2.2. Dialysis Experiments
In this section, the experimental results of dialysis without photocatalysis are analyzed in view of the utilization of the PEBAX membranes in the photocatalytic membrane reactor.
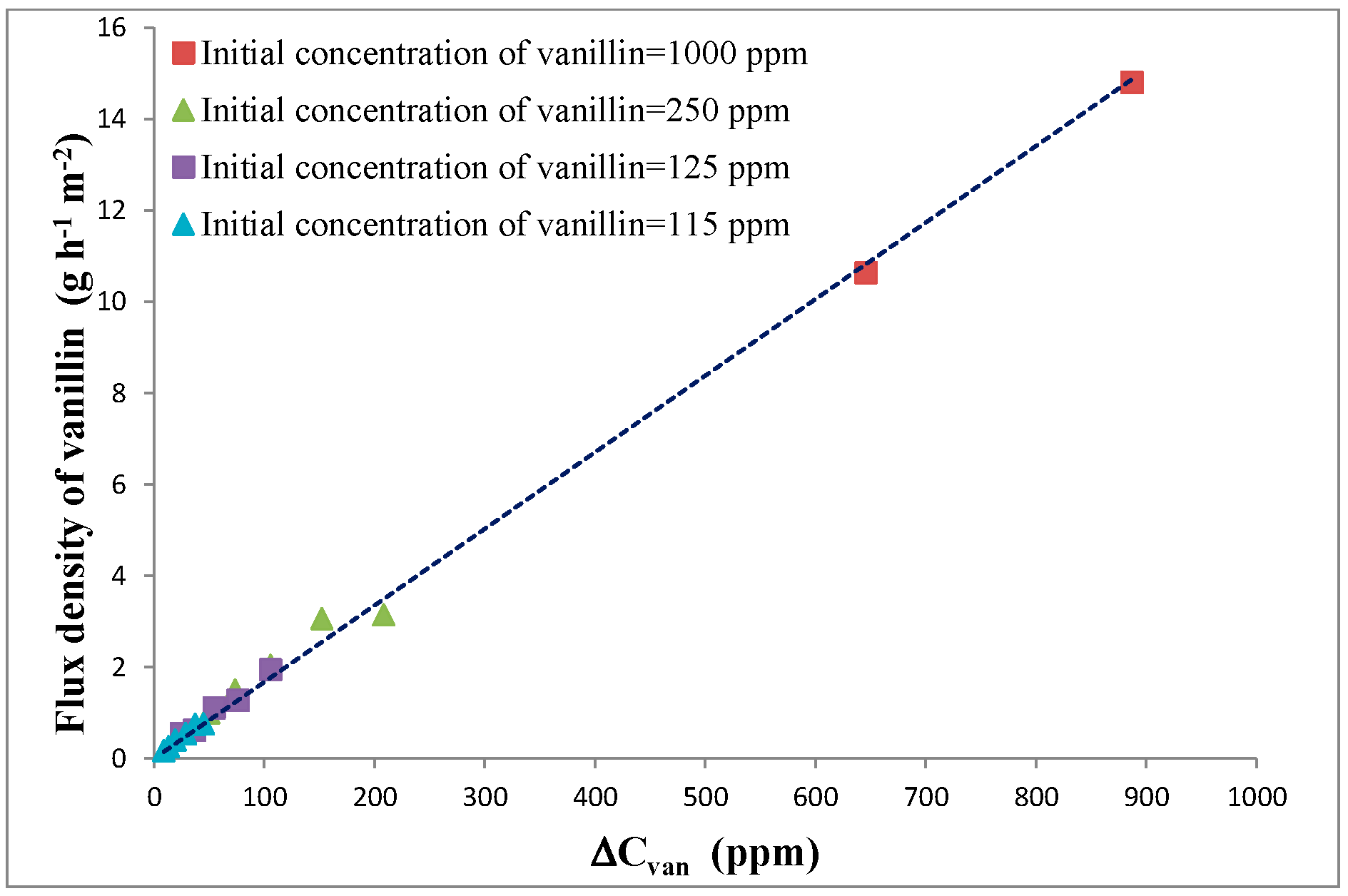
The difference between the chemical potential of vanillin between the two phases at the opposite side of the membrane (Liquid 1, upstream, and Liquid 2, downstream) is the driving force for permeation. The concentration of vanillin in the tested solutions is relatively low and the value of the activity coefficient even at the highest tested concentration (about 1000 ppm) is close to the one at infinite dilution. In fact, according to the NRTL model with the values of the intervening parameters reported by De Albuquerque and Mazzotti [
13], the activity coefficient of vanillin in aqueous solution is about 131 at 1000 ppm and 132 at 1 ppm. Consequently, the driving force is directly proportional to the difference of the vanillin concentration (ΔC
van = C
1,van−C
2,van), as confirmed by the experimental results shown in
Figure 3.
Even if vanillin permeates just by diffusion through a non-porous membrane and concentration polarization (as discussed below) affects the rate of permeation, the flux is more than 10 times higher than the one obtained at the same concentration and temperature in pervaporation. For the sake of comparison, in pervaporation, the vanillin flux density is about 1.1 g/(m
2 h) (pervaporation at 60 °C with C
van,upstream the membrane =1000 ppm, P
permeate =2 mbar), whereas, in dialysis, it is 15 g/(m
2 h) (ΔC
van ≈ 1000 ppm, operating conditions in the
Figure 4 description). Moreover, in dialysis, the flux does not change significantly in the temperature range from 20 to 60 °C, whereas, in pervaporation, the flux is approximately halved every 10 °C decrease of the temperature. The possibility of operating with a higher flux allows for a substantial decrease in the membrane area required to obtain the desired recovery of vanillin. Moreover, the flux remains high even at ambient temperature and dialysis can also be utilized effectively in bioreactors. The permeation of water is irrelevant because the solutions are so diluted that the driving force for water permeation is negligible.
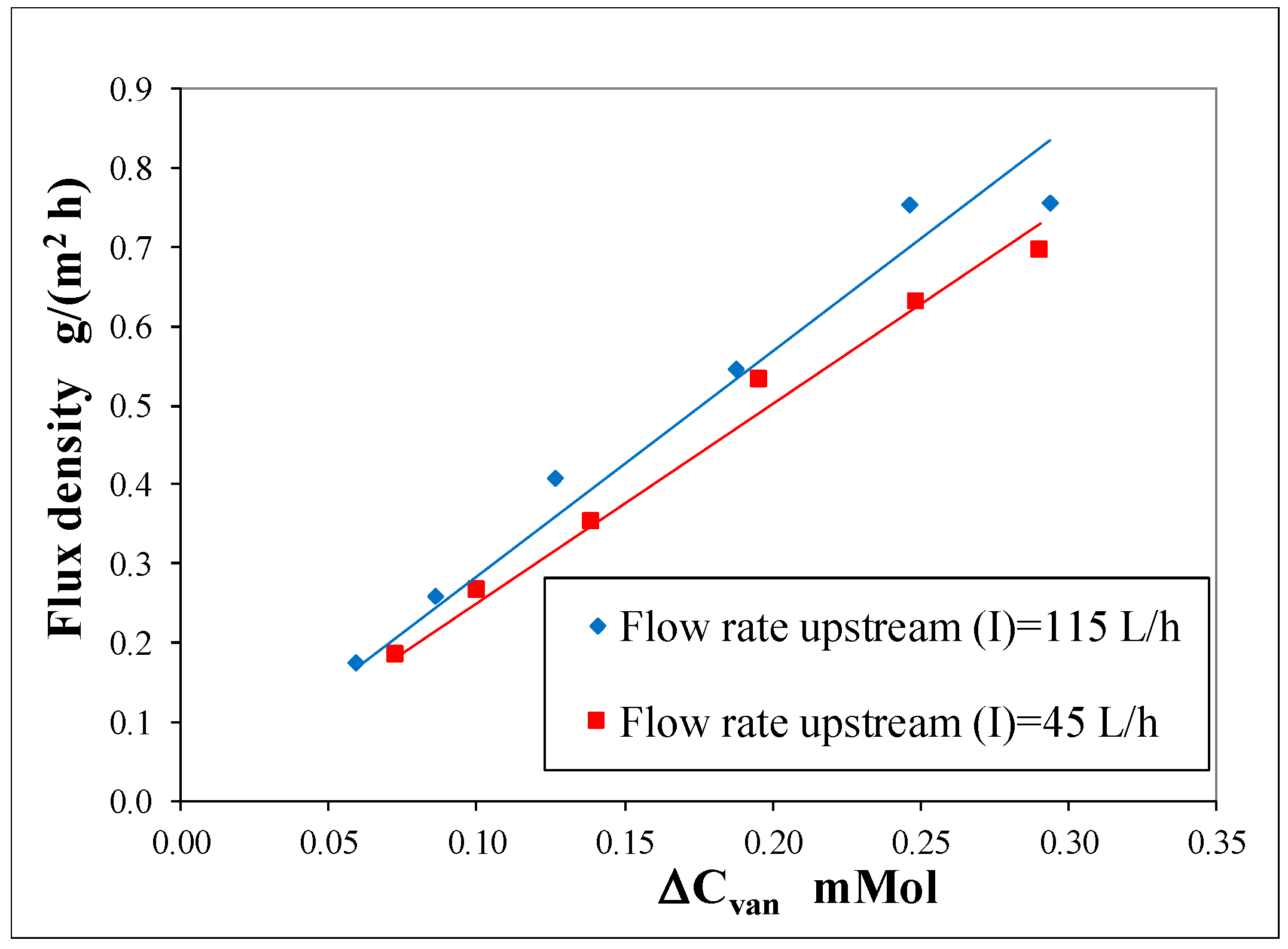
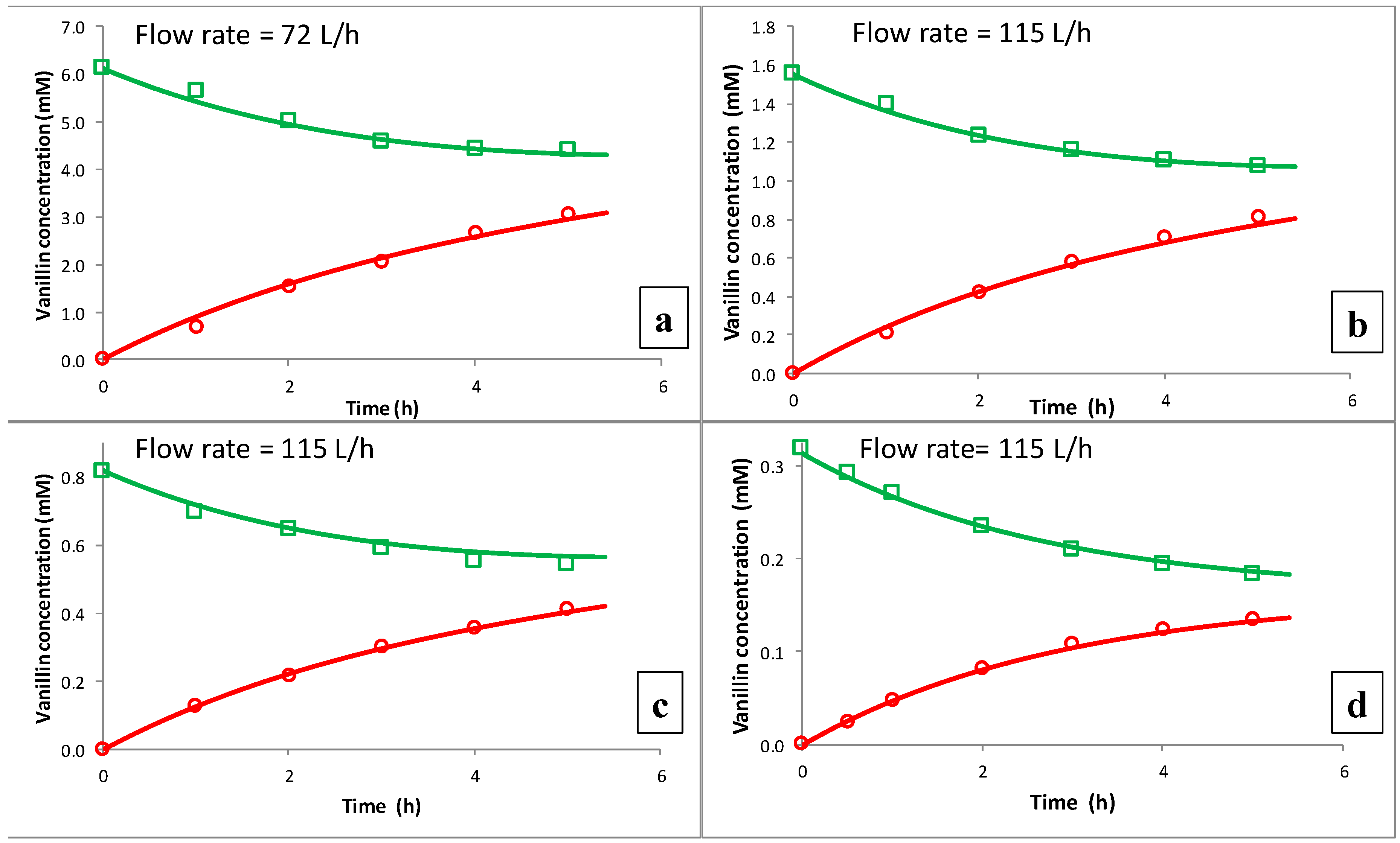
Figure 4 shows that the flux density is also affected by the flow rate of the fluid passing through the module. This outcome reveals that concentration polarization cannot be neglected. At higher flow rates, the flux density of vanillin increases because the mass transfer of vanillin from the bulk of Liquid 1 to the surface of the membrane is favored by the higher tangential velocity of the fluid and concentration polarization diminishes.
To get a better understanding of concentration polarization and of the phenomena which take place in dialysis, the transient behavior of vanillin permeation was experimentally studied by varying the initial concentration of vanillin and the upstream flow rate. The results were analyzed through a mathematical model, which is substantially based on a solution–diffusion mechanism.
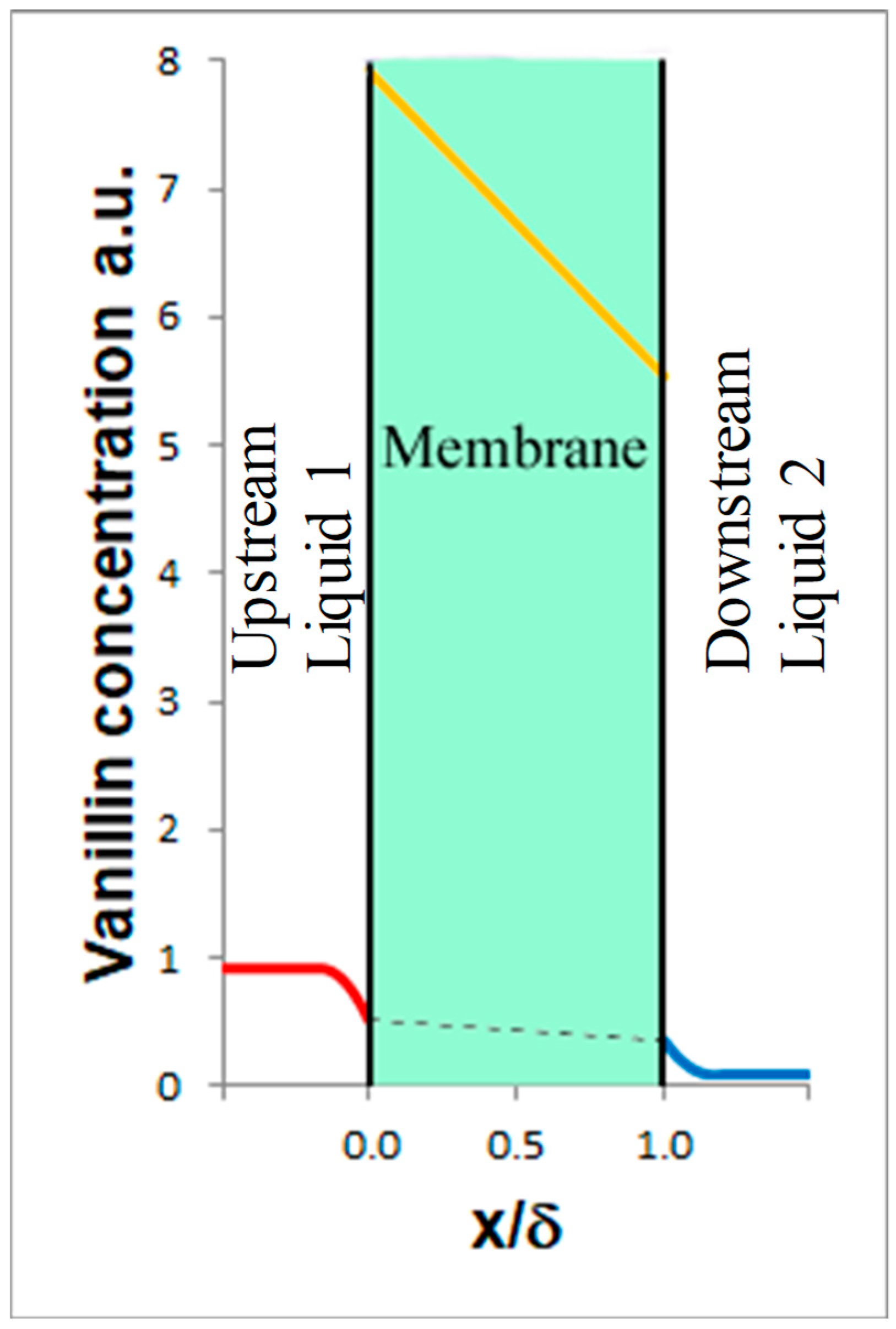
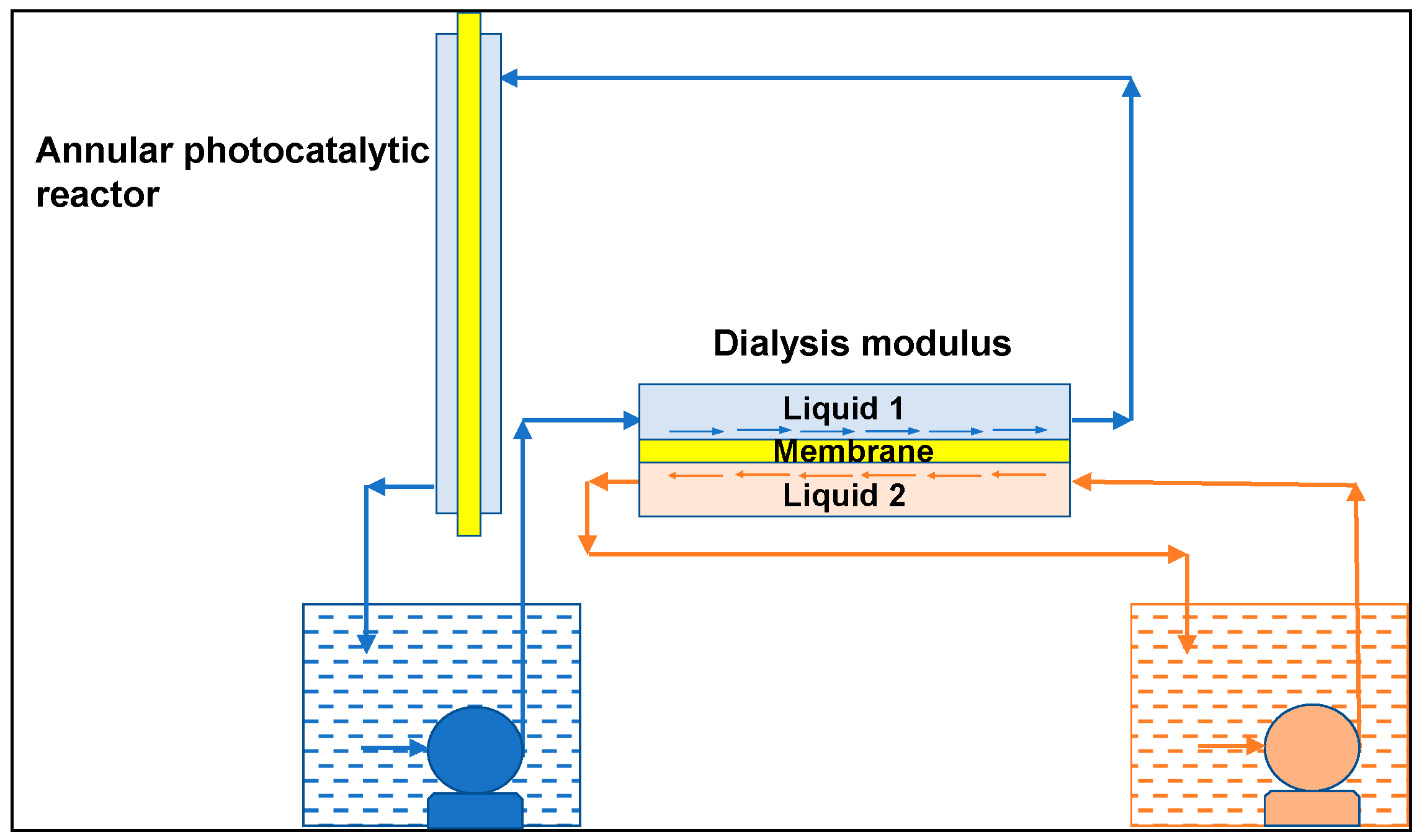
Referring to
Figure 5, which schematically shows the system comprising the upstream Liquid 1, the membrane, and the downstream Liquid 2, the model takes into account (see also [
11]):
• Mass transport upstream of the membrane (Liquid 1):
, where is the vanillin concentration in the bulk of Liquid 1, t is the time, is the membrane area, the volume of Liquid 1, is the mass transfer coefficient of vanillin in Liquid 1, and is the vanillin concentration at x = 0 in Liquid 1.
• Equilibrium at the interface between Liquid 1 and the membrane:
, where is the partition coefficient with the vanillin concentration in the membrane at x = 0.
• Mass transport inside the membrane:
, where is the vanillin concentration in the membrane, g is the so-called plasticization coefficient, and is the diffusion coefficient of vanillin in the membrane for →0.
• Equilibrium at the interface between the membrane and Liquid 2:
where is the vanillin concentration in the membrane at x = δ and is the vanillin concentration in Liquid 2 at x = δ.
• Mass transport downstream of the membrane (Liquid 2):
where is the vanillin concentration in the bulk of Liquid 2, is the volume of Liquid 2, and is the mass transfer coefficient of vanillin in Liquid 2.
The mass transfer coefficients k1 and k2 in Liquids 1 and 2 were predicted by the following well-known analogy:
, with the Sherwood number, the Reynolds number, the Schmidt number, Dh the hydraulic diameter, L the length of the channel, the vanillin diffusivity in the aqueous solution, ν the kinematic viscosity of the solution, and vm the average velocity.
To evaluate the partition coefficients (), experiments on the adsorption in the membrane were carried out until reaching equilibrium. The partition coefficient of vanillin resulted to be Kvan = 21.3 and 15.1 at 30 and 60 °C, respectively. In an analogous way, the partition coefficient of ferulic acid at 30 °C was found to be 29.9.
The equations of the model were solved numerically by the volume control method with a Crank–Nicolson time integration scheme with the initial conditions, at t = 0, , , (at 0 ≤ x ≤ δ), , and , where is the initial concentration of vanillin in Liquid 1. In this way, the trends over time of all the unknown concentrations were evaluated.
The experimental data, obtained from several dialysis experiments carried out at different initial concentrations of vanillin in Liquid 1 and different flow rates through the module, were used to validate the mathematical model. All parameters were independently measured, taken from the literature, or evaluated by established relationships (as the previously presented analogy for the mass transfer coefficients). The only exceptions were the vanillin diffusivity in the membrane and the correlated plasticization coefficient, which were estimated by a least squares non-linear fitting procedure. The best fit (see
Figure 6) was obtained with
= 9 × 10
−11 m
2/s and g = 0. The high values of both the diffusivity and the partition coefficient contribute to the high permeability of vanillin through the membrane.
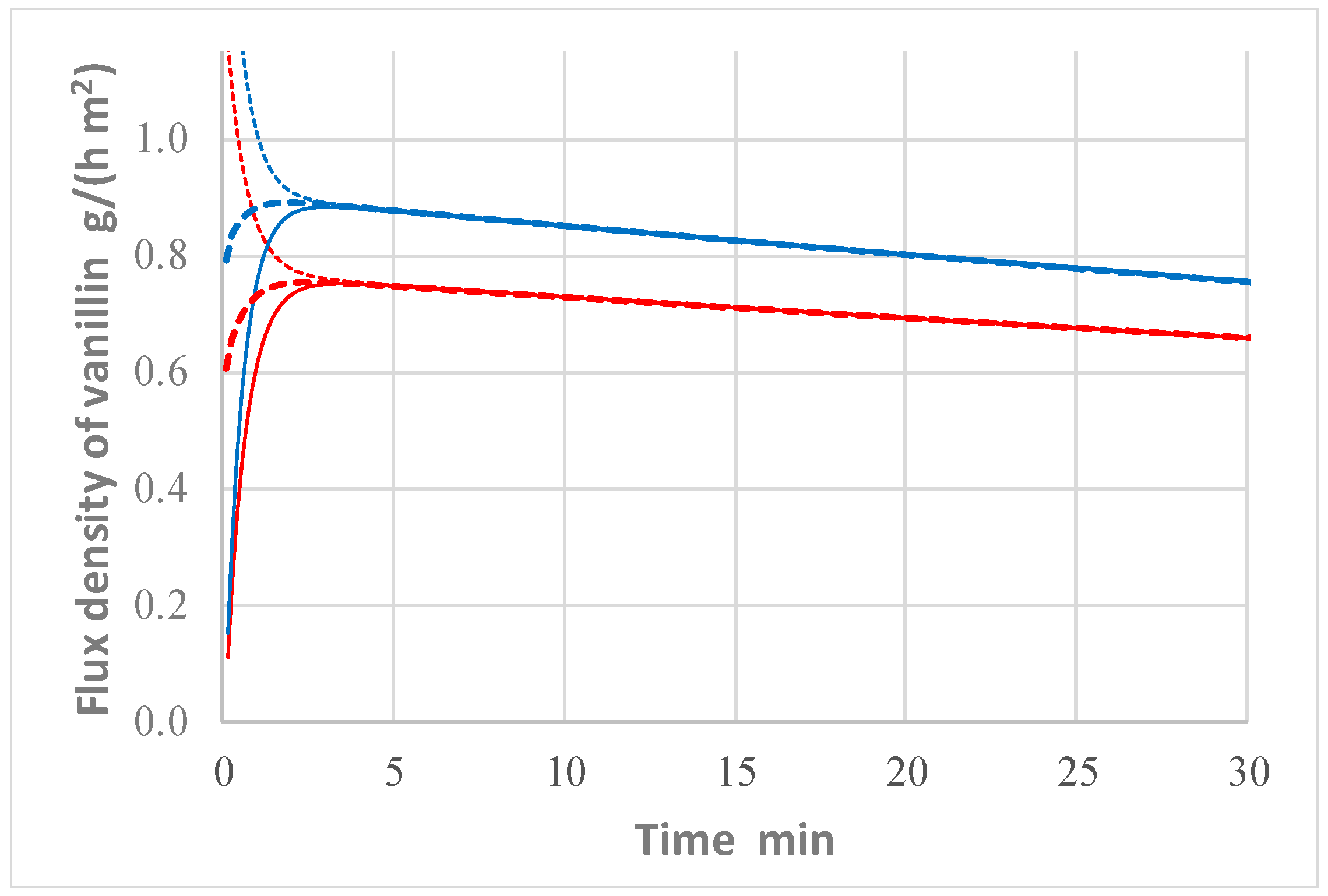
The model reveals that, after a relatively short initial period (less than 5 min), the system approaches a pseudo-steady-state condition, which is characterized, at a given time, by the same value, J, for the flux of vanillin upstream of the membrane, at any cross section inside the membrane and downstream of the membrane. This phenomenon is apparent in
Figure 7, which reports the flux density of vanillin, calculated by the model, upstream of the membrane, in the middle of the membrane (at x = δ/2), and downstream of the membrane vs. time in two cases, which simulate two experiments carried out with the same initial concentration of vanillin, but different flow rates of Liquid 1.
When the pseudo-steady state is reached, the following equalities hold:
, where Rupstream, Rmembrane, and Rdownstream indicate the resistances to vanillin mass transfer upstream, through, and downstream of the membrane, respectively, and Rtotal is the total resistance (). It follows that:
; ; and . Alternatively, the resistance to mass transfer at pseudo-steady state can be evaluated directly from the mass transport equations as:
; (valid only if g = 0, as in the present case); and .
Therefore, it is possible to use the values of the concentrations (
) calculated by the model to estimate the ratios of the various resistances to the total resistance. Some values of these ratios are also reported in [
11]. Notably,
turns out to be just a small fraction of the total resistance. For instance, when the flow rate of Liquid 1 is 45 L/h,
is just 16% of the total resistance. It is clear that concentration polarization upstream and downstream of the membrane controls the permeation. It is rather surprising that, even if the membrane is non-porous and relatively thick (45 µm) and permeation takes place only due to a diffusion mechanism, membrane resistance is minor. Contrary to pervaporation, concentration polarization can limit the rate of permeation because of the much higher permeate flux. Therefore, there is extensive room for improvement of vanillin flux simply by designing the module for a high tangential velocity to limit concentration polarization. It is worth observing that the flow was laminar in all presented experiments. In fact, for Liquid 2 it is Re = 1070 (flow rate = 75 L/h), while for Liquid 1 it is Re = 620 (flow rate = 45 L/h) and Re = 1600 (flow rate = 115 L/h). Besides, in the operating conditions indicated in the
Figure 7 description, the permeate flow rate of vanillin increases by about 15% simply by augmenting the flow rate of Liquid 1 through the module from 45 to 115 L/h.
2.3. Membrane Reactor, MR (Dialysis Integrated with Photocatalysis)
Camera-Roda et al. [
8] established that the main reason for the high retention of ferulic acid (FA) observed in pervaporation is not the dissociation of FA, as hypothesized by Brazinha et al. [
6], but rather the low volatility of FA, whose evaporation is negligible in the permeate even at the very low permeate pressure and relatively high temperature utilized in pervaporation. Given the similar molecular structure, it is expected that the solubility and diffusivity of FA in the membrane are analogous to those of vanillin. This implies that, in dialysis, without the evaporative step, FA can also permeate relatively easily through the membrane. The same may hold for most of the other organic intermediate compounds that are produced. These suppositions were confirmed in dialysis experiments. Therefore, in dialysis, it becomes possible to feed the substrate to the reactor by permeating it from the opposite side of the membrane as long as it is consumed by the reaction. In this way, the concentration of FA and, consequently, the rate of reaction can be maintained higher in the reactor, thus enhancing the production. Given these considerations, experimental runs with the MR were carried out with the same initial concentration of FA (1 mM) in both the aqueous solutions at the two opposite sides of the membrane. In fact, if FA is initially present in Liquid 2, this latter acts as a substrate reservoir for the replenishment of the FA consumed by the reaction [
14].
Table 1 reports the values of the concentration of the main organic compounds after 5 h of reaction on the two sides (reactor and the opposite side) of the membrane.
The values in
Table 1 demonstrate that all organic compounds produced by the reaction permeate. The presence of cis-ferulic acid is due to the UVA illumination in the reactor, which transforms trans-FA into cis-FA [
3]. This latter permeates to Liquid 2 along with all the intermediate organic compounds. Trans-ferulic acid permeates from Liquid 2 to the reactor, because in the reactor TFA disappears due to the reaction, so that the concentration of TFA is higher in Liquid 2.
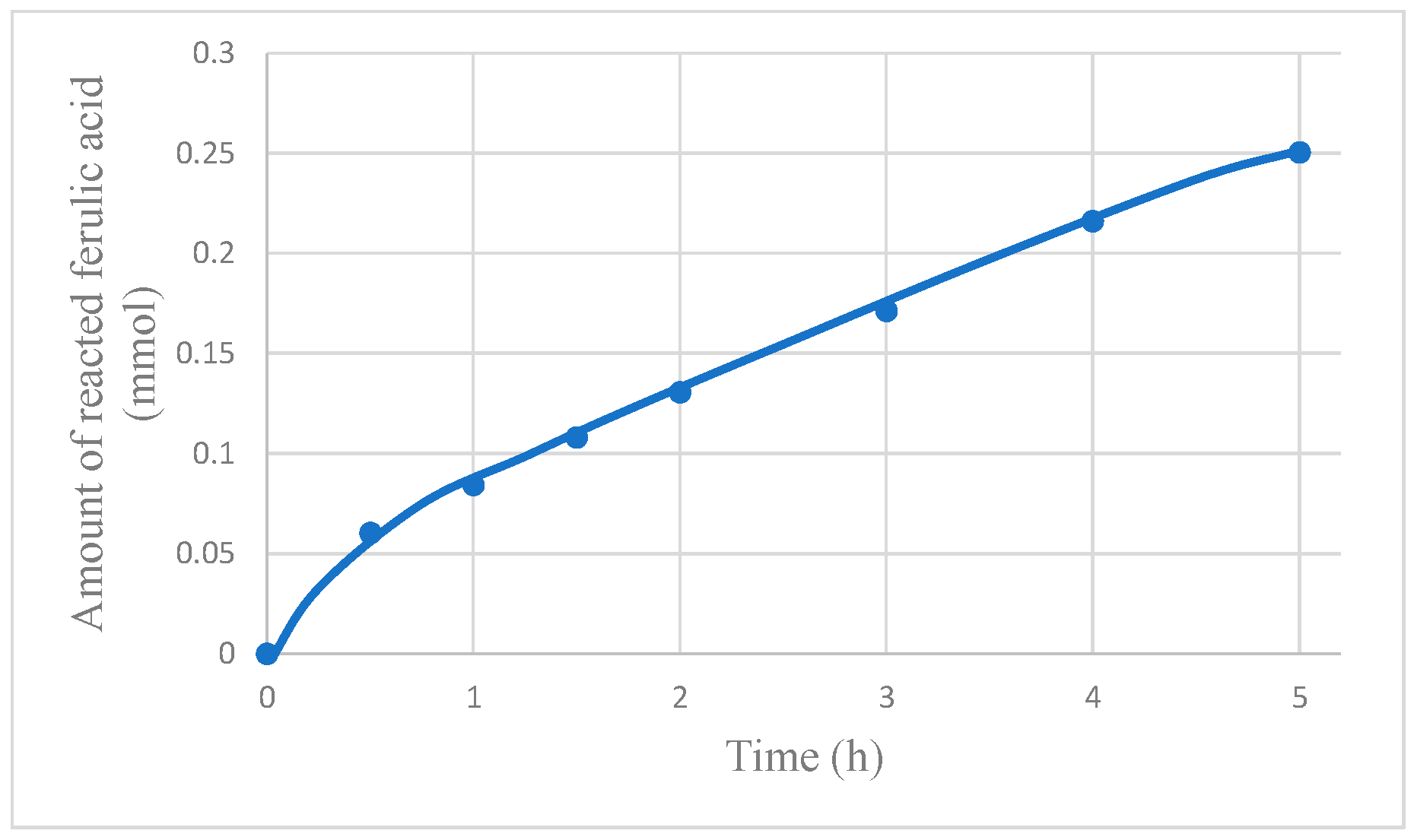
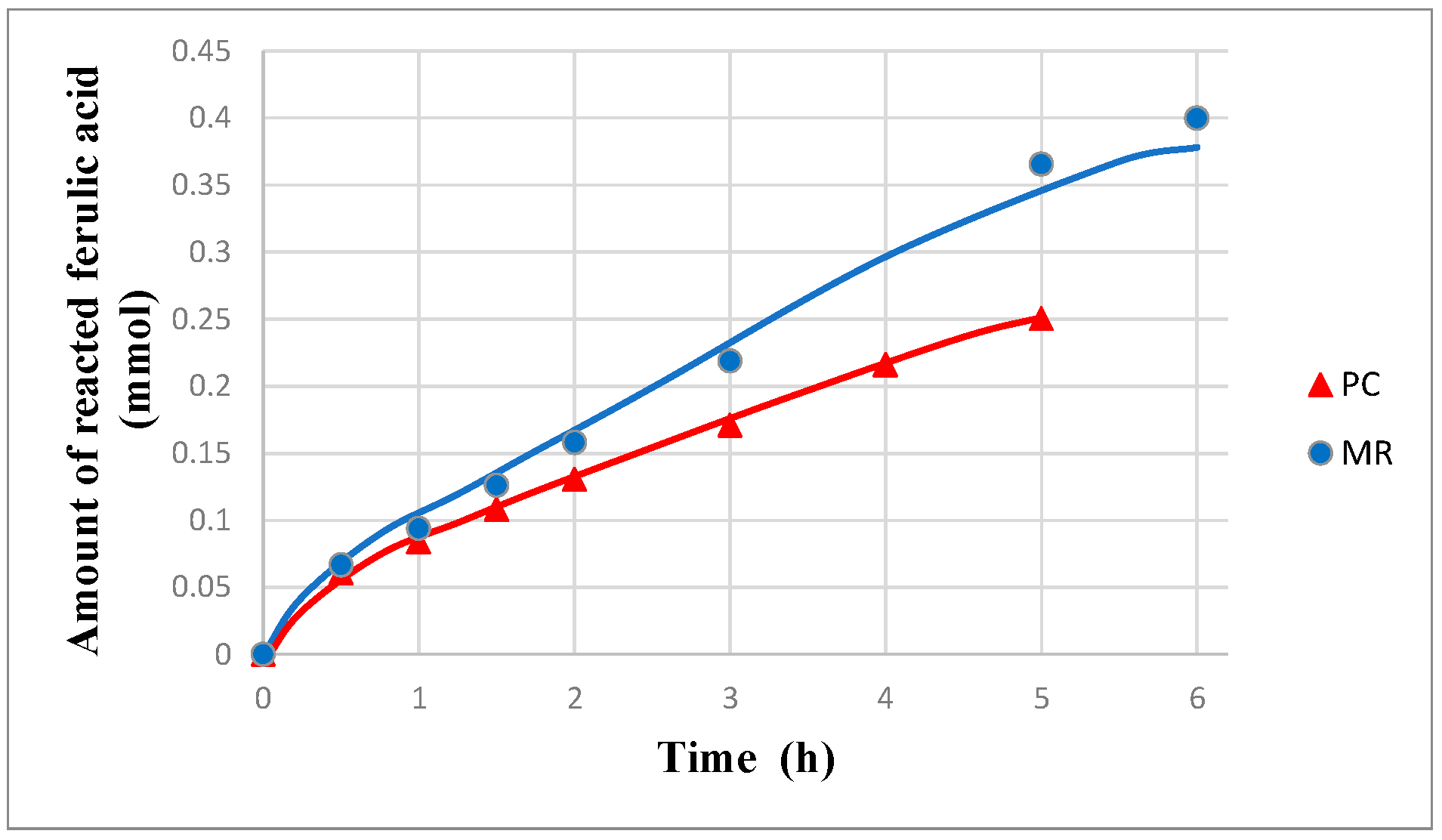
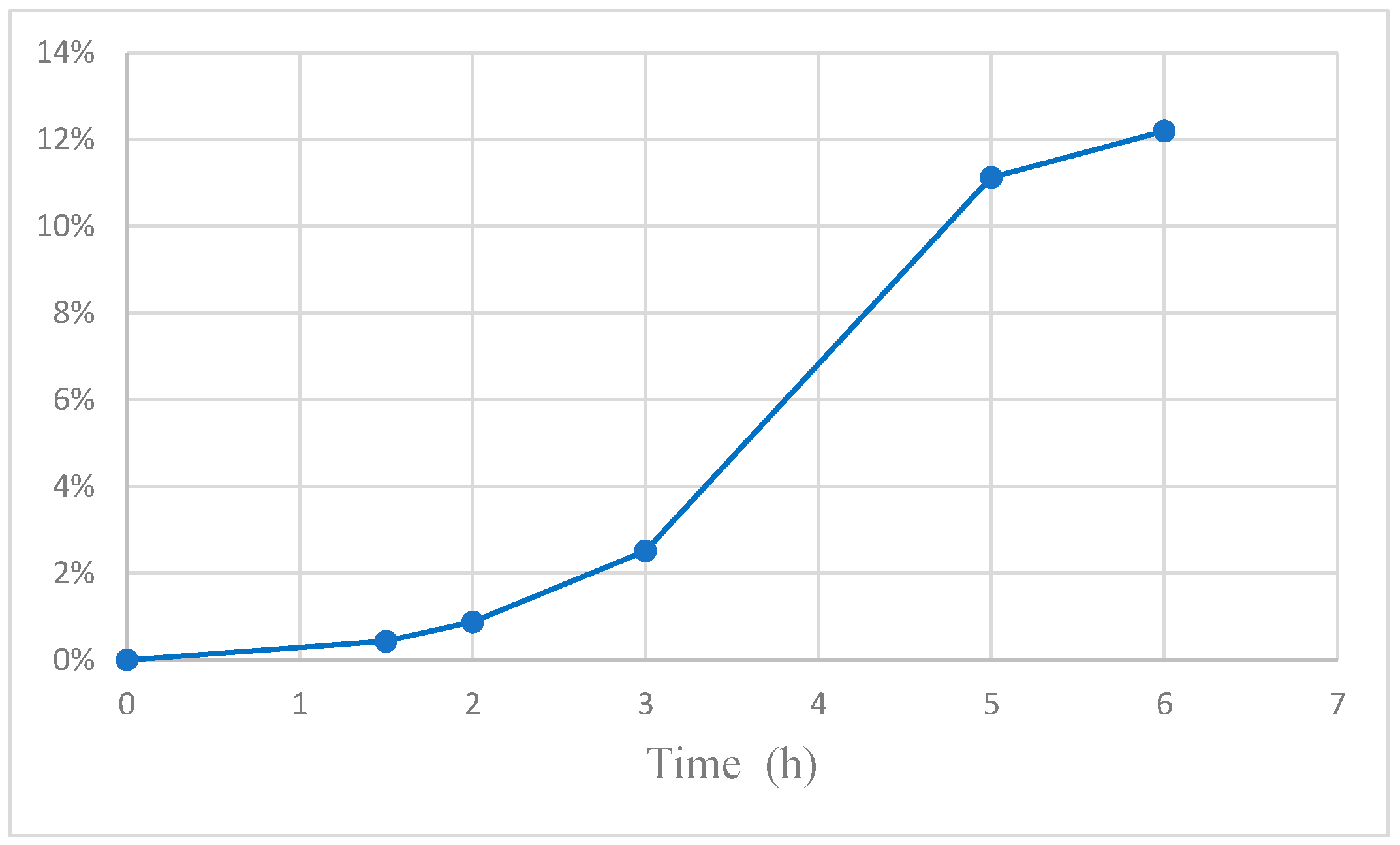
Interestingly, as shown in
Figure 8, the amount of reacted FA (regardless if cis-FA or trans-FA) is higher in the MR than in PC (i.e., without dialysis). There are two mechanisms for the increase of FA transformation:
(1) The recovery of vanillin and the other intermediate compounds reduces the inhibition of the photocatalytic oxidation of FA caused by the presence of these compounds.
(2) The substrate consumed by the reaction is partially replenished by the permeation of FA from the opposite side of the membrane.
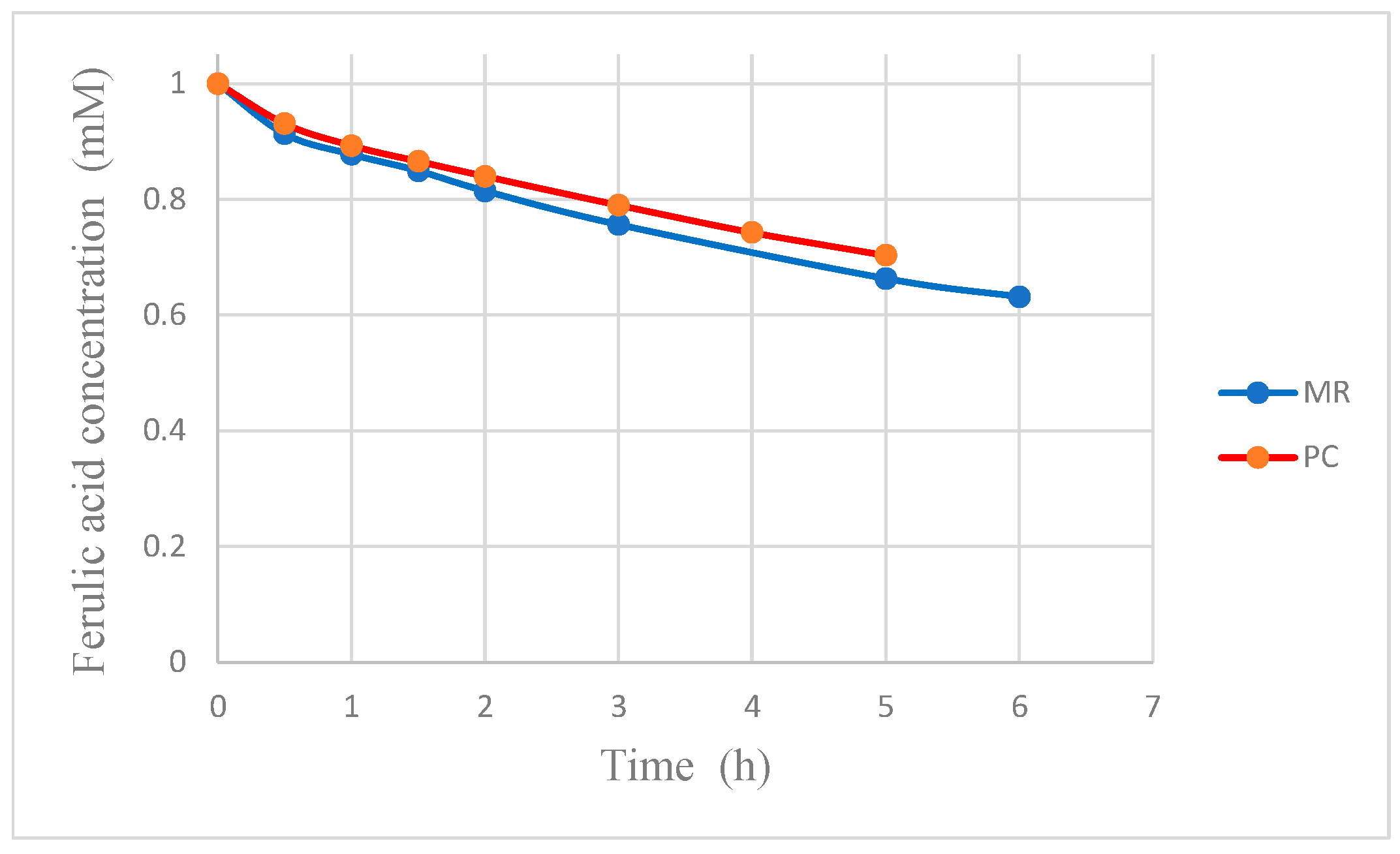
If the first phenomenon were negligible, the FA concentration would be higher in the MR than in photocatalysis alone (PC). However, in
Figure 9, it is apparent that the opposite is true, thus showing that the removal of the organics effectively increases the reaction rate by reducing the inhibition caused by these compounds. It is worth noticing that, in the case of pervaporation, only vanillin is removed from the reacting solution, whereas almost all the other intermediate compounds do not permeate, due to their low volatility, and their presence hinders the rate of oxidation of FA.
Figure 10 confirms that the replenishment of FA from Liquid 2 into the reactor by permeation is efficient and the permeation of FA rises when the concentration of FA in the reactor tends to decrease because the driving force for permeation increases.
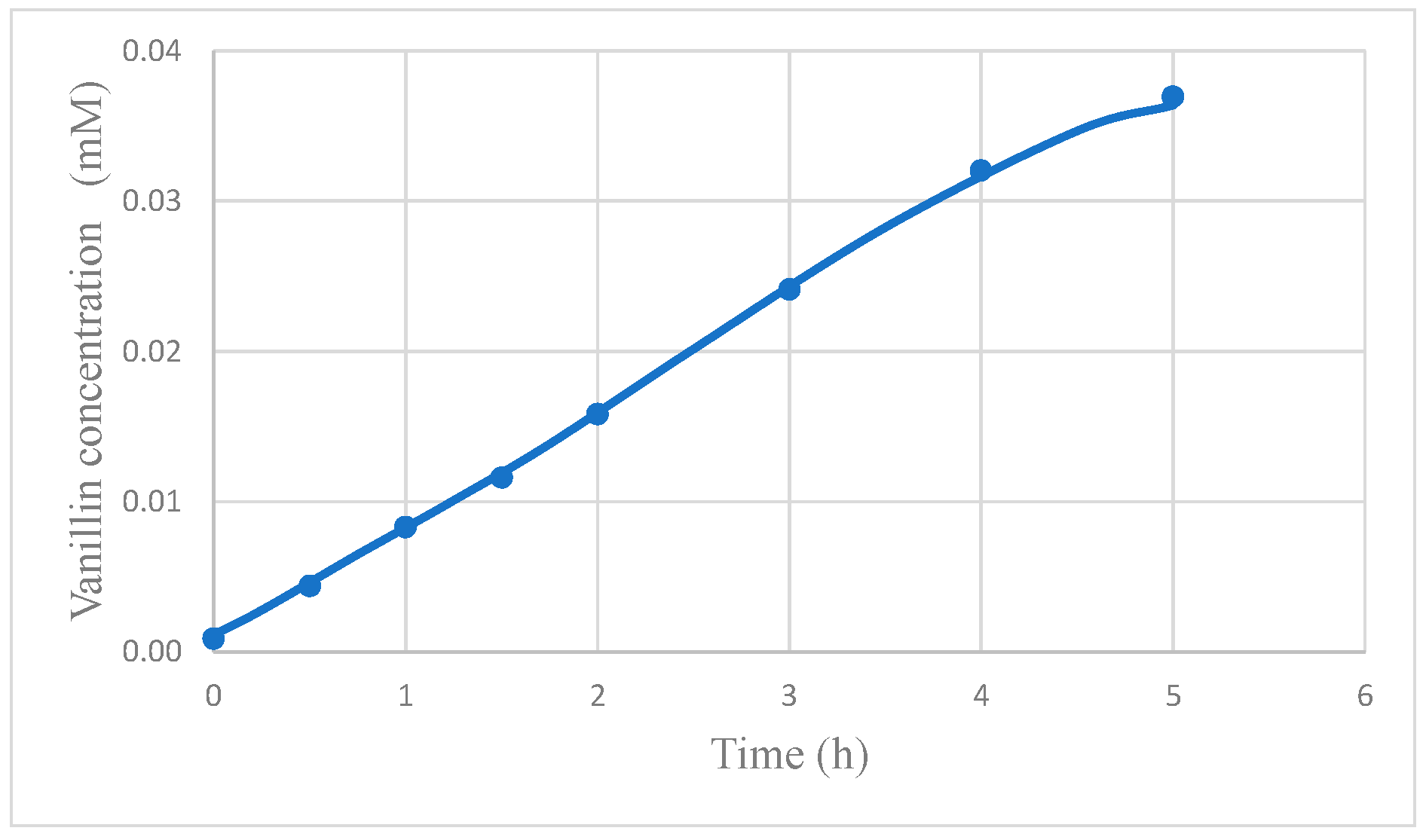
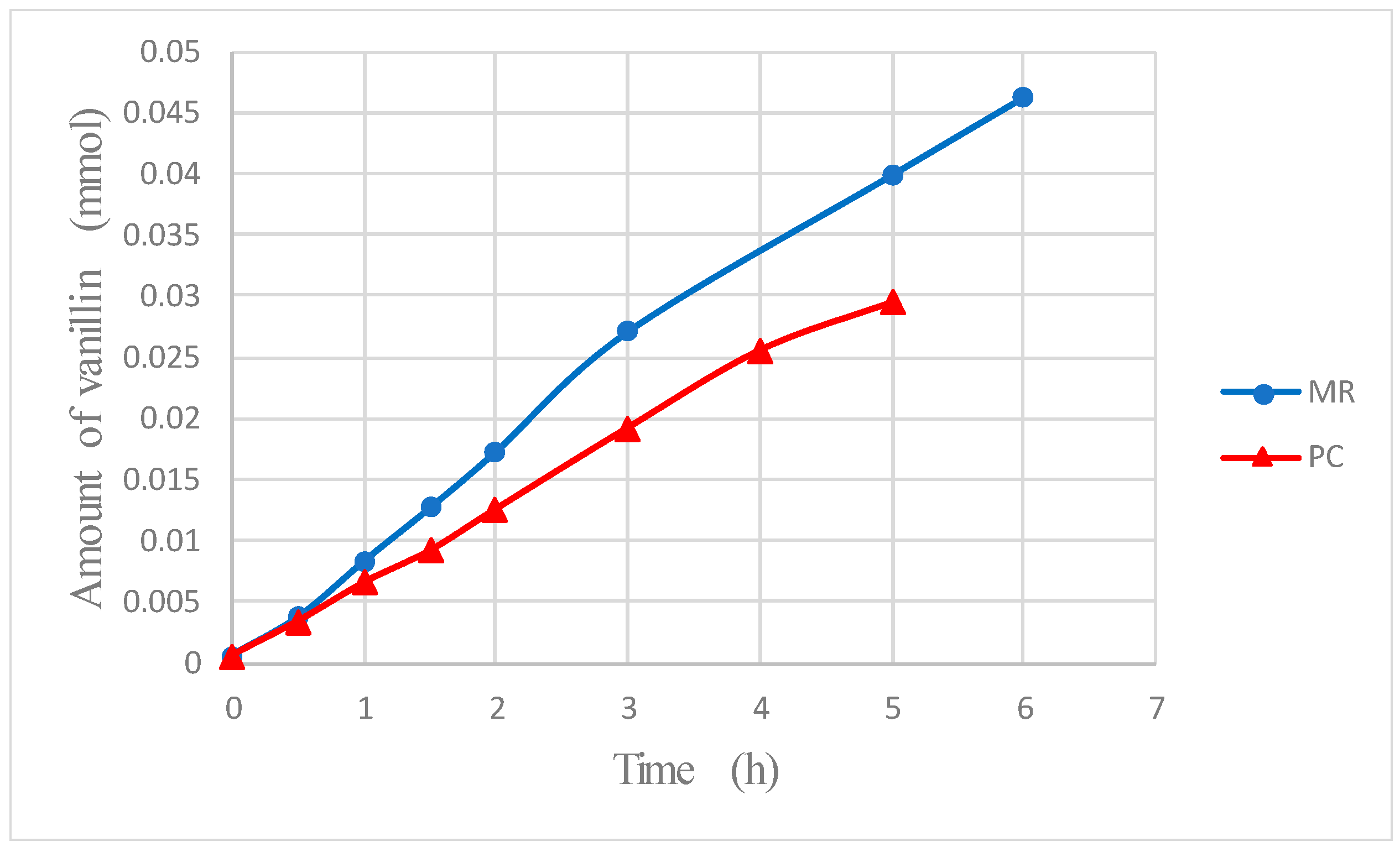
The final result of the increase of the reaction rate and of the lowering of vanillin degradation obtained by its recovery by dialysis is the improvement of vanillin production. In fact,
Figure 11 shows that the amount of produced vanillin is significantly higher in the MR. For instance, with the present experimental system, the total amount of vanillin produced after 5 h in the membrane reactor was more than one third higher than in the photocatalytic reactor without dialysis. It is expected that the higher is the ratio, Φ, between the characteristic rate of vanillin permeation to the rate of reaction [
10], i.e., the higher is the ratio of the membrane area to the reactor volume, the more important is the improvement of vanillin production. With the values of Da and 1/Pe, which are reported in
Section 3, this ratio is Φ =
= 0.87. Thus, the order of magnitude of Φ is 1, a value which is worth improving [
10] by increasing the membrane area to obtain a further enhancement of the vanillin yield.
Finally, it was observed that the photocatalytic powders do not permeate (the membrane is not porous) and that no decay of the permeability takes place even after several experimental runs.