1. Introduction
Energy is an important material basis for human survival and development. Due to environmental problems and energy shortages caused by excessive use of fossil energy, biomass as a renewable energy source has received more and more attention [
1,
2,
3]. Lignin is one of the three main components of lignocellulosic biomass, and the other two components are cellulose and hemicellulose. It is mainly located between cellulose fibers, and plays a role in resisting pressure. Among woody plants, lignin is the second largest organic substance in the world, accounting for 25% [
4]. It is a major by-product of second-generation bioethanol production, and is the main impurity for separating cellulose from pulp and wood for papermaking. Because of its aromatic qualities, it has potential as biofuel and chemical aromatic hydrocarbon feedstock [
5]. Lignin is a complex phenolic polymer formed from three alcohol monomers (p-coumarol alcohol, coniferyl alcohol, and sinapyl alcohol, see
Figure 1a). This polymerization is a chemically controlled random free-radical coupling reaction [
6]. Due to different monomers, lignin can be divided into 3 types: syringin lignin (S-lignin), guaiacyl lignin (G-lignin), and p-hydroxyphenyl lignin (H-lignin) (
Figure 1b). They are generally connected by β-O-4, α-O-4 and 5-5 bonds, of which β-O-4 bonds are dominant and account for more than half of the lignin bond structure [
7,
8].
A large number of studies on lignin pyrolysis have been conducted in recent years. The pyrolysis of lignin and model compounds has been frequently studied by many researchers using methods like thermogravimetric analysis (TGA), Fourier Transform infrared spectroscopy (FTIR), gas chromatography (GC), mass spectrometry (MS), and others [
9,
10,
11,
12,
13,
14]. These extensive studies have reached a generally accepted hypothesis that pyrolysis follows complex reaction paths and that each reaction path is dominated by free radical reactions. Some of these radicals may be the cause of char formation [
15,
16,
17]. The study of free radicals involved in lignin pyrolysis is mainly limited to ex situ measurements and inference based on reactants and products. Only recently, researchers have started to detect the generation of free radicals during lignin pyrolysis by using electron paramagnetic resonance (EPR) [
18,
19,
20,
21,
22]. In order to further explore the reaction mechanism of the pyrolysis of lignin, the pyrolysis process of guaiacol (o-methoxyphenol) as a lignin model compound was studied by free radical detection technology (electron paramagnetic resonance) in this paper.
2. Results
2.1. Py-GC/MS Experiments
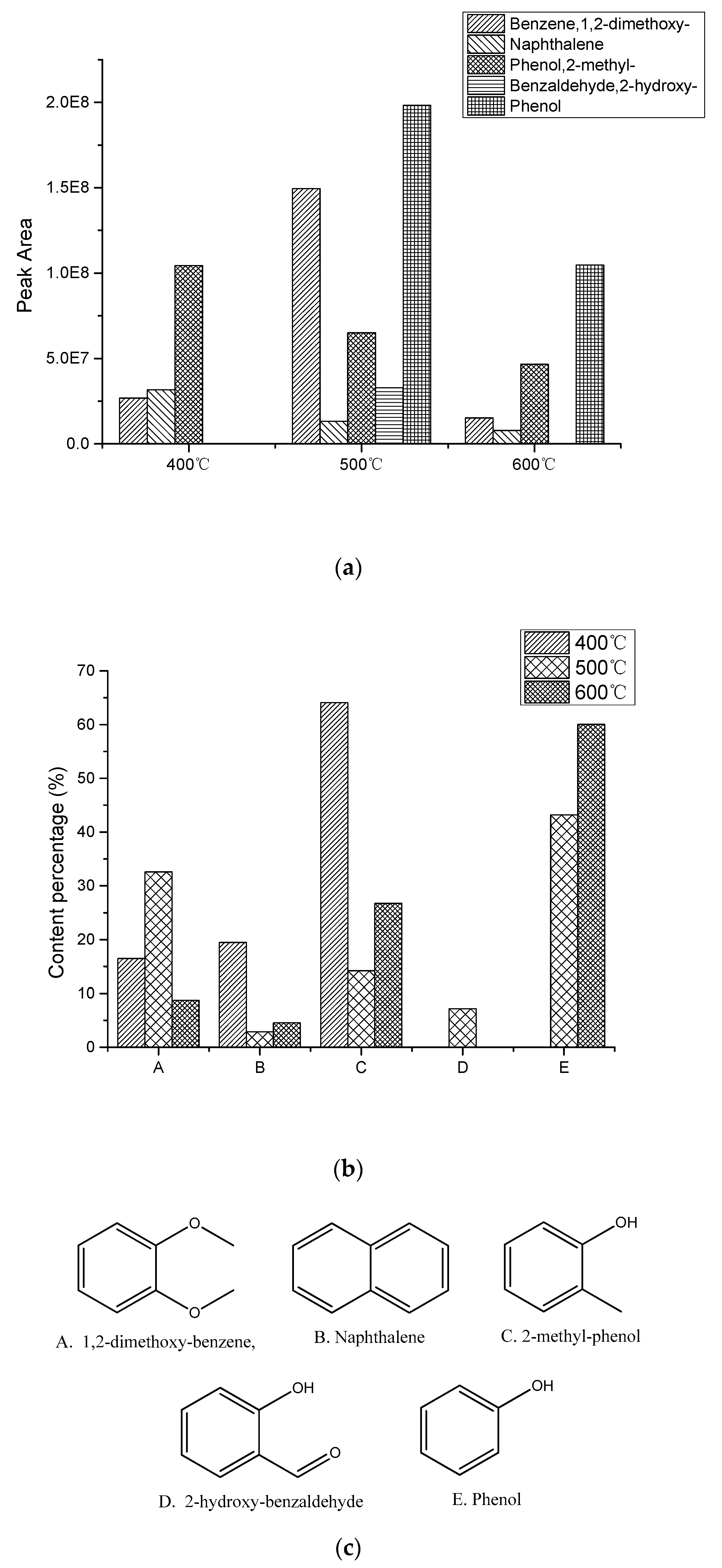
The data seen in
Figure 2 was obtained by normalizing the results of Py-GC/MS experiments. As is shown in
Figure 2a, the total amount of guaiacol pyrolysis products reached a maximum at 500 °C, while the yield at 400 °C was substantially similar to that at 600 °C. It can be seen in
Figure 2a that the yields of both naphthalene and2-methyl-phenol decreased with increasing temperature, and the same applies to phenol, although no formation of phenol was detected at 400 °C. Moreover, the generation of 2-hydroxy-benzaldehyde was only detected at 500 °C. The yield of 1,2-dimethoxy-benzene reached the maximum at 500 °C. According to the product analysis, at 400 °C, the pyrolysis reaction of guaiacol may only be carried out by simple functional group replacement, including hydrogen radical and methyl substitution. And as the temperature increases, demethoxylation becomes easier. When the temperature reaches 600 °C, the reduction in liquid phase product may be due to an increase in the product of the gas phase. A discussion of this mechanism will be mentioned later.
2.2. In Situ Observation of Pyrolysis
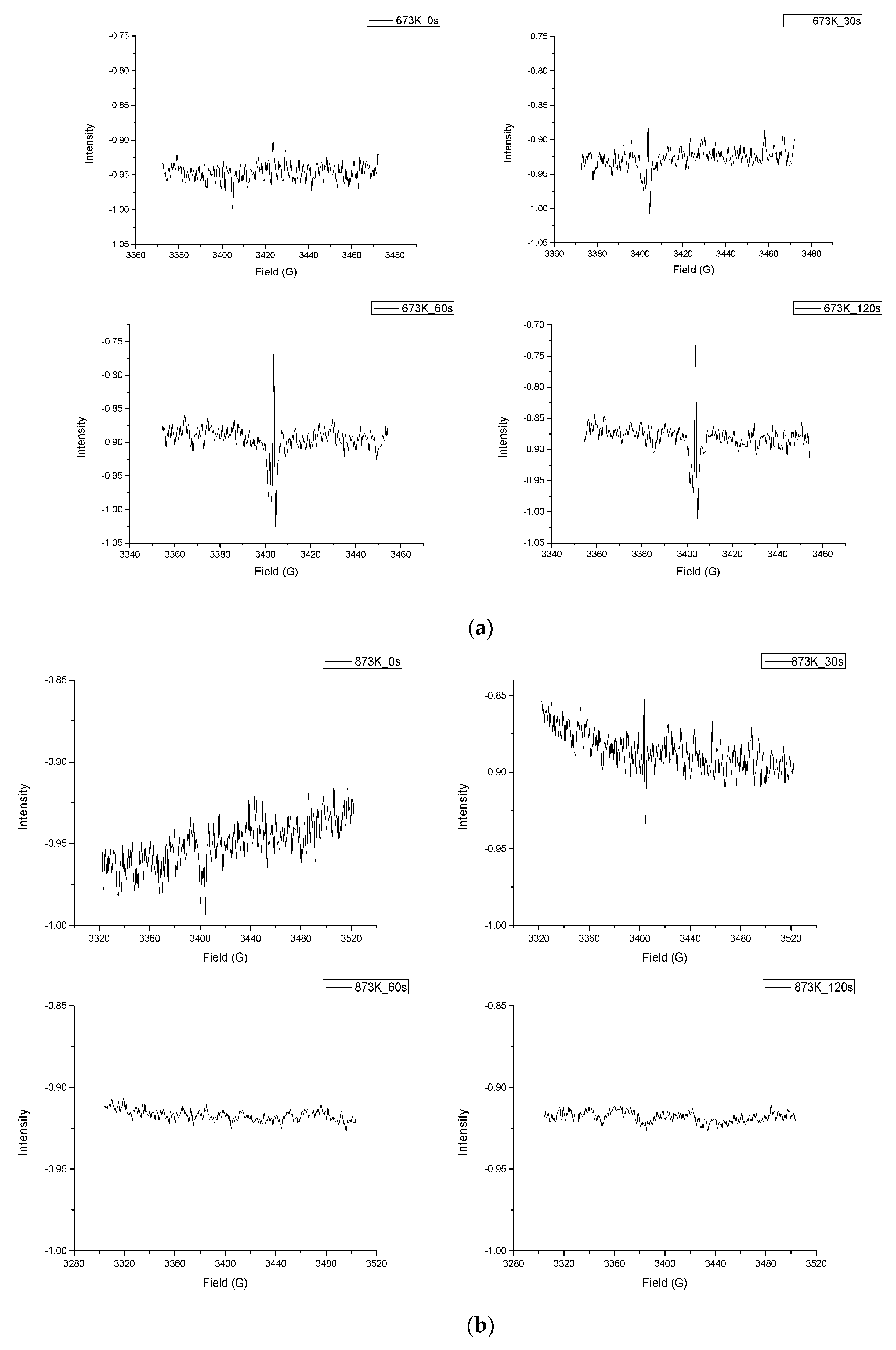
To further investigate the effect of temperature on free radical formation, we performed isothermal pyrolysis of guaiacol at 400 °C and 600 °C. And the pyrolysis process and radical formation were observed by in situ EPR experiments. We observed every 30 s after the pyrolysis reaction reached the set temperature. The result of in situ EPR experiments can be seen in
Figure 3.
As shown in
Figure 3a, a signal of free radicals was detected under the conditions of center field 3400 G and sweep width 100 G. After reaching the pyrolysis temperature 673 K for 30 s, an obvious signal peak appeared in the electron paramagnetic resonance spectrometer, indicating that free radicals were generated during pyrolysis with the g-factor = 2.00018. We reselected 3420 G as the center field for observation. In the subsequent testing process, the free radicals were still stable. Similarly, in
Figure 3b, a significant signal peak appeared after the reaction reached the preset temperature 873 K for 30 s, as seen in the first two figures of
Figure 3b, while the center field was 3450 G and the sweep width was 100 G. The g-factor of this free radicals signal also equaled 2.00018. But the difference is that in the subsequent tests, the signal peak disappeared. The above results indicated that free radicals were produced within 30 s of the pyrolysis of guaiacol.
2.3. The Mechanism of Pyrolysis
In order to study the reaction mechanism of free radicals in the process of pyrolysis of guaiacol in more detail, we used nitrone-based 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) as a spin trap, which reacted with free radicals generated during the guaiacol pyrolysis reaction. Then, the adduct formed by the reaction was detected by EPR. The pyrolysis experiment was performed in a tube furnace with the reaction temperature of 873 K and the EPR detection was performed at room temperature.
As seen in
Figure 4, the electron paramagnetic resonance spectrum is extremely complicated because it characterizes all the free radicals detected, so many characteristic peaks of free radicals may overlap, which makes our spectrum difficult. Under the detection conditions of the center field of 3363 G and the sweep width of 900 G, we obtained the signal. The g-factor of the free radical signal shown in
Figure 4a equals 2.00036. We tried to de-split the signal and separate the characteristic peaks. In the EPR spectra (
Figure 4) obtained by spin trapping, the characteristic lines of methyl radicals can be clearly fitted, which indicates that a large amount of methyl radicals are generated during pyrolysis. Free radicals are always generated in pairs. Correspondingly, it can be seen that a large amount of free radicals 1(C
6H
4(OH)O*) (
Figure 5) are produced at the same time when a large amount of methyl radicals are produced by homogenization. Therefore, free radicals 1(C
6H
4(OH)O*) and methyl radicals play important roles in the pyrolysis reaction of guaiacol. Secondly, methyl radicals that are not quenched by free radicals are also likely to act as initiators for other radical reactions, inducing changes in other chemical structures.
3. Discussion
3.1. In Situ Observation of Pyrolysis
The small molecule radicals generated during pyrolysis are generally highly active, so they easily combine with each other in the reaction process to exhibit poor stability. The stable and distinct signal peak after 60 s in
Figure 3a may be derived from a relatively stable, paramagnetic material. It is similar to the signal peak of coke, which has been observed in our earlier EPR experiments. The formation of polycyclic aromatic hydrocarbons may be the main cause of coking of the pyrolysis reaction pathway. As shown in
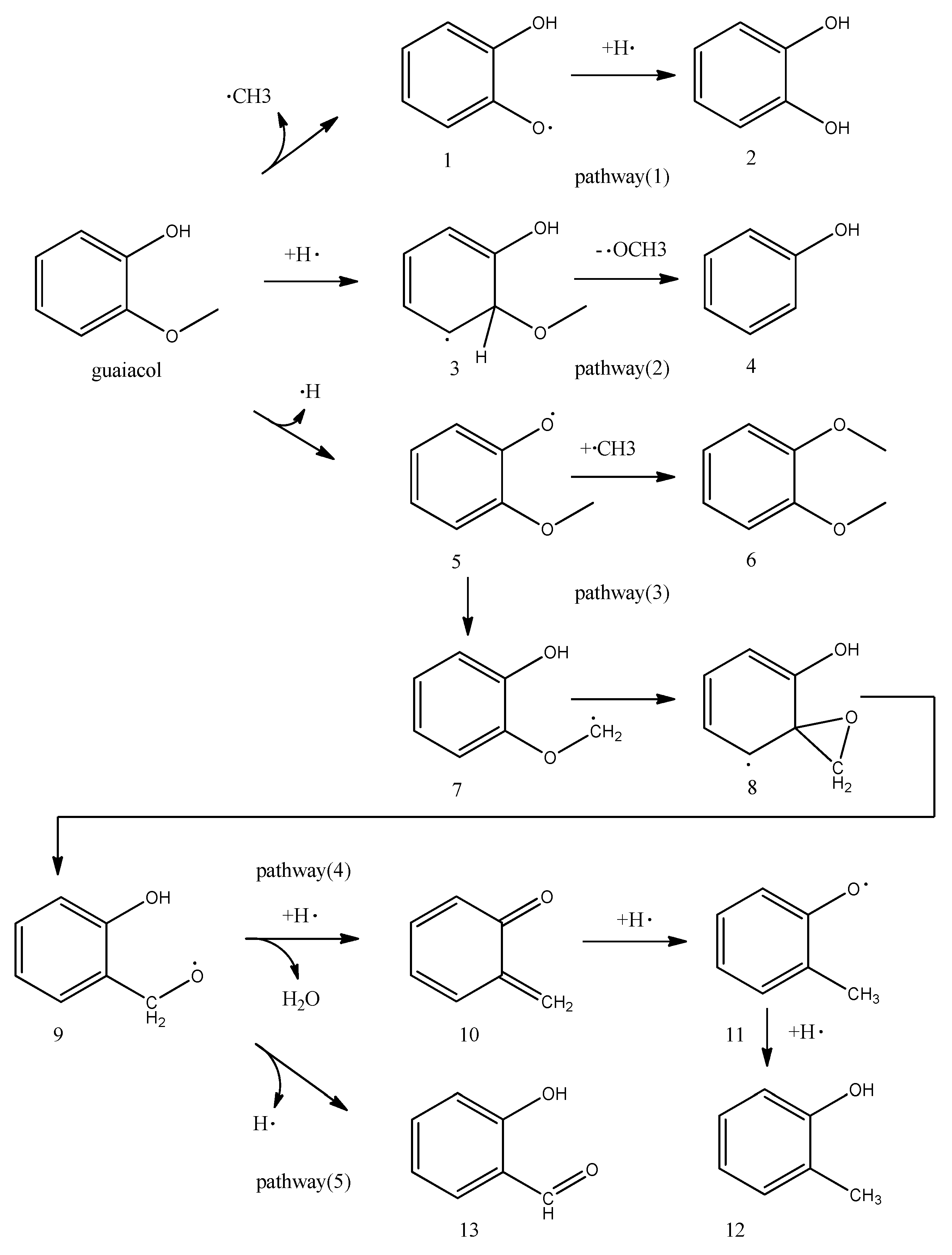
Figure 2b, the main part of the product is 2-methyl-phenol at 400 °C, which means at lower temperatures, the reaction is more likely to proceed in pathway (4) (
Figure 5). A part of the research on guaiacol pyrolysis charring, it has been pointed out that o-quinonemethide (6-methylene-2,4-cyclohexadien-1-one)(compound 10) was the key intermediate. These results showed that o-quinonemethide produced at about 350 °C vanished rapidly at around 420 °C in the vapor phase, indicating char formation [
23]. The experimental data in this paper agree well with these results. The process of generating 2-methyl-phenol may be accompanied by the generation of o-quinonemethide. We speculate that this signal peak may be derived from the coking intermediates produced in the guaiacol pyrolysis process or the coke in the product. It was generated around 400 °C and remained stable near this temperature range and vanished rapidly at higher temperatures. At higher temperatures, we can see from
Figure 2b that the proportion of 1,2-dimethoxy-benzene and phenol in the pyrolysis product of guaiacol is larger, which indicates that the priority of reaction pathway (2) and pathway (3) is higher. At 600 °C, the 2-hydroxy-benzaldehyde generated from pathway (5) may also undergo a decarbonylation reaction to remove CO under the action of high temperature, thereby generating phenol. Different from the products of 2-methyl-phenol at 400 °C, which is the main part, the distribution of products at 500 and 600 °C shows that the reaction of pathway (5) occurs with more difficulty at higher temperatures, so the formation of o-quinonemethide is reduced and the coke is also reduced. The above results may indicate that the signal shown in
Figure 3 may be the signal peak of the o-quinonemethide, but there is still a lot of research that has to be done to prove that.
3.2. The Mechanism of Pyrolysis.
In reaction pathway (1) (
Figure 5), methyl radicals and free radicals 1 are produced by homogenization, and then methyl, as an inducer, participates in other reactions or directly converts to methane. Subsequently, the free radicals 1 combine with the hydrogen radical generated in the reaction to form 1,2-dihydroxybenzene.
In reaction pathway (2) (
Figure 5), guaiacol and hydrogen radicals in the reaction form intermediates through the transition state. Then phenol is formed by demethoxylation, which has been proven to be the lowest reaction energy barrier path by many density functional theory studies.
In theory, the Gibbs free energy of generating (2) is the lowest, but (2) is not detected in the results of Py-GC/MS, and is replaced by 6. The existence of the reaction pathway (3) (
Figure 5) indicates that the hydrogen radical has a higher priority in reacting with other radicals than the reaction pathway (1) in the case where the external hydrogen source is insufficient. Reaction pathway (1) and reaction pathway (3) are in a competitive relationship.
Combining the results of Py-GC/MS with
Figure 5, it can be seen that pathway (3) and pathways (4) and (5) (
Figure 5) are competitive reactions. At lower temperatures, the reaction of pathway (4) is more likely to occur. Guaiacol undergoes an isomerization reaction after losing hydrogen radicals to form intermediates 9. Key compounds 10 (o-quinonemethide) are formed after hydrogenation and dehydration. When the temperature rises to 500 °C, the reaction of pathway (5) starts. Intermediate 9 loses hydrogen radicals to form 2-hydroxy-benzaldehyde and then undergoes a decarbonylation reaction at a higher temperature to be converted into phenol.
In the pyrolysis process of guaiacol, there are reactions in which radicals 1, radicals 5, methyl radicals, and hydrogen radicals are continuously aligned and recombined. Therefore, methyl radicals play a key role in the pyrolysis process of guaiacol.
4. Materials and Methods
Materials. The chemical materials including guaiacol, high-puritty nitrogen, and hydrogen used for this study were all commercially available and were used without further treatment.
Pyrolysis and in-situ pyrolysis. Two pyrolysis experiments were used: (1) continuous pyrolysis of the same guaiacol sample in a tube furnace at different temperatures; and (2) in situ pyrolysis, by using an EPR high-temperature cavity, guaiacol was pyrolyzed at different temperatures.
Py-GC/MS experiments. Additional pyrolysis and analytical experiments were performed on a micro pyrolyzer (PY-3030D, FRONTIER LABORATORIES LTD, JAP) connected with gas chromatography mass spectrometers (ThermalFisher, TRACE2000-DSQII, USA). Pyrolysis experiments were carried out at 400, 500, and 600 °C with the heating rate of 1000 K/s. To ensure a complete reaction, the residence time of the reactor at the set temperature was set to 30 s.
EPR experiments. A Bruker EMX spectrometer was used to record X-band continuous wave EPR at both room temperature and high temperature in pyrolysis of guaiacol. The typical instrument parameters were as follows: center field 3200–3400 G, sweep width 100–1000 G, sweep time 15–30 s, time constant 10 ms, MW power 5 mW, modulation 100 kHz, and modulation amplitude 1 G. The spectrum obtained by the detection was simulated by the simulation software supporting the EPR instrument. In order to study the reaction mechanism of free radicals in the process of pyrolysis of guaiacol in more detail, we employed the EPR spin trapping methodology using 5,5-dimethyl-1-pyrroline-N-oxide (DMPO), which is a nitrone-based trap.
5. Conclusions
The pyrolysis reaction of guaiacol is a free radical reaction, and the radical which can be detected mainly by EPR is a methyl radical, and the corresponding free radical 1. The intermediate of guaiacol pyrolysis coking is formed at about 400 °C and decomposes rapidly at higher temperatures. The addition of hydrogen radicals to other reaction pathways takes precedence over the reaction pathway for the production of catechol. In the pyrolysis process of guaiacol, there are reactions in which radicals 1, radicals 5, methyl radicals, and hydrogen radicals are continuously aligned and recombined.
Author Contributions
Conceptualization, G.L. and Z.L.; methodology, G.L.; software, G.L.; validation, G.L., W.W. and J.C.; formal analysis, G.L.; investigation, G.L.; resources, Z.L.; data curation, G.L. and W.W.; writing—original draft preparation, G.L.; writing—review and editing, G.L.; visualization, G.L.; supervision, J.C.; project administration, Z.L.; funding acquisition, Z.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research and the APC were funded by the National Key Technologies R&D Program of China [No.2018YFB1501405].
Acknowledgments
This work was supported by the National Key Technologies R&D Program of China [No.2018YFB1501405].
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gallezot, P. Conversion of biomass to selected chemical products. Chem. Soc. Rev. 2012, 41, 1538–1558. [Google Scholar] [CrossRef]
- Bridgwater, A.V. Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenerg. 2012, 38, 68–94. [Google Scholar] [CrossRef]
- Bulushev, D.A.; Ross, J.R.H. Catalysis for conversion of biomass to fuels via pyrolysis and gasification: A review. Catal. Today 2011, 171, 1–13. [Google Scholar] [CrossRef]
- Naik, S.N.; Goud, V.V.; Rout, P.K.; Dalai, A.K. Production of first and second generation biofuels: A comprehensive review. Renew. Sust. Energ. Rev. 2010, 14, 578–597. [Google Scholar] [CrossRef]
- Czernik, S.; Bridgwater, A.V. Overview of applications of biomass fast pyrolysis oil. Energy Fuels 2004, 18, 590–598. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U.; Steele, P.H. Pyrolysis of wood/biomass for bio-oil: A critical review. Energy Fuels 2006, 20, 848–889. [Google Scholar] [CrossRef]
- Zakzeski, J.; Bruijnincx, P.C.A.; Jongerius, A.L.; Weckhuysen, B.M. The Catalytic Valorization of Lignin for the Production of Renewable Chemicals. Chem. Rev. 2010, 110, 3552–3599. [Google Scholar] [CrossRef] [PubMed]
- French, R.; Czernik, S. Catalytic pyrolysis of biomass for biofuels production. Fuel Process. Technol. 2010, 91, 25–32. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Y.; Huang, X. Study of guaiacol pyrolysis mechanism based on density function theory. Fuel Process. Technol. 2014, 123, 159–165. [Google Scholar] [CrossRef]
- Nguyen, T.S.; Zabeti, M.; Lefferts, L.; Brem, G.; Seshan, K. Catalytic upgrading of biomass pyrolysis vapours using faujasite zeolite catalysts. Biomass Bioenerg. 2013, 48, 100–110. [Google Scholar] [CrossRef]
- Jeon, M.-J.; Jeon, J.-K.; Suh, D.J.; Park, S.H.; Sa, Y.J.; Joo, S.H.; Park, Y.-K. Catalytic pyrolysis of biomass components over mesoporous catalysts using Py-GC/MS. Catal. Today 2013, 204, 170–178. [Google Scholar] [CrossRef]
- Huang, J.; Li, X.; Wu, D.; Tong, H.; Li, W. Theoretical studies on pyrolysis mechanism of guaiacol as lignin model compound. J. Renew. Sustain. Energy 2013, 5, 043112. [Google Scholar] [CrossRef]
- Carlson, T.R.; Cheng, Y.-T.; Jae, J.; Huber, G.W. Production of green aromatics and olefins by catalytic fast pyrolysis of wood sawdust. Energy Environ. Sci. 2011, 4, 145–161. [Google Scholar] [CrossRef]
- Alonso, D.M.; Bond, J.Q.; Dumesic, J.A. Catalytic conversion of biomass to biofuels. Green Chem. 2010, 12, 1493–1513. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, J.; Hou, X.; Shao, J.; Geng, W. Study on CO2 gasification properties and kinetics of biomass chars and anthracite char. Bioresour. Technol. 2015, 177, 66–73. [Google Scholar] [CrossRef]
- Gil, M.V.; Riaza, J.; Álvarez, L.; Pevida, C.; Rubiera, F. Biomass devolatilization at high temperature under N2 and CO2: Char morphology and reactivity. Energy 2015, 91, 655–662. [Google Scholar] [CrossRef]
- Ellis, N.; Masnadi, M.S.; Roberts, D.G.; Kochanek, M.A.; Ilyushechkin, A.Y. Mineral matter interactions during co-pyrolysis of coal and biomass and their impact on intrinsic char co-gasification reactivity. Chem. Eng. J. 2015, 279, 402–408. [Google Scholar] [CrossRef]
- Hemberger, P.; Custodis, V.B.F.; Bodi, A.; Gerber, T.; van Bokhoven, J.A. Understanding the mechanism of catalytic fast pyrolysis by unveiling reactive intermediates in heterogeneous catalysis. Nat. Commun. 2017, 8, 15946. [Google Scholar] [CrossRef]
- Wang, M.; Liu, C.; Xu, X.; Li, Q. Theoretical study of the pyrolysis of vanillin as a model of secondary lignin pyrolysis. Chem. Phys. Lett. 2016, 654, 41–45. [Google Scholar] [CrossRef]
- Bährle, C.; Custodis, V.; Jeschke, G.; van Bokhoven, J.A.; Vogel, F. The influence of zeolites on radical formation during lignin pyrolysis. ChemSusChem 2016, 9, 2397–2403. [Google Scholar] [CrossRef]
- Sankar, M.; Nowicka, E.; Carter, E.; Murphy, D.M.; Knight, D.W.; Bethell, D.; Hutchings, G.J. The benzaldehyde oxidation paradox explained by the interception of peroxy radical by benzyl alcohol. Nat. Commun. 2014, 5, 3332. [Google Scholar] [CrossRef] [PubMed]
- Bährle, C.; Custodis, V.; Jeschke, G.; van Bokhoven, J.A.; Vogel, F. In situ observation of radicals and molecular products during lignin pyrolysis. ChemSusChem 2014, 7, 2022–2029. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Wu, S.; Huang, J.; Zhang, X. Direct evidence from in situ FTIR spectroscopy that o-quinonemethide is a key intermediate during the pyrolysis of guaiacol. Anal. Bioanal. Chem. 2017, 409, 2531–2537. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).