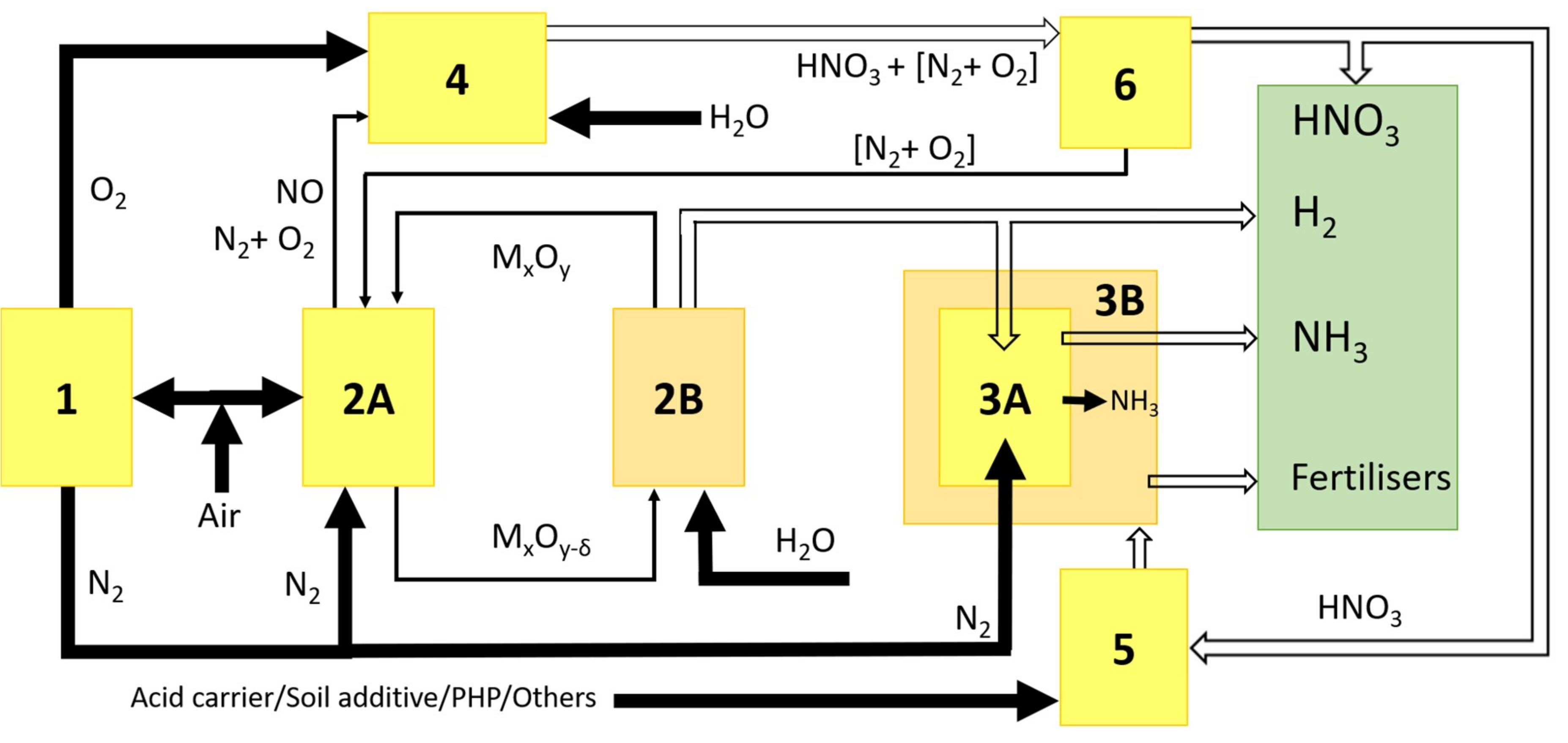
2.1. Critical Microwave Power P*
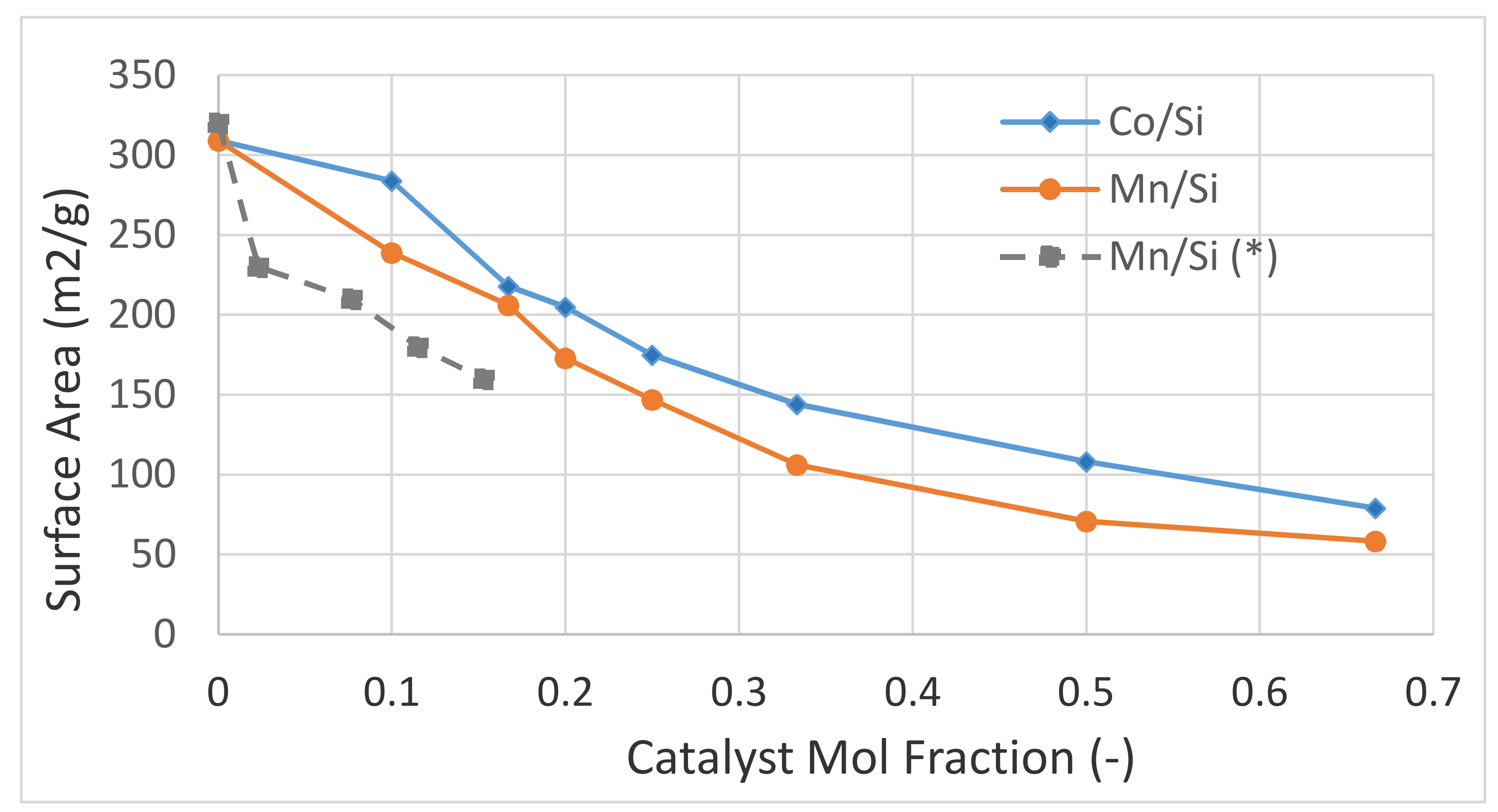
The critical power P* was evaluated when M/Si = 1/3 (i.e., 25 mol% catalyst) for several catalysts or co-catalysts. Because the microwave power could only be changed stepwise, these values are not accurate but clearly demonstrate the existence of a critical power. Here P* refers to the machine power input when it is possible to achieve catalyst nitrate salt decomposition below which decomposition does not take place unless the temperature is increased to ca. 600 °C to achieve thermal decomposition. These results are shown in
Table 1 for selected d-block (transition) metal catalysts and
Table 2 for selected s- and p-block metals (catalyst promoters) as a function of atomic number.
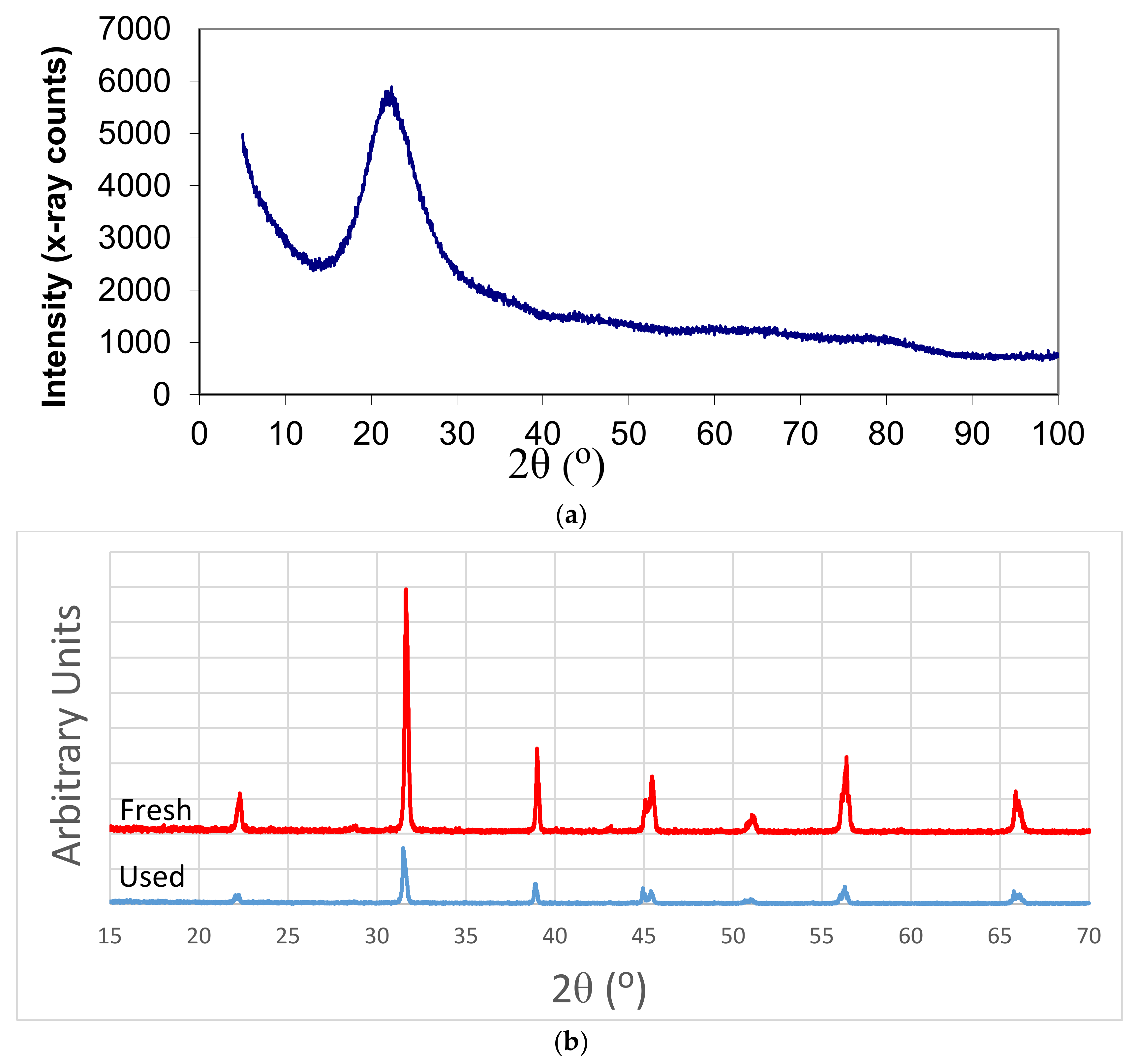
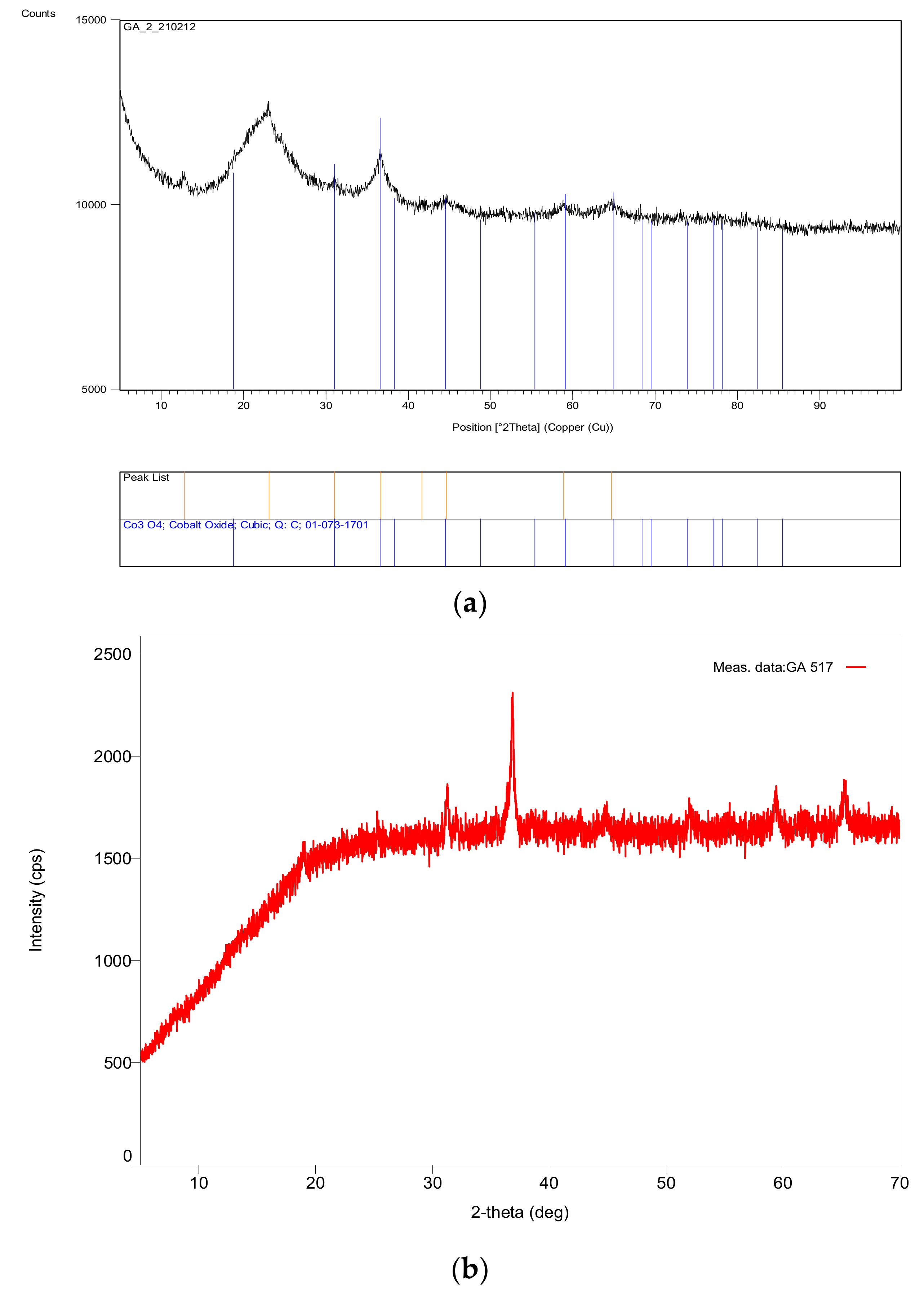
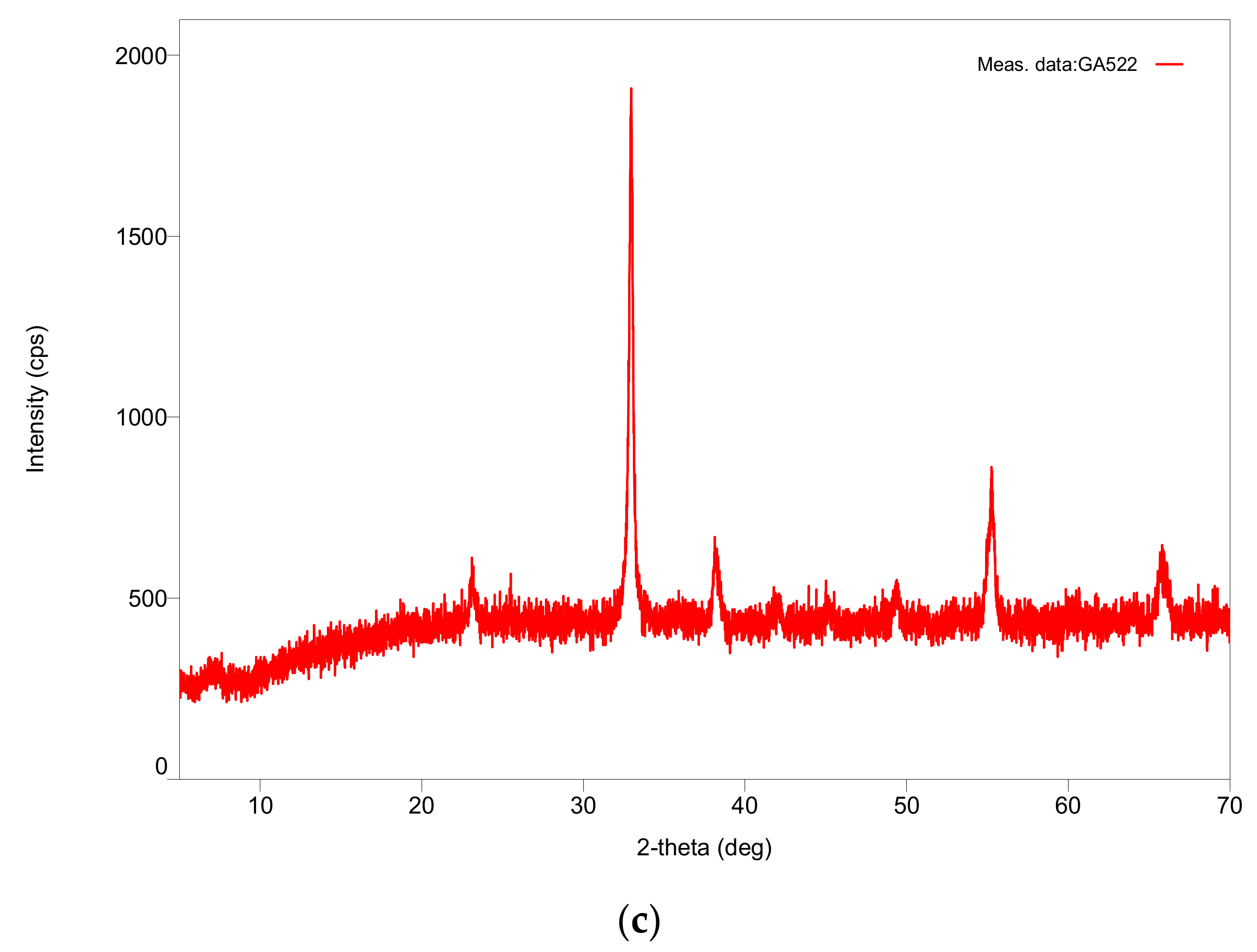
Table 1 shows that, for transition d-metal catalysts, the critical power increases with the atomic number, although the minimum microwave power was 340 W. It was also found that with the exception of Fe, the catalyst precursor fluids were stable even after 4 years of storage. There were no discernable differences in P* values, as well as with respect to the catalyst properties such as crystallite size and surface area, between the catalysts synthesized from the fresh and stored (four years) catalyst precursor fluids. As shown previously [
1], in the case of Fe/Si, the catalyst precursor fluid gradually gels and NO
x starts to evolve due to the decomposition of ferric nitrate, especially when the fluid is exposed to light.
We also present the tap density (which indicates porosity) of the freshly prepared porous expanded solid material obtained after microwave radiation following the evaporation of water and nitrate decomposition. These experiments were carried out at 900 W microwave power input because it was found that the tap density is also dependent on the microwave power. Note that in the case of Cu, at Cu/Si = 1/3, there is no nitrate decomposition but a porous structure is still obtained after water evaporation. It can be seen from
Table 1, the tap density for the transition metal oxide catalysts is approximately constant at ca. 0.065 g/cm
3.
Table 2 indicates that the critical power is relatively low for the catalyst promoters; P* = 450 W for Mg, Ca, Ba, Zn, Bi, La and P* is < 340 W for Sr and Al. Similarly, the tap density is approximately constant at 0.18 g/cc for Mg, Ca, Sr, Ba, Zn, Bi while for Al and La, the tap density is ca. 0.057 g/cc, similar to those of the transition metal catalyst oxides as shown in
Table 1. The concentration dependence of P* is investigated for Mn, Co and Cu. The results are tabulated in
Table 3.
Table 3 indicates that for Mn, P* is less than 340 W at all concentrations studied (16.7–66.7 mol%) whereas for Cu, P* increases with concentration from 1.26 kW at 16.7 mol% to P* > 1.8 kW when catalyst concentration is 25 mol% or above. However, it was found that when a binary catalyst system was used in which one of the metals has a very high critical power, such as Cu, the resulting catalyst precursor has a considerably lower critical power. For example, P* for Cu/Si = 1/3, is above 1800 W and for Co/Si = 1/3, P* = 720 W, while for Zn/Si = 1/3 P* = 900 W. For the binary catalysts, while keeping the total metal-silica ratio constant at 1/3 (i.e., M
T/Si = 1/3) Co/Cu/Si = 1/1/6 and Co/Zn/Si = 1/1/6, P* < 340 W.
Most importantly, it can be seen from
Table 3, that for cobalt, P* reaches maximum at 720 W when Co/Si = 1/3. Unlike Cu, further increase in Co concentration results in decrease in P*. This behavior is associated with plasma generation during microwave irradiation of certain types of catalyst oxides (spinels) when the total catalyst concentration (M
T) and silica molar ratio, M
T/Si, is above a critical level.
2.10. Barium Titanate SEM and EDS
Structural changes in BaTiO
3 as a result of prolonged plasma irradiation has been investigated [
68] when Dielectric Barrier Discharge (DBD) plasma was generated through the application of high voltage across two electrodes using the equipment described in [
14,
16,
68]. It was shown that although [O]/([Ba] + [Ti]) ratio was initially 1.75 (theoretical value is 1.5 indicating the presence of excess TiO
2 as concluded by the fact that [Ti]/[Ba] was also high at 1.07), this ratio was reduced to 1.13 after 80 h on stream during direct CH
4 conversion to H
2 and higher hydrocarbons. The reduction in lattice oxygen can also be attributed to the oxidation of carbon deposits and CH
4.
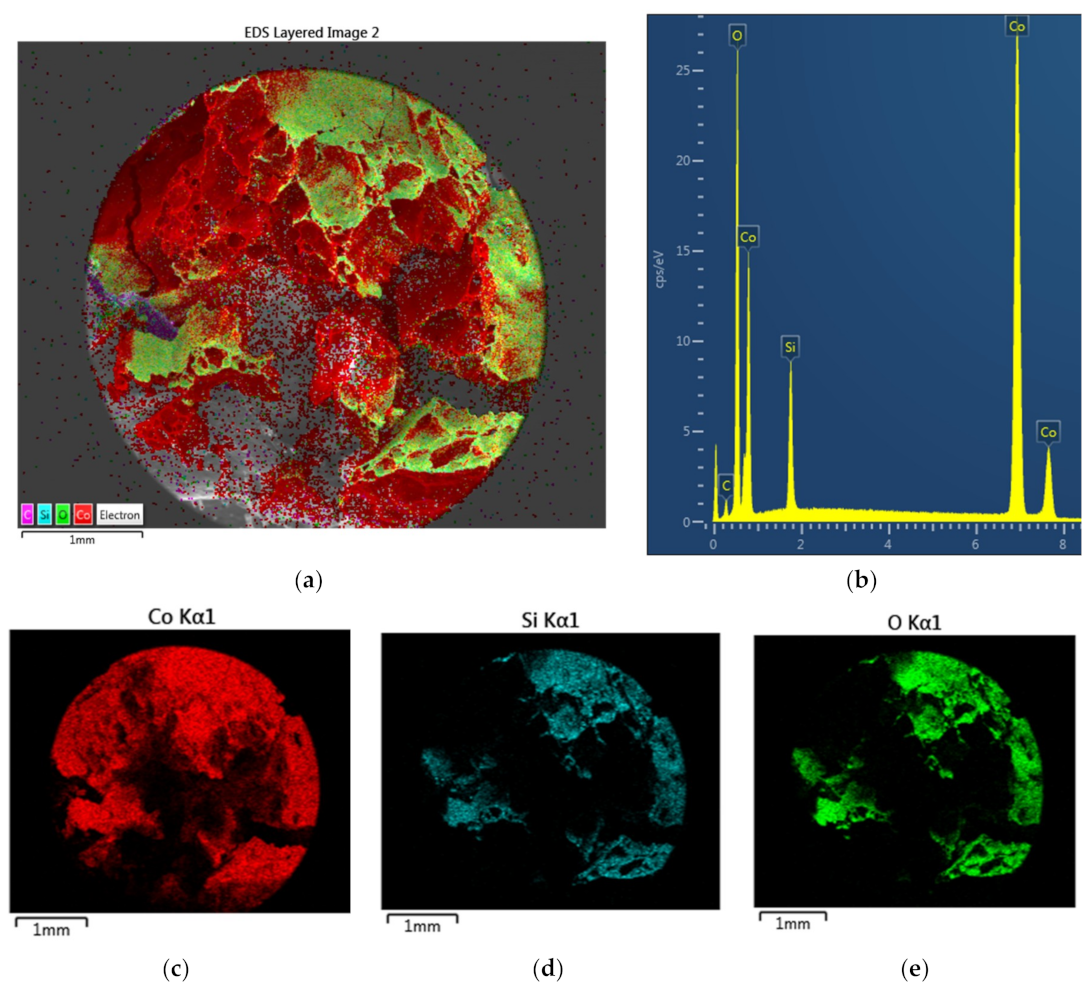
Figure 6 illustrates the Energy Dispersive X-ray Spectroscopy (EDS) analysis of a fresh BaTiO
3 particle fracture surface with a large amount of carbon due to coating and impurities. The atomic fractions are; [O] = 0.611; [Ti] = 0.196 and [Ba] = 0.193 with [Ti]/[Ba] = 1.02; and [O]/([Ba] + [Ti]) = 1.57 very close to the theoretical values. The corresponding EDS layered image and EDS of the surface are shown in
Figure 6a,b respectively.
Figure 7 illustrates the fine structure of a plasma exposed BaTiO
3 particle at two magnifications (1k and 100k) as a reference. Plasma exposure of BaTiO
3 was carried out at 100 W for 50 h in air stream using the experimental set-up described previously [
14,
16].
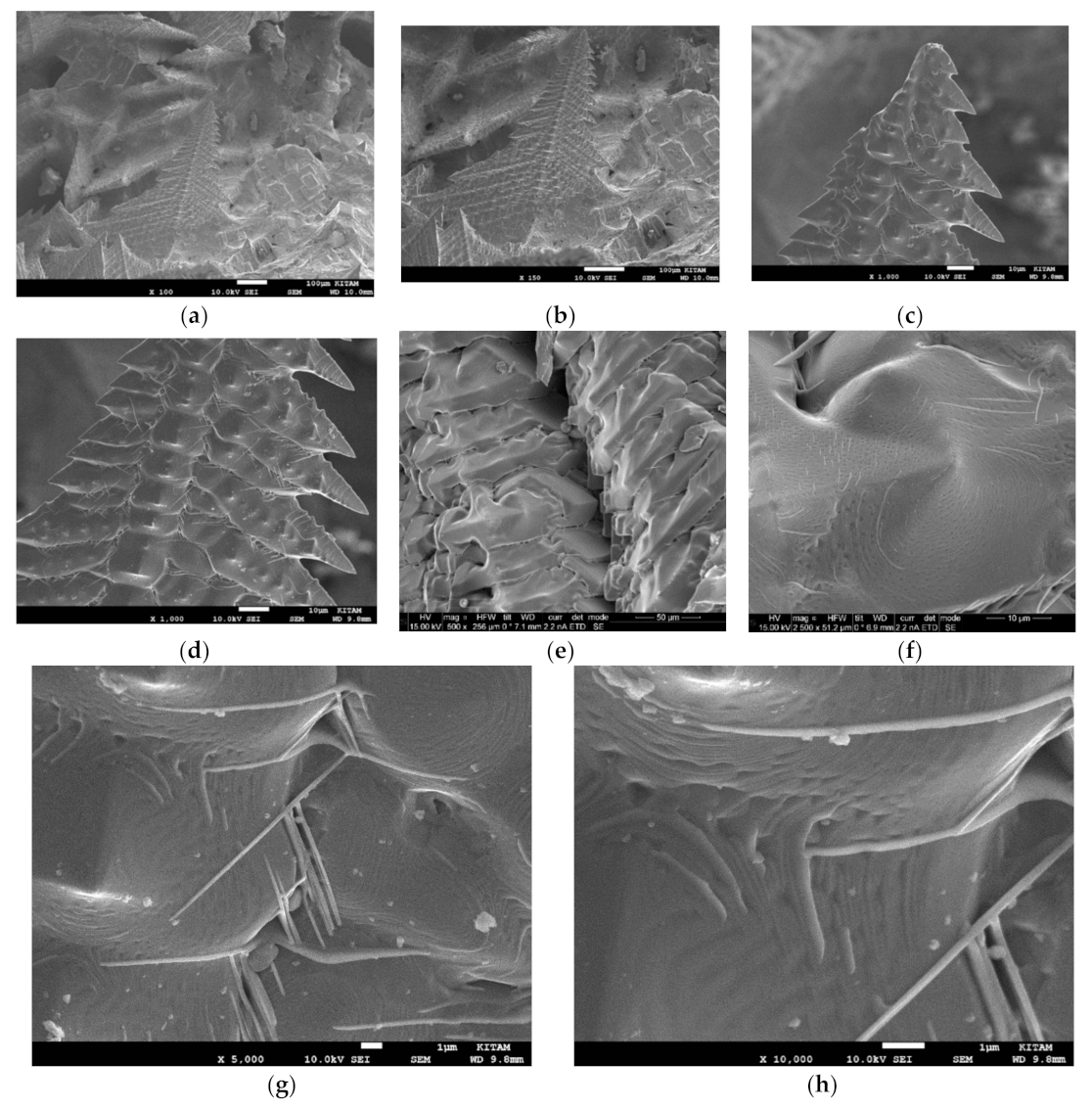
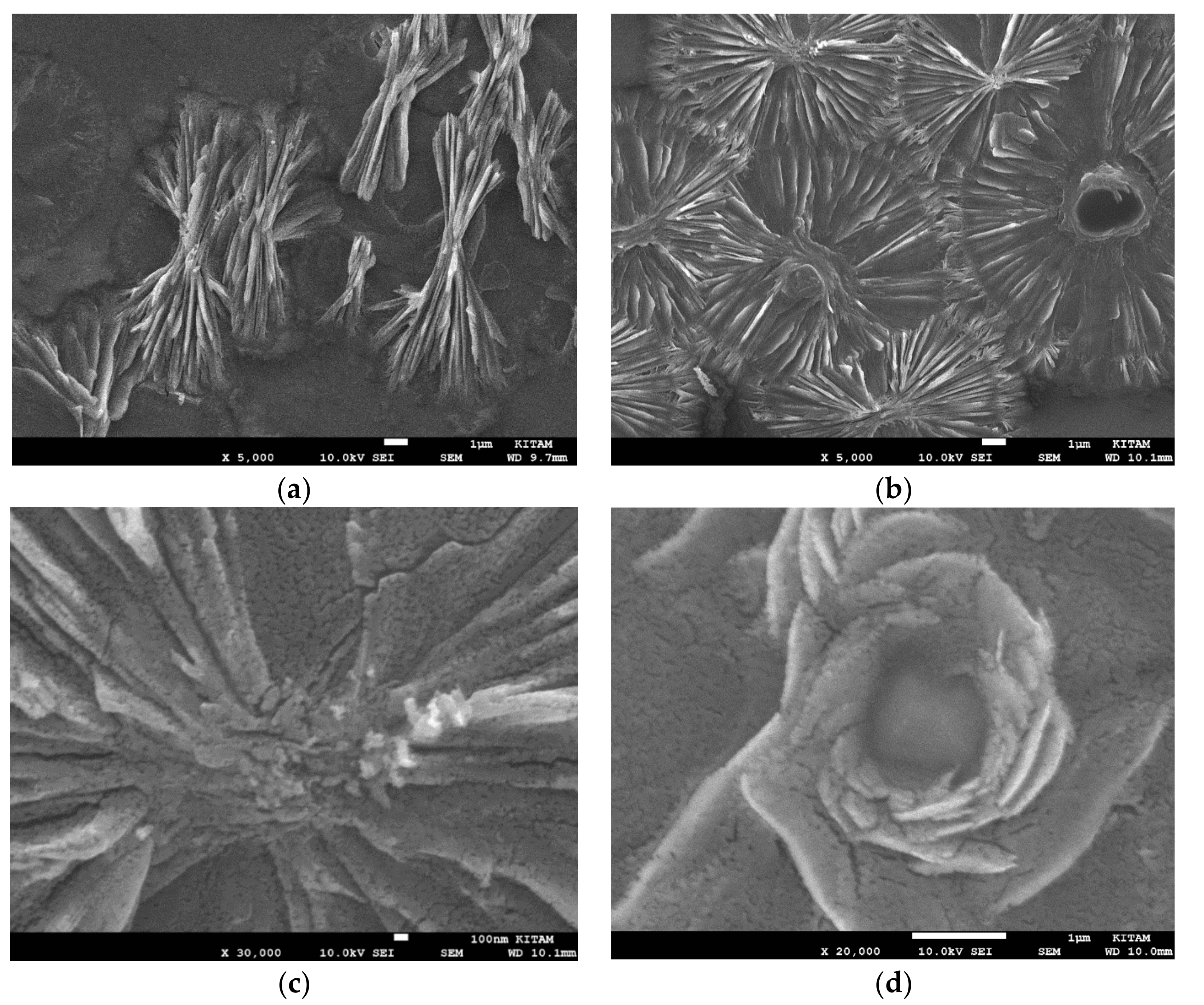
Figure 7a shows the presence of large BaTiO
3 grains (ca. 100 μm) and the development of layered/herringbone structures which are typical of the tetragonal phase [
78,
80]. EDX-spectra of these structures in
Figure 7 do not indicate any chemical modification such as the presence of nitrogen. Their chemical structure at different locations and magnification are very similar with [Ti]/[Ba] = 1.04 and [O]/([Ba] + [Ti]) = 2.12. On the other hand, when BaTiO
3 particles are subjected to microwave irradiation with plasma generation, in addition to the presence of structures similar to those shown in
Figure 7, additional crystal structures are observed with highly significant spatial variations reflected in changes in their chemical composition, including the presence of nitrogen.
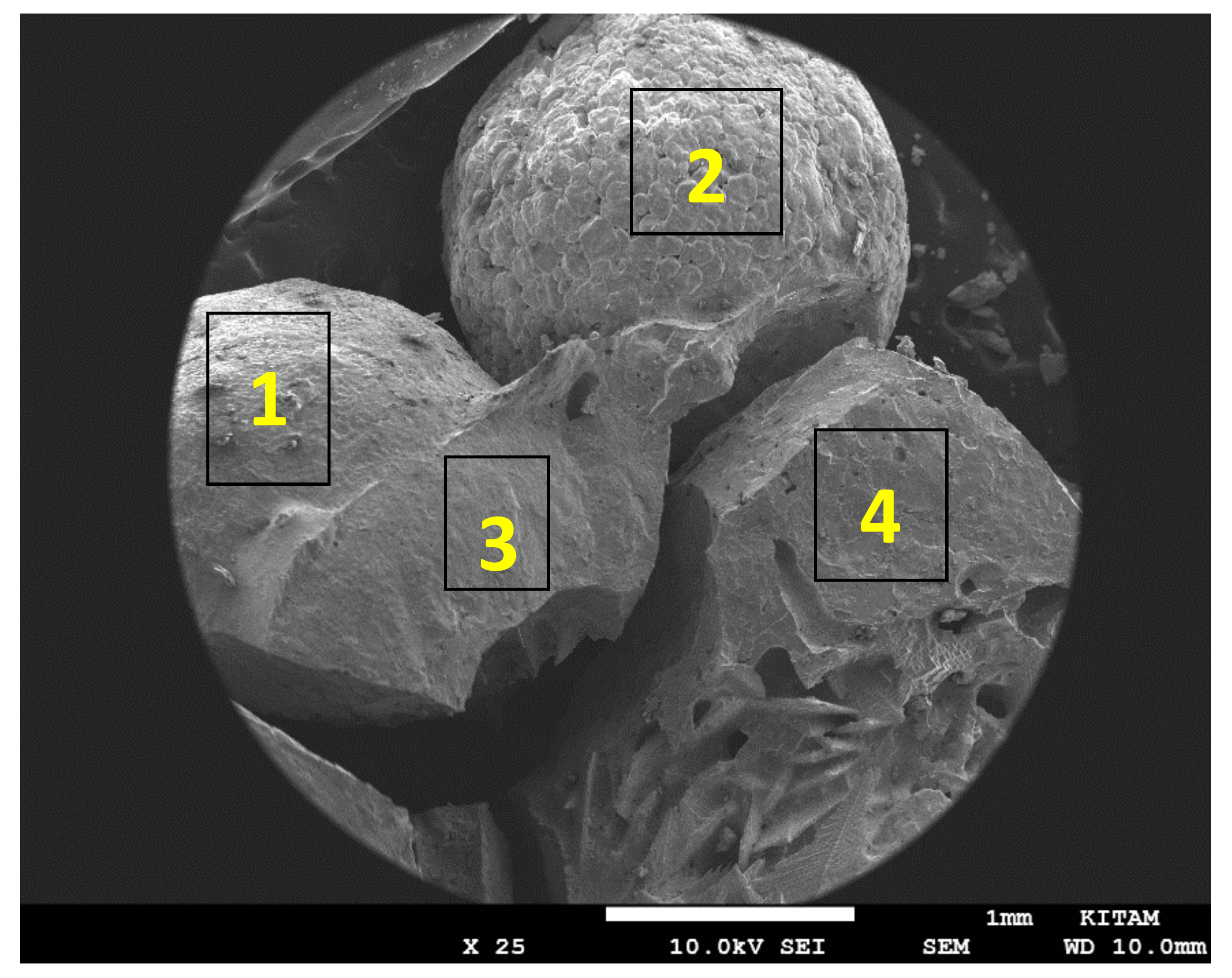
Figure 8 is a SEM image of a collection of 4 BaTiO
3 particles (Particles 1–4) with diameter of 3mm, following microwave irradiation in air at 1800 W for 10 min with plasma generation. These particles represent all the features seen in individual particles examined. They show the intact particle surface, fractured particle surface, sintering and the effect of microwave induced plasma generation in BaTiO
3 through void formation and electrical treeing (Lichtenberg phenomenon) [
81,
82] as a result of dielectric breakdown.
The effects of microwave irradiation with plasma generation are described in
Figure 9,
Figure 10,
Figure 11 and
Figure 12.
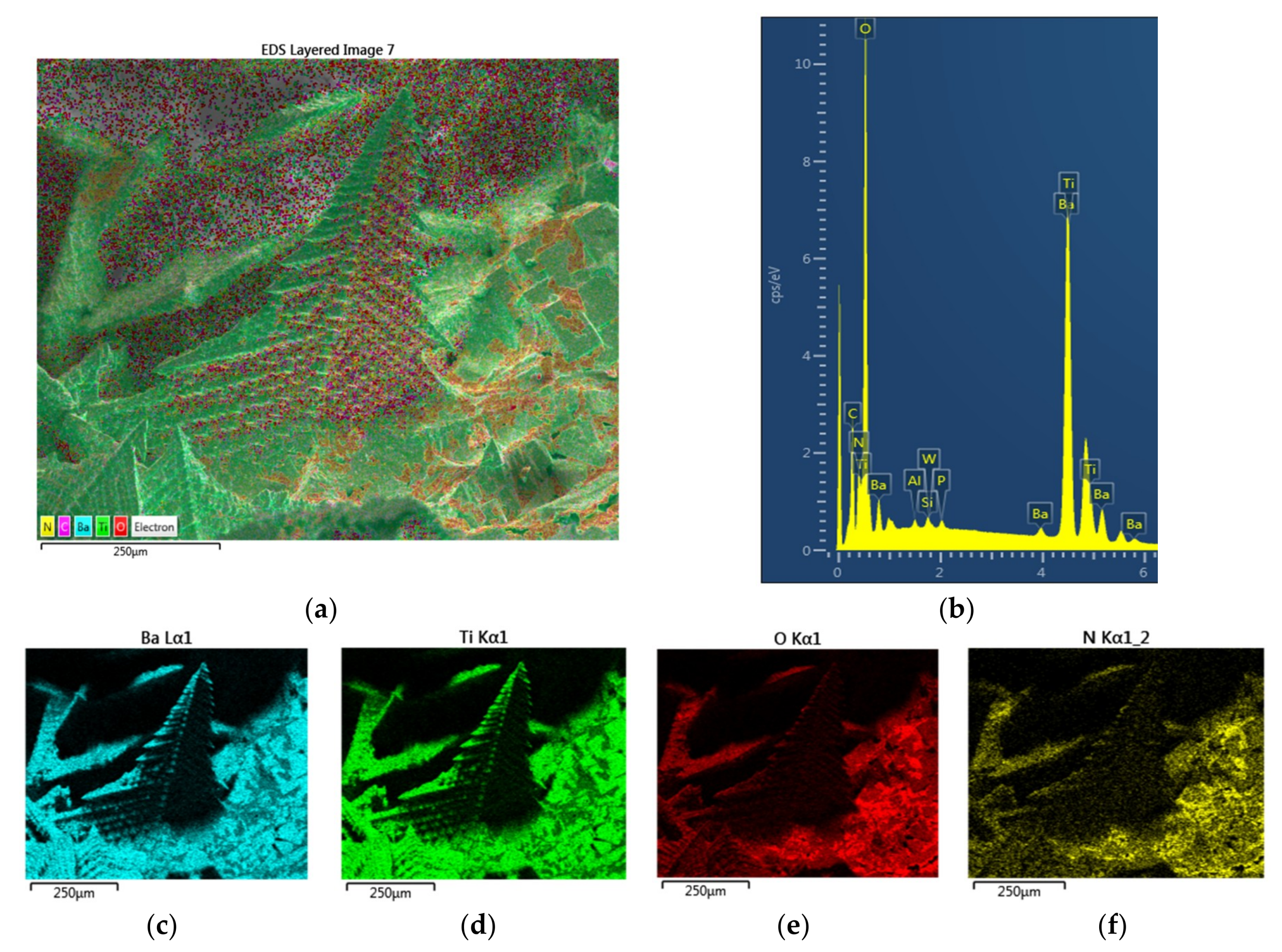
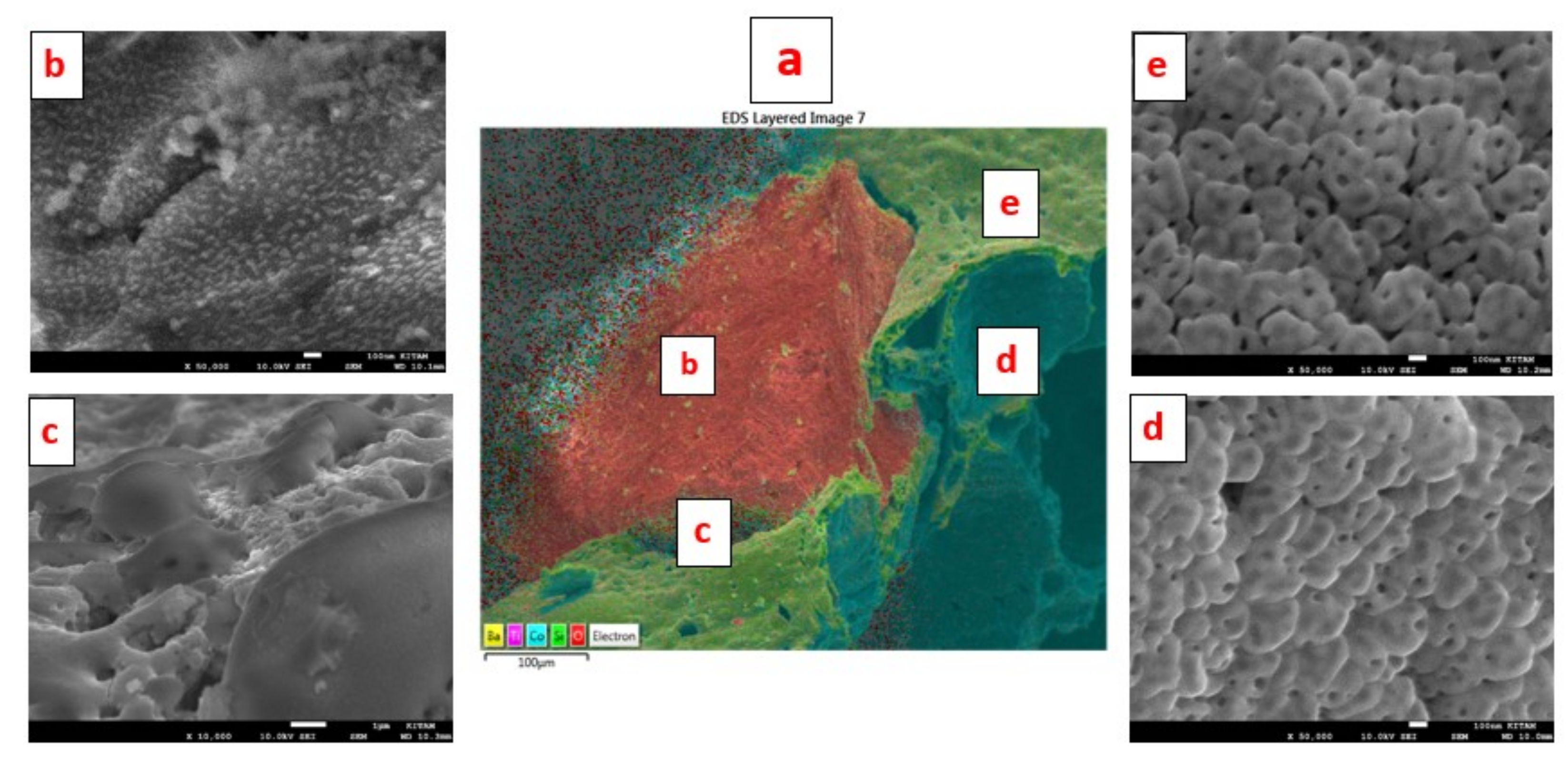
Figure 9a is the EDS layered image of Particle-1, showing the distribution of the elements of BaTiO
3. It is clear from this image that there are regions where oxygen appears to be in excess compared with the theoretical composition. The presence of excess oxygen is confirmed through the EDX-spectra of the surface, such as shown in
Figure 9b as well as the elemental mapping of Ba, Ti and O. EDX-spectrum of the surface yields: [Ba] = 0.123; [Ti] = 0.094; [O] = 0.682; [N] = 0.101 with [Ba]/[Ti] = 1.31 and [O]/([Ba] + [Ti]) = 3.14. These results indicate that the particle surface has Ba-O termination accommodating O-rich BaO
3 composition and a negatively charged surface. Here each Ba ion is coordinated by six O ions. It is thus possible to have bonding between N and BaO
3 surface structure which accounts for the presence of nitrogen. The distribution of oxygen and nitrogen on this sample, as well as other samples are further investigated below (
Figure 12) and it is shown that N-rich and O-rich regions always overlap. It is known that graphene can be doped with nitrogen through plasma treatment to modify its electronic properties [
83]. It is therefore not surprising to have nitrogen incorporation into the BaTiO
3 lattice through microwave induced plasma.
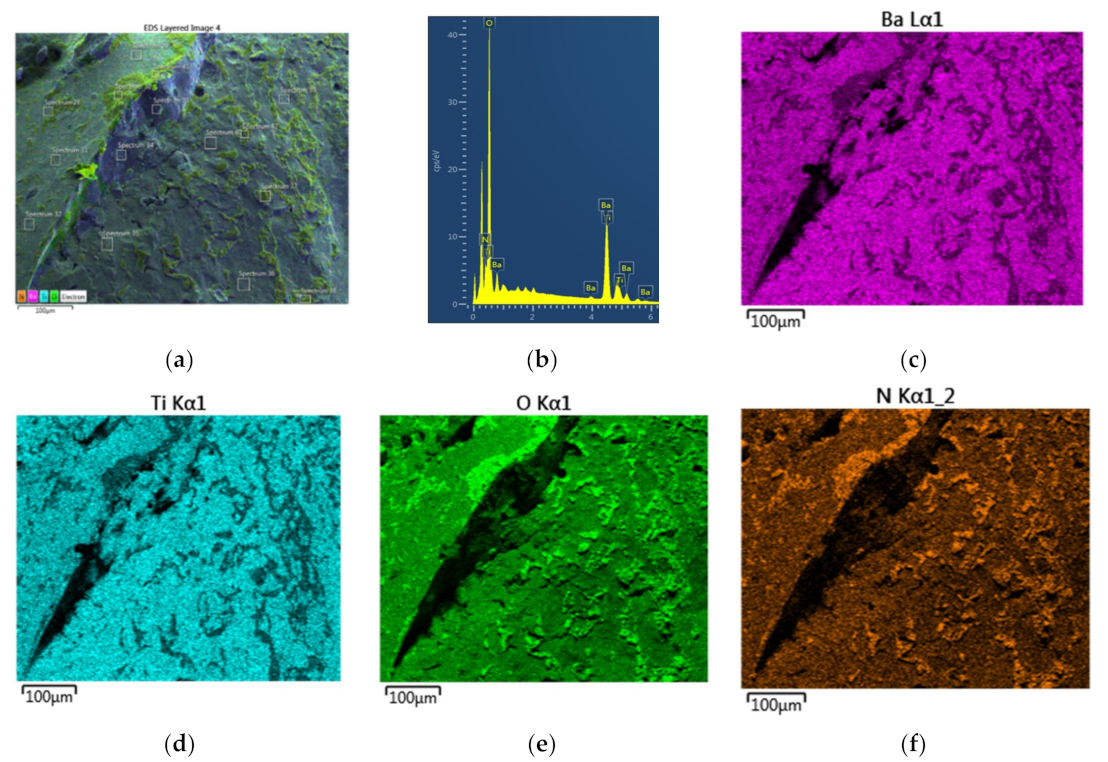
Figure 10 shows that there are only spots of O-rich regions on the BaTiO
3 surface (Particle-2) which is also reflected on the EDX-spectrum of the surface as shown in
Figure 10b. This spectrum yields the following molar fractions: [O] = 0.676, [Ba] = 0.165, [Ti] = 0.159 leading to Ba/Ti = 1.03 which is very close to its theoretical value and [O]/([Ba] + [Ti]) = 2.08 which is over 50% smaller than the previous case for Particle-1 (
Figure 9b). These results also indicate that the BaTiO
3 surface is O-rich due to Ba-O termination. As seen in
Figure 10c–e, the BaTiO
3 grains are not fused due to low level electrical activity on its surface.
Figure 11 and
Figure 12 show how the inner regions of BaTiO
3 particles are affected by plasma.
Figure 11a is the EDS-layered image of the fracture surface of Particle-4 shown in
Figure 8. It shows an electrical treeing image in which regions of O-rich and N-rich regions co-exist. EDX-spectrum yields the following molar fractions: [O] = 0.660; [N] = 0.096; [Ba] = 0.131; [Ti] = 0.112; [Ba]/[Ti] = 1.17 and [O]/([Ba] + [Ti]) = 2.72. These data indicate that the chemical structure in this region is similar to that shown in
Figure 9. Electrical treeing is further investigated in
Section 2.12.
Figure 12 is the summary of the EDS results for the interface region containing both surface of the Particle-1 and the fractured region of Particle-3. EDS layered image for this particle shown in
Figure 12a indicates the presence of O-rich and N-rich regions which are superimposed and scattered throughout the sample. The summed map of the EDX-spectrum of the same area shown in
Figure 12b reflects the presence of nitrogen which is further illustrated in the mapping of Ba, Ti, O, and N as shown in
Figure 12c–f.
In this particle region, EDX-spectrum yields the following molar fractions: [O] = 0.642; [N] = 0.034; [Ba] = 0.176; [Ti] = 0.148; [Ba]/[Ti] = 1.19; [O]/([Ba] + [Ti]) = 1.98 which show that the chemical structure in this region is similar to that shown in
Figure 9 and
Figure 11. In confirmation with the mapping of oxygen and nitrogen, mappings shown in
Figure 11e,f and
Figure 12e,f clearly confirms that oxygen and nitrogen co-exist in the same region. As seen in
Figure 12a, several local EDX-spectra are also taken in order to examine more closely chemical compositions, in particular the presence and location of nitrogen.
As seen in
Figure 12a, the distribution of the elements of BaTiO
3 and nitrogen on the surface or fracture surface of the particle is not homogeneous. We therefore investigate the locations where there appears to be: (a) No nitrogen; (b) Low concentration of nitrogen and (c) High level of nitrogen is present. Typical EDX-spectrum for each of these regions are shown in
Figure 13 which clearly indicates the highly significant differences in compositions on and within BaTiO
3 following the microwave irradiation with plasma generation.
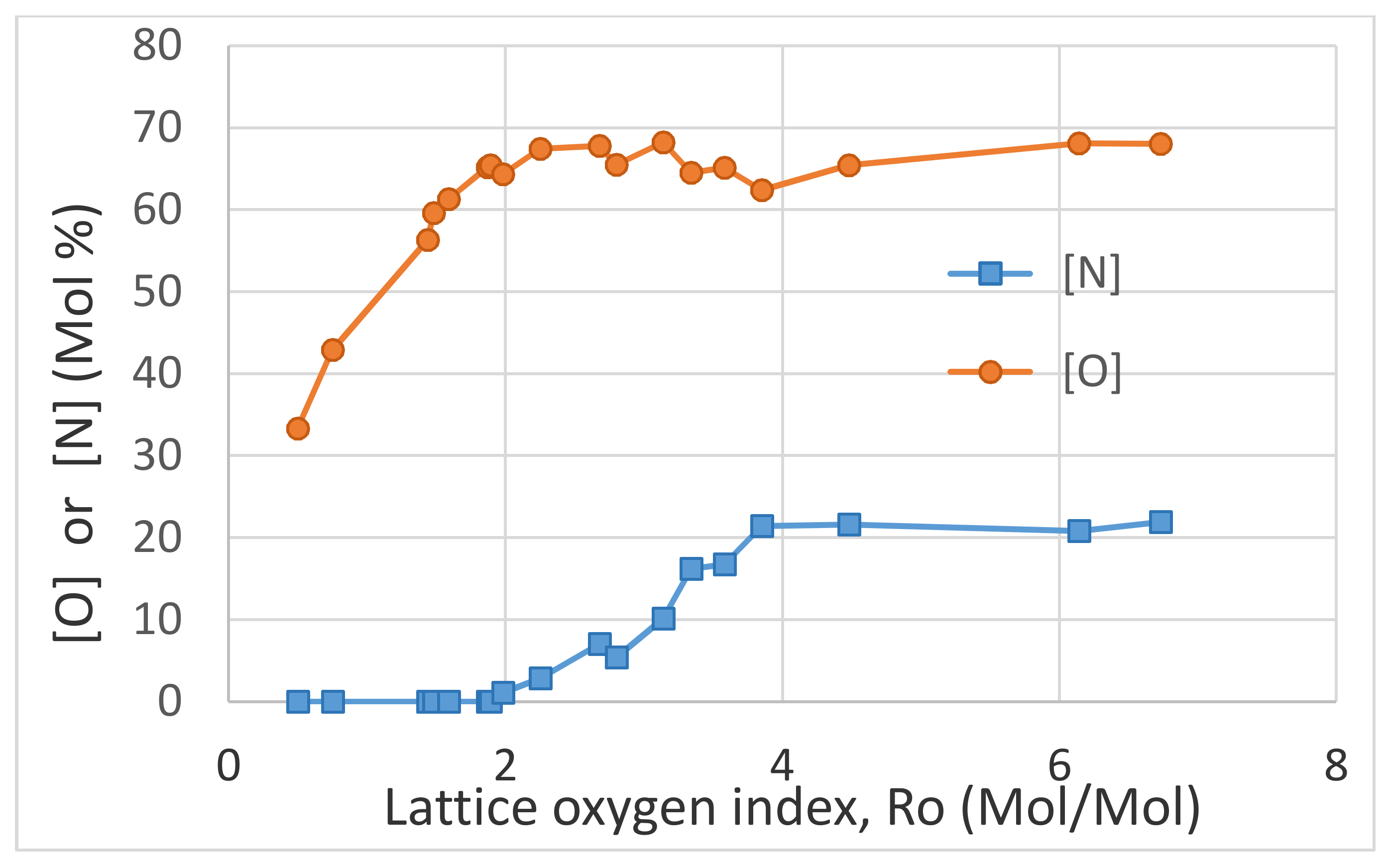
The EDS data is summarized in
Figure 14. The oxygen-rich barium terminated characteristics of BaTiO
3 surfaces is represented as the molar ratio of oxygen and the sum of Ba and Ti by the lattice oxygen index, R
o defined as R
o = [O]/([Ba] + [Ti]). The stoichiometric value of R
o = 1.5. At R
o = 1.5, [O] = 0.6 as expected.
Figure 14 shows that nitrogen is absent when R
o < 2 and its concentration increases rapidly with R
o reaching an asymptotic value of ≈22 mol% when R
o > 4. Oxygen concentration increases with R
o reaching an asymptotic value of ≈68 mol%. As seen in
Figure 14, when R
o < 1.5, [O] < 0.6. These results indicate that microwave radiation induced plasma results in structural and compositional inhomogeneity on the surface and within BaTiO
3. It is likely that both microwave heating and plasma are responsible for the creation of these changes [
68,
84,
85].
Compared with the XRD-studies, EDS studies yield valuable information about the mechanism of nitrogen oxide formation during microwave induced plasma generation in air. It is clear that this process is a surface phenomenon, rather than a bulk reaction involving lattice oxygen and plasma activated nitrogen. In order to investigate any structural changes in BaTiO3, next, we investigate the Scanning Electron Microscopy of the samples for which EDS studies have been carried out in this section.
2.12. Catalyst and Lattice Oxygen Heterogeneity Indices
In the foregoing sections, the generation of plasma during microwave irradiation of the perovskite type catalyst in air and evolution of nitric oxide have been demonstrated using BaTiO3. It is shown that nitric oxide evolution is through the consumption of lattice oxygen which is subsequently replenished by oxygen from air. When the oxygen supply is limited, the lattice oxygen is consumed by the activated nitrogen thus reducing the catalyst oxide. This results in the formation of oxygen-depleted regions interspersed within the catalyst particle which also has regions of high oxygen due to chemical bonding of nitrogen in BaTiO3 resulting in changes in crystal structure. In this section we investigate the effects of microwave irradiation with plasma generation in Co/Si = X catalyst using extensive EDS and SEM studies in order to supplement the XRD investigation carried out in previous sections.
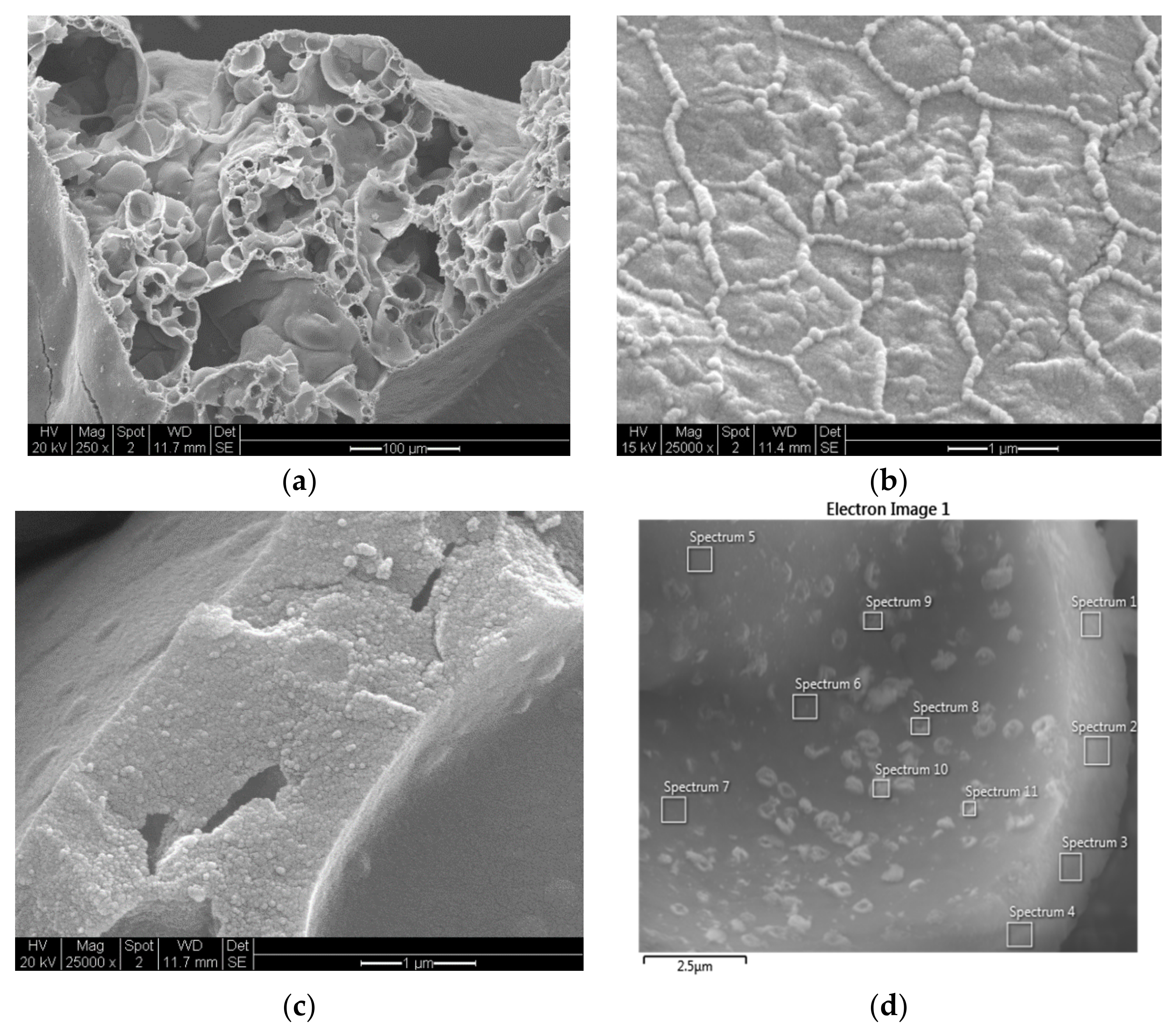
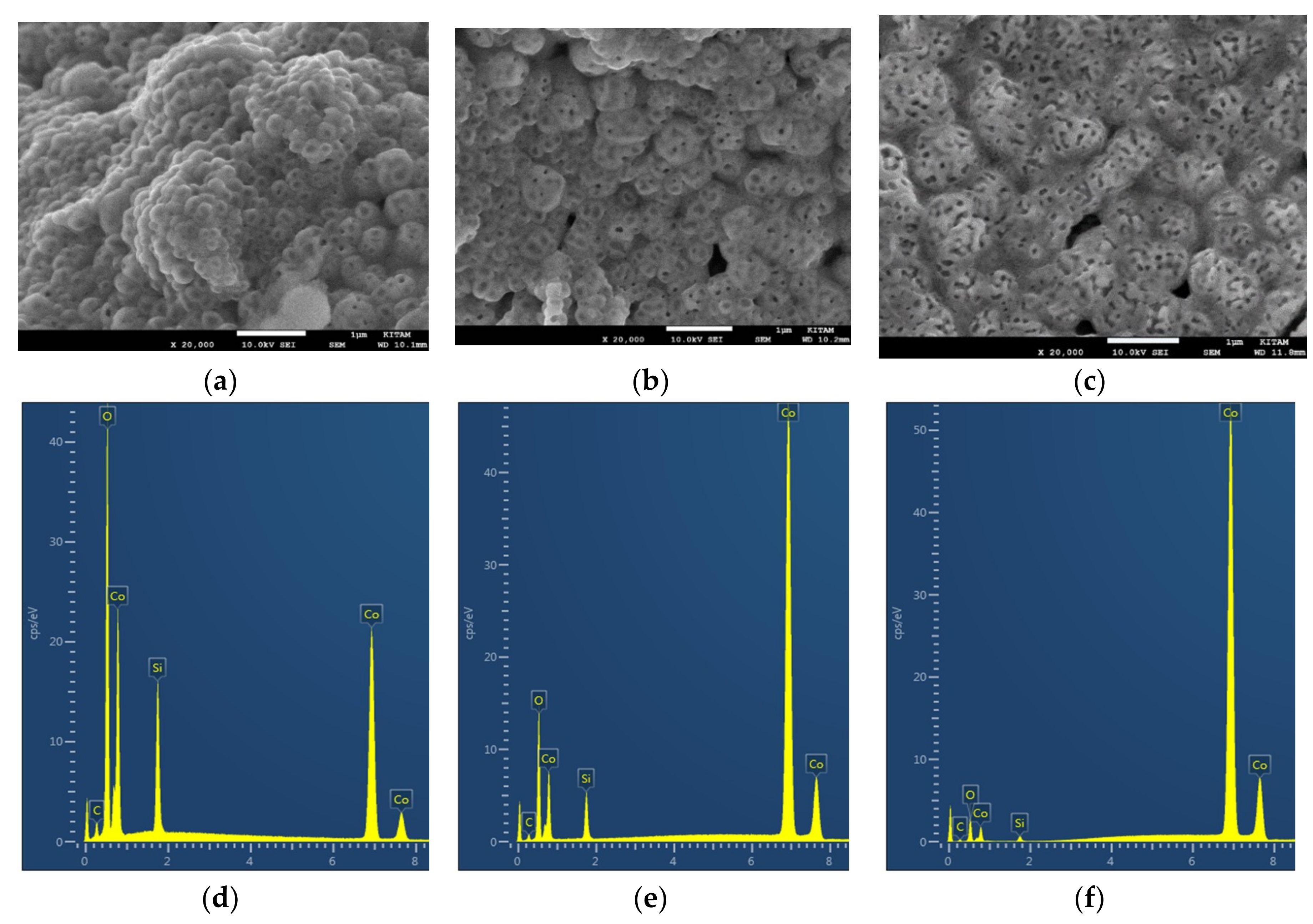
The SEM images in
Figure 17a–c illustrate the structure of the Co/Si = 1/4 catalyst in which the overall porous structure, dominant surface structure and the wall structure are shown. The structure shown in
Figure 17b indicates the presence of Co-rich domains which disappear if the catalyst is subjected to heat treatment at temperatures ≥ 550 °C. Co/Si = X catalyst has been studied previously [
1] when X ≤ 1/4. In this process [
1], the organic coating layer on the catalyst is also burnt off.
In the present study, our aim is to investigate the heterogeneity of the catalyst structure which appears to be induced when X > 1/3. This is achieved through EDS and SEM investigations. The spatial heterogeneity of the catalyst chemical structure is carried out by EDS-spot analysis at several locations. Four of the largest and another four of the smallest catalyst (Co) concentration regions are then chosen and the compositions averaged within each group. The catalyst heterogeneity is essentially represented by the standard deviation of the concentrations across the catalyst domain. In the current study, we consider the variation of the Catalyst-Support Index (A
n) and Catalyst-Oxygen Index (B
n) which are defined as:
where M = Co or Mn in the present study. [M], [Si] and [O] represent the molar concentrations of the catalyst (M), support (Si) and oxygen respectively. Here the subscript n = 0, 1, 2, T represents the mean concentrations in the M/Si = X catalyst where the catalyst concentration [M]
n is at average (n = 0); or the lowest/minimum (n = 1) or the highest/ maximum (n = 2). n = T represents the theoretical/stoichiometric concentrations and concentration ratios based on the feed M(NO
3)
m and SiO
2 in the catalyst support fluid. Hence, for the supported catalyst system M/Si = X, A
T = X and the theoretical catalyst concentration is given by:
The average, minimum and maximum values of the catalyst/support ratio (A
n) and lattice oxygen/catalyst + support ratio (B
n) are denoted by A
0, A
1, A
2, B
0, B
1, B
2 respectively. We define the catalyst heterogeneity (H
A) and oxygen heterogeneity (H
B) as:
The theoretical value of B
T requires the knowledge of the chemical structure of the catalyst oxide after the processing of the catalyst and catalyst support fluid. The XRD studies indicated that the dominant structure is spinel, M
3O
4 (M = Co, Mn) while the support is SiO
2. Hence the theoretical oxygen concentration [O]
T and theoretical oxygen index B
T in Equations (5) and (8) are calculated from:
The theoretical ranges of AT an BT are 0 ≤ AT ≤ ∞; 2 ≥ BT ≥ 4/3 corresponding to 0 ≤ X ≤ ∞.
The mapping of the constituent elements of the catalyst (Co, Si and O) is carried out by EDS at small magnifications (30–100 x) to obtain the average atomic concentrations in a given location. At least two particles were examined. Afterwards, using the elemental mapping in the layered EDS images, several (usually 20) spot analysis were performed both at catalyst (Co or Mn)-rich and catalyst-depleted areas. In four of the regions with the highest (and the lowest) catalyst, concentrations of the catalyst, support and lattice oxygen were averaged to obtain A0, A1, A2, B0, B1, B2 as well as the catalyst and oxygen heterogeneity indices, HA and HB using Equations (7) and (8) were evaluated.
2.13. SEM and EDS of Cobalt/Silica = X Catalyst Processed under Microwave Radiation with Plasma Generation
In the EDS analysis, carbon concentration was also evaluated for each set of data. Although the catalyst samples are not coated with carbon in SEM-EDS investigations, carbon appears in the silica supported catalyst because silica is coated with epoxy silane (γ-Glycidoxypropyltrimethoxysilane), which contains five oxygen and six carbon atoms after the silanation reaction on the silica support surface. Therefore, in addition to the appearance of carbon in the EDX-spectra, oxygen content was also increased. The effect of silane on measured oxygen concentration decreases with increasing catalyst concentration. Although we have not considered the effect of the organic coating on the catalyst structure, we have corrected the lattice oxygen concentration to take into account of the silane oxygen. We note that the processing temperature is not sufficiently high for the burning of the silane.
The compositions within the pores and pore walls of the catalyst Co/Si = 1/4 are evaluated using the EDS-images similar to that shown in
Figure 17d.
Figure 18 illustrates the spatial chemical heterogeneity of the Co/Si = 1 catalyst on the surface of the catalyst which generated plasma during microwave processing at 1800 W, where the total processing time was 1 min. It can be seen from
Figure 18a that the particle surface has regions with enhanced cobalt concentration with [Co]/[Si] = 6.17 instead of the theoretical value of [Co]/[Si] = 1.0. The EDX-spectrum is shown in
Figure 18b, whereas the mapping of Co, Si and O are shown in
Figure 18c–e.
In the absence of any plasma generation during processing, the chemical heterogeneity results from the phase separation which appear as decorations on the catalyst and pore surfaces as show in
Figure 17b–d. As shown previously [
1], Co/Si = X catalysts show phase separation in the form of decorations illustrated in
Figure 17. These decorations cover the pore surfaces, which are otherwise featureless. Therefore, heterogeneity is enhanced when the surface decorations are extensive and dispersed on the featureless background surface. This type of heterogeneity disappears if the catalyst is subjected to heat treatment to burn off the silica surface coating at ca. 600 °C [
1], while annealing is taking place.
The heterogeneity in Co/Si = 1/4 in different regions is evaluated from EDS studies and summarized in
Table 7. Heterogeneity with respect to Cobalt/Silica ratio (H
A) and catalyst lattice oxygen (H
B) within the walls, on smooth surfaces and decorated surfaces are shown in
Table 7. H
A = 0.808 for the walls is similar to the smooth surfaces H
A = 0.901 while for the decorated surfaces H
A = 0.432. However, if the whole surface is treated as a single entity, H
A = 0.932, a value similar to the wall and smooth surface heterogeneity. Heterogeneity in the lattice oxygen (H
B) is smaller and the highest heterogeneity H
B is observed in the walls and the lowest in the decorated surfaces. Nevertheless, the chemical heterogeneity in the absence of plasma is not significant compared with the case when Co/Si ≥ 1, which is accompanied by plasma generation during processing with microwave irradiation. Therefore, in the subsequent sections, when evaluating the heterogeneity indices, we will only consider the surface rather than the walls, which do not contribute significantly to the catalysis compared with the catalyst surfaces.
The characteristics of the catalysts Co/Si = 1 and Co/Si = 2, which are produced when plasma is generated during microwave irradiation are shown in
Figure 18,
Figure 19,
Figure 20 and
Figure 21.
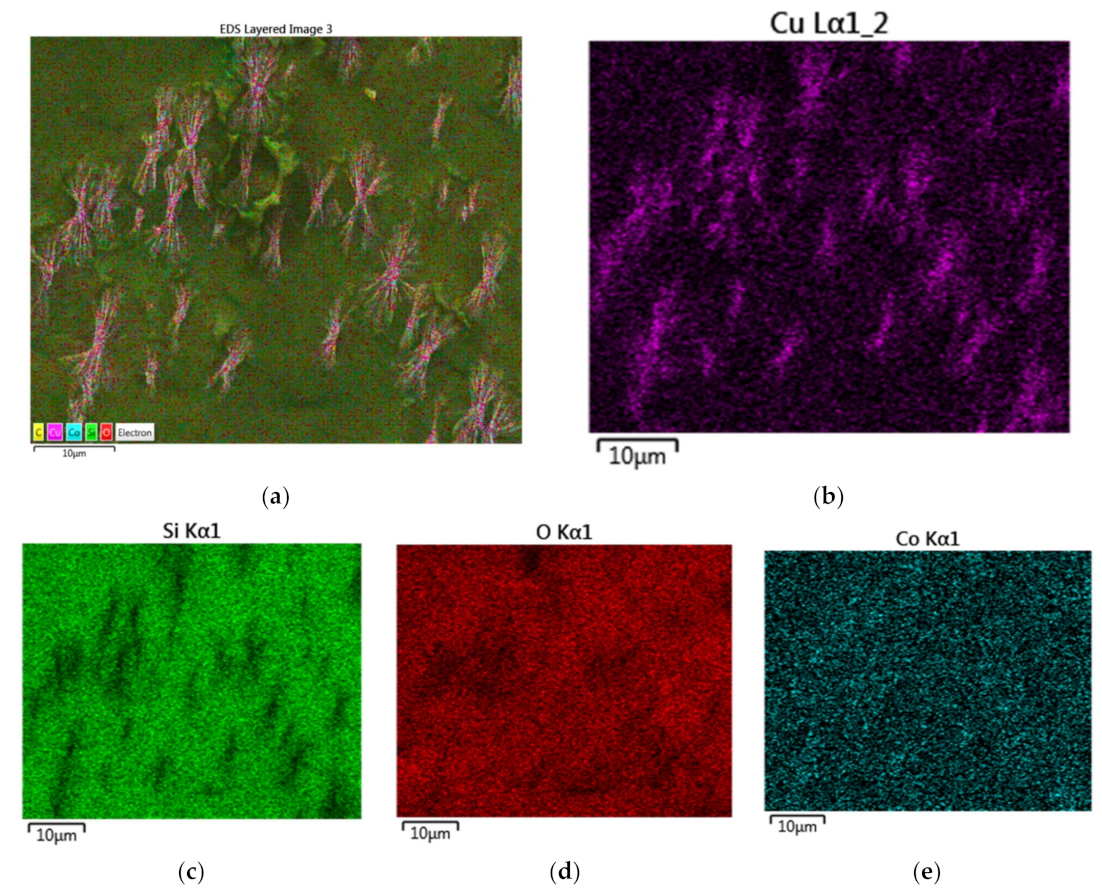
Figure 18a–e illustrates the elemental mapping on the surface of a Co/Si = 1 catalyst particle. It can be seen from
Figure 18a,c–e that the catalyst surface is highly inhomogeneous and that although cobalt is present across the whole catalyst domain (
Figure 18c). Silica (
Figure 18d) and oxygen (
Figure 18e) rich-domains are closely associated indicating that the oxygen within the catalyst is mainly associated with silica, rather than with cobalt in the form of Co
3O
4. The average molar concentrations for this particle shown in
Figure 18a are: [Co] = 0.413; [Si] = 0.067 and [O] = 0.520 as reflected by the EDX-spectrum shown in
Figure 18b. The comparison of the EDX-spectra for Co/Si = 1/4 (
Figure 17e) and Co/Si = 1 (
Figure 18b) show a large enhancement of the Co peaks due to Co concentration increase, as well as cobalt distribution across the catalyst surface.
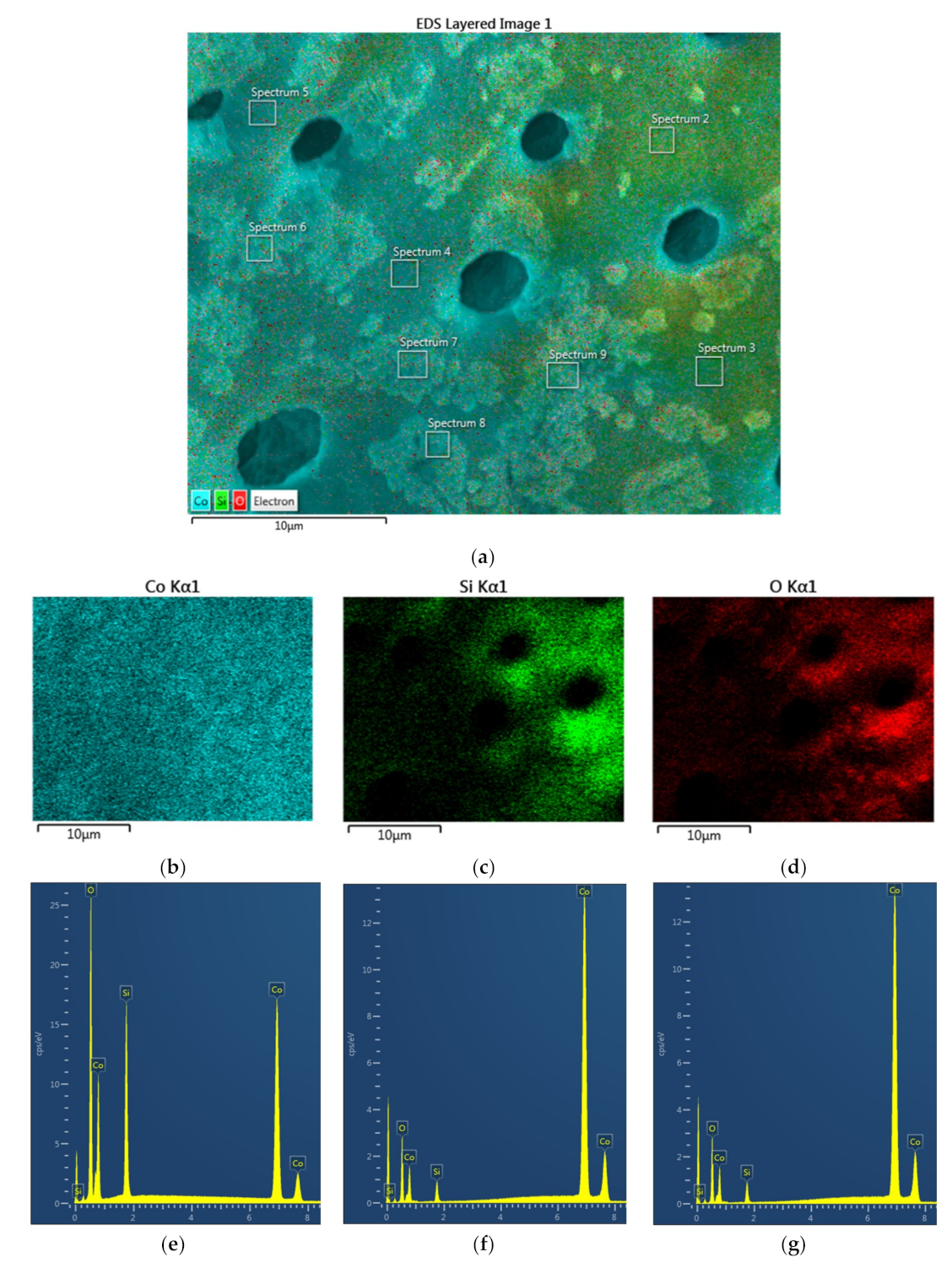
Figure 19a–i illustrates the region where the average cobalt concentration is below that shown for the whole particle illustrated in
Figure 18a for Co/Si = 1. This is determined from the EDS layered image (
Figure 19b) and confirmed by the spectrum of the whole area as shown in
Figure 19c. The mapping of Co, Si and O shown in
Figure 19d–f indicates the existence of some heterogeneity in chemical composition.
The SEM image of the Co/Si = 1 catalyst surface shown in
Figure 19a illustrates the surface structure for this catalyst. It shows two regions that are similar to that shown in
Figure 17b,c for Co/Si = 1/4 except that the surface decorations are now extensive. These two structures are further magnified in
Figure 19g,i respectively. It has craters surrounded by loosely formed small platelets resulting from the aggregation of particles with size ca. 50 nm. As shown below, these two distinct structures present in
Figure 19g,i is associated with low and high cobalt concentrations, respectively. These craters are formed when phase separation takes place within the walls and nano-sized platelets are ejected from the walls.
Figure 19b is also the site of the spot analysis. At this site, based on the spectrum in
Figure 19c, the overall composition is [Co] = 0.329, [Si] = 0.109, [O] = 0.562 indicating that [Co] is below the overall composition for the whole catalyst particle shown in
Figure 18a. The highest and lowest cobalt compositions are tabulated in
Table 8 and compared with the average for this site.
Table 8 also includes the concentration data for the whole particle as evaluated from
Figure 18.
Table 8 indicates that, as the scale of the scrutiny becomes finer, the concentration heterogeneity for the catalyst increases. At high Co concentrations, Si concentration decreases and as a result the catalyst/silica ratio increases. On the other hand, [O]/([Co] + [Si]) remains constant at 1.73. The surface structure at the lowest cobalt concentration is shown in
Figure 19g and that with the highest cobalt composition is shown in
Figure 19i.
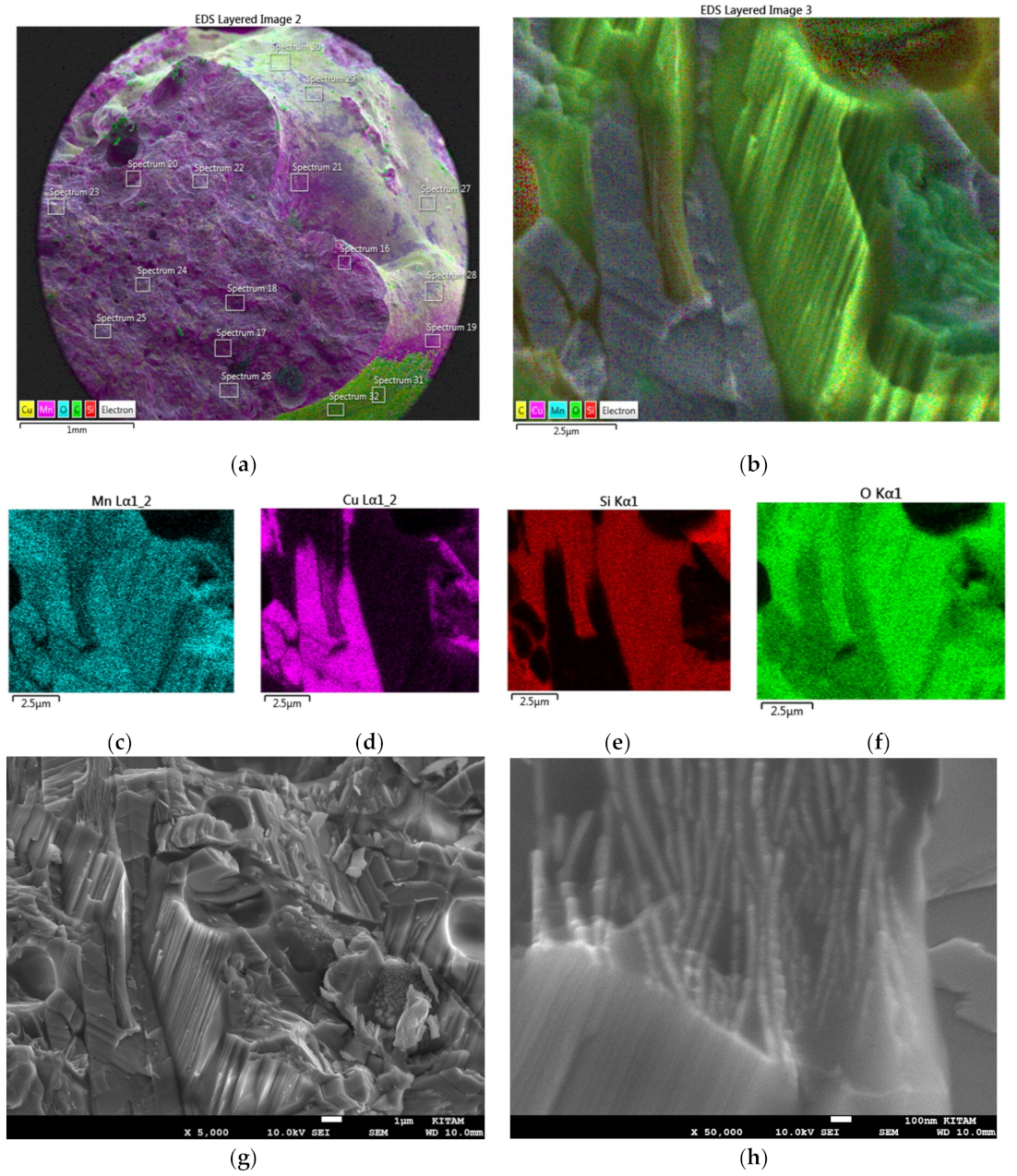
The heterogeneity analysis for Co/Si = 1 catalyst is also carried out in regions where the cobalt concentration is higher or lower than the average cobalt concentration of the catalyst particle, as exemplified in
Figure 20a–i. The EDS image of such a location is shown in
Figure 20a while the mapping of Co, Si and O are illustrated in
Figure 20b–d.
Figure 20e–g is the EDX-spectra of the locations where cobalt concentration is minimum (e); average (f) or maximum (g). The EDX-spectrum and the SEM image of the location with the lowest cobalt concentration is shown in
Figure 20e,h where the composition is: [Co] = 0.496; [Si] = 0.126; [O] = 0.378. The EDX-spectrum and SEM image of the region, where cobalt concentration is average as evaluated from the EDS layered image, are shown in
Figure 20a,f where the composition is: [Co] = 0.720; [Si] = 0.068; [O] = 0.212.
Figure 20g,i shows the EDX-spectrum and SEM of the location where cobalt concentration is maximum with composition: [Co] = 0.908; [Si] = 0.018; [O] = 0.074. The proximity of the locations with the lowest and highest cobalt locations are shown in SEM and EDS images of
Figure 20j,k where the decorative regions as well as the tips of the craters are rich in cobalt. These cobalt rich regions have nearly 90 mol% phase separated reduced metallic cobalt with a distinct platelet morphology whereas the region with the lowest cobalt consists of fused grains with primary particle size of ca. 50 nm with cobalt still in an oxidized state.
A summary of these results is shown in
Table 9, where the variation of the catalyst/support and lattice oxygen heterogeneity indices H
A and H
B are tabulated as a function of catalyst concentration. It shows that H
A increases rapidly from H
A = 0.425 when Co/Si = 1/5 to H
A = 41 when Co/Si = 1 which is accompanied by plasma generation. Further increase in the catalyst concentration with plasma generation results in reduction in H
A. By definition, under thermodynamic equilibrium conditions, in the limiting cases H
A → 0 and H
B → 0 when [Co] → 0 or 1. However, in practice, equilibrium may take a long time to reach as it requires high heat and mass transfer rates throughout the catalyst. Nevertheless, the current form of the supported catalyst, with its very high porosity and connectivity, allows enhanced heat and mass transfer rates and reaches equilibrium faster compared with non-porous catalysts.
As seen from
Table 9, the lattice oxygen heterogeneity index (H
B) increases gradually with increasing catalyst concentration, even when plasma is generated. However, this does not mean that the lattice oxygen remains stable. It indicates that the catalyst oxide reduces to metal or low valency oxides with an overall reduction of oxygen concentration across the catalyst surface. The remaining oxygen in the supported catalyst is due to the silica support.
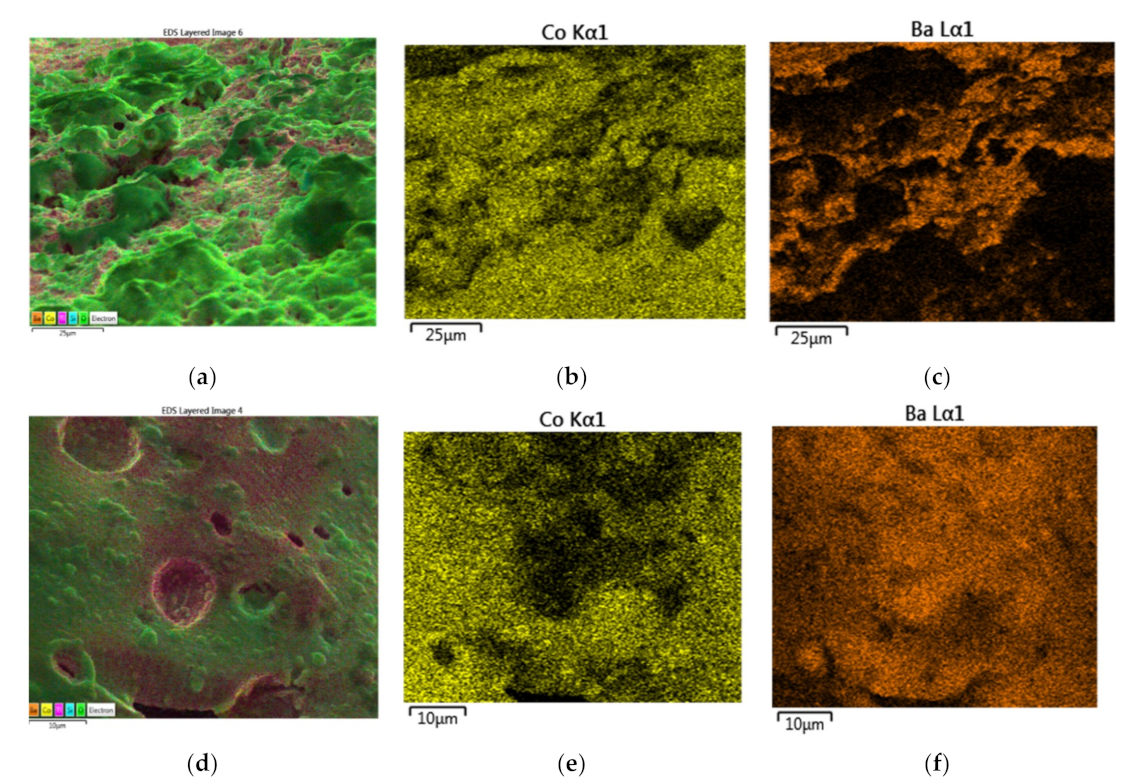
In
Figure 21, the results for the catalyst Co/Si = 2 are summarized. The EDS layered image at two magnifications clearly show that the catalyst surface predominantly consists of cobalt, whereas silica is mainly confined to the pore walls, as seen in
Figure 21a,b. Elemental mapping of Co, Si, and O shown in
Figure 21c–e confirm the above conclusions. EDS image at low magnification in
Figure 21a can be used for the evaluation of the average surface cobalt concentration for this catalyst particle whereas the EDS image in
Figure 21b has regions of high and low cobalt concentration, suitable for the evaluation of H
A and H
B.
After identifying regions of below-average and above-average cobalt concentrations, EDS spot analysis was used to determine the sites with the highest and the lowest cobalt concentrations where SEM images were also acquired. These SEM images and the corresponding spectra are shown in
Figure 22a–f.
Figure 22d–f are the EDX-spectra of the sites with the lowest, average and the highest cobalt concentration respectively.
Figure 22a–c are the SEM images corresponding to the lowest, average and the highest cobalt concentrations respectively.
These SEM images indicate that the surface is covered by particles with one or two holes per particle at low cobalt concentration [Co]
1 = 0.248 (
Figure 22a,d). As cobalt concentration increased, there appears to be a particle agglomeration resulting in approximate doubling of the size and the corresponding increase in the number of holes per particle (
Figure 22b,e). At the highest cobalt concentration [Co]
2 = 0.933 (97.9 wt%) a large scale particle fusing appears to take place with further modification in particle pore structure (
Figure 22c,f). In this high cobalt region, the corresponding Si- and O-concentrations are very low [Si] = 0.007 (0.4 wt%) and [O] = 0.060 (1.7 wt%). It is therefore clear that Co exists mainly as Co
0 and the available oxygen is mainly from SiO
2. At this location where Co concentration is very high, [Co]/[Si] = 131 (theoretical value = 2) and [O]/([Co] + [Si]) = 0.064 indicating that partial phase separation between Co and Si is present and oxygen depletion is due to Co reduction and subsequent NO
x formation. It is interesting to observe that the agglomeration of the single-hole particles in
Figure 22a results in multiple-hole agglomerates covering the surface of the pores with holes taking the shape of slits (
Figure 22c).
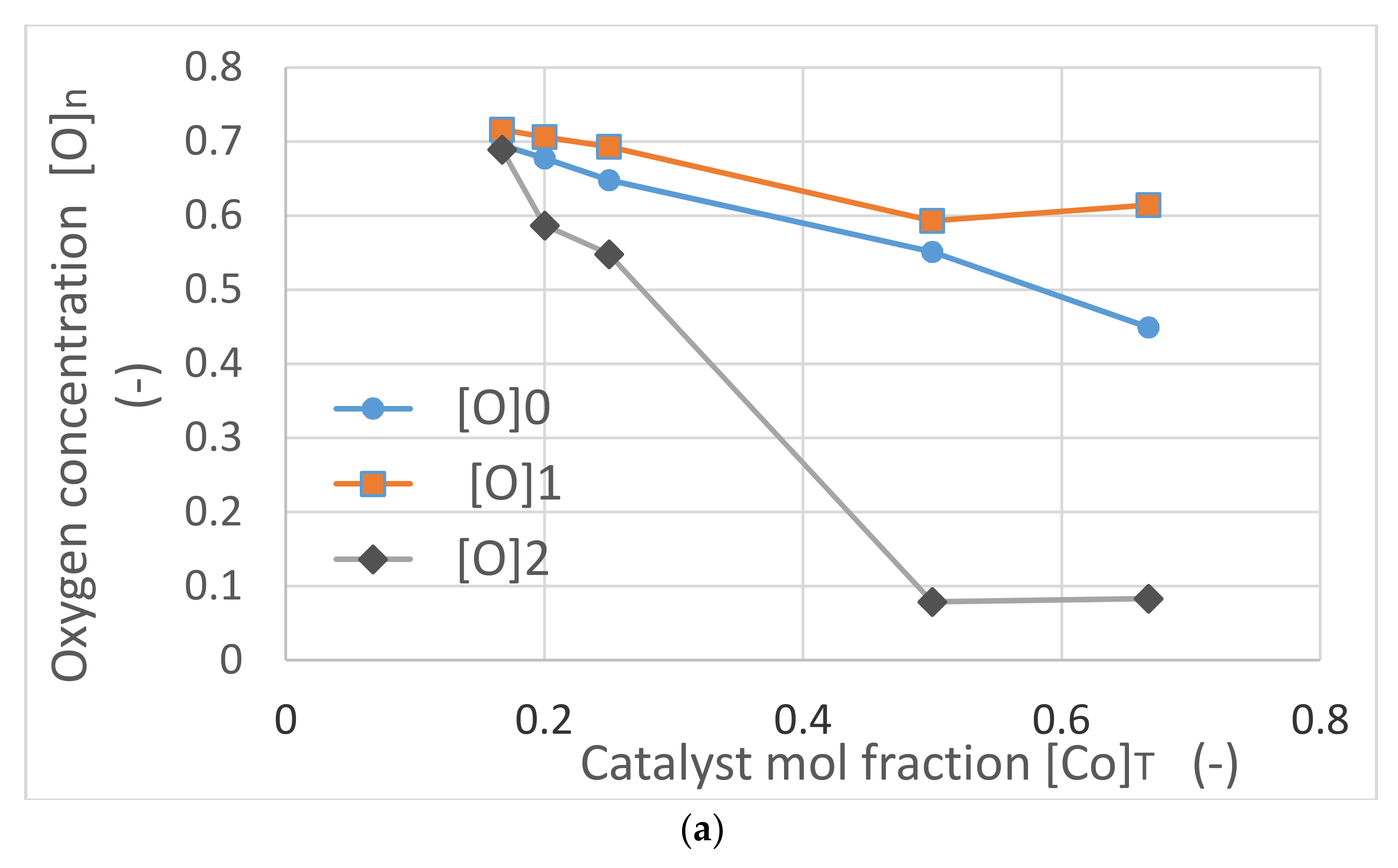
Figure 23 illustrates the characteristics of Co/Si = X catalysts as a function of catalyst concentration [Co]
T. The variation of average oxygen concentration [O]
0, oxygen concentrations in the regions with the lowest cobalt, [O]
1 and the highest cobalt, [O]
2 are shown in
Figure 23a. It can be seen that the oxygen levels decrease with increasing catalyst concentration in all regions. When [Co]
T ≥ 1, which is associated with plasma generation, there is a sharp decay in oxygen concentration in the regions where Co concentration is the highest. In these regions, catalyst oxygen concentration is mainly due to catalyst support and the catalyst is mainly present as Co
0.
In
Figure 23a, the oxygen concentrations [O]
0 and [O]
1 are higher than the theoretical concentration [O]
T. Freshly synthesized silica supported catalyst is in the form of M
3O
4/SiO
2 and hence the range of [O]
T is 0.667 ≥ [O]
T ≥ 0.571 corresponding to 0 ≤ [Co]
T ≤ 1. This indicates that silica supported catalyst has anionic silica clusters [
86] in the form of Si
pO
q where p = 3 – 8 and (2p − 1) ≤ q ≤ (2p + 2). Such clusters can react with water [
86] to form SiO
3(OH). Therefore, the presence of localized excess oxygen in the region with low cobalt and high oxygen regions ([Co]
1 and [O]
1), excess oxygen is ca. 10% for Co/Si = 1/5, which can be explained by SiO
2 cluster formation.
Figure 23b represents the variation of catalyst/support ratio (A
n = [Co]
n/[Si]
n) as a function of catalyst concentration [Co]
T at three locations corresponding to n = 0, 1, 2. Due to the spread of the data, the results are shown on a logarithmic scale for A
n. It can be seen that the cobalt/silica concentration ratio increases rapidly and plasma is generated when [Co]
T ≥ 1. It can be concluded that when the catalyst is reduced to Co
0, the catalyst surface also becomes significantly richer in Co
0 and the heterogeneity becomes more significant.
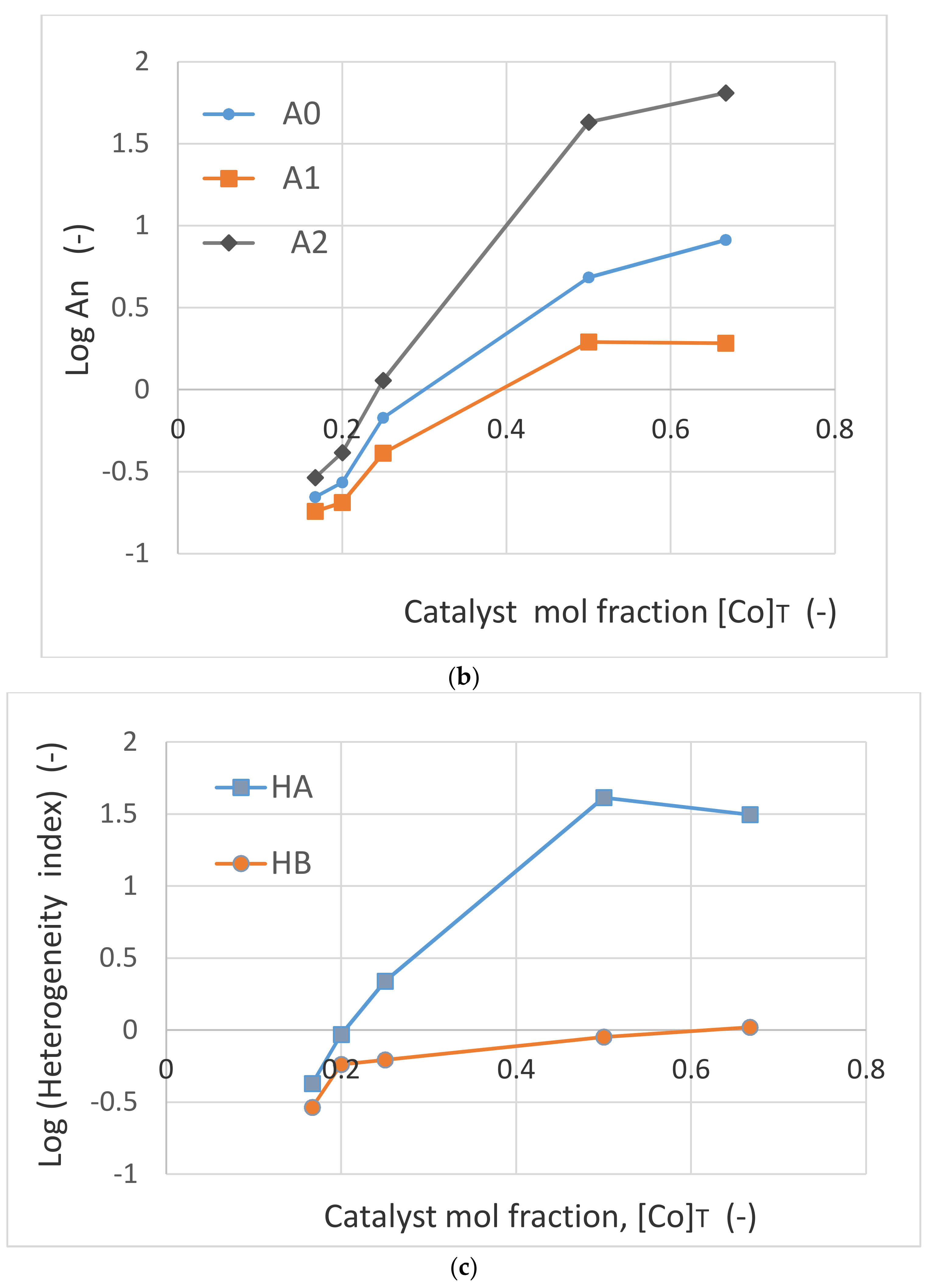
Figure 23c shows the variation (in logarithmic scale), of the catalyst and oxygen heterogeneity indices, Log (H
A) and Log (H
B) as a function of feed cobalt concentration [Co]
T. Within the experimental range of [Co]
T, oxygen heterogeneity index increases modestly compared with the cobalt heterogeneity index which increases by nearly 100-fold when [Co]
T is increased from [Co]
T = 0.167 to [Co]
T = 0.5, followed by a decrease with further increase in catalyst concentration.
2.14. SEM and EDS study of Manganese/Silica Catalyst Processed under Microwave Radiation with Plasma Generation
The SEM and EDS analysis carried out for Co/Si= X (X = 1, 2) is replicated for Mn/Si = X (X = 1, 2) catalyst. For Mn/Si = 1 catalyst it is found that the structural and chemical heterogeneity of this catalyst, is relatively low compared with Co/Si = 1 catalyst.
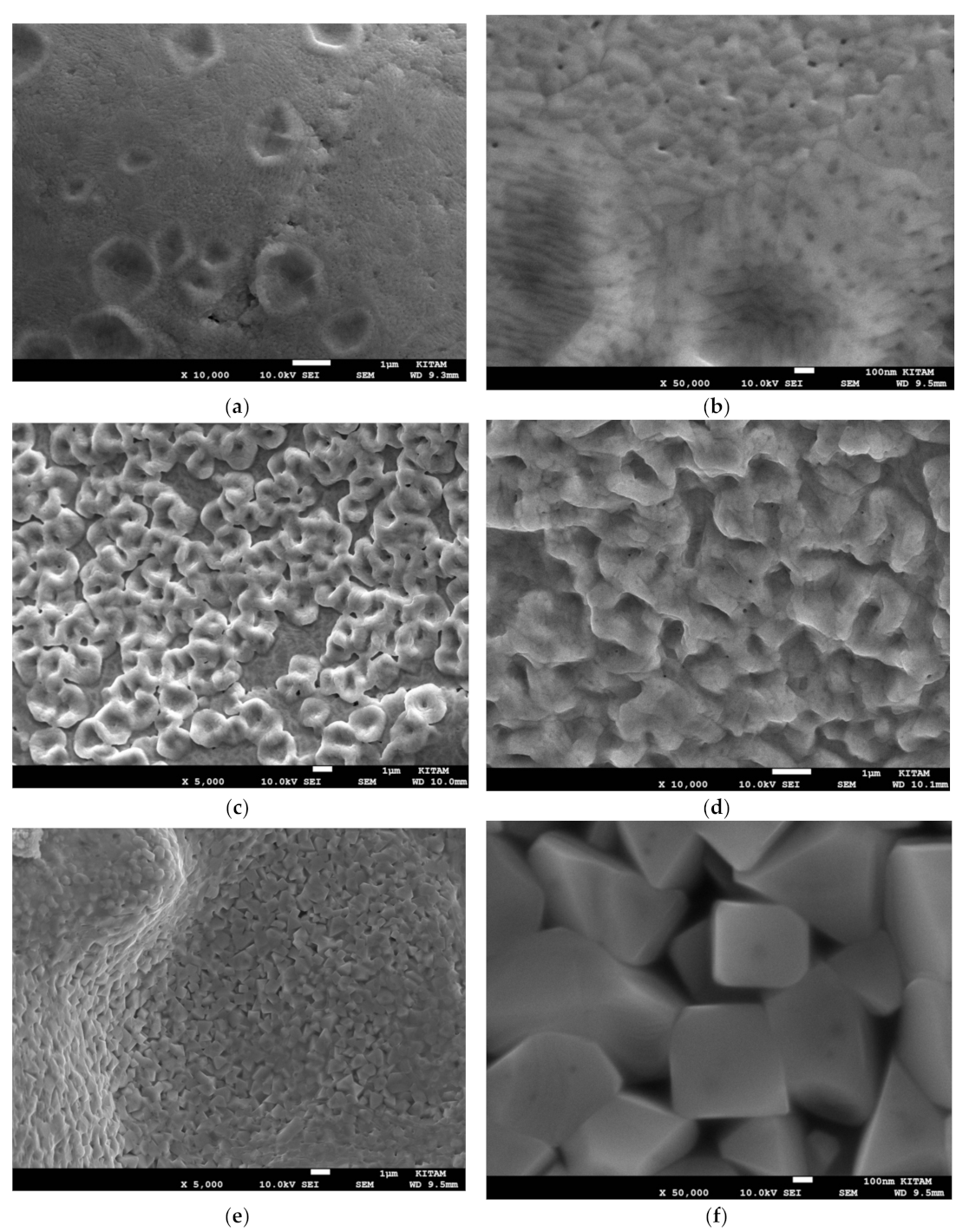
Figure 24a–d illustrates the morphology of Mn/Si = 1 catalyst. The catalyst particle surface as well as the surface of the pores consist of ca. 500 nm sized fused particles, while the walls contain similar size closely packed discrete particles. The electron images of the catalyst site shown in
Figure 24a is illustrated in
Figure 25a–e, which indicates that the Mn, Si, O distributions are inhomogeneous but not as marked as the inhomogeneity in Co/Si = 1 catalyst. The overall composition of the region shown in
Figure 25a represents the average Mn concentration with: [Mn]
0 = 0.384; [Si]
0 = 0.149; [O]
0 = 0.467.
On the other hand, the physical and chemical heterogeneity in Mn/Si = 2 catalyst is highly significant, as shown in
Figure 26.
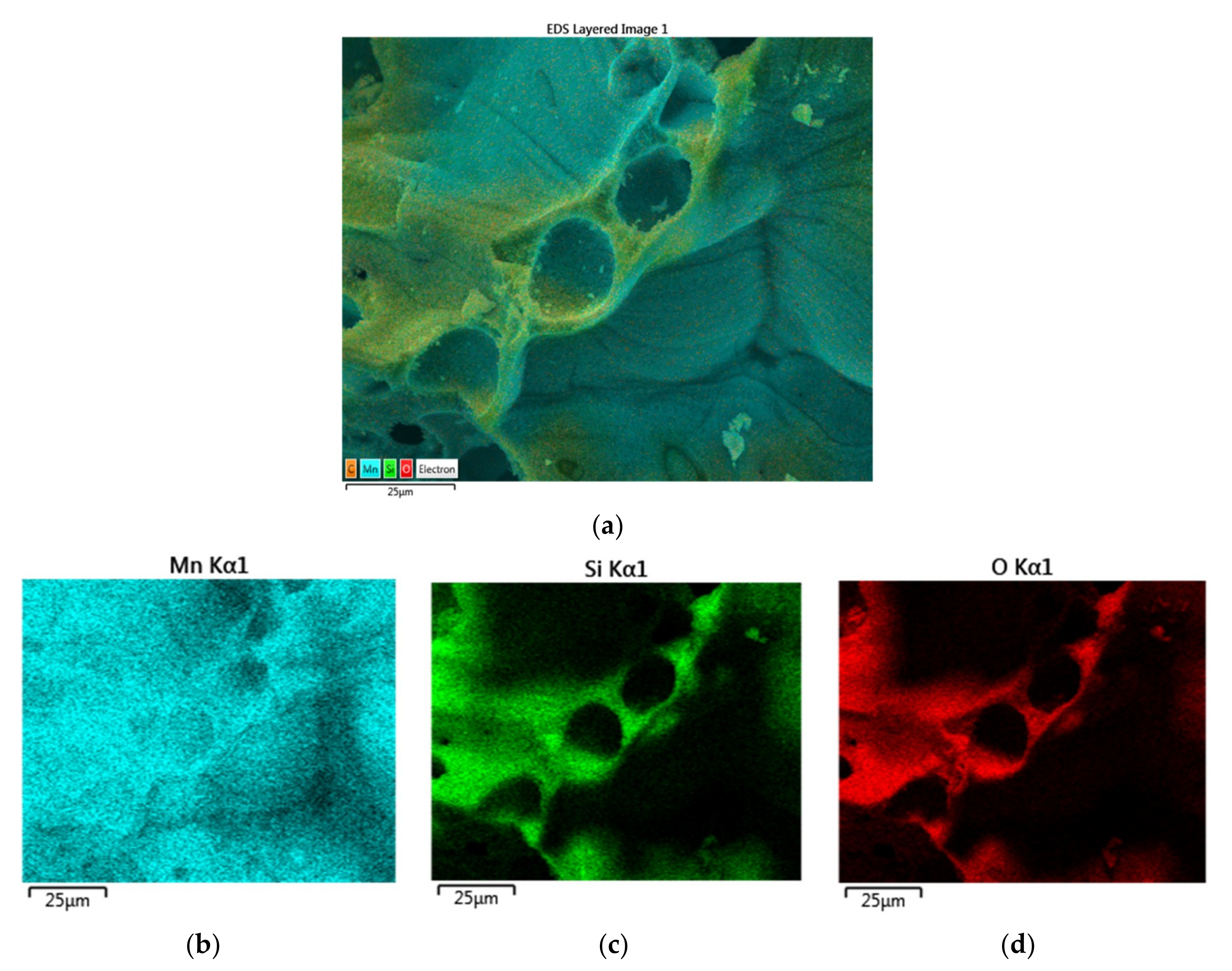
Figure 26a,b illustrates the electron image of two sites at two different magnifications.
Figure 26a shows the presence of chemical heterogeneity on the particle surface while
Figure 26b illustrates the local heterogeneity in the inner pore walls and surfaces. The elemental mapping of Mn, Si, O corresponding to the electron images are shown in
Figure 26c–h respectively. These figures show that Mn is present throughout the sample while Si and O are localized especially within the pore walls.
Figure 26a is used to determine the average concentrations while
Figure 26b is used to determine the locations of the below- and above-average Mn concentrations in evaluating the heterogeneity indices, H
A and H
B as well as in evaluating the catalyst morphology as a function of Mn concentration.
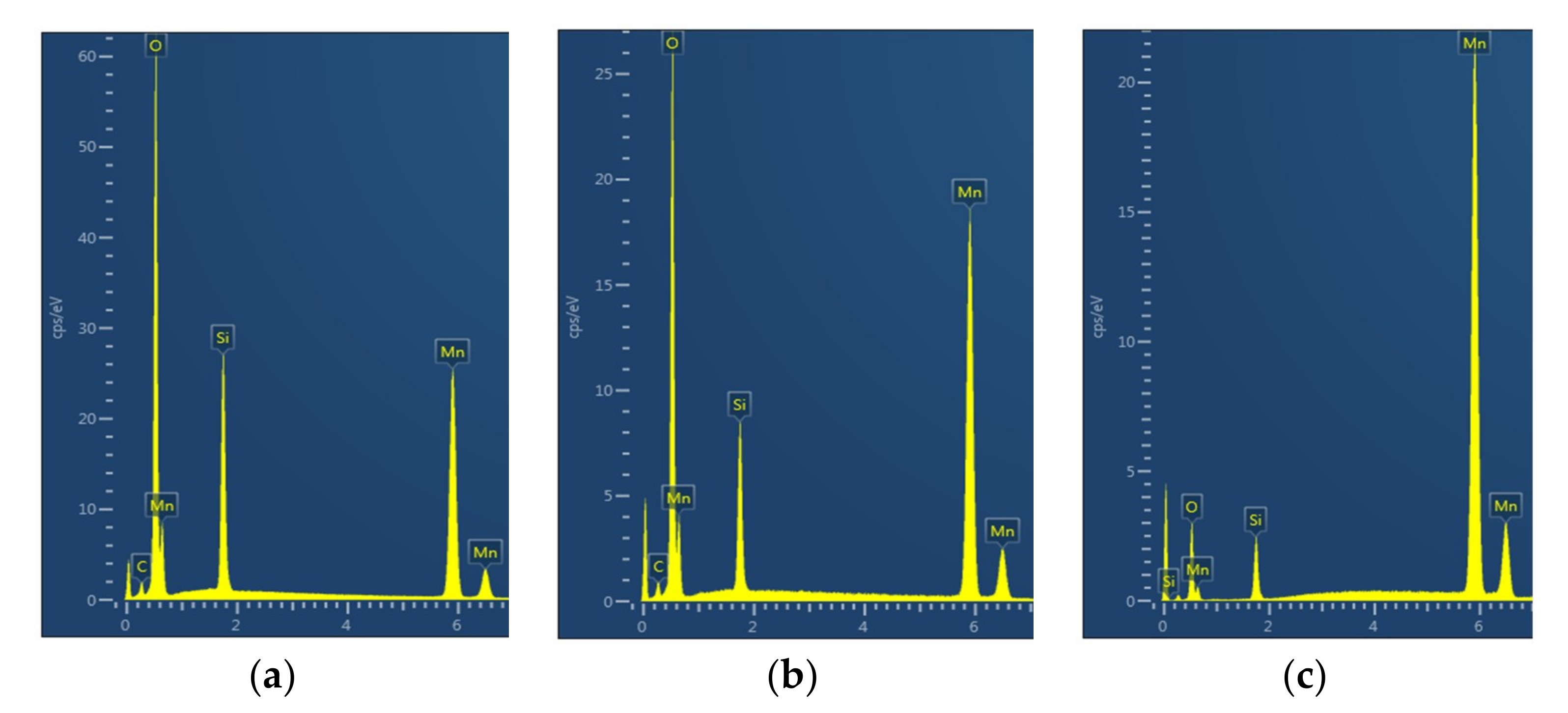
Figure 27a–c shows the EDS-spectra at three locations representing: (a) the average, n = 0; (b) the lowest, n = 1; and (c) the highest, n = 2, Mn concentration for Mn/Si = 2 catalyst. Similar to Co/Si = X it was observed that carbon was not present in the regions with the highest Mn concentration. Also shown are the corresponding results when the fresh catalyst was further subjected to microwave irradiation for 1 extra minute at a power rating of 1800 W.
Molar concentrations of [Mn]
n, [Si]
n, and [O]
n as well as catalyst/ support ratio, A
n, catalyst oxygen capacity B
n together with the corresponding heterogeneity indices H
A and H
B are tabulated in
Table 10. It can be seen from
Table 10 that the highest Mn concentration ([Mn]
2 = 0.732) is not as high as that observed for Co ([Co]
2 = 0.903) for Co/Si = 2 catalyst even after an extra microwave irradiation when [Mn]
2 = 0.815. As a result, the catalyst/support heterogeneity index H
A for Mn/Si = 2 is significantly lower than those observed for Co/Si = 2. Lattice oxygen heterogeneity index H
A for Mn/Si = 2 is slightly higher for Mn compared with Co. This can be attributed to the fact that Mn has several more oxidation states than Co.
The morphological variations in catalyst (Mn/Si = 2) structure as a function of Mn, Si, O concentrations are shown in
Figure 28. Mn/Si = X catalyst particles are porous with a large surface area exposed to air during processing. Below this surface skin, pores are present, with pore wall thickness of ca. 1 μm. Both the catalyst skin and the pore surfaces have morphologically distinct structures depending on the plasma exposure (time span and intensity) during processing while the pore walls have proto-structures but mainly consist of nano-sized porous spheres. The porous wall structures are common in all the catalysts described here and previously [
1].
Figure 28 shows the surface skin structure as a function of local catalyst concentration. It can be seen that as Mn concentration increases (with corresponding decrease in Si and O), surface structures (decorations) appear with increasing dominance.
Figure 28a,b shows the background silica rich surface layer (with composition [Mn] = 0.201; [Si] = 0.115; [O] = 0.684) which is porous and has nano-sized (ca.10–20 nm) structures especially within the surface pores.
The observed surface decorations are associated with higher Mn concentrations as seen in
Figure 28c where [Mn] = 0.317; [Si] = 0.075 and [O] = 0.606. As a result of stronger plasma generation, surface shown in
Figure 28d consists of sintered particles with very large Mn and smaller oxygen concentration with composition [Mn] = 0.772; [Si] = 0.059; [O] = 0.169. The dominant structure on the surface of the inner pores of Mn/Si = 2 catalyst, the surface is covered ca. 500 nm crystals with partial sintering and enhanced Mn concentration with composition: [Mn] = 0.843, [Si] = 0.023 and [O] = 0.134.
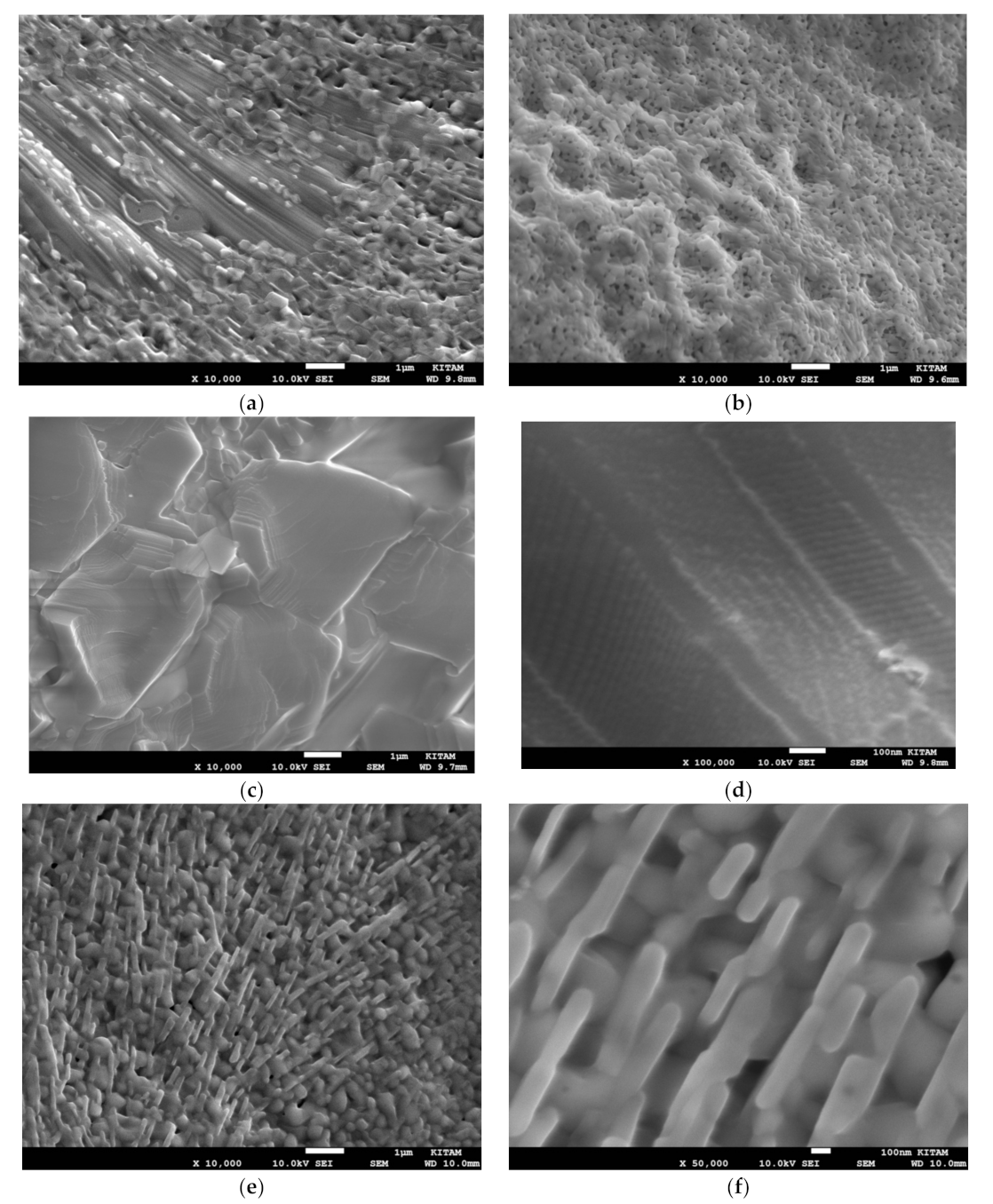
The effect of further microwave irradiation and plasma generation is illustrated in
Figure 29a–f. Here, the fresh Mn/Si = 2 catalyst was microwave irradiated for another 60 s at 1800 W with plasma generation to obtain Mn/Si = 2* catalyst. The surface structures change significantly while Mn concentration increases as seen in
Figure 29a–d. The concentration of Mn rich decorations across the surface increases with further microwave radiation. Sintering and porosity of the surface decorations increases with pore size ca. 100–200 nm as seen in
Figure 28a–d. At the highest Mn concentration (when [Mn] = 0.840; [Si] = 0.023; [O] = 0.137) the nano-structure of the material has a layered configuration shown in
Figure 29d. In fact, the fine structure of these crystals is similar to that of microwave irradiated BaTiO
3 structure (see
Figure 15 and
Figure 16) except that in Mn/Si = 2 catalyst, the grains are smaller (ca. 10 μm) and the layers are thinner (ca. 10 nm) compared with BaTiO
3 grain layers of ca. 100 nm. Some of the cuboidal nano-crystals observed (see
Figure 28e,f) on the pores of the fresh Mn/Si = 2 catalyst appear to be partially transformed into ca. 100 nm diameter and ca. 500 nm long rods (with composition: [Mn] = 0.804; [Si] = 0.019; [O] = 0.177) as seen in
Figure 29e,f. These results indicate that it is possible to obtain several different catalyst morphologies through microwave induced plasma. These structures are likely to be catalytically more active under plasma.
2.15. Catalyst Reduction, Surface Transition from Spinel to Silicate Perovskite (MSiO3) Structure and Formation of Olivines (M2SiO4)
The foregoing analysis of the spinel and perovskite catalysts primarily investigated by EDS analysis indicate that within the same catalysts there exists reduced and oxidized domains with distinct crystallite structures separated by micrometer-scale distances. Therefore, such catalysts can simultaneously catalyze two sequential chemical reactions forming a micro-scale chemical-looping system.
In many cases, after synthesis, the catalyst oxides need to be reduced in hydrogen stream at ca. 500–600 °C before use. Clearly, the present technique can be used as a means of catalyst reduction. Furthermore, in plasma reactors, high concentrations of metal catalysts are not tolerated due to electrical discharges, whereas in the current technique, plasma is generated at high metal catalyst concentrations. This also provides a cost advantage because cheap catalysts at high concentrations can economically replace expensive catalysts.
It is often assumed that the support in supported catalysts is inert and does not undergo any chemical change during catalyst preparation or chemical reaction. This is indeed highly desirable as such chemical changes could result in catalyst deactivation and unpredictability. The supported catalyst system introduced by the author [
1] does appear to satisfy this criterion partly because, although they have been produced using microwave (or indeed using UV or thermal energy) no plasma generation was observed even when Nickel/Silica system (Ni/Si = X) was synthesized at high Ni concentrations, X = 2. The undesirability of catalyst activation loss due to chemical reactions during preparation or catalytic reactions would be circumvented if the reaction media also helped catalyst renewal. The reversibility of catalyst chemical/physical structure would also provide certain advantages as discussed next.
As shown previously, in M/Si = X (M = Co, Mn) at high catalyst loadings when X ≥ 1, extra reflections appear in the XRD spectra (See
Section 2.7). These extra reflections can be explained by the transfer of catalyst spinel structure, M
3O
4 (M = Co, Mn in the present case) to silicate perovskite structure MSiO
3 and/or olivine structure M
2SiO
4 at high catalyst concentration with microwave induced plasma generation. The preparation and XRD studies [
87,
88,
89,
90,
91,
92] of MSiO
3 and M
2SiO
4 show that several of the emergent reflections in M/Si = X (X ≥ 1) catalysts are present in MSiO
3 and M
2SiO
4 compounds. This explains the formation of surface decorations shown in both Co/Si = X and Mn/Si = X catalysts when X ≥ 1. As shown in
Figure 24,
Figure 25,
Figure 26,
Figure 27,
Figure 28 and
Figure 29, such structures are dispersed in the main catalyst structure which results in high oxygen (H
B) and catalyst (H
A) heterogeneities.
Chemical and physical heterogeneities are formed as a result of microwave induced plasma. Therefore, this combination can be used to achieve surface modification. MSiO
3 and M
2SiO
4 compounds provide an alternative and more efficient anode for lithium ion batteries which represent the most common electrical energy storage technology. Currently, anode of these batteries is made from intercalating graphite. In order to enhance anode energy density capacity, metal silicates such as M
2SiO
4 (M = Co, Fe, Mn) have been studied [
87,
88,
89,
90,
91,
92].