Novel Submerged Photocatalytic Membrane Reactor for Treatment of Olive Mill Wastewaters
Abstract
:1. Introduction
2. Results and Discussion
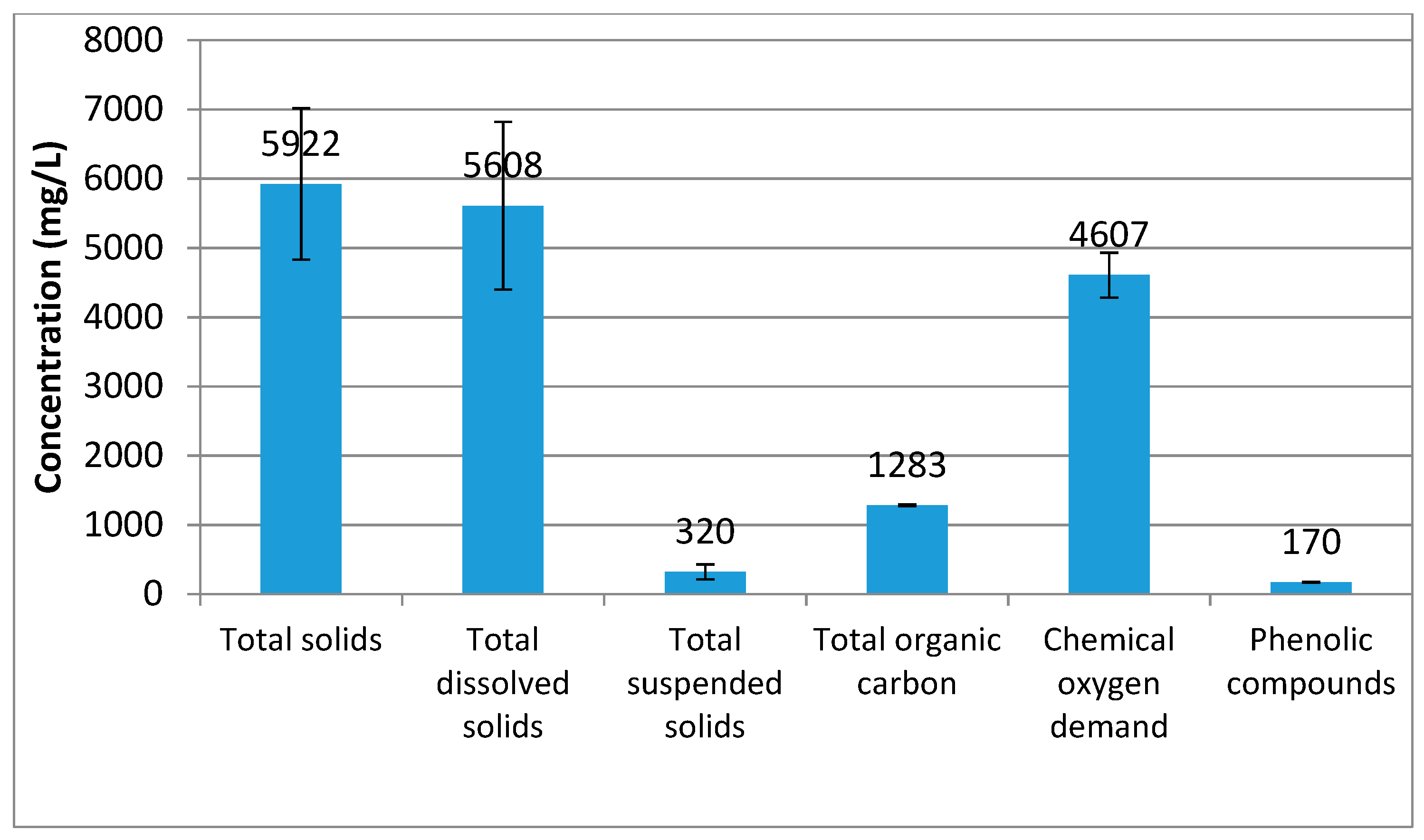
2.1. Characterization of the Wastewater Matrix
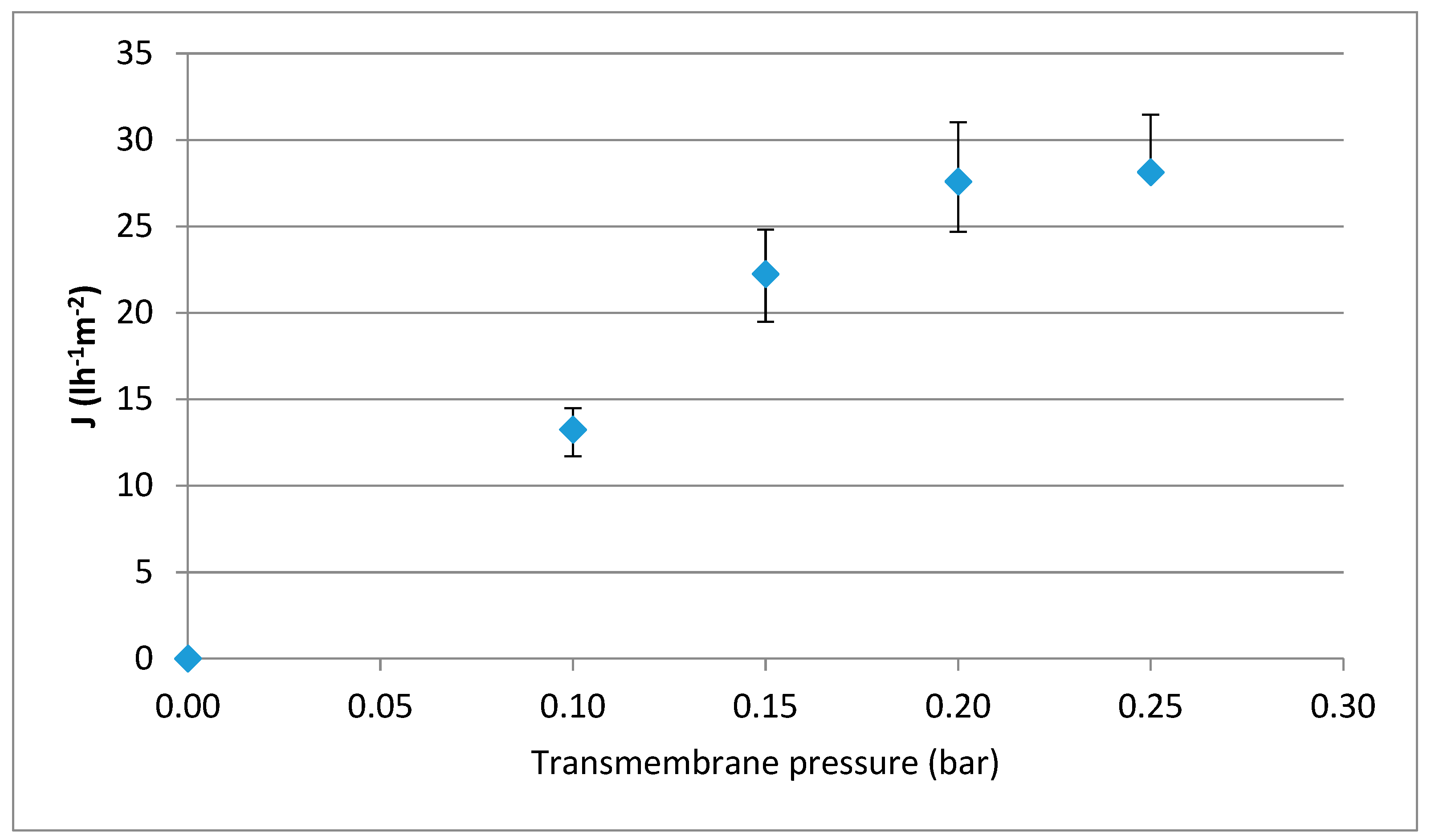
2.2. Determination of the Hydraulic Permeability and the Optimal Transmembrane Pressure Conditions
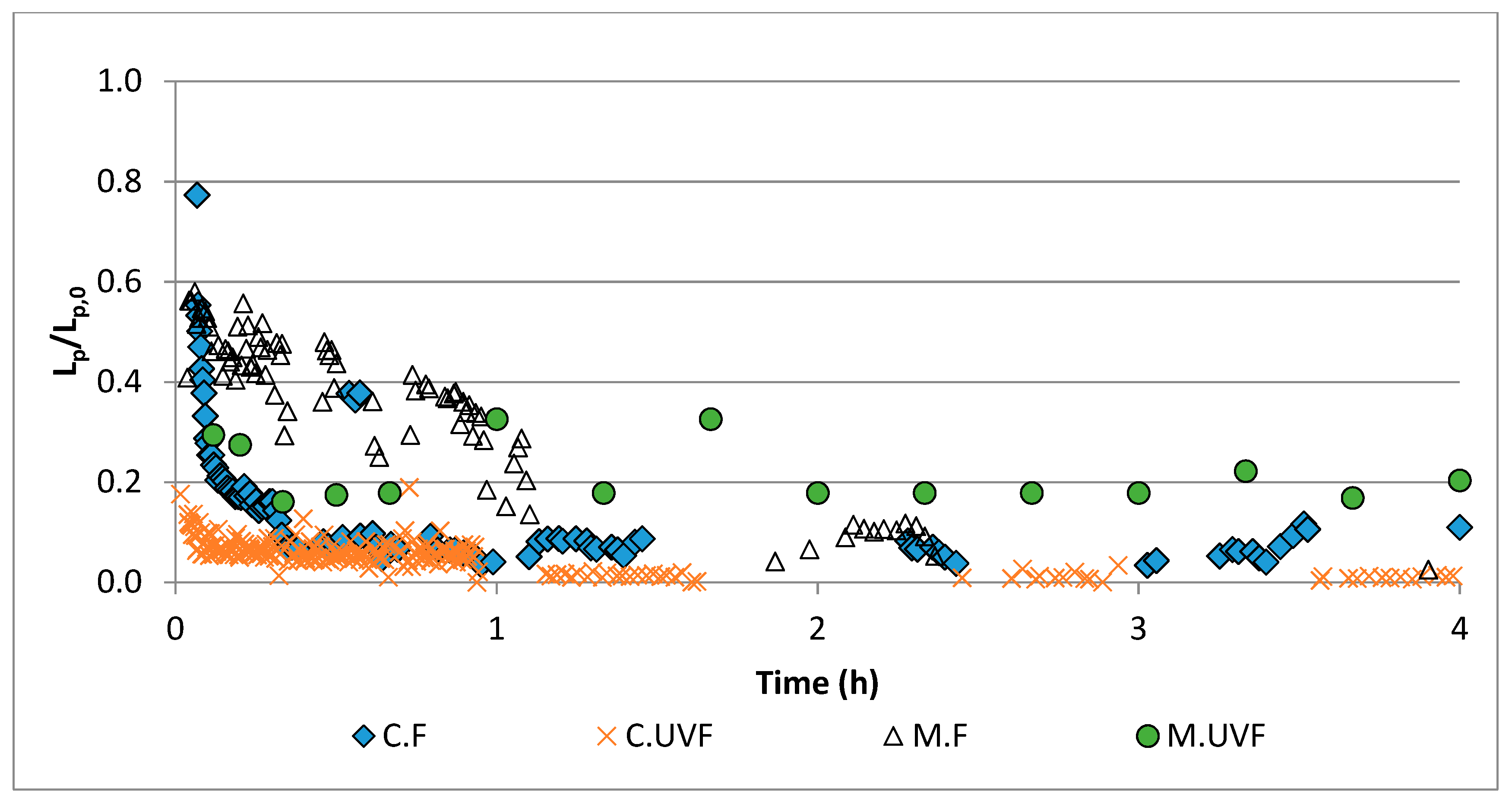
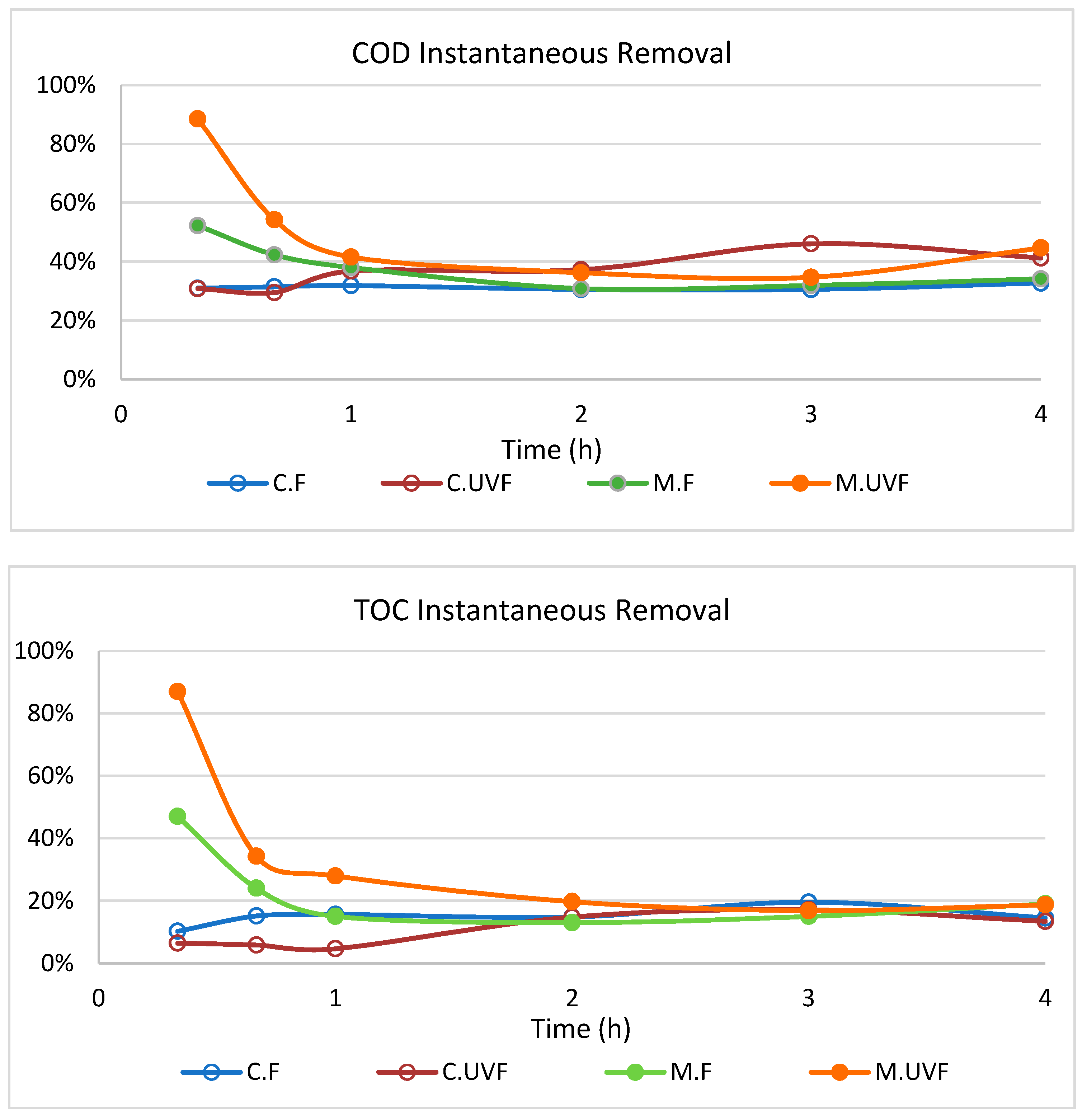
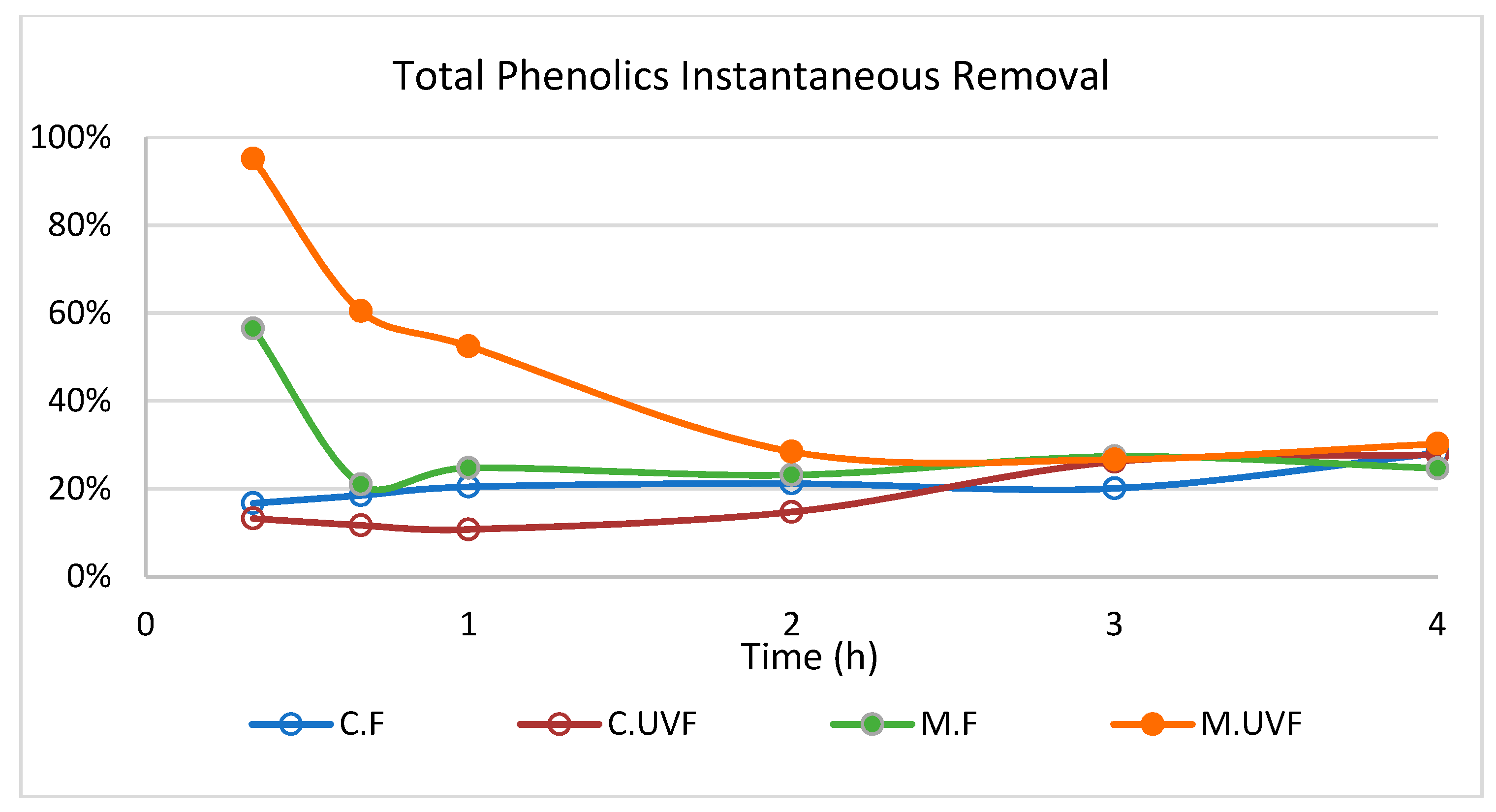
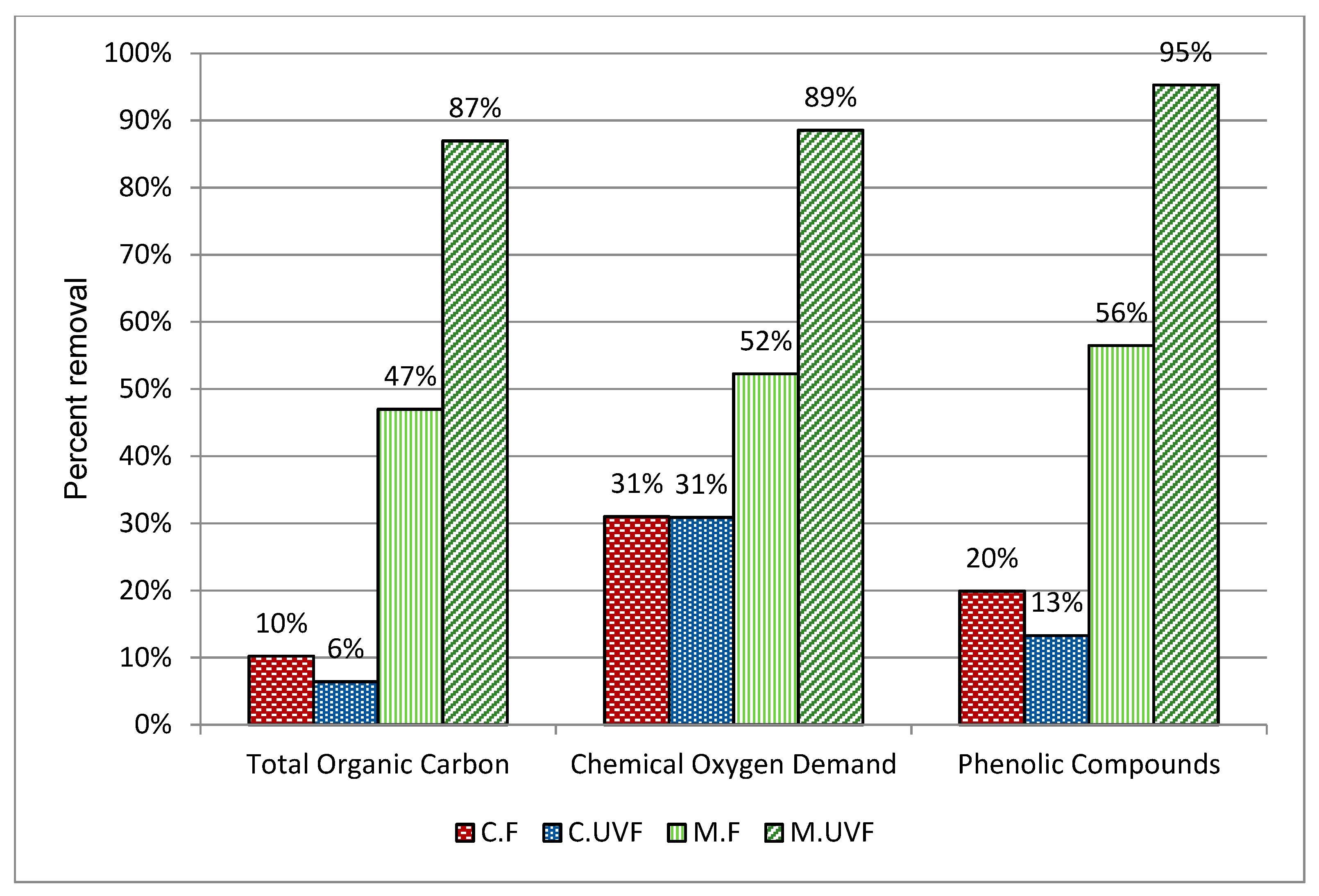
2.3. Wastewater Treatment in the Photocatalytic Membrane Reactor
3. Material and Methods
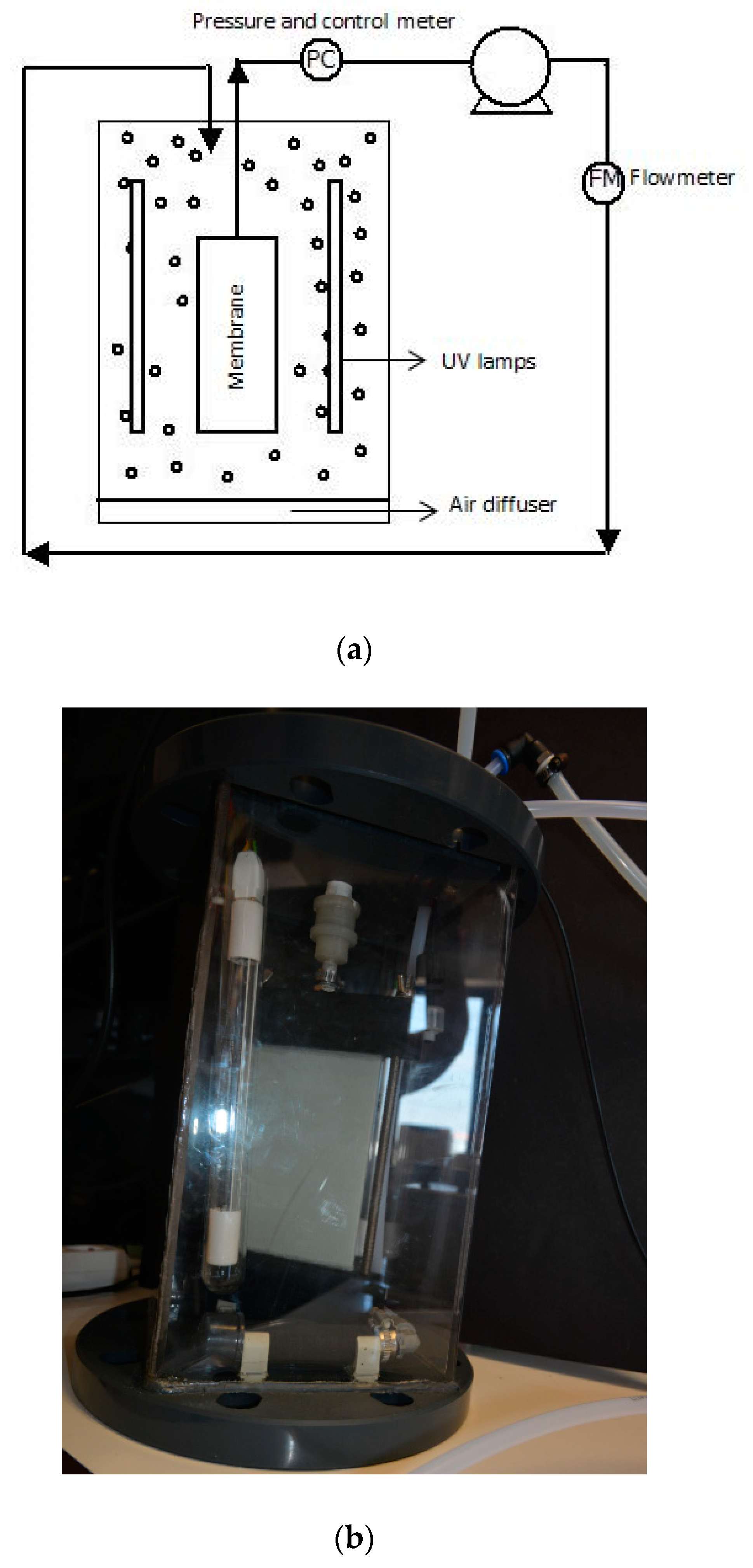
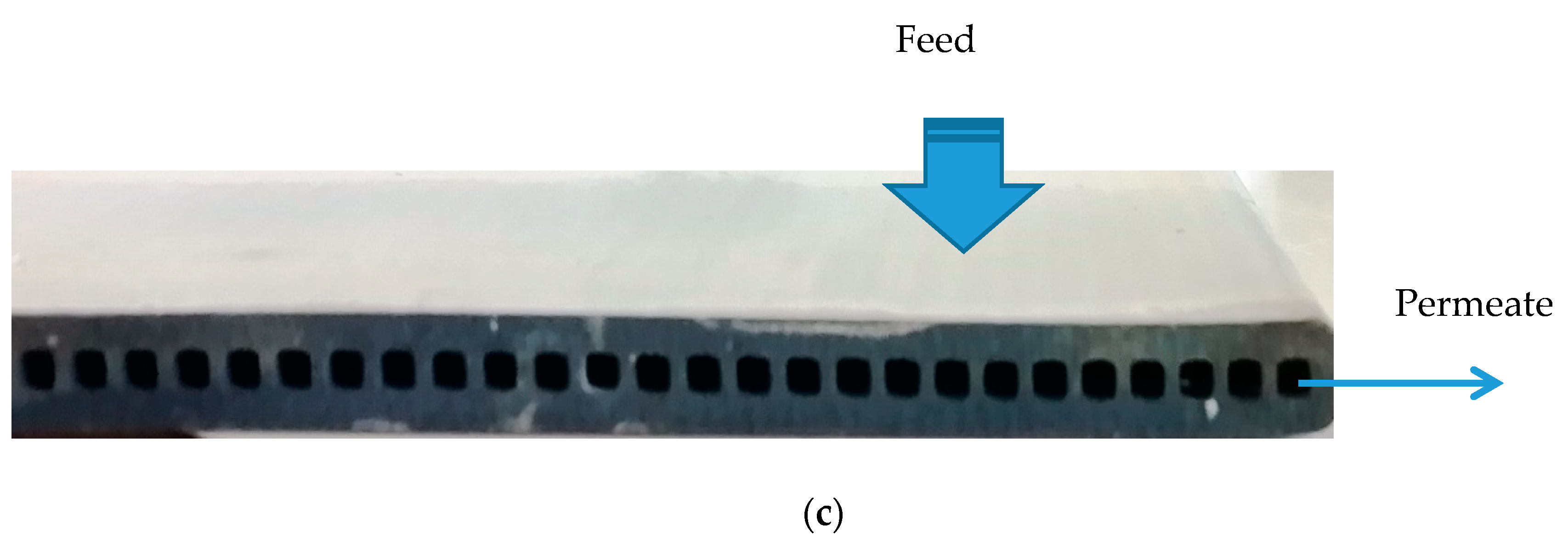
3.1. Submerged Photocatalytic Membrane Reactor
3.2. Wastewater Matrix and Analytical Methods
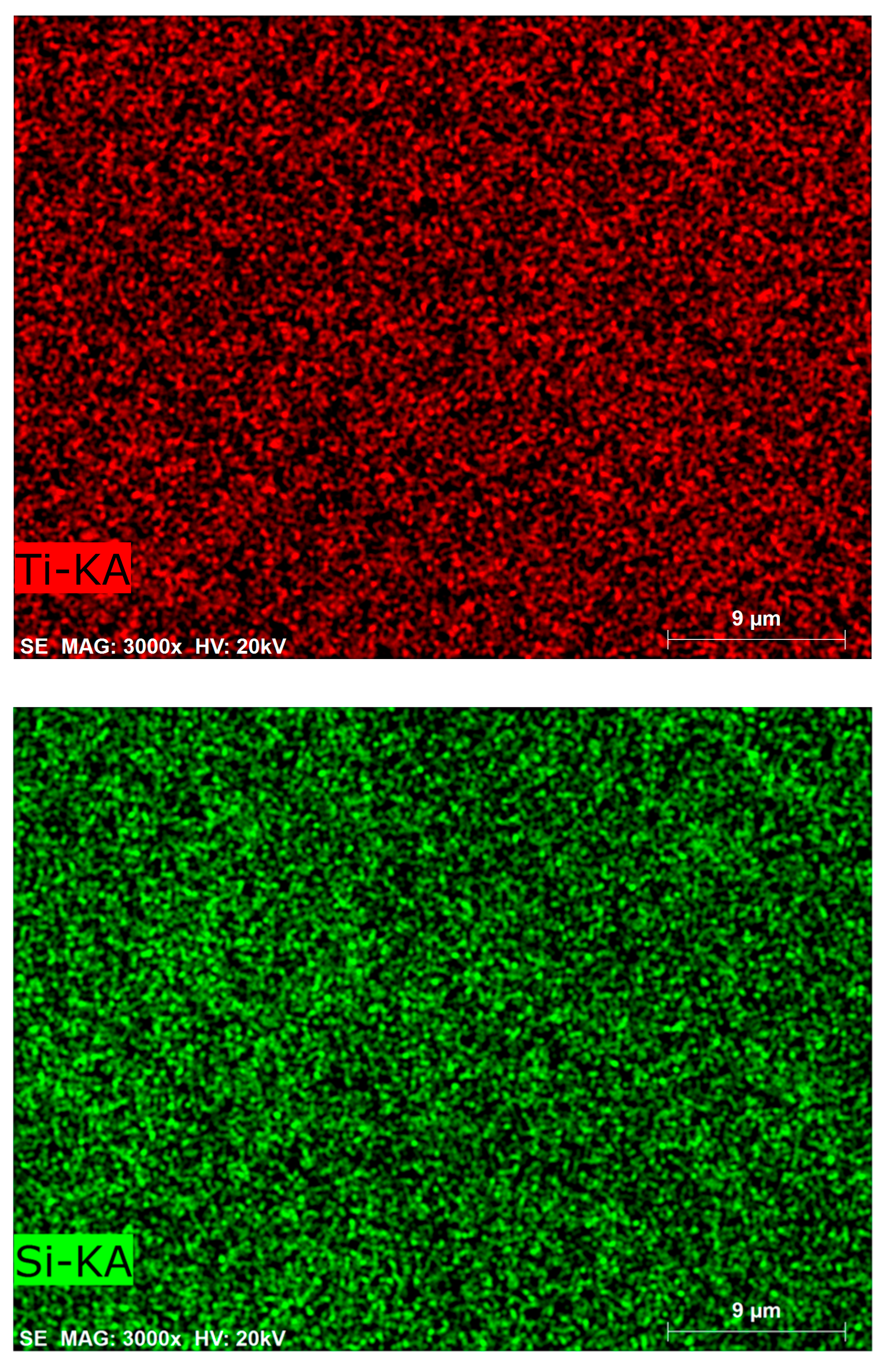
3.3. Preparation of the Photocatalytic Membrane
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ochando-Pulido, M.J.; Martinez-Ferez, A. On the Recent Use of Membrane Technology for Olive Mill Wastewater Purification. Membranes 2015, 5, 513–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ochando-Pulido, J.M.; Pimentel-Moral, S.; Verardo, V.; Martinez-Ferez, A. A focus on advanced physico-chemical processes for olive mill wastewater treatment. Sep. Purif. Technol. 2017, 179, 161–174. [Google Scholar] [CrossRef]
- Fraga, C.M.; Sanches, S.; Crespo, G.J.; Pereira, J.V. Assessment of a New Silicon Carbide Tubular Honeycomb Membrane for Treatment of Olive Mill Wastewaters. Membranes 2017, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Sanches, S.; Fraga, M.C.; Silva, N.A.; Nunes, P.; Crespo, J.G.; Pereira, V.J. Pilot scale nanofiltration treatment of olive mill wastewater: A technical and economical evaluation. Environ. Sci. Pollut. Res. 2017, 24, 3506–3518. [Google Scholar] [CrossRef] [PubMed]
- Pulido, J.M.O. A review on the use of membrane technology and fouling control for olive mill wastewater treatment. Sci. Total Environ. 2016, 563–564, 664–675. [Google Scholar] [CrossRef]
- Chen, Y.; Dong, B.Z.; Gao, N.Y.; Fan, J.C. Effect of coagulation pretreatment on fouling of an ultrafiltration membrane. Desalination 2007, 204, 181–188. [Google Scholar] [CrossRef]
- Yoo, S.S.; Chu, K.H.; Choi, I.-H.; Mang, J.S.; Ko, K.B. Operating cost reduction of UF membrane filtration process for drinking water treatment attributed to chemical cleaning optimization. J. Environ. Manag. 2018, 206, 1126–1134. [Google Scholar] [CrossRef]
- Kovács, I.; Veréb, G.; Kertész, S.; Hodúr, C.; László, Z. Fouling mitigation and cleanability of TiO2 photocatalyst-modified PVDF membranes during ultrafiltration of model oily wastewater with different salt contents. Environ. Sci. Pollut. Res. 2018, 25, 34912–34921. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Wang, R.; Hashimoto, K.; Fujishima, A.; Chikuni, M.; Kojima, E.; Kitamura, A.; Shimohigoshi, M.; Watanabe, T. Light-induced amphiphilic surfaces. Nature 1997, 388, 431. [Google Scholar] [CrossRef]
- Wold, A. Photocatalytic properties of titanium dioxide (TiO2). Chem. Mater. 1993, 5, 280–283. [Google Scholar] [CrossRef]
- Mozia, S. Photocatalytic membrane reactors (PMRs) in water and wastewater treatment. A review. Sep. Purif. Technol. 2010, 73, 71–91. [Google Scholar] [CrossRef]
- Iglesias, O.; Rivero, M.J.; Urtiaga, A.M.; Ortiz, I. Membrane-based photocatalytic systems for process intensification. Chem. Eng. J. 2016, 305, 136–148. [Google Scholar] [CrossRef]
- Le-Clech, P.; Lee, E.-K.; Chen, V. Hybrid photocatalysis/membrane treatment for surface waters containing low concentrations of natural organic matters. Water Res. 2006, 40, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Plakas, K.V.; Sarasidis, V.C.; Patsios, S.I.; Lambropoulou, D.A.; Karabelas, A.J. Novel pilot scale continuous photocatalytic membrane reactor for removal of organic micropollutants from water. Chem. Eng. J. 2016, 304, 335–343. [Google Scholar] [CrossRef]
- Patsios, S.I.; Sarasidis, V.C.; Karabelas, A.J. A hybrid photocatalysis–ultrafiltration continuous process for humic acids degradation. Sep. Purif. Technol. 2013, 104, 333–341. [Google Scholar] [CrossRef]
- Damodar, R.A.; You, S.-J.; Ou, S.-H. Coupling of membrane separation with photocatalytic slurry reactor for advanced dye wastewater treatment. Sep. Purif. Technol. 2010, 76, 64–71. [Google Scholar] [CrossRef]
- Ong, C.S.; Lau, W.J.; Goh, P.S.; Ng, B.C.; Ismail, A.F. Investigation of submerged membrane photocatalytic reactor (sMPR) operating parameters during oily wastewater treatment process. Desalination 2014, 353, 48–56. [Google Scholar] [CrossRef]
- Sanches, S.; Penetra, A.; Rodrigues, A.; Cardoso, V.V.; Ferreira, E.; Benoliel, M.J.; Barreto Crespo, M.T.; Crespo, J.G.; Pereira, V.J. Removal of pesticides from water combining low pressure UV photolysis with nanofiltration. Sep. Purif. Technol. 2013, 115, 73–82. [Google Scholar] [CrossRef]
- Erdei, L.; Arecrachakul, N.; Vigneswaran, S. A combined photocatalytic slurry reactor–immersed membrane module system for advanced wastewater treatment. Sep. Purif. Technol. 2008, 62, 382–388. [Google Scholar] [CrossRef]
- Zhang, W.; Ding, L.; Luo, J.; Jaffrin, M.Y.; Tang, B. Membrane fouling in photocatalytic membrane reactors (PMRs) for water and wastewater treatment: A critical review. Chem. Eng. J. 2016, 302, 446–458. [Google Scholar] [CrossRef]
- Chin, S.S.; Lim, T.M.; Chiang, K.; Fane, A.G. Factors affecting the performance of a low-pressure submerged membrane photocatalytic reactor. Chem. Eng. J. 2007, 130, 53–63. [Google Scholar] [CrossRef]
- Bae, T.-H.; Tak, T.-M. Effect of TiO2 nanoparticles on fouling mitigation of ultrafiltration membranes for activated sludge filtration. J. Membr. Sci. 2005, 249, 1–8. [Google Scholar] [CrossRef]
- Mustafa, G.; Wyns, K.; Vandezande, P.; Buekenhoudt, A.; Meynen, V. Novel grafting method efficiently decreases irreversible fouling of ceramic nanofiltration membranes. J. Membr. Sci. 2014, 470, 369–377. [Google Scholar] [CrossRef]
- Younas, H.; Bai, H.; Shao, J.; Han, Q.; Ling, Y.; He, Y. Super-hydrophilic and fouling resistant PVDF ultrafiltration membranes based on a facile prefabricated surface. J. Membr. Sci. 2017, 541, 529–540. [Google Scholar] [CrossRef]
- Mustafa, G.; Wyns, K.; Janssens, S.; Buekenhoudt, A.; Meynen, V. Evaluation of the fouling resistance of methyl grafted ceramic membranes for inorganic foulants and co-effects of organic foulants. Sep. Purif. Technol. 2018, 193, 29–37. [Google Scholar] [CrossRef]
- Pi, J.-K.; Yang, H.-C.; Wan, L.-S.; Wu, J.; Xu, Z.-K. Polypropylene microfiltration membranes modified with TiO2 nanoparticles for surface wettability and antifouling property. J. Membr. Sci. 2016, 500, 8–15. [Google Scholar] [CrossRef]
- Lee, G.R.; Crayston, J.A. Sol-gel processing of transition-metal alkoxides for electronics. Adv. Mater. 1993, 5, 434–442. [Google Scholar] [CrossRef]
- Goei, R.; Lim, T.-T. Asymmetric TiO2 hybrid photocatalytic ceramic membrane with porosity gradient: Effect of structure directing agent on the resulting membranes architecture and performances. Ceram. Int. 2014, 40, 6747–6757. [Google Scholar] [CrossRef]
- Goei, R.; Dong, Z.; Lim, T.-T. High-permeability pluronic-based TiO2 hybrid photocatalytic membrane with hierarchical porosity: Fabrication, characterizations and performances. Chem. Eng. J. 2013, 228, 1030–1039. [Google Scholar] [CrossRef]
- Huertas, R.M.; Fraga, M.C.; Crespo, J.G.; Pereira, V.J. Sol-gel membrane modification for enhanced photocatalytic activity. Sep. Purif. Technol. 2017, 180, 69–81. [Google Scholar] [CrossRef]
- 91/271/EEC. Council Directive of 21 May 1991 Concerning Urban Waste Water Treatment; EEC: Brussels, Belgium, 1991. [Google Scholar]
- Paraskeva, P.; Diamadopoulos, E. Technologies for olive mill wastewater (OMW) treatment: A review. J. Chem. Technol. Biotechnol. 2006, 81, 1475–1485. [Google Scholar] [CrossRef]
- Angerosa, F. Influence of volatile compounds on virgin olive oil quality evaluated by analytical approaches and sensor panels. Eur. J. Lipid Sci. Technol. 2002, 104, 639–660. [Google Scholar] [CrossRef]
- Sabatini, N.; Marsilio, V. Volatile compounds in table olives (Olea Europaea L., Nocellara del Belice cultivar). Food Chem. 2008, 107, 1522–1528. [Google Scholar] [CrossRef]
- Madaeni, S.S.; Ghaemi, N.; Alizadeh, A.; Joshaghani, M. Influence of photo-induced superhydrophilicity of titanium dioxide nanoparticles on the anti-fouling performance of ultrafiltration membranes. Appl. Surf. Sci. 2011, 257, 6175–6180. [Google Scholar] [CrossRef]
- Song, Z.; Fathizadeh, M.; Huang, Y.; Chu, K.H.; Yoon, Y.; Wang, L.; Xu, W.L.; Yu, M. TiO2 nanofiltration membranes prepared by molecular layer deposition for water purification. J. Membr. Sci. 2016, 510, 72–78. [Google Scholar] [CrossRef]
- Kim, S.B.; Hong, S.C. Kinetic study for photocatalytic degradation of volatile organic compounds in air using thin film TiO2 photocatalyst. Appl. Catal. B Environ. 2002, 35, 305–315. [Google Scholar] [CrossRef]
- Selishchev, D.S.; Kolobov, N.S.; Pershin, A.A.; Kozlov, D.V. TiO2 mediated photocatalytic oxidation of volatile organic compounds: Formation of CO as a harmful by-product. Appl. Catal. B Environ. 2017, 200, 503–513. [Google Scholar] [CrossRef]
- Rice, E.W.; Baird, R.B.; Eaton, A.D. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA; American Water Works Association: Denver, CO, USA; Water Environment Federation: Alexandria, VA, USA, 1995; Volume 19. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
| Compound | % Similarity * |
|---|---|
| n-buthyl-eter | 97 |
| 2-Hexanone | 95 |
| 2-octanone | 87 |
| 2-heptanone | 95 |
| trans-3,4-Epoxyoctane | 83 |
| Acetic acid | 93 |
| 2-nonanone | 94 |
| 2-buthoxyethanol | 96 |
| Propionic acid | 94 |
| Butyric Acid | 87 |
| Pentanoic acid | 93 |
| Hexanoic Acid | 96 |
| Heptanoic Acid | 94 |
| Cyclohexanecarboxylic acid | 89 |
| Test ID | Membrane Type | UV Light | Filtration | Objective |
|---|---|---|---|---|
| C.UV | Control | Yes | No | Evaluate direct photolysis using low pressure UV lamps |
| C.F | No | Yes | Evaluate the filtration performance of the control membrane | |
| C.UVF | Yes | Yes | Evaluate the combined effect of the control membrane | |
| M.UV | Modified | Yes | No | Evaluate the photocatalytic properties of the modified membrane surface |
| M.F | No | Yes | Evaluate the filtration performance of the modified membrane | |
| M.UVF | Yes | Yes | Evaluate the combined effect of the modified membrane |
| Test | % Removals after 4 h of Filtration Test | ||
|---|---|---|---|
| Total Solids | Total Suspended Solids | Total Dissolved Solids | |
| C.F | 14 | 93 | 8 |
| C.UVF | 14 | 92 | 10 |
| M.F | 17 | 89 | 13 |
| M.UVF | 20 | 98 | 18 |
| Compound | C.F | C.UVF | M.F | M.UVF | ||||
|---|---|---|---|---|---|---|---|---|
| Similarity (%) | Removal (%) | Similarity (%) | Removal (%) | Similarity (%) | Removal (%) | Similarity (%) | Removal (%) | |
| n-buthyl-eter | 97 | 100 | 97 | 100 | 96 | 100 | 97 | 100 |
| 2-Hexanone | 96−98 | 35 | 95−98 | 62 | 98 | 68 | 98 | 92 |
| 2-octanone | 87−91 | 37 | 87−95 | 100 | 98 | 98 | 87 | 100 |
| 2-heptanone | 95 | 100 | 91−96 | −104 | 98 | 79 | 97−98 | 44 |
| trans-3,4-epoxyoctane | 94 | 100 | 83 | 100 | 83 | 100 | 84 | 100 |
| Acetic acid | 93−98 | −25 | 98 | −25 | 95−98 | −72 | 94−98 | 86 |
| 2-nonanone | 94 | 100 | 97 | 100 | 98 | 100 | 98 | 100 |
| 2-buthoxyethanol | 96−98 | −27 | 96−97 | −46 | 95−98 | 67 | 98 | 100 |
| Propionic acid | 94−95 | −11 | 95−98 | −6 | 94−96 | 18 | 94−98 | 79 |
| Butiric Acid | 89−94 | 100 | 94 | 100 | 87−95 | 30 | 90−97 | 88 |
| Pentanoic acid | 93−96 | −8 | 94 | −2 | na | na | 96−98 | 91 |
| Hexanoic Acid | 94−96 | 6 | 96 | −22 | 96−98 | 85 | 96 | 94 |
| Heptanoic Acid | 98 | −57 | 94−96 | −123 | 96 | 61 | 94 | 100 |
| Cyclohexanecarboxylic acid | 84−89 | 2 | 83−84 | −3 | 89−90 | 42 | 89−90 | 89 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fraga, M.C.; Huertas, R.M.; Crespo, J.G.; Pereira, V.J. Novel Submerged Photocatalytic Membrane Reactor for Treatment of Olive Mill Wastewaters. Catalysts 2019, 9, 769. https://doi.org/10.3390/catal9090769
Fraga MC, Huertas RM, Crespo JG, Pereira VJ. Novel Submerged Photocatalytic Membrane Reactor for Treatment of Olive Mill Wastewaters. Catalysts. 2019; 9(9):769. https://doi.org/10.3390/catal9090769
Chicago/Turabian StyleFraga, Maria C., Rosa M. Huertas, João G. Crespo, and Vanessa J. Pereira. 2019. "Novel Submerged Photocatalytic Membrane Reactor for Treatment of Olive Mill Wastewaters" Catalysts 9, no. 9: 769. https://doi.org/10.3390/catal9090769
APA StyleFraga, M. C., Huertas, R. M., Crespo, J. G., & Pereira, V. J. (2019). Novel Submerged Photocatalytic Membrane Reactor for Treatment of Olive Mill Wastewaters. Catalysts, 9(9), 769. https://doi.org/10.3390/catal9090769





