Catalytic Properties of Microporous Zeolite Catalysts in Synthesis of Isosorbide from Sorbitol by Dehydration
Abstract
1. Introduction
2. Results and Discussion
2.1. Characteristics of the Catalysts
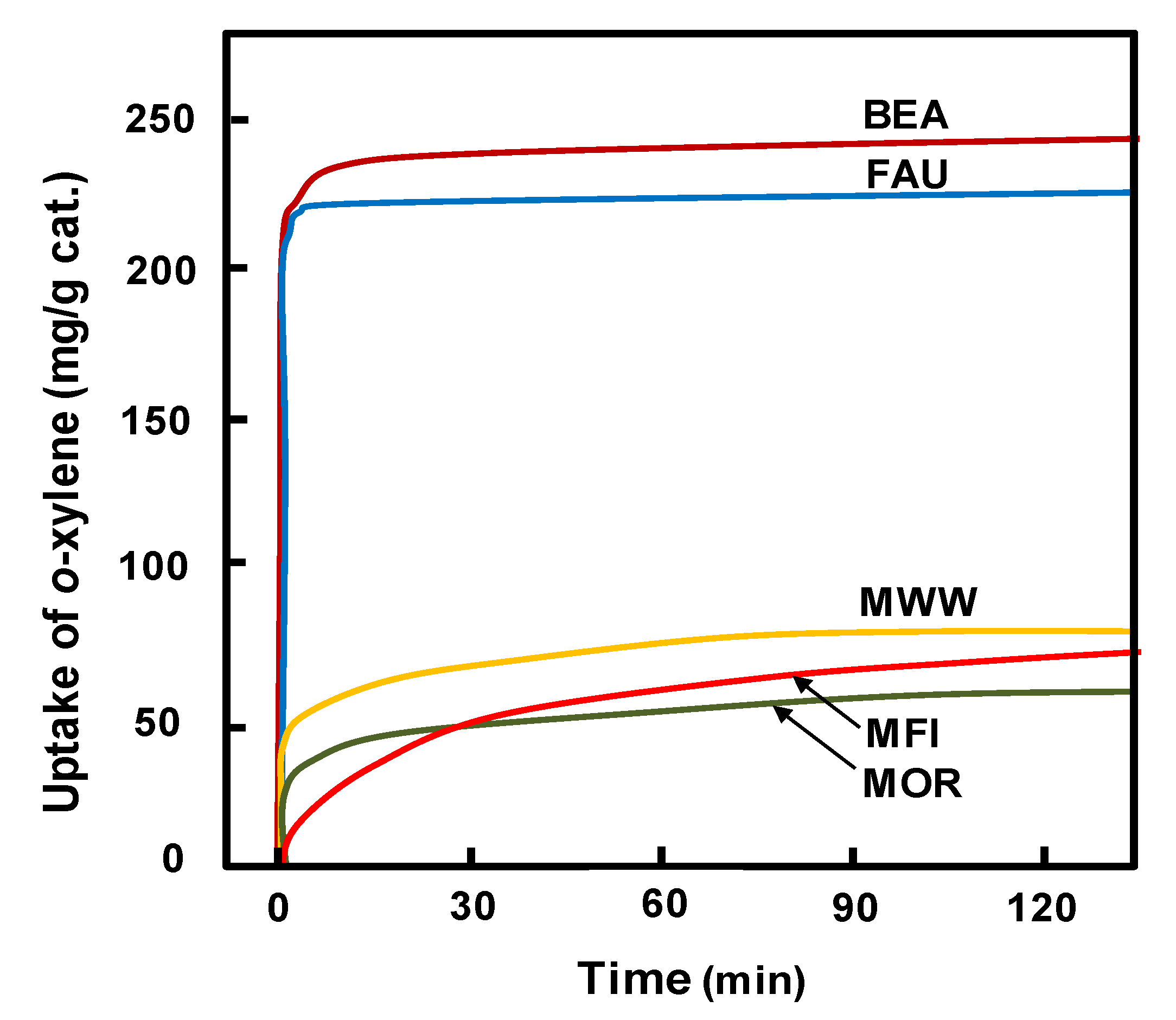
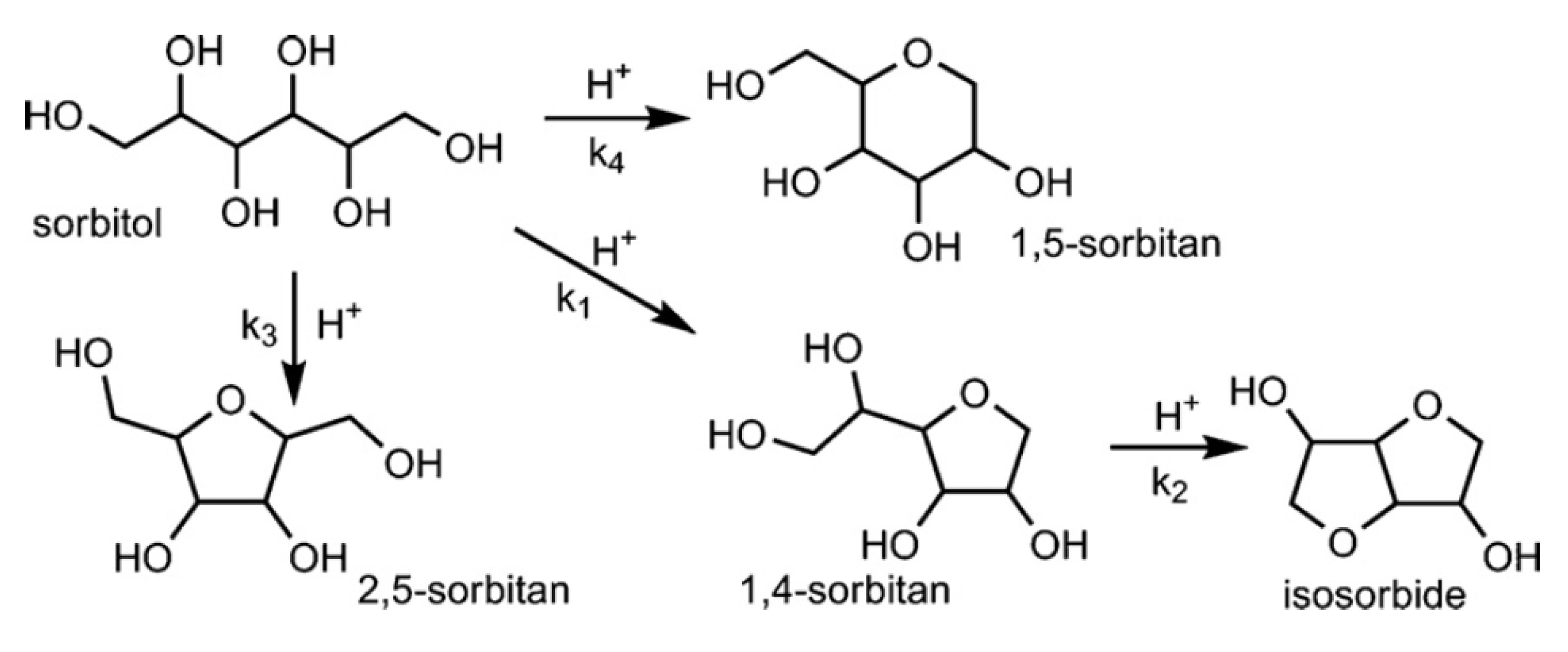
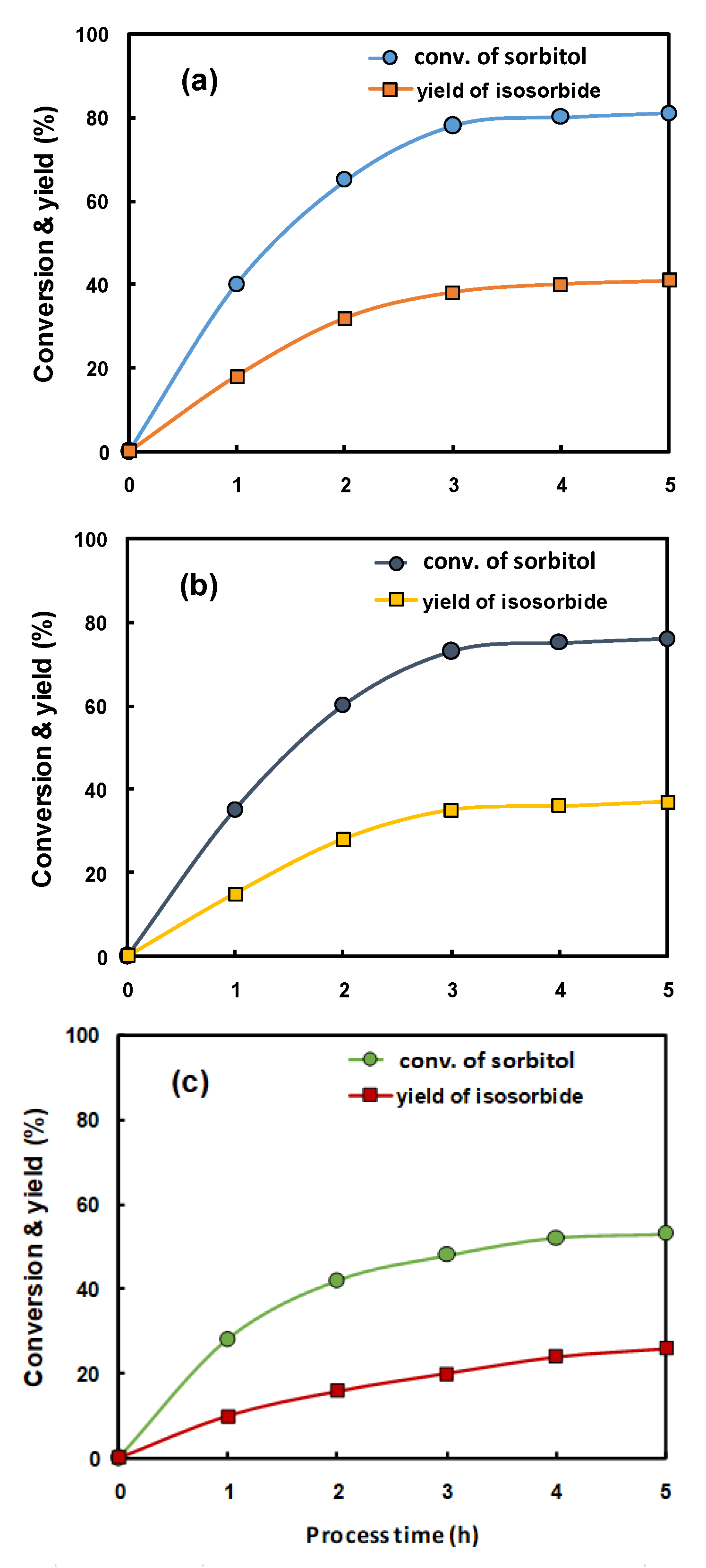
2.2. Reaction Characteristics of Isosorbide Synthesis on the Catalysts
3. Experimental
3.1. Materials and Catalysts
3.2. Preparation of Isosorbide from Sorbitol
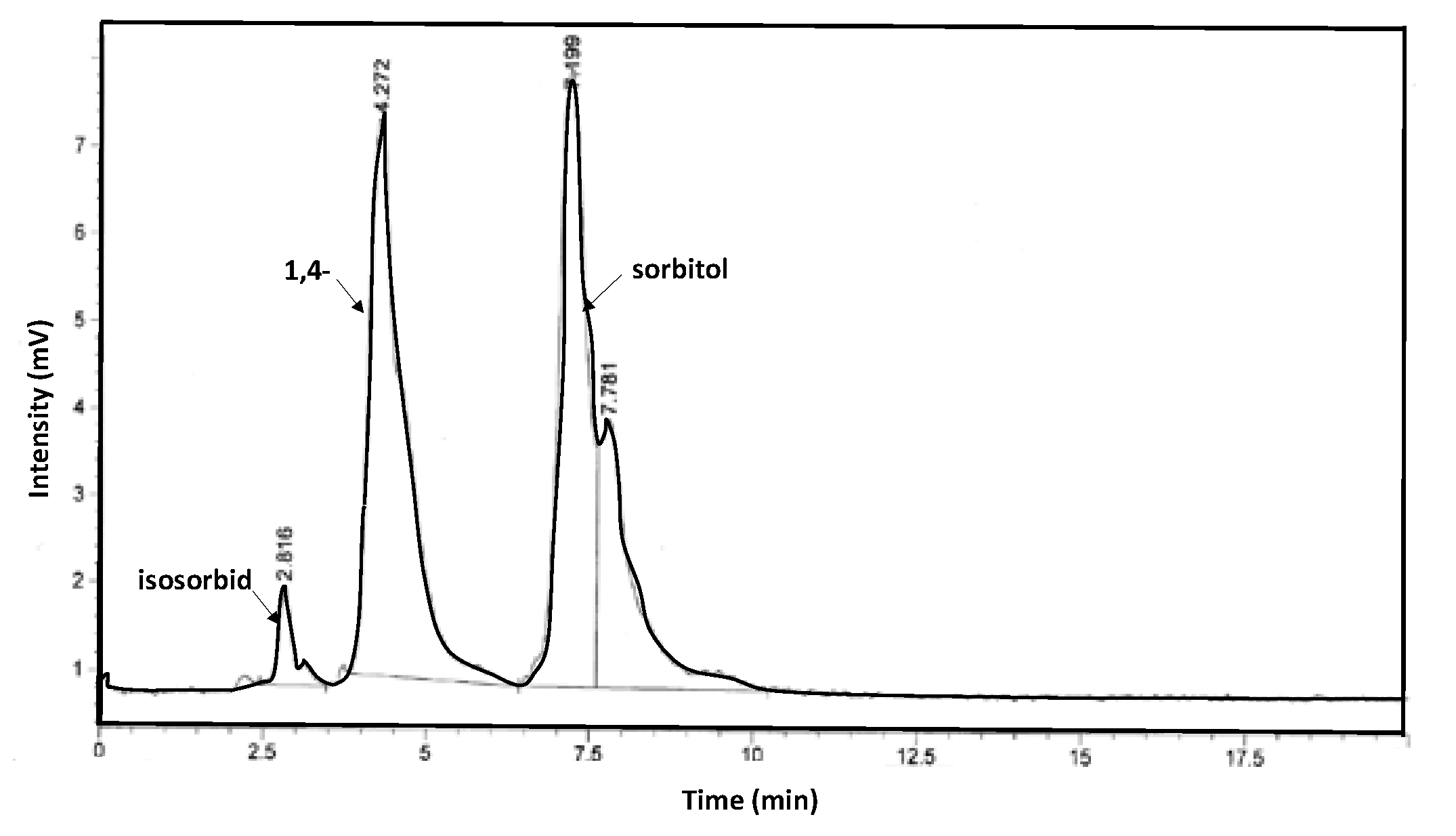
3.3. Characterization of the Catalysts and Products
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nagel, S.C.; vom Saal, F.S.; Thayer, K.A.; Dhar, M.G.; Boechler, M.; Welshons, W.V. Relative binding affinity-serum modified access (RBA-SMA) assay predicts the relative in vivo bioactivity of the xenoestrogens bisphenol A and octylphenol. Environ. Health Perspect. 1997, 105, 70–76. [Google Scholar] [CrossRef]
- Moriyama, K.; Tagami, T.; Akamizu, T.; Usui, T.; Saijo, M.; Kanamoto, N.; Hataya, Y.; Shimatsu, A.; Kazuya, H.; Nakao, K. Thyroid hormone action is disrupted by bisphenol A as an antagonist. J. Clin. Endocrinol. Metab. 2002, 87, 5185–5190. [Google Scholar] [CrossRef]
- Maffini, M.V.; Rubin, B.S.; Sonnenschein, C.; Soto, A.M. Endocrine disruptors and reproductive health: The case of bisphenol-A. Mol. Cell Endocrinol. 2006, 254, 179–186. [Google Scholar] [CrossRef]
- Staples, C.A.; Dome, P.B.; Klecka, G.M.; Oblock, S.T.; Harris, L.R. A review of the environmental fate, effects, and exposures of bisphenol A. Chemosphere 1998, 36, 2149–2173. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Hauser, R.; Marcus, M.; Olea, N.; Welshons, W.V. Human exposure to bisphenol A (BPA). Reprod. Toxicol. 2007, 24, 139–177. [Google Scholar] [CrossRef]
- Bae, S.; Kim, J.H.; Lim, Y.H.; Park, H.Y.; Hong, Y.C. Associations of bisphenol A exposure with heart rate variability and blood pressure. Hypertension 2012, 60, 786–793. [Google Scholar] [CrossRef]
- Rochester, J.R.; Bolden, A.L. Bisphenol S and F: A systematic review and comparison of the hormonal activity of bisphenol A substitutes. Environ. Health Perspect. 2015, 123, 643–650. [Google Scholar] [CrossRef]
- Nah, W.H.; Park, M.J.; Gye, M.C. Effects of early prepubertal exposure to bisphenol A on the onset of puberty, ovarian weights, and estrous cycle in female mice. Clin. Exp. Reprod. Med. 2011, 38, 75–81. [Google Scholar] [CrossRef]
- Braun, J.M.; Kalkbrenner, A.E.; Calafat, A.M.; Yolton, K.; Ye, X.; Dietrich, K.N.; Lanphear, B.P. Impact of early-life bisphenol A exposure on behavior and executive function in children. Pediatrics 2011, 128, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Baba, K.; Okada, K.; Kinoshita, T.; Imaoka, S. Bisphenol A disrupts notch signaling by inhibiting gamma-secretase activity and causes eye dysplasia of Xenopuslaevis. Toxicol. Sci. 2009, 108, 344–355. [Google Scholar] [CrossRef]
- Cao, X.L.; Popovic, S. Bisphenol A and three other bisphenol analogues in canned fish products from the Canadian market 2014. J. Food Prot. 2015, 78, 1402–1407. [Google Scholar] [CrossRef] [PubMed]
- Rocha, B.A.; Azevedo, L.F.; Gallimberti, M.; Campiglia, A.D.; Barbosa, A. High levels of bisphenol A and bisphenol S in brazilian thermal paper receipts and estimation of daily exposure. J. Toxicol. Environ. Health Part A 2015, 78, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Ruan, T.; Liang, D.; Song, S.; Song, M.; Wang, H.; Jiang, G. Evaluation of the in vitro estrogenicity of emerging bisphenol analogs and their respective estrogenic contributions in municipal sewage sludge in China. Chemosphere 2015, 124, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Richter, C.A.; Taylor, J.A.; Ruhlen, L.; Welshons, W.V.; vomSaal, F.S. Estradiol and bisphenol A stimulate androgen receptor and estrogen receptor gene expression in fetal mouse prostate mesenchyme cells. Environ. Health Perspect. 2007, 115, 902–908. [Google Scholar] [CrossRef]
- Li, J.; Ma, M.; Wang, A. A two-hybrid yeast assay to quantify the effects of xenobiotics on retinoid X receptor- mediated gene expression. Toxicol. Lett. 2008, 176, 198–206. [Google Scholar] [CrossRef]
- Montes-Grajales, D.; Olivero-Verbel, J. Computer-aided identification of novel protein targets of bisphenol A. Toxicol. Lett. 2013, 222, 312–320. [Google Scholar] [CrossRef]
- Boucher, J.G.; Gagné, R.; Rowan-Carroll, A.; Boudreau, A.; Yauk, C.L.; Atlas, E. Bisphenol A and bisphenol S induce distinct transcriptional profiles in differentiating human primary preadipocytes. PLoS ONE 2016, 11, e0163318. [Google Scholar] [CrossRef]
- Kinch, C.D.; Ibhazehiebo, K.; Jeong, J.H.; Habibi, H.R.; Kurrasch, D.M. Low-Dose exposure to bisphenol A and replacement bisphenol S induces precocious hypothalamic neurogenesis in embryonic zebrafish. Proc. Natl. Acad. Sci. USA 2015, 112, 1475–1480. [Google Scholar] [CrossRef]
- Pivnenko, K.; Pedersen, G.A.; Eriksson, E.; Astrup, T.F. Bisphenol A and its structural analogues in household waste paper. Waste Manag. 2015, 44, 39–47. [Google Scholar] [CrossRef]
- Corma, A.; Iborra, S.; Velty, A. Chemical routes for the transformation of biomass into chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef]
- Chheda, J.N.; Huber, G.W.; Dumesic, J.A. Liquid-Phase catalytic processing of biomass-derived oxygenated hydrocarbons to fuels and chemicals. Angew. Chem. Int. Ed. 2007, 46, 7164–7183. [Google Scholar] [CrossRef] [PubMed]
- Gallezot, P. Conversion of biomass to selected chemical products. Chem. Soc. Rev. 2012, 41, 1538–1558. [Google Scholar] [CrossRef] [PubMed]
- Alonso, D.M.; Bond, J.Q.; Dumesic, J.A. Catalytic conversion of biomass to biofuels. Green Chem. 2010, 12, 1493–1513. [Google Scholar] [CrossRef]
- Huber, W.; Iborra, S.; Corma, A. Synthesis of transportation fuels from biomass: Chemistry, catalysts, and engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef]
- Kobayashi, H.; Fukuoka, A. Synthesis and utilization of sugar compounds derived from lignocellulosic biomass. Green Chem. 2013, 15, 1740–1763. [Google Scholar] [CrossRef]
- Fukuoka, A.; Dhepe, P.L. Catalytic conversion of cellulose into sugar alcohols. Angew. Chem. Int. Ed. 2006, 45, 5161–5163. [Google Scholar] [CrossRef]
- Bai, F.; Wang, D.; Huo, Z.; Chen, W.; Liu, L.; Liang, X.; Chen, C.; Wang, X.; Peng, Q.; Li, Y. A versatile bottom-up assembly approach to colloidal spheres from nanocrystals. Angew. Chem. Int. Ed. 2007, 46, 6650–6653. [Google Scholar] [CrossRef]
- Geboers, J.; Van de Vyver, S.; Carpentier, K.; de Blochouse, K.; Jacobs, P.A.; Sels, B.F. Efficient catalytic conversion of concentrated cellulose feeds to hexitols with heteropoly acids and Ru on carbon. Chem. Commun. 2010, 46, 3577–3579. [Google Scholar] [CrossRef]
- Palkovits, R.; Tajvidi, K.; Ruppertc, A.M.; Procelewska, J. Heteropoly acids as efficient acid catalysts in the one-step conversion of cellulose to sugar alcohols. Chem. Commun. 2011, 47, 576–578. [Google Scholar] [CrossRef]
- Kobayashi, H.; Ito, Y.; Komanoya, T.; Hosaka, Y.; Dhepe, P.L.; Kasai, K.; Hara, K.; Fukuoka, A. Synthesis of sugar alcohols by hydrolytic hydrogenation of cellulose over supported metal catalysts. Green Chem. 2011, 13, 326–333. [Google Scholar] [CrossRef]
- Geboers, J.; Van de Vyver, S.; Carpentier, K.; Jacobs, P.A.; Sels, B.F. Hydrolytic hydrogenation of cellulose with hydrotreated caesium salts of heteropoly acids and Ru/C. Chem. Commun. 2011, 47, 5590–5592. [Google Scholar] [CrossRef]
- Han, J.W.; Lee, H. Direct conversion of cellulose into sorbitol using dual-functionalized catalysts in neutral aqueous solution. Catal. Commun. 2012, 19, 115–118. [Google Scholar] [CrossRef]
- Yang, P.; Kobayashi, H.; Hara, K.; Fukuoka, A. Phase change of nickel phosphide catalysts in the conversion of cellulose into sorbitol. ChemSusChem 2012, 5, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Hilgert, J.; Meine, N.; Rinaldi, R.; Schuth, F. Mechano catalytic depolymerization of cellulose combined with hydrogenolysis as a highly efficient pathway to sugar alcohols. Energy Environ. Sci. 2013, 6, 92–96. [Google Scholar] [CrossRef]
- Negoi, A.; Triantafyllidis, K.; Parvulescu, V.I.; Coman, S.M. The hydrolytic hydrogenation of cellulose to sorbitol over M (Ru, Ir, Pd, Rh)-BEA-zeolite catalysts. Catal. Today 2014, 223, 122–128. [Google Scholar] [CrossRef]
- Mishra, D.K.; Dabbawala, A.A.; Park, J.J.; Jhung, S.H.; Hwang, J.-S. Selective hydrogenation of d-glucose to d-sorbitol over HY zeolite supported ruthenium nanoparticles catalysts. Catal. Today 2014, 232, 99–107. [Google Scholar] [CrossRef]
- Rose, M.; Palkovits, R. Isosorbide as a renewable platform chemical for versatile applications—Quo vadis. ChemSusChem 2012, 5, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.D.; Parker, J.O. Nitrate therapy for stable angina pectoris. N. Engl. J. Med. 1998, 338, 520–531. [Google Scholar] [CrossRef]
- Kricheldorf, H.R. “Sugar diols” as building blocks of polycondensates. J. Macromol. Chem. Phys. 1997, 37, 599–631. [Google Scholar] [CrossRef]
- Fenouillot, F.; Rousseau, A.; Colomines, G.; Saint-Loup, R.; Pascault, J.-P. Polymers from renewable 1,4:3,6-dianhydrohexitols (isosorbide, isomannide and isoidide): A review. Prog. Polym. Sci. 2010, 35, 578–622. [Google Scholar] [CrossRef]
- Gohil, R.M. Properties and strain hardening character of polyethylene terephthalate containing isosorbide. Polym. Eng. Sci. 2009, 49, 544–553. [Google Scholar] [CrossRef]
- Chatti, S.; Schwarz, G.; Kricheldorf, H.R. Cyclic and noncyclic polycarbonates of isosorbide (1,4:3,6-Dianhydro-D-glucitol). Macromolecules 2006, 39, 9064–9070. [Google Scholar] [CrossRef]
- Feng, X.; East, A.J.; Hammond, W.B.; Zhang, Y.; Jaffe, M. Overview of advances in sugar-based polymers. Polym. Adv. Technol. 2011, 22, 139–150. [Google Scholar] [CrossRef]
- Jeong, J.M.; Park, J.H.; Baek, J.H.; Hwang, R.H.; Jeon, S.G.; Yi, K.B. Effects of acid treatment of Fe-BEA zeolite on catalytic N2O conversion. Korean J. Chem. Eng. 2017, 34, 81–86. [Google Scholar] [CrossRef]
- Davoodpour, M.; Tafreshi, R.; Khodadadi, A.A.; Mortazavi, Y. Two-Stage cracking catalyst of amorphous silica-alumina on Y zeolite for enhanced product selectivity and suppressed coking. Korean J. Chem. Eng. 2017, 34, 681–691. [Google Scholar] [CrossRef]
- Treacy, M.M.J.; Higgins, J.B. Collection of Simulated XRD Powder Patterns for Zeolites; Elsevier: Amsterdam, the Netherlands, 2007; pp. 174–302. [Google Scholar]
- Katada, N.; Igi, H.; Kim, J.-H.; Niwa, M. Determination of the acidic properties of zeolite by theoretical analysis of temperature-programmed desorption of ammonia based on adsorption equilibrium. J. Phys. Chem. B 1997, 101, 5969–5977. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Katada, N.; Niwa, M. Acidity of β zeolite with different Si/Al2 ratio as measured by temperature programmed desorption of ammonia. Micropor. Mesopor. Mater. 2000, 40, 271–281. [Google Scholar] [CrossRef]
- Zhu, M.; Liu, Y.; Yao, Y.; Jiang, J.; Zhang, F.; Yang, Z.; Lu, Z.; Kumakiri, I.; Chen, X.; Kita, H. Preparation and catalytic performance of Ti-MWW zeolite membrane for phenol hydroxylation. Micropor. Mesopor. Mater. 2018, 268, 84–87. [Google Scholar] [CrossRef]
- Zholobenko, V.L.; Khodakov, A.Y.; Impéror-Clerc, M.; Durand, D.; Grillo, I. Initial stages of SBA-15 synthesis: An overview. Adv. Colloid Interface Sci. 2008, 142, 67–74. [Google Scholar] [CrossRef]
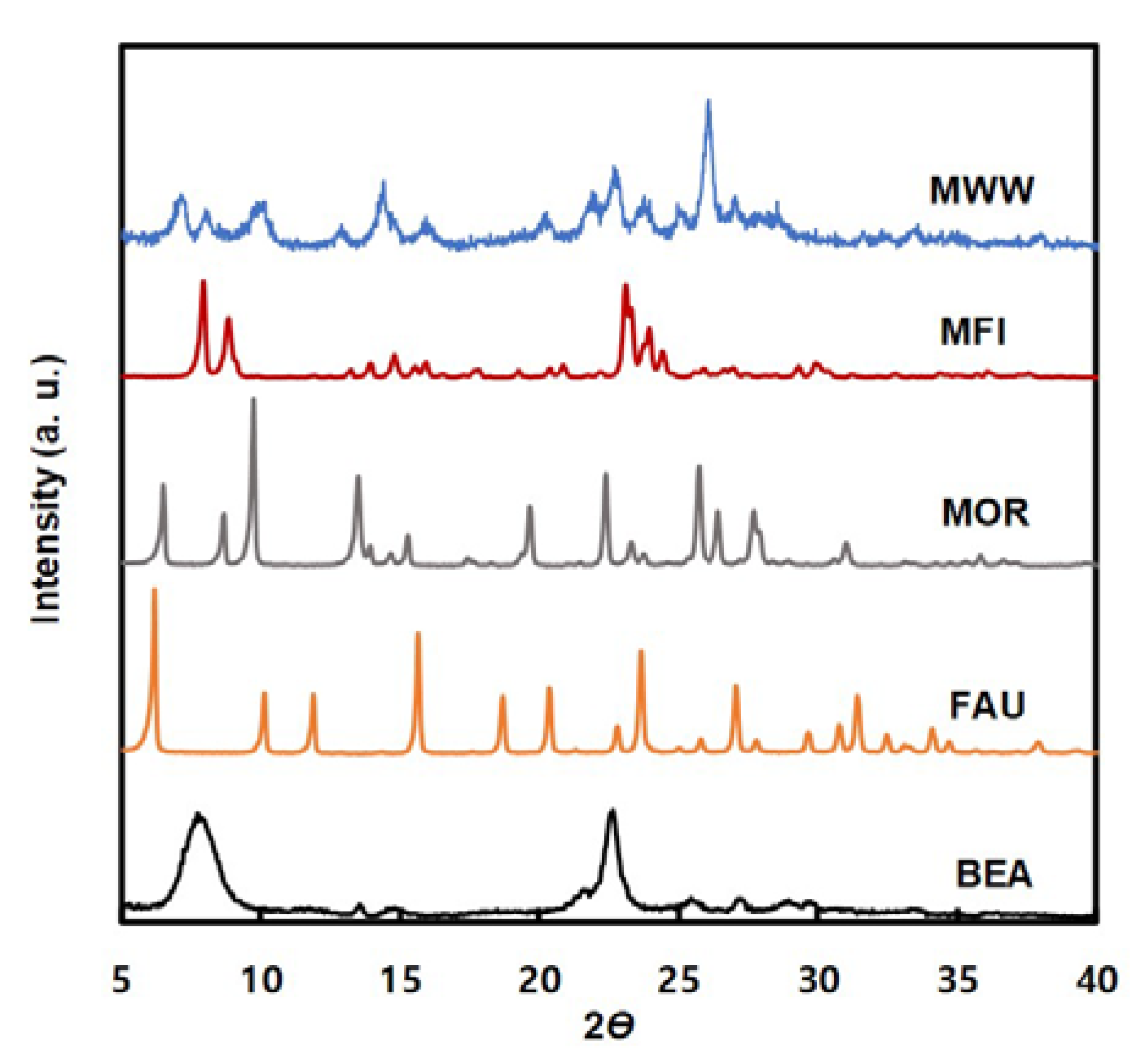
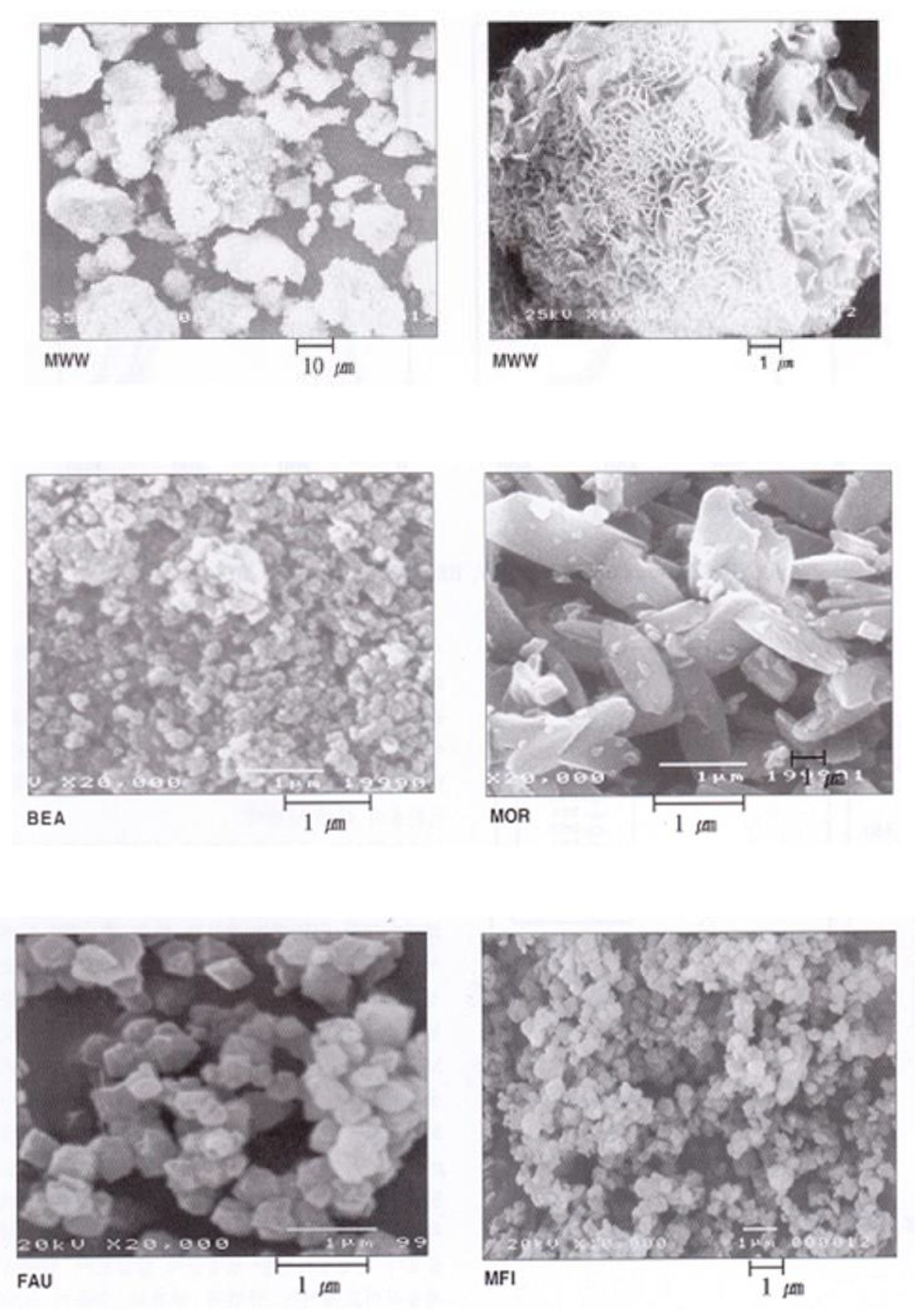
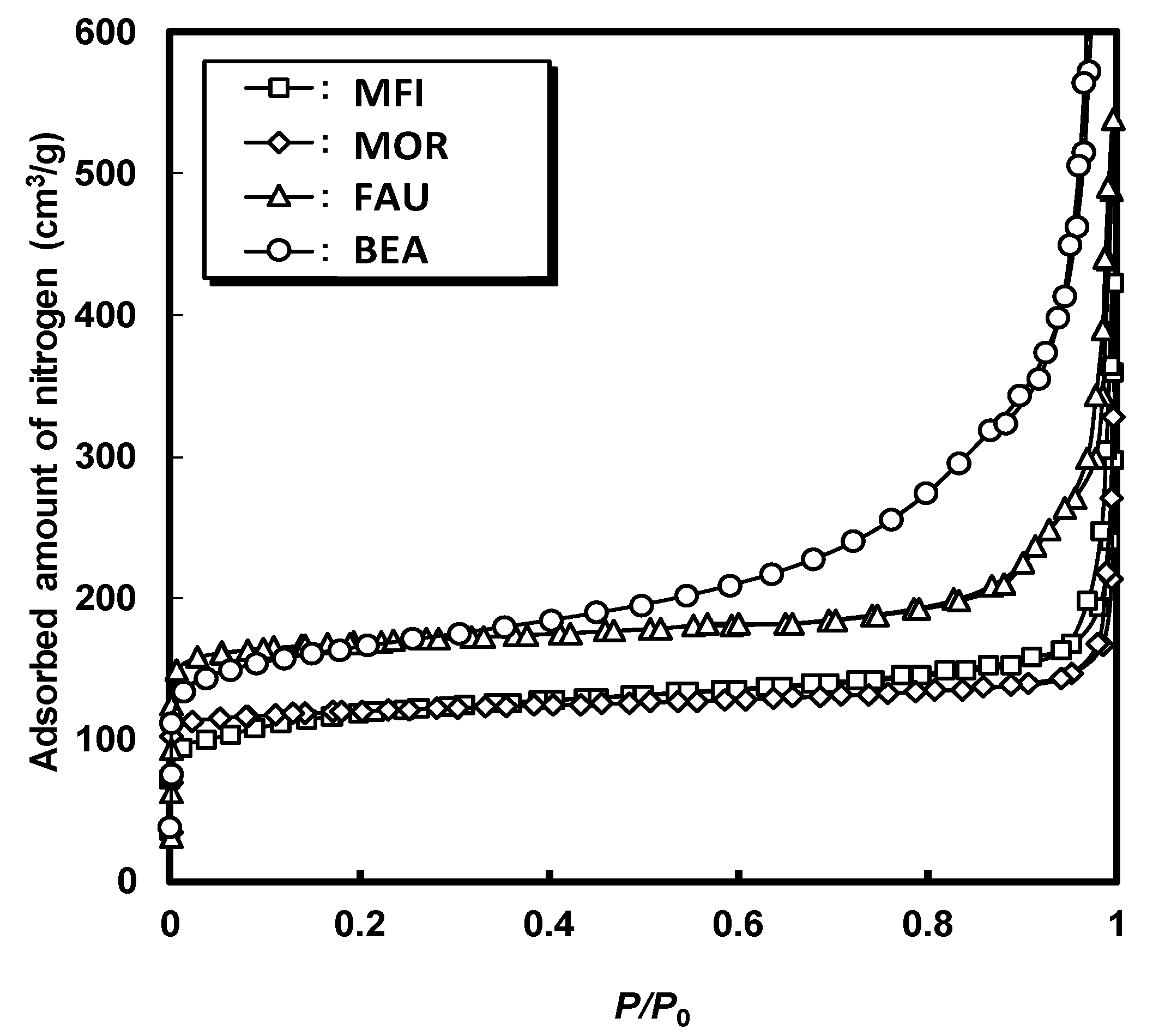


| Zeolite | Si/Al Molar Ratio (-) | Pore Diameter (Å) | BET Surface Area (m2/g) | Micropore Volume 1 (cm3/g) |
|---|---|---|---|---|
| MWW | 13 | 5.6 × 5.6 | 420 | 0.15 |
| BEA | 13 | 7.6 × 6.4, 5.5 × 5.5 | 690 | 0.19 |
| FAU | 5 | 7.4 × 7.4 | 700 | 0.24 |
| MOR | 10 | 6.5 × 7.0, 2.6 × 5.7 | 410 | 0.13 |
| MFI | 50 | 5.3 × 5.6, 5.1 × 5.5 | 260 | 0.12 |
| Catalyst | Conversion (%) | Yield (%) |
|---|---|---|
| BEA | 81.1 | 41.2 |
| MFI | 39.1 | 18.5 |
| FAU | 53.0 | 26.1 |
| SBA-15 | 10.1 | 5.2 |
| MWW | 15.7 | 11.2 |
| Amberlyst 35 | 76.1 | 36.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, S.; Jeon, K.-J.; Park, Y.-K.; Kim, B.-J.; Chung, K.-H.; Jung, S.-C. Catalytic Properties of Microporous Zeolite Catalysts in Synthesis of Isosorbide from Sorbitol by Dehydration. Catalysts 2020, 10, 148. https://doi.org/10.3390/catal10020148
Jeong S, Jeon K-J, Park Y-K, Kim B-J, Chung K-H, Jung S-C. Catalytic Properties of Microporous Zeolite Catalysts in Synthesis of Isosorbide from Sorbitol by Dehydration. Catalysts. 2020; 10(2):148. https://doi.org/10.3390/catal10020148
Chicago/Turabian StyleJeong, Sangmin, Ki-Joon Jeon, Young-Kwon Park, Byung-Joo Kim, Kyong-Hwan Chung, and Sang-Chul Jung. 2020. "Catalytic Properties of Microporous Zeolite Catalysts in Synthesis of Isosorbide from Sorbitol by Dehydration" Catalysts 10, no. 2: 148. https://doi.org/10.3390/catal10020148
APA StyleJeong, S., Jeon, K.-J., Park, Y.-K., Kim, B.-J., Chung, K.-H., & Jung, S.-C. (2020). Catalytic Properties of Microporous Zeolite Catalysts in Synthesis of Isosorbide from Sorbitol by Dehydration. Catalysts, 10(2), 148. https://doi.org/10.3390/catal10020148