First-Principles Mechanistic Insights into the Hydrogen Evolution Reaction on Ni2P Electrocatalyst in Alkaline Medium
Abstract
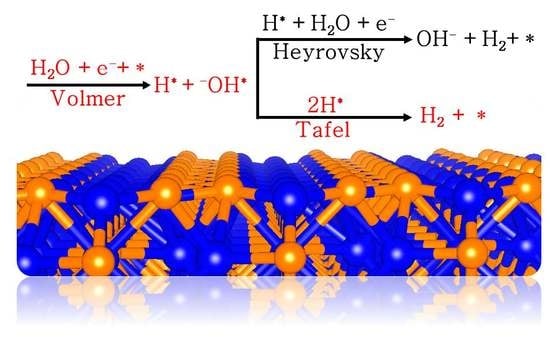
1. Introduction
2. Results and Discussion
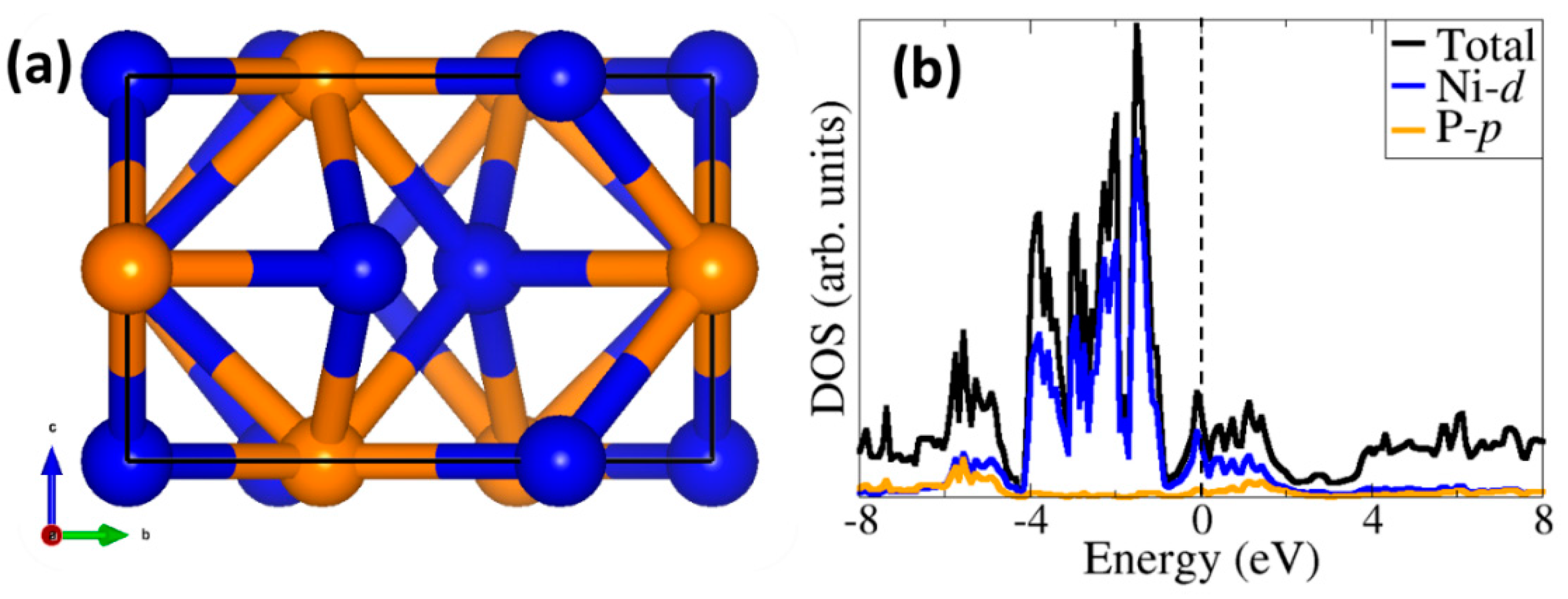
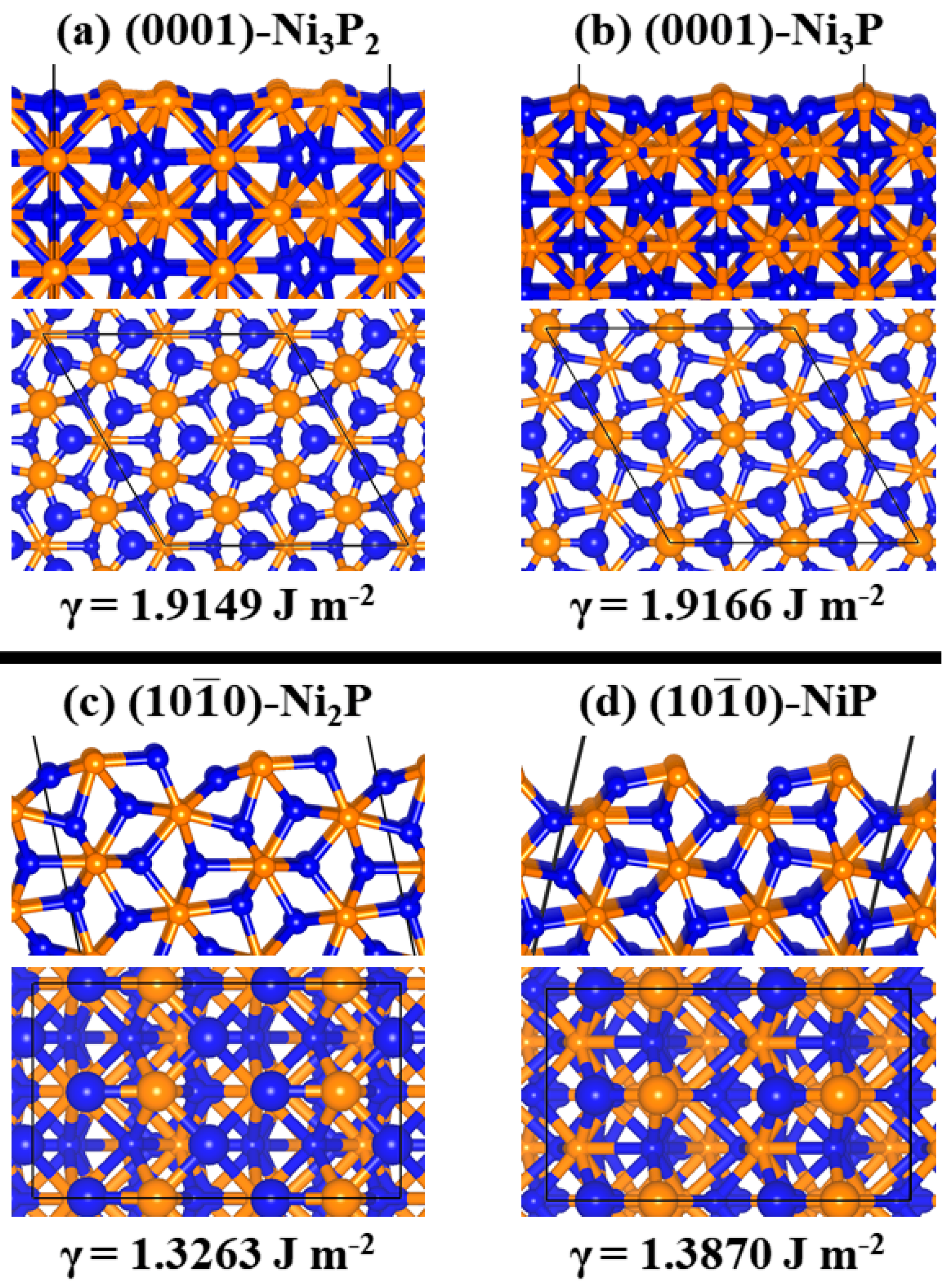
2.1. Ni2P Bulk and Surface Characterization
2.2. Molecular H2O Adsorption on Ni2P (0001) and (100) Surfaces
2.3. Dissociative H2O Adsorption on Ni2P (0001) and (100) Surfaces
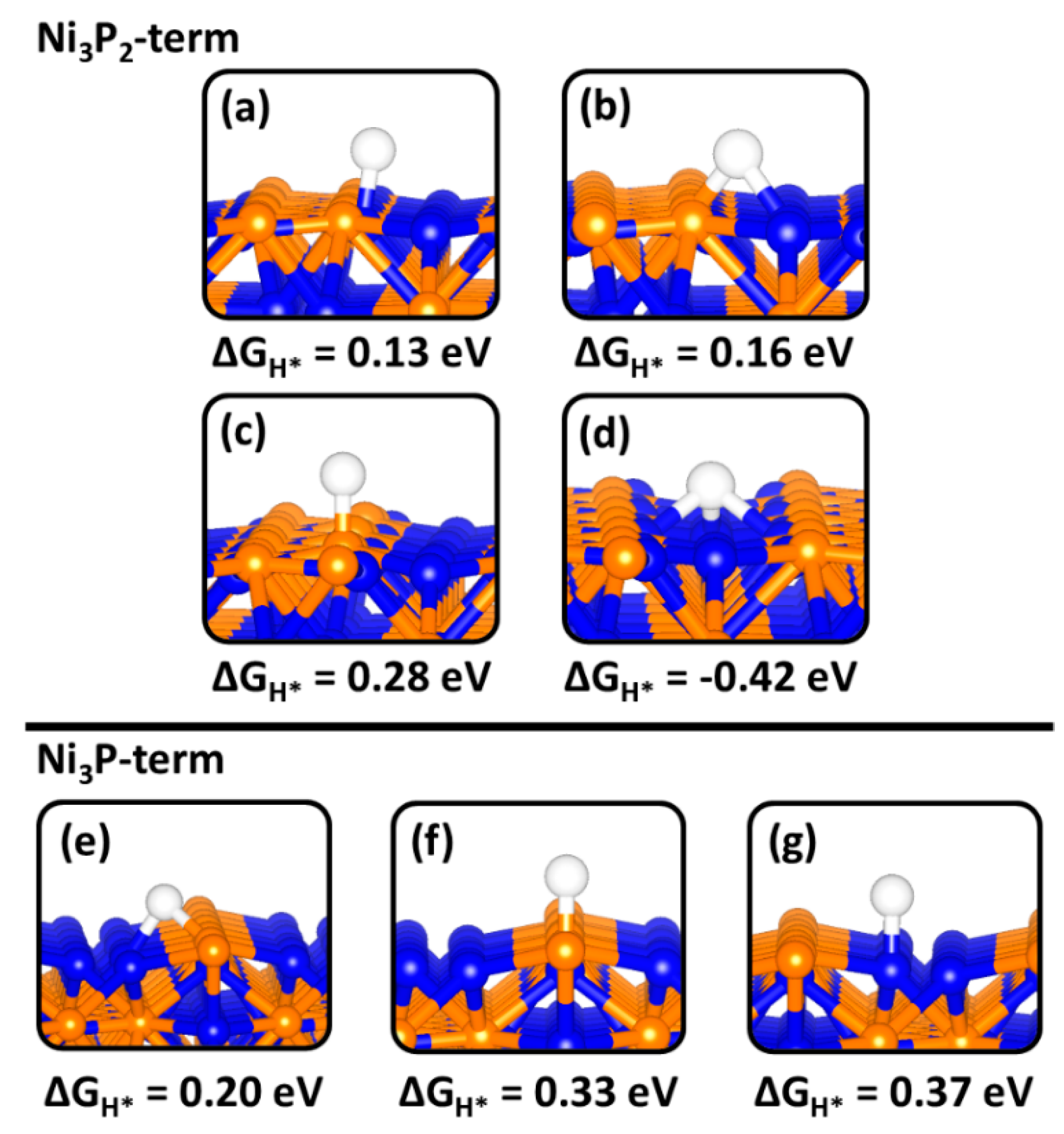
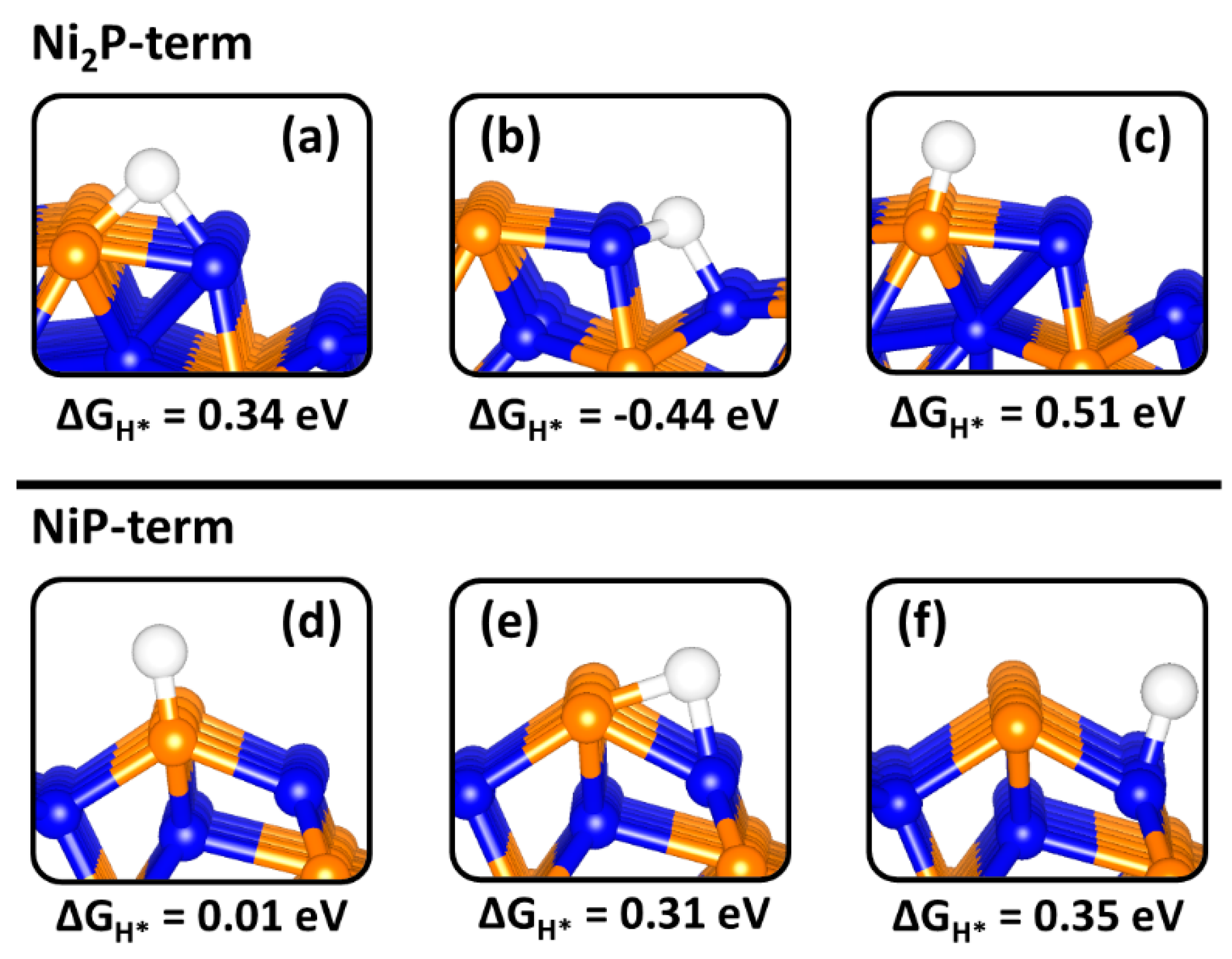
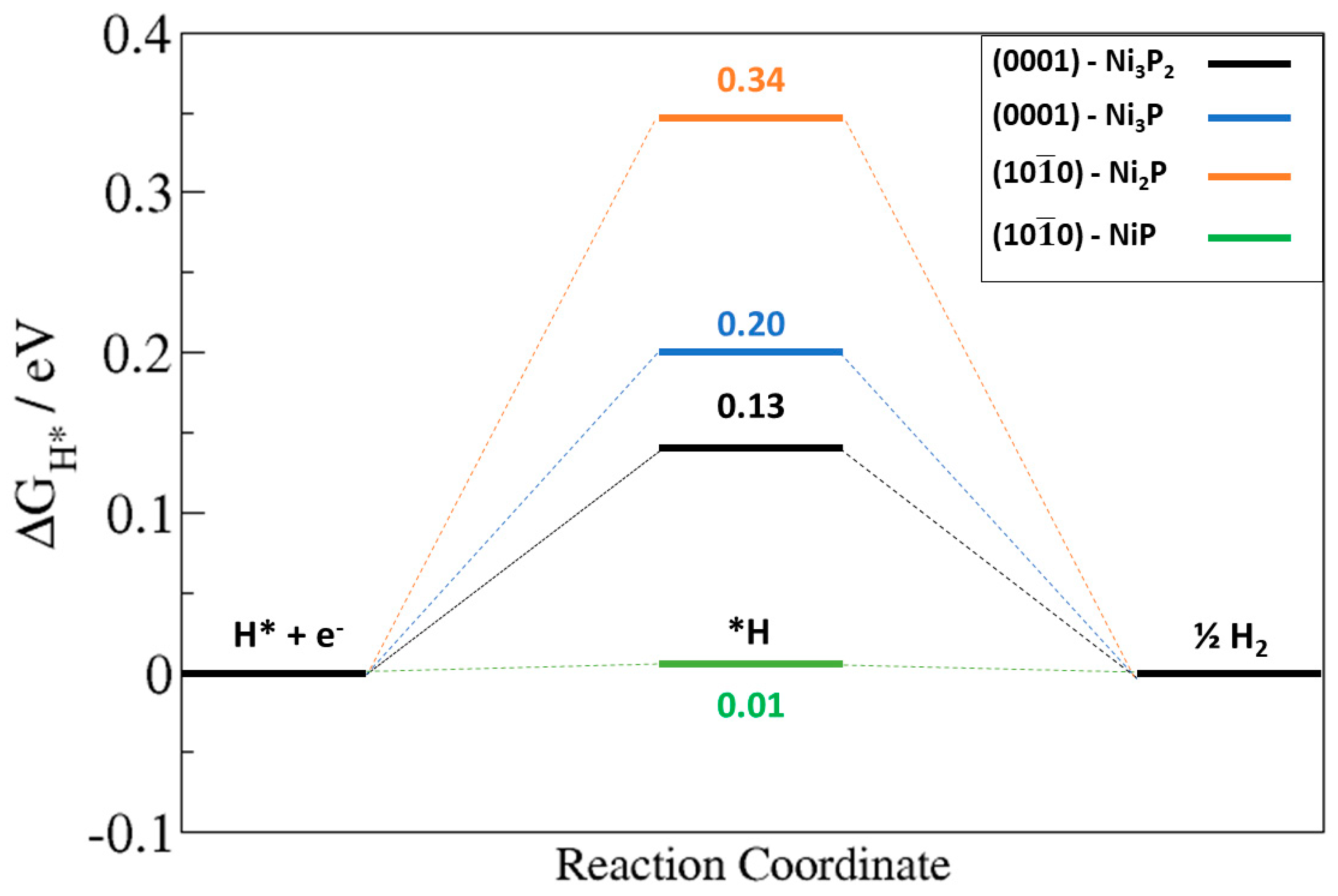
2.4. H Adsorption on Ni2P (0001) and (100) Surfaces
3. Summary and Conclusions
4. Computational Details
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dunn, S. Hydrogen futures: Toward a sustainable energy system. Int. J. Hydrogen Energy 2002, 27, 235–264. [Google Scholar] [CrossRef]
- Turner, J.; Sverdrup, G.; Mann, M.K.; Maness, P.C.; Kroposki, B.; Ghirardi, M.; Evans, J.R.; Blake, D. Renewable hydrogen production. Int. J. Energy Res. 2008, 32, 379–407. [Google Scholar] [CrossRef]
- Holladay, J.D.; Hu, J.; King, D.L.; Wang, Y. An overview of hydrogen production technologies. Catal. Today 2009, 139, 244–260. [Google Scholar] [CrossRef]
- Greeley, J.; Jaramillo, T.F.; Bonde, J.; Chorkendorff, I.; Nørskov, J.K. Computational high throughput screening of electrocatalytic materials for hydrogen evolution. Nat. Mater. 2006, 5, 909–913. [Google Scholar] [CrossRef]
- Ojha, K.; Saha, S.; Dagar, P.; Ganguli, A.K. Nanocatalysts for hydrogen evolution reactions. Phys. Chem. Chem. Phys. 2018, 20, 6777–6799. [Google Scholar] [CrossRef]
- McCrory, C.C.L.; Jung, S.; Ferrer, I.M.; Chatman, S.M.; Peters, J.C.; Jaramillo, T.F. Benchmarking hydrogen evolving reaction and oxygen evolving reaction electrocatalysts for solar water splitting devices. J. Am. Chem. Soc. 2015, 137, 4347–4357. [Google Scholar] [CrossRef]
- Noerskov, J.K.; Bligaard, T.; Logadottir, A.; Kitchin, J.R.; Chen, J.G.; Pandelov, S.; Stimming, U.; Nørskov, J.K.; Bligaard, T.; Logadottir, A.; et al. Trends in the exchange current for hydrogen evolution. J. Electrochem. Soc. 2005, 152, J23–J26. [Google Scholar] [CrossRef]
- Stamenkovic, V.R.; Mun, B.S.; Arenz, M.; Mayrhofer, K.J.J.; Lucas, C.A.; Wang, G.; Ross, P.N.; Markovic, N.M. Trends in electrocatalysis on extended and nanoscale Pt-bimetallic alloy surfaces. Nat. Mater. 2007, 6, 241. [Google Scholar] [CrossRef]
- Kong, D.; Cha, J.J.; Wang, H.; Lee, H.R.; Cui, Y. First-row transition metal dichalcogenide catalysts for hydrogen evolution reaction. Energy Environ. Sci. 2013, 6, 3553–3558. [Google Scholar] [CrossRef]
- Wan, C.; Regmi, Y.N.; Leonard, B.M. Multiple phases of molybdenum carbide as electrocatalysts for the hydrogen evolution reaction. Angew. Chem. Int. Ed. 2014, 53, 6407–6410. [Google Scholar] [CrossRef]
- Chen, W.F.; Sasaki, K.; Ma, C.; Frenkel, A.I.; Marinkovic, N.; Muckerman, J.T.; Zhu, Y.; Adzic, R.R. Hydrogen-evolution catalysts based on non-noble metal nickel–molybdenum nitride nanosheets. Angew. Chem. Int. Ed. 2012, 51, 6131–6135. [Google Scholar] [CrossRef]
- Stern, L.A.; Feng, L.; Song, F.; Hu, X. Ni2P as a Janus catalyst for water splitting: The oxygen evolution activity of Ni2P nanoparticles. Energy Environ. Sci. 2015, 8, 2347–2351. [Google Scholar] [CrossRef]
- Pan, Y.; Lin, Y.; Chen, Y.; Liu, Y.; Liu, C. Cobalt phosphide-based electrocatalysts: Synthesis and phase catalytic activity comparison for hydrogen evolution. J. Mater. Chem. A 2016, 4, 4745–4754. [Google Scholar] [CrossRef]
- Cabán-Acevedo, M.; Stone, M.L.; Schmidt, J.R.; Thomas, J.G.; Ding, Q.; Chang, H.C.; Tsai, M.L.; He, J.-H.; Jin, S. Efficient hydrogen evolution catalysis using ternary pyrite-type cobalt phosphosulphide. Nat. Mater. 2015, 14, 1245. [Google Scholar] [CrossRef]
- Mukherjee, D.; Austeria, P.M.; Sampath, S. Two-Dimensional, Few-Layer Phosphochalcogenide, FePS3: A New Catalyst for Electrochemical Hydrogen Evolution over Wide pH Range. ACS Energy Lett. 2016, 1, 367–372. [Google Scholar] [CrossRef]
- Callejas, J.F.; Read, C.G.; Popczun, E.J.; McEnaney, J.M.; Schaak, R.E. Nanostructured Co2P Electrocatalyst for the Hydrogen Evolution Reaction and Direct Comparison with Morphologically Equivalent CoP. Chem. Mater. 2015, 27, 3769–3774. [Google Scholar] [CrossRef]
- Schipper, D.E.; Zhao, Z.; Thirumalai, H.; Leitner, A.P.; Donaldson, S.L.; Kumar, A.; Qin, F.; Wang, Z.; Grabow, L.C.; Bao, J.; et al. Effects of Catalyst Phase on the Hydrogen Evolution Reaction of Water Splitting: Preparation of Phase-Pure Films of FeP, Fe2P, and Fe3P and Their Relative Catalytic Activities. Chem. Mater. 2018, 30, 3588–3598. [Google Scholar] [CrossRef]
- Chu, S.; Chen, W.; Chen, G.; Huang, J.; Zhang, R.; Song, C.; Wang, X.; Li, C.; Ostrikov, K. In situ engineering bi-metallic phospho-nitride bi-functional electrocatalysts for overall water splitting. Appl. Catal. B Environ. 2019, 243, 537–545. [Google Scholar] [CrossRef]
- Morales-García, Á.; He, J.; Lyu, P.; Nachtigall, P. Exploring the stability and reactivity of Ni2P and Mo2C catalysts using ab initio atomistic thermodynamics and conceptual DFT approaches. Biomass Convers. Biorefin. 2017, 7, 377–383. [Google Scholar] [CrossRef]
- Popczun, E.J.; McKone, J.R.; Read, C.G.; Biacchi, A.J.; Wiltrout, A.M.; Lewis, N.S.; Schaak, R.E. Nanostructured nickel phosphide as an electrocatalyst for the hydrogen evolution reaction. J. Am. Chem. Soc. 2013, 135, 9267–9270. [Google Scholar] [CrossRef]
- Pei, Y.; Cheng, Y.; Chen, J.; Smith, W.; Dong, P.; Ajayan, P.M.; Ye, M.; Shen, J. Recent developments of transition metal phosphides as catalysts in the energy conversion field. J. Mater. Chem. A 2018, 6, 23220–23243. [Google Scholar] [CrossRef]
- Hansen, M.H.; Stern, L.A.; Feng, L.; Rossmeisl, J.; Hu, X. Widely available active sites on Ni2P for electrochemical hydrogen evolution–insights from first principles calculations. Phys. Chem. Chem. Phys. 2015, 17, 10823–10829. [Google Scholar] [CrossRef]
- Moon, J.S.; Jang, J.H.; Kim, E.G.; Chung, Y.H.; Yoo, S.J.; Lee, Y.K. The nature of active sites of Ni2P electrocatalyst for hydrogen evolution reaction. J. Catal. 2015, 326, 92–99. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Kim, J.Y.; Hanson, J.C.; Sawhill, S.J.; Bussell, M.E. Physical and Chemical Properties of MoP, Ni2P, and MoNiP Hydrodesulfurization Catalysts: Time-Resolved X-ray Diffraction. J. Phys. Chem. B 2003, 107, 6276–6285. [Google Scholar] [CrossRef]
- Kawai, T.; Bando, K.K.; Lee, Y.K.; Oyama, S.T.; Chun, W.-J.; Asakura, K. EXAFS measurements of a working catalyst in the liquid phase: An in situ study of a Ni2P hydrodesulfurization catalyst. J. Catal. 2006, 241, 20–24. [Google Scholar] [CrossRef]
- Wang, W.; Li, X.; Sun, Z.; Wang, A.; Liu, Y.; Chen, Y.; Duan, X. Influences of calcination and reduction methods on the preparation of Ni2P/SiO2 and its hydrodenitrogenation performance. Appl. Catal. A Gen. 2016, 509, 45–51. [Google Scholar] [CrossRef]
- Sun, Z.; Li, X.; Wang, A.; Wang, Y.; Chen, Y. The Effect of CeO2 on the Hydrodenitrogenation Performance of Bulk Ni2P. Top. Catal. 2012, 55, 1010–1021. [Google Scholar] [CrossRef]
- Krishnan, P.S.; Ramya, R.; Umasankar, S.; Shanthi, K. Promotional effect of Ni2P on mixed and separated phase MoS2/Al-SBA-15 (10) catalyst for hydrodenitrogenation of ortho-Propylaniline. Microporous Mesoporous Mater. 2017, 242, 208–220. [Google Scholar] [CrossRef]
- Oyama, S.T. Novel catalysts for advanced hydroprocessing: Transition metal phosphides. J. Catal. 2003, 216, 343–352. [Google Scholar] [CrossRef]
- Chen, J.; Chen, Y.; Yang, Q.; Li, K.; Yao, C. An approach to preparing highly dispersed Ni2P/SiO2 catalyst. Catal. Commun. 2010, 11, 571–575. [Google Scholar] [CrossRef]
- Liu, P.; Rodriguez, J.A.; Takahashi, Y.; Nakamura, K. Water-gas-shift reaction on a Ni2P (001) catalyst: Formation of oxy-phosphides and highly active reaction sites. J. Catal. 2009, 262, 294–303. [Google Scholar] [CrossRef]
- Mishra, I.K.; Zhou, H.; Sun, J.; Qin, F.; Dahal, K.; Bao, J.; Chen, S.; Ren, Z. Hierarchical CoP/Ni5P4/CoP microsheet arrays as a robust pH-universal electrocatalyst for efficient hydrogen generation. Energy Environ. Sci. 2018, 11, 2246–2252. [Google Scholar] [CrossRef]
- Han, A.; Jin, S.; Chen, H.; Ji, H.; Sun, Z.; Du, P. A robust hydrogen evolution catalyst based on crystalline nickel phosphide nanoflakes on three-dimensional graphene/nickel foam: High performance for electrocatalytic hydrogen production from pH 0–14. J. Mater. Chem. A 2015, 3, 1941–1946. [Google Scholar] [CrossRef]
- Liu, P.; Rodriguez, J.A. Catalysts for Hydrogen Evolution from the [NiFe] Hydrogenase to the Ni2P(001) Surface: The Importance of Ensemble Effect. J. Am. Chem. Soc. 2005, 127, 14871–14878. [Google Scholar] [CrossRef]
- Li, Q.; Hu, X. First-principles study of Ni2P(0001) surfaces. Phys. Rev. B 2006, 74, 35414. [Google Scholar] [CrossRef]
- Hernandez, A.B.; Ariga, H.; Takakusagi, S.; Kinoshita, K.; Suzuki, S.; Otani, S.; Oyama, S.T.; Asakura, K. Dynamical LEED analysis of Ni2P(0001)-1× 1: Evidence for P-covered surface structure. Chem. Phys. Lett. 2011, 513, 48–52. [Google Scholar] [CrossRef][Green Version]
- Zhang, L.; Ren, X.; Guo, X.; Liu, Z.; Asiri, A.M.; Li, B.; Chen, L.; Sun, X. Efficient Hydrogen Evolution Electrocatalysis at Alkaline pH by Interface Engineering of Ni2P–CeO2. Inorg. Chem. 2018, 57, 548–552. [Google Scholar] [CrossRef]
- Yu, L.; Mishra, I.K.; Xie, Y.; Zhou, H.; Sun, J.; Zhou, J.; Ni, Y.; Luo, D.; Yu, F.; Yu, Y.; et al. Ternary Ni2(1-x)Mo2xP nanowire arrays toward efficient and stable hydrogen evolution electrocatalysis under large-current-density. Nano Energy 2018, 53, 492–500. [Google Scholar] [CrossRef]
- Subbaraman, R.; Tripkovic, D.; Chang, K.C.; Strmcnik, D.; Paulikas, A.P.; Hirunsit, P.; Chan, M.; Greeley, J.; Stamenkovic, V.; Markovic, N.M. Trends in activity for the water electrolyser reactions on 3 d M (Ni, Co, Fe, Mn) hydr (oxy) oxide catalysts. Nat. Mater. 2012, 11, 550. [Google Scholar] [CrossRef]
- Rundqvist, S. X-ray investigations of Mn3P, Mn2P and Ni2P. Acta Chem. Scand. 1962, 16, 992–998. [Google Scholar] [CrossRef]
- Lee, Y.K.; Oyama, S.T. Bifunctional nature of a SiO2-supported Ni2P catalyst for hydrotreating: EXAFS and FTIR studies. J. Catal. 2006, 239, 376–389. [Google Scholar] [CrossRef]
- He, J.; Morales-García, Á.; Bludský, O.; Nachtigall, P. The surface stability and equilibrium crystal morphology of Ni2P nanoparticles and nanowires from an ab-initio atomistic thermodynamic approach. CrystEngComm 2016, 18, 3808–3818. [Google Scholar] [CrossRef]
- Moula, M.G.; Suzuki, S.; Chun, W.J.; Otani, S.; Oyama, S.T.; Asakura, K. Surface structures of Ni2P (0001)—Scanning tunneling microscopy (STM) and low-energy electron diffraction (LEED) characterizations. Surf. Interface Anal. 2006, 38, 1611–1614. [Google Scholar] [CrossRef]
- Edamoto, K.; Inomata, H.; Ozawa, K.; Nakagawa, Y.; Asakura, K.; Otani, S. Electronic structure of the Ni2P(100) surface: Angle-resolved photoemission study. Solid State Commun. 2010, 150, 1120–1123. [Google Scholar] [CrossRef]
- Feng, J.X.; Xu, H.; Dong, Y.T.; Lu, X.F.; Tong, Y.-X.; Li, G.R. Efficient hydrogen evolution electrocatalysis using cobalt nanotubes decorated with titanium dioxide nanodots. Angew. Chem. Int. Ed. 2017, 56, 2960–2964. [Google Scholar] [CrossRef]
- Zhang, F.S.; Wang, J.W.; Luo, J.; Liu, R.R.; Zhang, Z.M.; He, C.-T.; Lu, T.B. Extraction of nickel from NiFe-LDH into Ni2P@NiFe hydroxide as a bifunctional electrocatalyst for efficient overall water splitting. Chem. Sci. 2018, 9, 1375–1384. [Google Scholar] [CrossRef]
- Wexler, R.B.; Martirez, M.P.J.; Rappe, A.M. Active role of phosphorus in the hydrogen evolving activity of nickel phosphide (0001) surfaces. ACS Catal. 2017, 7, 7718–7725. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab-initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Watson, G.W.; Kelsey, E.T.; de Leeuw, N.H.; Harris, D.J.; Parker, S.C. Atomistic simulation of dislocations, surfaces and interfaces in MgO. J. Chem. Soc. Faraday Trans. 1996, 92, 433–438. [Google Scholar] [CrossRef]
- Tasker, P.W. The stability of ionic crystal surfaces. J. Phys. C Solid State Phys. 1979, 12, 4977–4984. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in Molecules—A Quantum Theory; Oxford University Press: Oxford, UK, 1990. [Google Scholar]
- Tang, W.; Sanville, E.; Henkelman, G. A grid-based Bader analysis algorithm without lattice bias. J. Phys. Condens. Matter 2009, 21, 84204. [Google Scholar] [CrossRef]
- Henkelman, G.; Uberuaga, B.P.; Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 2000, 113, 9901–9904. [Google Scholar] [CrossRef]
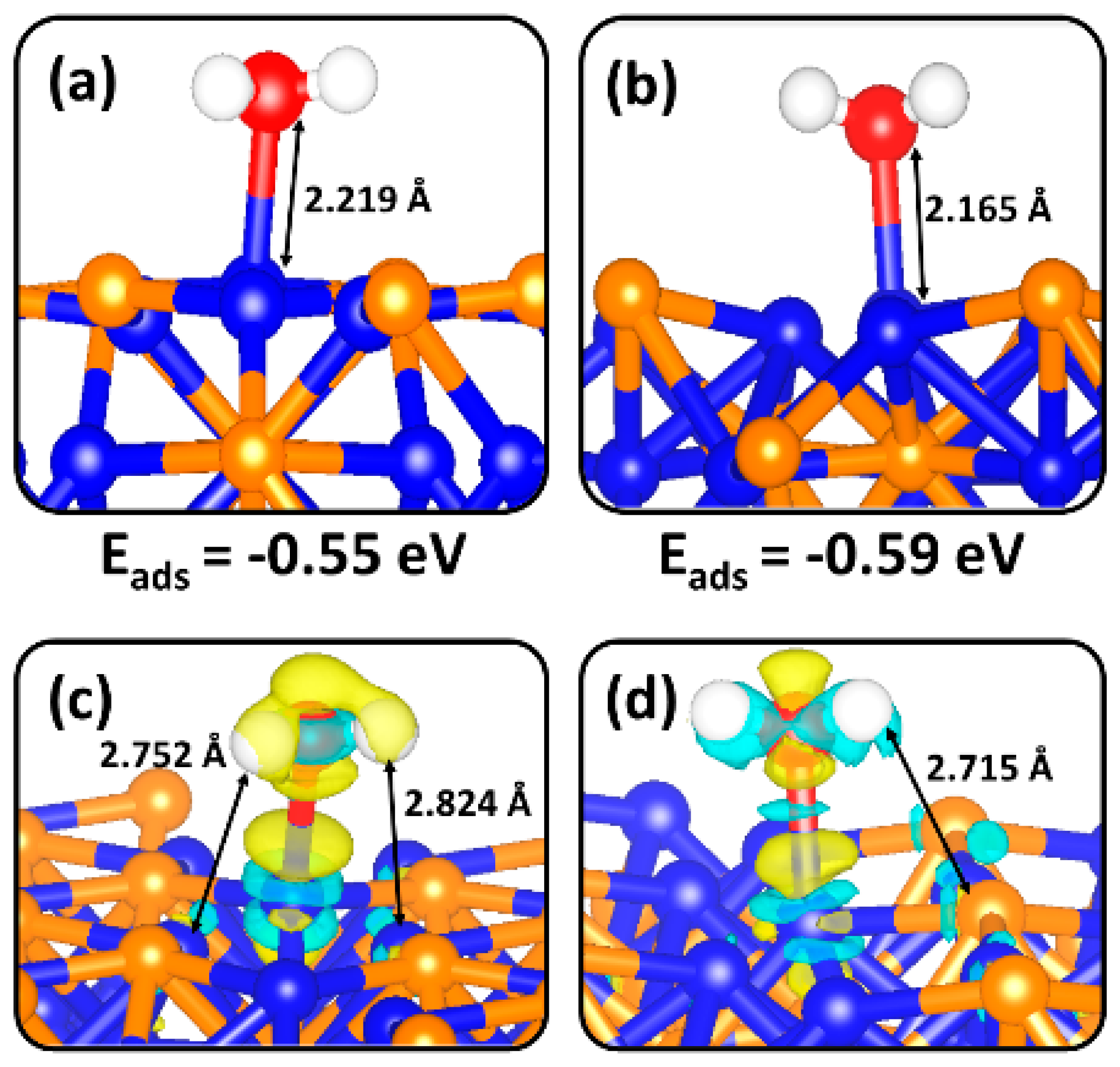
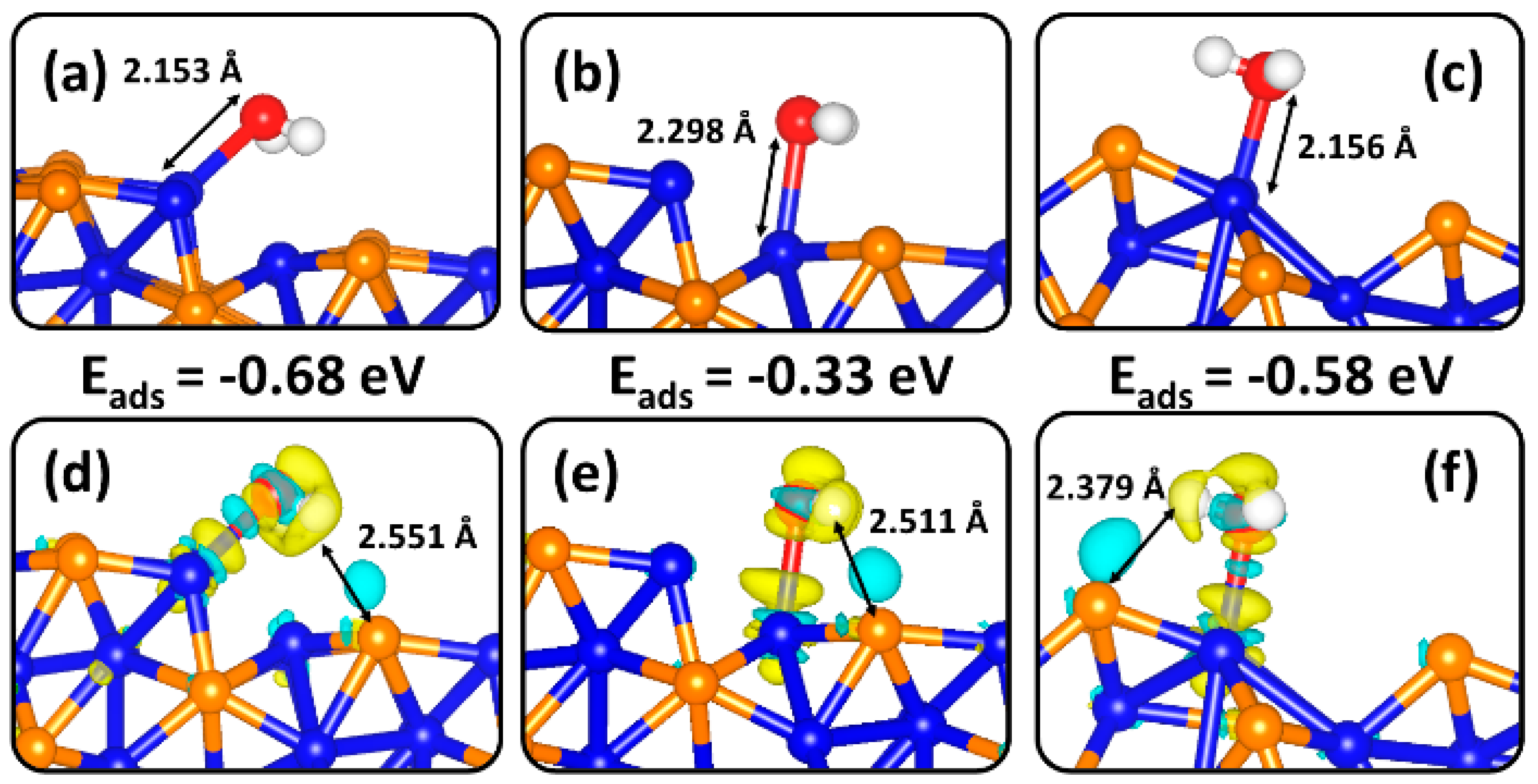


| Surface | Eads (eV) | d(Ni-O) (Å) | d(O-H) (Å) | α(H-O-H) (°) | q(H2O) (e-) | ν(O-H) (cm−1) |
|---|---|---|---|---|---|---|
| (0001)-Ni3P2 | −0.55 | 2.219 | 0.980 | 104.6 | 0.04 | 3820, 3705 |
| (0001)-Ni3P | −0.59 | 2.165 | 0.976 | 106.8 | 0.02 | 3829, 3735 |
| - | - | - | - | - | - | - |
| (100)-Ni2P | −0.68 | 2.153 | 0.988 | 101.8 | 0.01 | 3607, 3493 |
| (100)-Ni2P | −0.33 | 2.298 | 0.984 | 105.0 | -0.01 | 3632, 3540 |
| (100)-NiP | −0.58 | 2.156 | 0.995 | 104.5 | 0.03 | 3763, 3574 |
| Surface | Site (OH/H) | Eads(OH + H) (eV) | d(Ni–O) (Å) | d(O–H) (Å) | d(H–Ni/(P)) (Å) |
|---|---|---|---|---|---|
| (0001)-Ni3P2 | bridge-Ni/Ni3-hollow | −1.07 | 2.034 | 0.978 | 1.761 |
| (0001)-Ni3P | top-Ni/bridge-NiP | 0.45 | 1.860 | 0.977 | 1.746, 1.547 (P) |
| (100)-Ni2P | bridge-Ni/bridge-Ni | −0.69 | 1.969 | 0.975 | 1.582 |
| (100)- NiP | top-Ni/top-P | 0.05 | 1.841 | 0.980 | 1.427 (P) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cross, R.W.; Dzade, N.Y. First-Principles Mechanistic Insights into the Hydrogen Evolution Reaction on Ni2P Electrocatalyst in Alkaline Medium. Catalysts 2020, 10, 307. https://doi.org/10.3390/catal10030307
Cross RW, Dzade NY. First-Principles Mechanistic Insights into the Hydrogen Evolution Reaction on Ni2P Electrocatalyst in Alkaline Medium. Catalysts. 2020; 10(3):307. https://doi.org/10.3390/catal10030307
Chicago/Turabian StyleCross, Russell W., and Nelson Y. Dzade. 2020. "First-Principles Mechanistic Insights into the Hydrogen Evolution Reaction on Ni2P Electrocatalyst in Alkaline Medium" Catalysts 10, no. 3: 307. https://doi.org/10.3390/catal10030307
APA StyleCross, R. W., & Dzade, N. Y. (2020). First-Principles Mechanistic Insights into the Hydrogen Evolution Reaction on Ni2P Electrocatalyst in Alkaline Medium. Catalysts, 10(3), 307. https://doi.org/10.3390/catal10030307