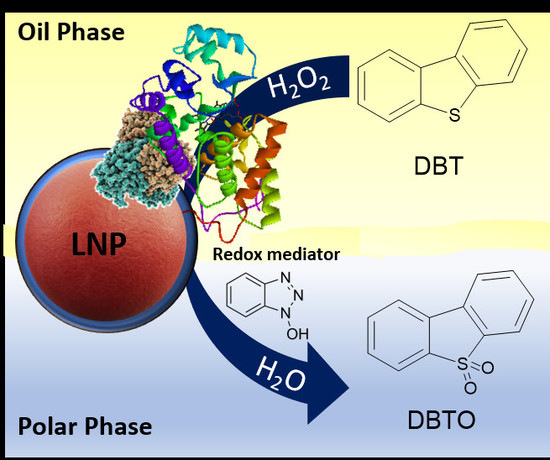
Oxidative Bio-Desulfurization by Nanostructured Peroxidase Mediator System
Abstract
1. Introduction
2. Results and Discussion
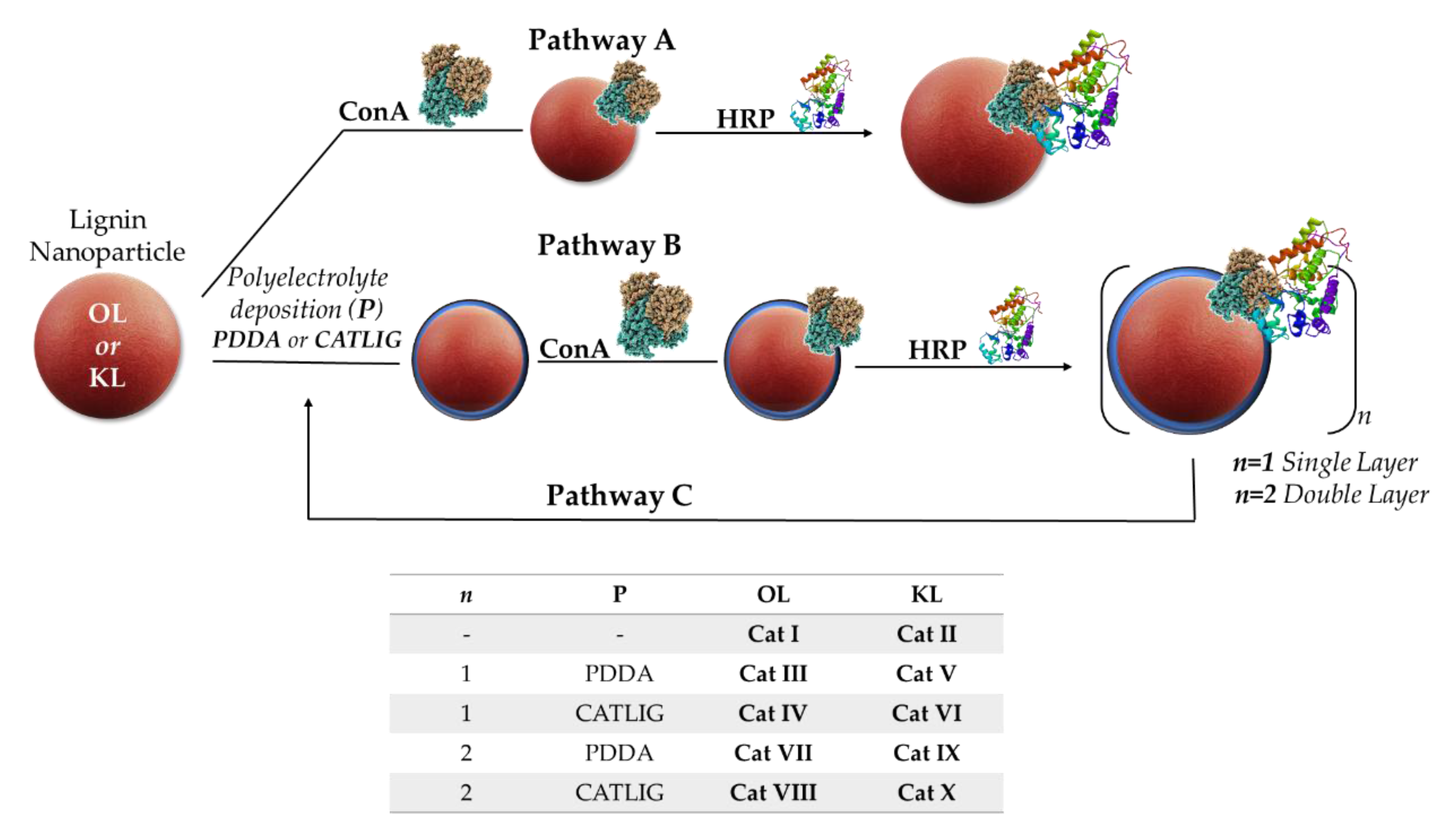
2.1. Preparation of Nanobiocatalysts
2.2. Activity Parameters and Kinetic Data of Biocatalysts I–X
2.3. Oxidation of DBT with Cat I–X
3. Materials and Methods
3.1. Materials
3.2. Preparation of Cat I-II
3.3. Preparation of Cat III-V and Cat IV-VI
3.4. Preparation of Cat VII-X
3.5. Scanning Electron Microscopy Analysis
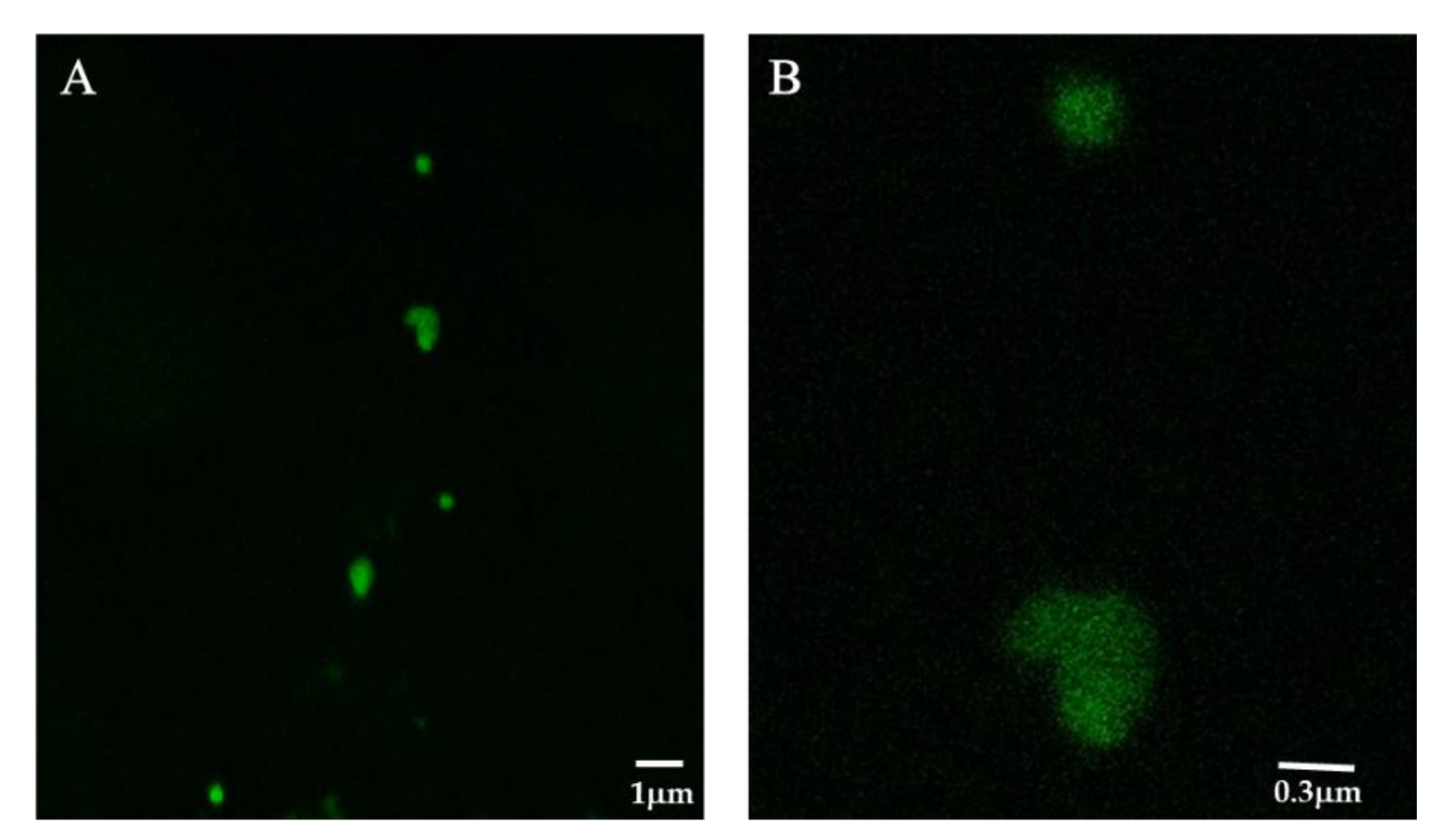
3.6. Synthesis of FITC-Labeled HRP and Laser Confocal Microscopy
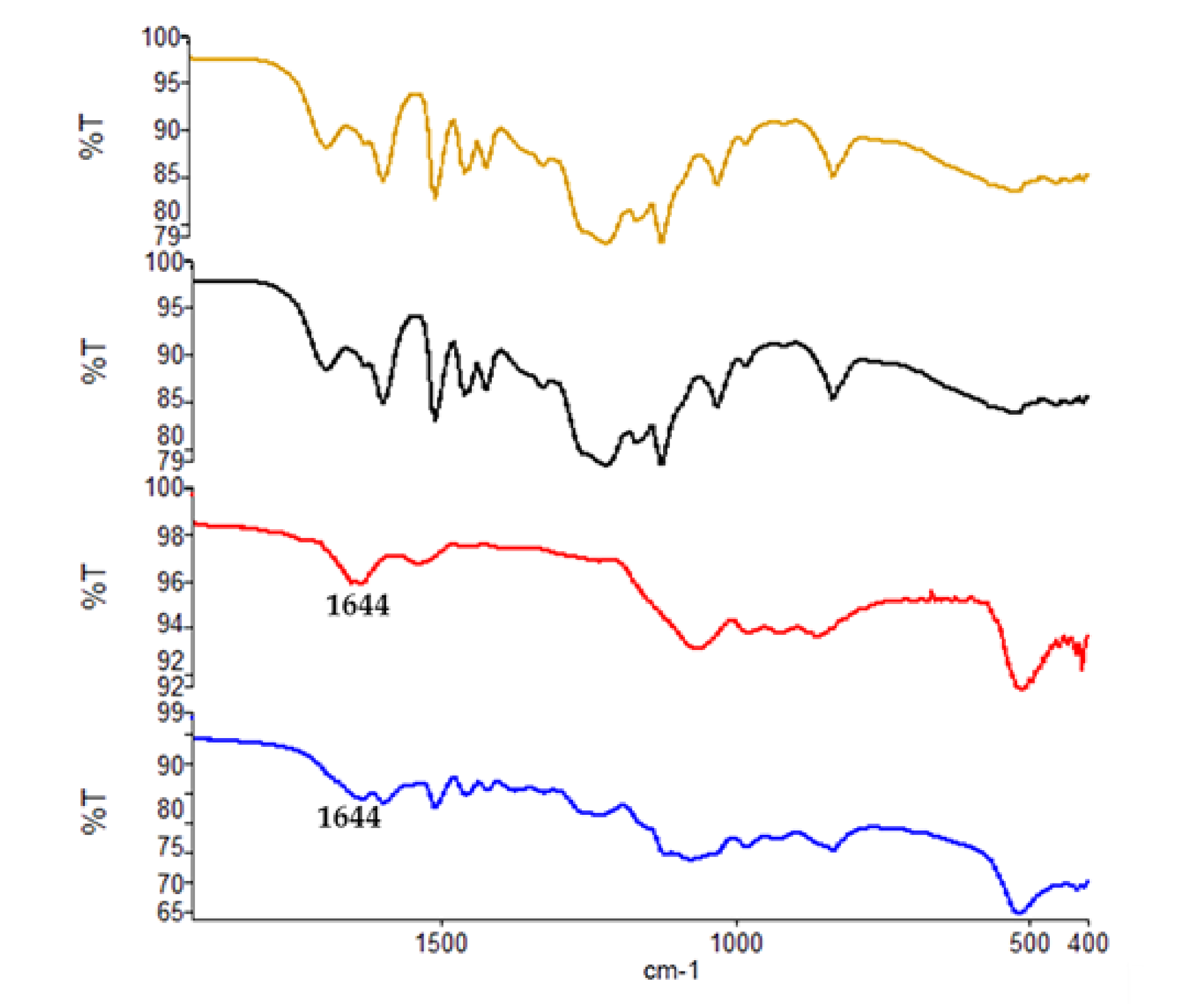
3.7. ATR-IR Characterization
3.8. Activity Parameters of Cat I-X
3.9. Kinetic Data of Cat I-X
3.10. Oxidation of DBT with Cat I-X and Redox Mediator Systems
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Souza, W.F.; Guimaraes, I.; Guerreiro, M.C.; Oliveira, L.C.A. Catalytic oxidation of sulfur and nitrogen compounds from diesel fuel. Appl. Catal. A General 2009, 360, 205–209. [Google Scholar] [CrossRef]
- Zannikos, F.; Lois, E.; Stournas, S. Desulfurization of petroleum fractions by oxidation and solvent extraction. Fuel Process. Technol. 1995, 42, 35–45. [Google Scholar] [CrossRef]
- Ismagilov, Z.; Yashnik, S.; Kerzhentsev, M.; Parman, V.; Bourane, A.; Al-Shahrani, F.M.; Haji, A.A.; Koseoglu, O.R. Oxidative desulfurization of hydrocarbon fuels. Catal. Rev. Sci. Eng. 2011, 53, 199–255. [Google Scholar] [CrossRef]
- Gatan, R.; Barger, P.; Gembicki, V.; Cavanna, A.; Molinari, D. Oxidative desulfurization: A new technology for ULSD. Am. Chem. Soc. Div. Fuel Chem. 2004, 49, 577–579. [Google Scholar]
- Li, J.; Yang, Z.; Li, S.; Jin, Q.; Zhao, J. Review on oxidative desulfurization of fuel by supported heteropolyacid catalysts. J. Ind. Eng. Chem. 2020, 82, 1–16. [Google Scholar] [CrossRef]
- Hossain, M.N.; Park, H.C.; Choi, H.S. A comprehensive review on catalytic oxidative desulfurization of liquid fuel oil. Catalysts 2019, 9, 229. [Google Scholar] [CrossRef]
- Crucianelli, M.; Bizzarri, B.M.; Saladino, R. SBA-15 Anchored Metal Containing Catalysts in the Oxidative Desulfurization Process. Catalysts 2019, 9, 984. [Google Scholar] [CrossRef]
- Houda, S.; Lancelot, C.; Blanchard, P.; Poinel, L.; Lamonier, C. Oxidative desulfurization of heavy oils with high sulfur content: A review. Catalysts 2018, 8, 344. [Google Scholar] [CrossRef]
- Sikarwar, P.; Gosu, V.; Subbaramaiah, V. An overview of conventional and alternative technologies for the production of ultra-low-sulfur fuels. Rev. Chem. Eng. 2019, 35, 669–705. [Google Scholar] [CrossRef]
- Mjalli, F.S.; Ahmed, O.U.; Al-Wahaibi, T.; Al-Wahaibi, Y.; Al-Nashef, I.M. Deep oxidative desulfurization of liquid fuels. Rev. Chem. Eng. 2014, 30, 337–378. [Google Scholar] [CrossRef]
- Saladino, R.; Botta, G.; Crucianelli, M. Advances in Nanostrostructured Transition Metal Catalysis Applied to Oxidative Desulfurization. In Applying Nanotechnology to the Desulfurization Process in Petroleum Engineering; Tawfik, A.S., Ed.; IGI Global: Hershey, PA, USA, 2016; pp. 180–215. [Google Scholar]
- Piccinino, D.; Abdalghani, I.; Botta, G.; Crucianelli, M.; Passacantando, M.; Di Vacri, M.L.; Saladino, R. Preparation of wrapped carbon nanotubes poly(4-vinylpyridine)/MTO based heterogeneous catalysts for the oxidative desulfurization (ODS) of model and synthetic diesel fuel. Appl. Catal. B-Environ. 2017, 200, 392–401. [Google Scholar] [CrossRef]
- Di Giuseppe, A.; Crucianelli, M.; De Angelis, F.; Crestini, C.; Saladino, R. Efficient Oxidation of Thiophene Derivatives with Homogeneous and Heterogeneous MTO/H2O2 systems: A novel approach for Oxidative Desulfurization (ODS) of Diesel Fuel. Appl. Catal. B-Environ. 2009, 89, 239–245. [Google Scholar] [CrossRef]
- Davoodi-Dehaghania, F.; Vosoughi, M.; Ziaee, A.A. Biodesulfurization of dibenzothiophene by a newly isolated Rhodococcus erythropolis strain. Bioresour. Technol. 2010, 101, 1102–1105. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Humphrey, A.E.; Krawiec, S. Kinetic Analyses of Desulfurization of Dibenzothiophene by Rhodococcus erythropolis in Continuous Cultures. Appl. Environ. Microbiol. 1996, 62, 3066–3068. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.H.; Chang, Y.K.; Cho, K.; Chang, H.N. Desulfurization of model and diesel oils by resting cells of Gordona sp. Biotechnol. Lett. 2000, 22, 193–196. [Google Scholar] [CrossRef]
- Villaseňor, F.; Loera, O.; Campero, A.; Viniegra-Gonzàlez, G. Oxidation of dibenzothiophene by laccase or hydrogen peroxide and deep desulfurization of diesel fuel by the later. Fuel Process. Technol. 2004, 86, 49–59. [Google Scholar] [CrossRef]
- Bressler, D.C.; Fedorak, P.M.; Pickard, M.A. Oxidation of carbazole, N-ethylcarbazole, fluorene, and dibenzothiophene by the laccase of Coriolopsis gallica. Biotechnol. Lett. 2000, 14, 1119–1125. [Google Scholar] [CrossRef]
- Klyachko, N.L.; Klibanov, A.M. Oxidation of dibenzothiophene catalyzed by hemoglobin and other hemoproteins in various aqueous—Organic media. Appl. Biochem. Biotechnol. 1992, 37, 53–68. [Google Scholar] [CrossRef]
- Vazquez-Duhalt, R.; Westlake, D.W.S.; Fedorak, P.M. Lignin Peroxidase Oxidation of Aromatic Compounds in Systems Containing Organic Solvents. Appl. Env. Micr. 1994, 60, 459–466. [Google Scholar] [CrossRef]
- Eibes, G.; Cajthaml, T.; Moreira, M.T.; Feijoo, J.; Lema, J.M. Enzymatic degradation of anthracene, dibenzothiophene and pyrene by manganese peroxidase in media containing acetone. Chemosphere 2006, 64, 408–414. [Google Scholar] [CrossRef]
- Ichinose, H.; Wariishi, H.; Tanaka, H. Effective oxygen transfer reaction catalyzed by microperoxidase-11 during sulfur oxidation of dibenzothiophene. Enzyme Microb. Technol. 2002, 30, 334–339. [Google Scholar] [CrossRef]
- Ayala, M.; Tinoco, R.; Hernandez, V.; Bremauntz, P.; Vazquez-Duhalt, R. Biocatalytic oxidation of fuel as an alternative to biodesulfurization. Fuel Process. Technol. 1998, 57, 101–111. [Google Scholar] [CrossRef]
- Doerge, D.R.; Cooray, N.M.; Brewster, M.E. Peroxidase-catalyzed S-oxygenation: Mechanism of oxygen transfer for lactoperoxidase. Biochemistry 1991, 30, 8960. [Google Scholar] [CrossRef] [PubMed]
- Stachyra, T.; Guillochon, D.; Pulvin, S.; Thomas, D. Hemoglobin, horseradish peroxidase, and heme-bovine serum albumin as biocatalyst for the oxidation of dibenzothiophene. Appl. Biochem. Biotech. 1996, 59, 231–244. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Madeira, L.; Ferreira-Leitaŏ, V.S.; da Silva Bon, E.P. Dibenzothiophene oxidation by horseradish peroxidase in organic media: Effect of the DBT:H2O2 molar ratio and H2O2 addition mode. Chemosphere 2008, 71, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Bhasarkar, J.; Borah, A.J.; Goswami, P.; Moholkar, V.S. Mechanistic analysis of ultrasound assisted enzymatic desulfurization of liquid fuels using horseradish peroxidase. Bioresour. Technol. 2015, 196, 88–98. [Google Scholar] [CrossRef]
- Ayala, M.; Verdin, J.; Vazquez-Duhalt, R. The prospects for peroxidase-based biorefining of petroleum fuels. Biocatal. Biotransform. 2007, 25, 114–129. [Google Scholar] [CrossRef]
- Terrès, E.; Montiel, M.; Le Borgne, S.; Torres, E. Immobilization of chloroperoxidase on mesoporous materials for the oxidation of 4,6-dimethyldibenzothiophene, a recalcitrant organic sulfur compound present in petroleum fractions. Biotechnol. Lett. 2008, 30, 173–179. [Google Scholar] [CrossRef]
- Wu, S.; Lin, J.; Chan, S. Oxidation of dibenzothiophene catalyzed by heme-containing enzymes encapsulated in sol-gel glass. Appl. Biochem. Biotechnol. 1994, 47, 11–20. [Google Scholar] [CrossRef]
- Li, Y.; Huang, X.; Qu, Y. A strategy for efficient immobilization of laccase and horseradish peroxidase on single-walled carbon nanotubes. J. Chem. Technol. Biotechnol. 2013, 88, 2227–2232. [Google Scholar] [CrossRef]
- Juarez-Moreno, K.; Díaz de León, J.N.; Zepeda, T.A.; Vazquez-Duhalt, R.; Fuentes, S. Oxidative transformation of dibenzothiophene by chloroperoxidase enzyme immobilized on (1D)-γ-Al2O3 nanorods. J. Mol. Catal. B-enzym. 2015, 115, 9095. [Google Scholar] [CrossRef]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Hu, T.Q. Chemical Modification, Properties, and Usage of Lignin; Kluwer Academic-Plenum Publishers: New York, NY, USA, 2002. [Google Scholar]
- Piccinino, D.; Capecchi, E.; Botta, L.; Bizzarri, B.M.; Bollella, P.; Antiochia, R.; Saladino, R. Layer-by-Layer Preparation of Microcapsules and Nanocapsules of Mixed Polyphenols with High Antioxidant and UV-Shielding Properties. Biomacromolecules 2018, 19, 3883–3893. [Google Scholar] [CrossRef] [PubMed]
- Capecchi, E.; Piccinino, D.; Bizzarri, B.M.; Avitabile, D.; Pelosi, C.; Colantonio, C.; Calabrò, G.; Saladino, R. Enzyme-Lignin Nanocapsules Are Sustainable Catalysts and Vehicles for the Preparation of Unique Polyvalent Bioinks. Biomacromolecules 2019, 20, 1975–1988. [Google Scholar] [CrossRef] [PubMed]
- Capecchi, E.; Piccinino, D.; Delfino, I.; Bollella, P.; Antiochia, R.; Saladino, R. Functionalized tyrosinase-lignin nanoparticles as sustainable catalysts for the oxidation of phenols. Nanomaterials 2018, 6, 438. [Google Scholar] [CrossRef] [PubMed]
- Piccinino, D.; Capecchi, E.; Botta, L.; Bollella, P.; Antiochia, R.; Crucianelli, M.; Saladino, R. Layer by layer supported laccase on lignin nanoparticles catalyzes the selective oxidation of alcohols to aldehydes. Catal. Sci. Technol. 2019, 9, 4125–4134. [Google Scholar] [CrossRef]
- Frommhagen, M.; Mutte, S.K.; Westphal, A.H.; Koetsier, M.J.; Hinz, S.W.A.; Visser, J.; Vincken, J.P.; Weijers, D.; Van Berkel, W.J.H.; Gruppen, H.; et al. Boosting LPMO-driven lignocellulose degradation by polyphenol oxidase-activated lignin building blocks. Biotechnol. Biofuels 2017, 10, 121. [Google Scholar] [CrossRef]
- Brenelli, L.; Squina, F.M.; Felby, C.; Cannella, D. Laccase-derived lignin compounds boost cellulose oxidative enzymes AA9. Biotechnol. Biofuels 2018, 11, 10. [Google Scholar] [CrossRef]
- Ortiza, E.; Gallay, P.; Galicia, L.; Eguílaz, M.; Rivas, G. Nanoarchitectures based on multi-walled carbon nanotubes non-covalently functionalized with Concanavalin A: A new building-block with supramolecular recognition properties for the development of electrochemical biosensors. Sens. Actuators B Chem. 2019, 292, 254–262. [Google Scholar] [CrossRef]
- Ferreira, J.A.; Taherzadeh, M.J. Improving the economy of lignocellulose-based biorefineries with organosolv pretreatment. Biores. Technol. 2020, 299, 122. [Google Scholar] [CrossRef]
- Demuner, I.F.; Colodette, J.L.; Demuner, A.J.; Jardim, C.M. Biorefinery review: Wide-reaching products through kraft lignin. Bioresources 2019, 14, 7543–7581. [Google Scholar]
- Leskinen, T.; Smyth, M.; Xiao, Y.; Lintinen, K.; Mattinen, M.L.; Kostiainen, M.A.; Oinas, P.; Österberg, M. Scaling Up Production of Colloidal Lignin Particles. Nord. Pulp Pap. Res. J. 2017, 32, 586. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, J. Oriented immobilization of proteins on solid supports for use in biosensors and biochips: A review. Microchim. Acta 2016, 183, 1–19. [Google Scholar] [CrossRef]
- Makky, A.; Michel, J.P.; Maillard, P.; Rosilio, V. Biomimetic liposomes and planar supported bilayers for the assessment of glycodendrimeric porphyrins interaction with an immobilized lectin. Biochim. Biophys. Acta Rev. Biomembr. 2011, 1808, 656–666. [Google Scholar] [CrossRef]
- Zhang, Y.; Yong, Y.; Ge, J.; Liu, Z. Lectin agglutinated multienzyme catalyst with enhanced substrate affinity and activity. ACS Catal. 2016, 6, 3789–3795. [Google Scholar] [CrossRef]
- Huang, J.; Zhuang, W.; Wei, C.; Mu, L.; Zhu, J.; Zhu, Y.; Jinglan, W.; Yong, C.; Ying, H. Concanavalin A induced orientation immobilization of Nuclease P1: The effect of lectin agglutination. Process Biochem. 2018, 64, 160–169. [Google Scholar] [CrossRef]
- Yong, Y.; Su, R.; Liu, X.; Xu, W.; Zhang, Y.; Wang, R.; Ouyang, P.; Wu, J.; Ge, J.; Liu, Z. Lectin corona enhances enzymatic catalysis on the surface of magnetic nanoparticles. Biochem. Eng. J. 2018, 129, 26–32. [Google Scholar] [CrossRef]
- Saladino, R.; Guazzaroni, M.; Crestini, C.; Crucianelli, M. Dye Degradation by Layer-by-Layer Immobilised Peroxidase/Redox Mediator Systems. ChemCatChem 2013, 5, 1407–1415. [Google Scholar] [CrossRef]
- Xu, X.; Yuan, Y.; Guixian, H.; Wang, X.; Qi, P.; Wang, Z.; Wang, Q.; Wang, X.; Fu, Y.; Li, Y.; et al. Exploiting pH-Regulated Dimer- Tetramer Transformation of Concanavalin A to Develop Colorimetric Biosensing of Bacteria. Sci. Rep. 2017, 7, 2045–2322. [Google Scholar] [CrossRef]
- Xu, W.; Yong, Y.; Wang, Z.; Jiang, G.; Wu, J.; Liu, Z. Concanavalin A Coated Activated Carbon for High Performance Enzymatic Catalysis. ACS Sustain. Chem. Eng. 2017, 5, 90–96. [Google Scholar] [CrossRef]
- Palazzo, G.; Colafemmina, G.; Guzzoni Iudice, C.; Mallardi, A. Three immobilized enzymes acting in series in layer by layer assemblies: Exploiting thetrehalase-glucoseoxidase-horseradish peroxidase cascade reactions for the optical determination of trehalose. Sens. Actuators B Chem. 2014, 202, 217–223. [Google Scholar] [CrossRef]
- Rong, J.; Zhou, Z.; Wang, Y.; Han, J.; Li, C.; Zhang, W.; Ni, L. Immobilization of Horseradish Peroxidase on Multi-Armed Magnetic Graphene Oxide Composite: Improvement of Loading Amount and Catalytic Activity. Food Technol. Biotech. 2019, 57, 1330–1334. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wang, M.; Liang, C.; Jiang, H.; Shen, J.; Li, H. Thin-Layer Polymer Wrapped Enzymes Encapsulated in Hierarchically Mesoporous Silica with High Activity and Enhanced Stability. Sci. Rep. 2014, 4, 4421. [Google Scholar] [CrossRef] [PubMed]
- Tischer, W. Immobilized enzymes: Crystals or carriers? Trends Biotechnol. 1999, 117, 326–335. [Google Scholar] [CrossRef]
- Won, K.; Kim, Y.H.; An, E.S.; Lee, Y.S.; Song, B.K. Horseradish Peroxidase-Catalyzed Polymerization of Cardanol in the Presence of Redox Mediators. Biomacromolecules 2004, 5, 1–4. [Google Scholar] [CrossRef]
- Razola, S.S.; Aktas, E.; Viré, J.-C.; Kauffmann, J.-M. Reagentless enzyme electrode based on phenothiazine mediation of horseradish peroxidase for subnanomolar hydrogen peroxide determination. Analyst 2000, 125, 79–85. [Google Scholar] [CrossRef]
- Baciocchi, E.; Gerini, M.F.; Lanzalunga, O.; Mancinelli, S. Lignin peroxidase catalysed oxidation of 4-methoxymandelic acid. The role of mediator structure. Tetrahedron 2002, 58, 8087–8093. [Google Scholar] [CrossRef]
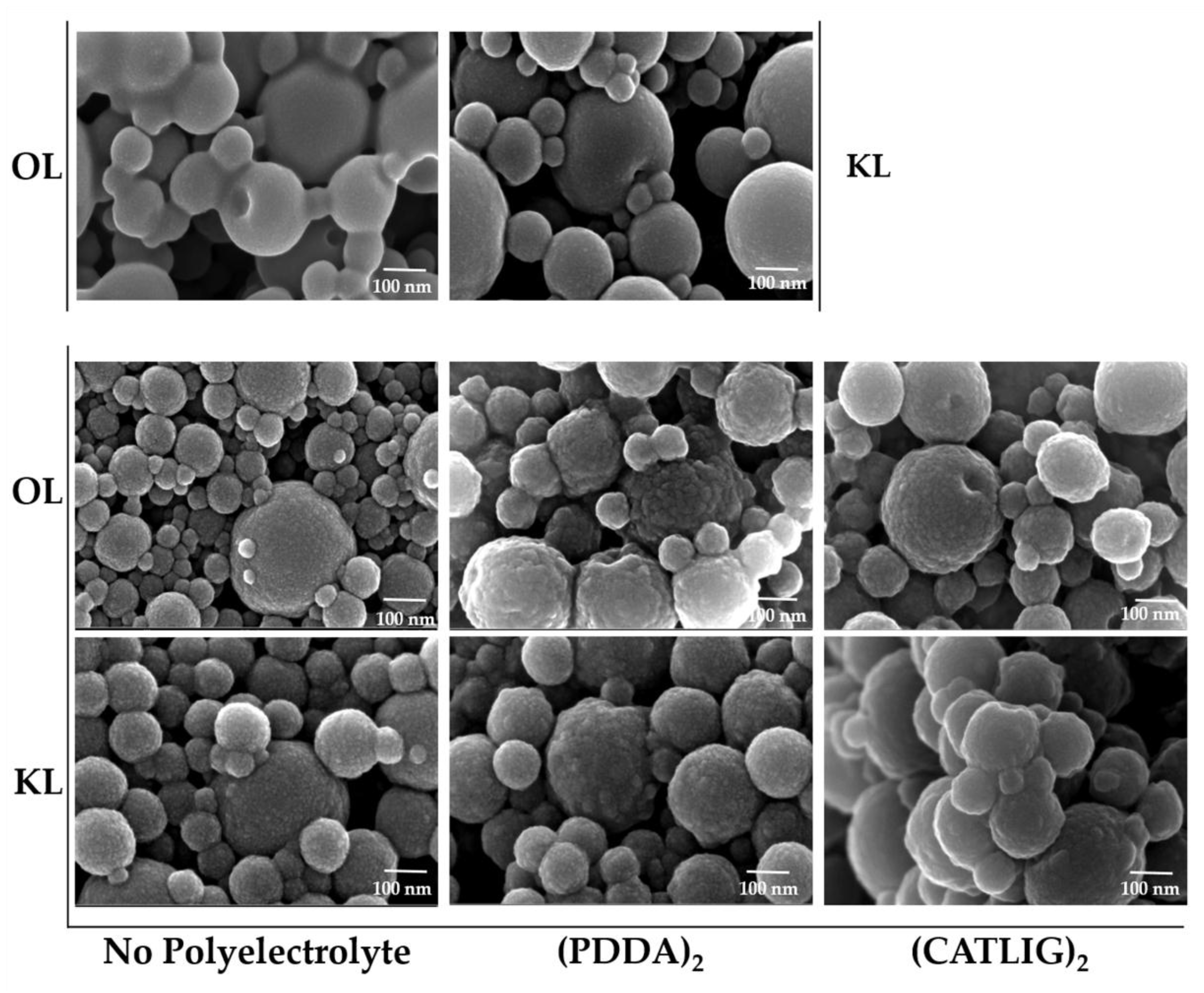
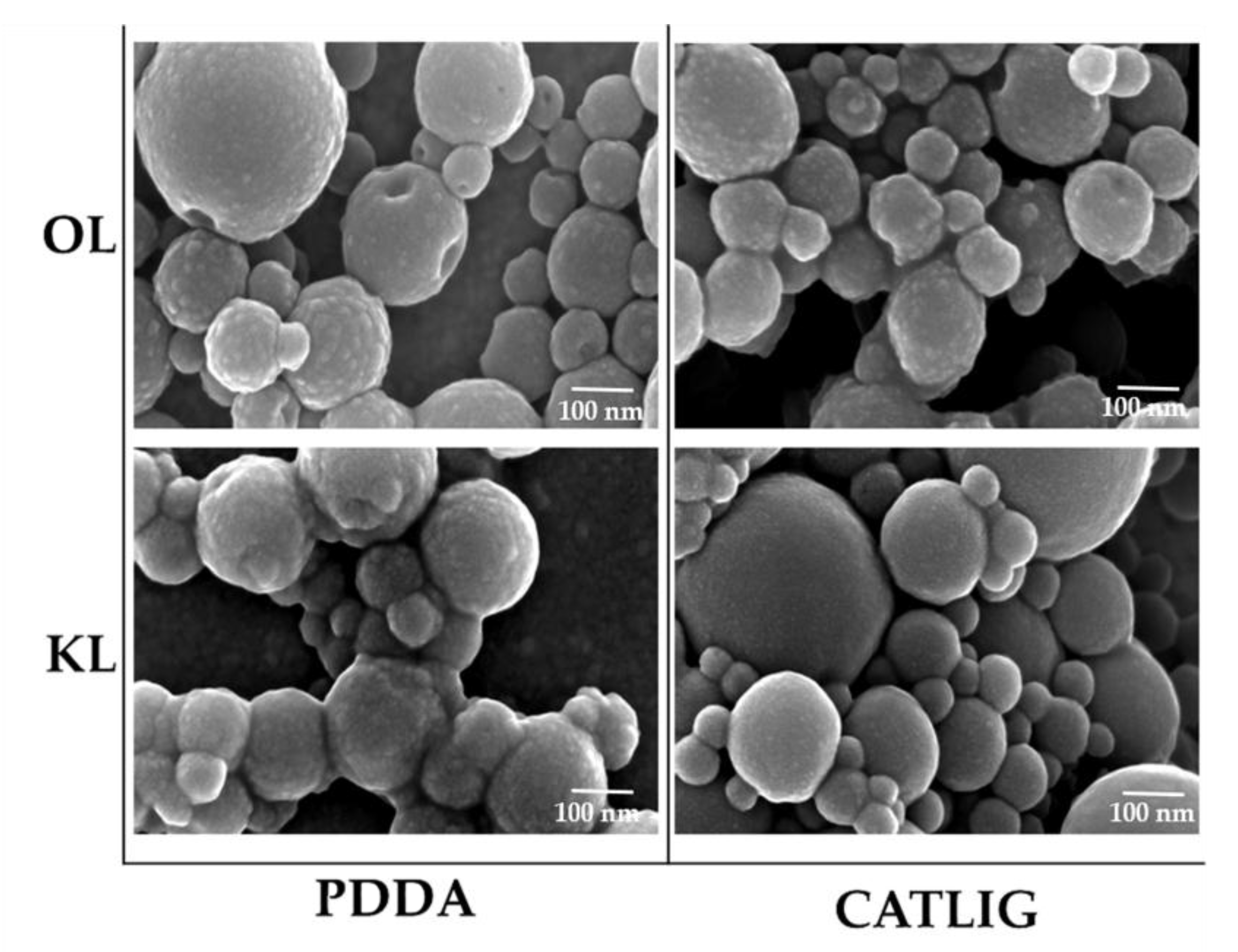
| Entry | Lignin/P | Cat | Immobilization Yield % | Activity Yield % | Activity (U/mg) | Km (mM) | Vmax (μM/s) |
|---|---|---|---|---|---|---|---|
| 1 | OL | I | 45 | 21 | 3.78 | 12.5 | 0.26 |
| 2 | KL | II | 53 | 63 | 11.34 | 11.3 | 0.28 |
| 3 | OL/PDDA | III | 56 | 43 | 7.74 | 9.9 | 0.30 |
| 4 | OL/CATLIG | IV | 57 | 64 | 11.52 | 9.7 | 0.33 |
| 5 | KL/PDDA | V | 62 | 72 | 12.96 | 9.8 | 0.32 |
| 6 | KL/CATLIG | VI | 64 | 86 | 15.48 | 9.4 | 0.34 |
| 7 | OL/(PDDA)2 | VII | 68 | 75 | 13.50 | 8.3 | 0.34 |
| 8 | OL/(CATLIG)2 | VIII | 69 | 76 | 13.68 | 8.1 | 0.36 |
| 9 | KL/(PDDA)2 | IX | 72 | 84 | 15.12 | 8.2 | 0.34 |
| 10 | KL/(CATLIG)2 | X | 73 | 85 | 15.30 | 7.9 | 0.35 |
| 11 | None | HRP | - | - | 18.00 | 9.5 | 0.35 |

| Entry | Lignin/P | Cat | Redox Mediator 1 | Conversion (%) | Yield (%) 2 |
|---|---|---|---|---|---|
| 1 | OL | I | none | 18 | >99 |
| 2 | KL | II | none | 22 | >99 |
| 3 | OL/PDDA | III | none | 20 | >99 |
| 4 | OL/CATLIG | IV | none | 23 | >99 |
| 5 | KL/PDDA | V | none | 30 | >99 |
| 6 | KL/CATLIG | VI | none | 32 | >99 |
| 7 | OL/(PDDA)2 | VII | none | 35 | >99 |
| 8 | OL/(CATLIG)2 | VIII | none | 36 | >99 |
| 9 | KL/(PDDA)2 | IX | none | 37 | >99 |
| 10 | KL/(CATLIG)2 | X | none | 38 | >99 |
| 11 | None | HRP | none | 78 | >99 |
| 12 | OL | I | VA | 25 | >99 |
| 13 | OL | I | HoBt | 34 | >99 |
| 14 | KL | II | VA | 32 | >99 |
| 15 | KL | II | HoBt | 46 | >99 |
| 15 | OL/PDDA | III | VA | 28 | >99 |
| 17 | OL/PDDA | III | HoBt | 33 | >99 |
| 18 | OL/CATLIG | IV | VA | 31 | >99 |
| 19 | OL/CATLIG | IV | HoBt | 36 | >99 |
| 20 | KL/PDDA | V | VA | 43 | >99 |
| 21 | KL/PDDA | V | HoBt | 52 | >99 |
| 22 | KL/CATLIG | VI | VA | 44 | >99 |
| 23 | KL/CATLIG | VI | HoBt | 53 | >99 |
| 24 | OL(PDDA)2 | VII | VA | 54 | >99 |
| 25 | OL(PDDA)2 | VII | HoBt | 64 | >99 |
| 26 | OL(CATLIG)2 | VIII | VA | 55 | >99 |
| 27 | OL(CATLIG)2 | VIII | HoBt | 65 | >99 |
| 28 | KL(PDDA)2 | IX | VA | 53 | >99 |
| 29 | KL(PDDA)2 | IX | HoBt | 64 | >99 |
| 30 | KL(CATLIG)2 | X | VA | 54 | >99 |
| 31 | KL(CATLIG)2 | X | HoBt | 65 | >99 |
| 32 | KL(CATLIG)2 | X | HoBt | 64 (Run no.1) 3 | >99 |
| 33 | KL(CATLIG)2 | X | HoBt | 60 (Run no.2) | >99 |
| 34 | KL(CATLIG)2 | X | HoBt | 58 (Run no.3) | >99 |
| 35 | KL(CATLIG)2 | X | HoBt | 42 (Run no.4) | >99 |
| 36 | KL(CATLIG)2 | X | HoBt | 45 (Run no.5) | >99 |
| 37 | None | HRP | VA | 55 | >99 |
| 38 | None | HRP | HoBt | 70 | >99 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capecchi, E.; Piccinino, D.; Bizzarri, B.M.; Botta, L.; Crucianelli, M.; Saladino, R. Oxidative Bio-Desulfurization by Nanostructured Peroxidase Mediator System. Catalysts 2020, 10, 313. https://doi.org/10.3390/catal10030313
Capecchi E, Piccinino D, Bizzarri BM, Botta L, Crucianelli M, Saladino R. Oxidative Bio-Desulfurization by Nanostructured Peroxidase Mediator System. Catalysts. 2020; 10(3):313. https://doi.org/10.3390/catal10030313
Chicago/Turabian StyleCapecchi, Eliana, Davide Piccinino, Bruno Mattia Bizzarri, Lorenzo Botta, Marcello Crucianelli, and Raffaele Saladino. 2020. "Oxidative Bio-Desulfurization by Nanostructured Peroxidase Mediator System" Catalysts 10, no. 3: 313. https://doi.org/10.3390/catal10030313
APA StyleCapecchi, E., Piccinino, D., Bizzarri, B. M., Botta, L., Crucianelli, M., & Saladino, R. (2020). Oxidative Bio-Desulfurization by Nanostructured Peroxidase Mediator System. Catalysts, 10(3), 313. https://doi.org/10.3390/catal10030313