyzed by bimetallic gold-copper catalyst. J. Catal. 2008, 260, 384–386. [Google Scholar] [CrossRef]
- Marelli, M.; Jouve, A.; Villa, A.; Psaro, R.; Balerna, A.; Prati, L.; Evangelisti, C. Hybrid Au/CuO Nanoparticles: Effect of Structural Features for Selective Benzyl Alcohol Oxidation. J. Phys. Chem. C 2019, 123, 2864–2871. [Google Scholar] [CrossRef]
- Liu, X.; Wang, A.; Li, L.; Zhang, T.; Mou, C.-Y.; Lee, J.-F. Structural changes of Au–Cu bimetallic catalysts in CO oxidation: In situ XRD, EPR, XANES, and FT-IR characterizations. J. Catal. 2011, 278, 288–296. [Google Scholar] [CrossRef]
- Amendola, V.; Pilot, R.; Frasconi, M.; Maragò, O.M.; Iatì, M.A. Surface plasmon resonance in gold nanoparticles: A review. J. Phys. Condens. Matter 2017, 29, 203002. [Google Scholar] [CrossRef] [PubMed]
- Daniel, M.C.; Astruc, D. Gold Nanoparticles: Assembly, Supramolecular Chemistry, Quantum-Size-Related Properties, and Applications Toward Biology, Catalysis, and Nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef] [PubMed]
- Creighton, J.A.; Eadon, D.G. Ultraviolet–visible absorption spectra of the colloidal metallic elements. J. Chem. Soc. Faraday Trans. 1991, 87, 3881–3891. [Google Scholar] [CrossRef]
- Lisiecki, I.; Pileni, M.P. Copper metallic particles synthesized “in situ” in reverse micelles: Influence of various parameters on the size of the particles. J. Phys. Chem. 1995, 99, 5077–5082. [Google Scholar] [CrossRef]
- Luo, Y.; Tu, Y.; Ren, Q.; Dai, X.; Xing, L.; Li, J. Surfactant-free fabrication of Cu2O nanosheets from Cu colloids and their tunable optical properties. J. Solid State Chem. 2009, 182, 182–186. [Google Scholar] [CrossRef]
- Xu, Z.; Lai, E.; Yang, S.H.; Hamad-Schifferli, K. Compositional dependence of the stability of AuCu alloy nanoparticles. Chem. Commun. 2012, 48, 5626–5628. [Google Scholar] [CrossRef]
- Wang, H.; Liu, D.; Xu, C. Directed synthesis of well dispersed and highly active AuCu and AuNi nanoparticle catalysts. Catal. Sci. Technol. 2016, 6, 7137–7150. [Google Scholar] [CrossRef]
- Schünemann, S.; Dodekatos, G.; Tüysüz, H. Mesoporous Silica Supported Au and AuCu Nanoparticles for Surface Plasmon Driven Glycerol Oxidation. Chem. Mater. 2015, 27, 7743–7750. [Google Scholar] [CrossRef]
- Casanova, O.; Iborra, S.; Corma, A. Biomass into chemicals: Aerobic oxidation of 5-hydroxymethyl-2-furfural into 2,5-furandicarboxylic acid with gold nanoparticle catalysts. ChemSusChem. 2009, 2, 1138–1144. [Google Scholar] [CrossRef] [PubMed]
- Albonetti, S.; Pasini, T.; Lolli, A.; Blosi, M.; Piccinini, M.; Dimitratos, N.; Lopez-Sanchez, J.A.; Morgan, D.J.; Carley, A.F.; Hutchings, G.J.; et al. Selective oxidation of 5-hydroxymethyl-2-furfural over TiO 2-supported gold-copper catalysts prepared from preformed nanoparticles: Effect of Au/Cu ratio. Catal. Today 2012, 195, 120–126. [Google Scholar] [CrossRef]
- Moore, A.D.; Holmes, S.M.; Roberts, E.P.L. Evaluation of porous carbon substrates as catalyst supports for the cathode of direct methanol fuel cells. RSC Adv. 2012, 2, 1669–1674. [Google Scholar] [CrossRef]


| Catalyst | Loading wt. % | Au/Cu mol/mol | ||
|---|---|---|---|---|
| Nominal | Actual | Nominal | Actual | |
| Au/Al2O3 | 3.0 | 2.7 | - | - |
| Au4Cu1/Al2O3 | 2.6 | 2.2 | 4.00 | 3.61 |
| Au1Cu1/Al2O3 | 2.0 | 1.8 | 1.00 | 0.88 |
| Au1Cu4/Al2O3 | 1.4 | 1.2 | 0.25 | 0.33 |
| Cu/Al2O3 | 1.0 | 1.4 | - | - |
| Au/C | 3.0 | 2.9 | - | - |
| Au4Cu1/C | 2.6 | 2.2 | 4.00 | 3.64 |
| Au1Cu1/C | 2.0 | 1.9 | 1.00 | 0.97 |
| Au1Cu4/C | 1.4 | 1.5 | 0.25 | 0.26 |
| Cu/C | 1.0 | 1.1 | - | - |
| Survey | HR | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Au 4f7/2 | |||||||||||
| Au (%At) | Cu (%At) | Au/Cu | Au0 | Auδ+ | Cu0-Cu+ | CuO | Cu(OH)2 | Cu(NO3)2 | Sat. | ||
| Au4Cu1/C | B.E. (eV) | 84.3 | 85.5 | - | 933.9 | - | - | ||||
| %At | 0.19 | 0.03 | 6.3 | 77 | 23 | - | 100 | - | - | yes | |
| Au1Cu1/C | B.E. (eV) | 84.4 | 85.4 | 932.2 | - | - | 935.8 | ||||
| %At | 0.10 | 0.11 | 0.91 | 74 | 26 | 65 | - | - | 35 | - | |
| Au1Cu4/C | B.E. (eV) | 83.3 | - | 932.9 | - | 934.5 | - | ||||
| %At | 0.07 | 0.09 | 0.77 | 100 | - | 78 | - | 22 | - | - | |
| Au4Cu1/Al2O3 | B.E. (eV) | 83.5 | 85.7 | - | 934.0 | - | - | ||||
| %At | 0.97 | 0.53 | 1.83 | 78 | 22 | - | 100 | - | - | yes | |
| Au1Cu1/Al2O3 | B.E. (eV) | 83.5 | 86.1 | 932.9 | - | - | 935.6 | ||||
| %At | 0.64 | 0.84 | 0.76 | 83 | 17 | 61 | - | - | 39 | - | |
| Au1Cu4/Al2O3 | B.E. (eV) | 83.7 | - | 932.7 | - | 934.6 | - | ||||
| %At | 0.16 | 1.36 | 0.12 | 100 | - | 70 | - | 30 | - | - | |
| Catalysts | Total Metal Amount (%At.) | Au (%At.) | Au0 (%At.) | Au0exp (%At.) | Conversion_1h (%) |
|---|---|---|---|---|---|
| Au4Cu1/Al2O3 | 1.50 | 97 | 78 | 76 | 25.6 |
| Au1Cu1/Al2O3 | 1.48 | 64 | 83 | 53 | 12.4 |
| Au1Cu4/Al2O3 | 1.52 | 16 | 100 | 16 | 7.27 |
| Catalyst Label | Au/Cu Nominal Molar Ratio | Metal Loading, wt. %a | AuCu Particle Size, nmb |
|---|---|---|---|
| Au/C | - | 3 | 2.7 ± 0.5 |
| Au4Cu1/C | 4 | 2.6 | 2.0 ± 0.6 |
| Au1Cu1/C | 1 | 2 | 2.3 ± 0.6 |
| Au1Cu4/C | 0.25 | 1.4 | 1.9 ± 0.6 |
| Cu/C | - | 1 | 2.9 ± 0.5 |
| Au/Al2O3 | - | 3 | 2.0 ± 0.5 |
| Au4Cu1/Al2O3 | 4 | 2.6 | 1.8 ± 0.5 |
| Au1Cu1/Al2O3 | 1 | 2 | 1.9 ± 0.4 |
| Au1Cu4/Al2O3 | 0.25 | 1.4 | 1.7 ± 0.4 |
| Cu/Al2O3 | - | 1 | 2.9 ± 0.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stucchi, M.; Capelli, S.; Cardaci, S.; Cattaneo, S.; Jouve, A.; Beck, A.; Sáfrán, G.; Evangelisti, C.; Villa, A.; Prati, L. Synergistic Effect in Au-Cu Bimetallic Catalysts for the Valorization of Lignin-Derived Compounds. Catalysts 2020, 10, 332. https://doi.org/10.3390/catal10030332
Stucchi M, Capelli S, Cardaci S, Cattaneo S, Jouve A, Beck A, Sáfrán G, Evangelisti C, Villa A, Prati L. Synergistic Effect in Au-Cu Bimetallic Catalysts for the Valorization of Lignin-Derived Compounds. Catalysts. 2020; 10(3):332. https://doi.org/10.3390/catal10030332
Chicago/Turabian StyleStucchi, Marta, Sofia Capelli, Simone Cardaci, Stefano Cattaneo, Andrea Jouve, Andrea Beck, György Sáfrán, Claudio Evangelisti, Alberto Villa, and Laura Prati. 2020. "Synergistic Effect in Au-Cu Bimetallic Catalysts for the Valorization of Lignin-Derived Compounds" Catalysts 10, no. 3: 332. https://doi.org/10.3390/catal10030332
APA StyleStucchi, M., Capelli, S., Cardaci, S., Cattaneo, S., Jouve, A., Beck, A., Sáfrán, G., Evangelisti, C., Villa, A., & Prati, L. (2020). Synergistic Effect in Au-Cu Bimetallic Catalysts for the Valorization of Lignin-Derived Compounds. Catalysts, 10(3), 332. https://doi.org/10.3390/catal10030332











