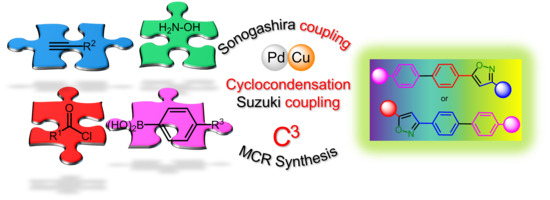
Rapid Sequentially Palladium Catalyzed Four-Component Synthesis of Novel Fluorescent Biaryl-Substituted Isoxazoles
Abstract
1. Introduction
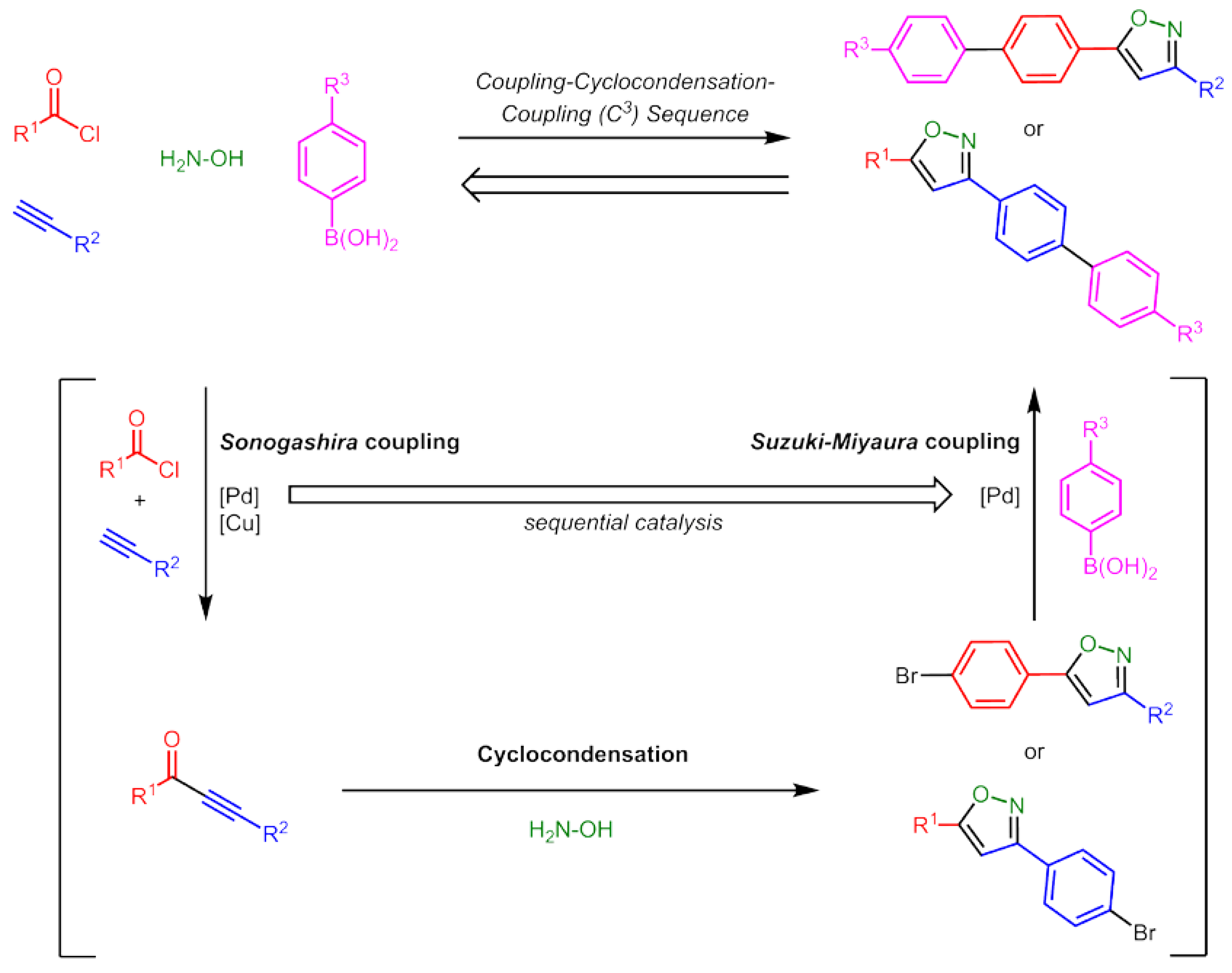
2. Results and Discussion
2.1. Synthesis and Structure
2.2. Photophysical Properties
2.3. Computational Studies and Electronic Structure
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References and Note
- Rajanarendar, E.; Rama Krishna, S.; Nagaraju, D.; Govardhan Reddy, K.; Kishore, B.; Reddy, Y.N. Environmentally benign synthesis, molecular properties prediction and anti-inflammatory activity of novel isoxazolo[5,4-d]isoxazol-3-yl-aryl-methanones via vinylogous Henry nitroaldol adducts as synthons. Bioorg. Med. Chem. Lett. 2015, 25, 1630–1634. [Google Scholar] [CrossRef]
- Banoglu, E.; Çelikoglu, E.; Volker, S.; Olgac, A.; Gerstmeier, J.; Garscha, U.; Caliskan, B.; Schubert, U.S.; Carotti, A.; Macchiarulo, A.; et al. 4,5-Diarylisoxazol-3-carboxylic acids: A new class of leukotriene biosynthesis inhibitors potentially targeting 5-lipoxygenaseactivating protein (FLAP). Eur. J. Med. Chem. 2016, 113, 1–10. [Google Scholar] [CrossRef]
- Siddiqui, N.; Idrees, M.; Khati, N.T.; Dhonde, M.G. Synthesis and antimicrobial activities of some new pyrazoles, oxadiazoles and isoxazole bearing benzofuran moiety. S. Afr. J. Chem. 2013, 66, 248–253. [Google Scholar]
- Basha, S.S.; Divya, K.; Padmaja, A.; Padmavathi, V. Synthesis and antimicrobial activity of thiazolyl pyrazoles and isoxazoles. Res. Chem. Intermed. 2015, 41, 10067–10083. [Google Scholar] [CrossRef]
- Lee, Y.S.; Park, S.M.; Kim, B.H. Synthesis of 5-isoxazol-5-yl-2′-deoxyuridines exhibiting antiviral activity against HSV and several RNA viruses. Bioorg. Med. Chem. Lett. 2009, 19, 1126–1128. [Google Scholar] [CrossRef]
- Kumbhare, R.M.; Kosurkar, U.B.; Janaki Ramaiah, M.; Dadmal, T.L.; Pushpavalli, S.N.C.V.L.; Pal-Bhadra, M. Synthesis and biological evaluation of novel triazoles and isoxazoles linked 2-phenyl benzothiazole as potential anticancer agents. Bioorg. Med. Chem. Lett. 2012, 22, 5424–5427. [Google Scholar] [CrossRef]
- Tzanetou, E.; Liekens, S.; Kasiotis, K.M.; Melagraki, G.; Afantitis, A.; Fokialakis, N.; Haroutounian, S.A. Antiproliferative novel isoxazoles: Modeling, virtual screening, synthesis, and bioactivity evaluation. Eur. J. Med. Chem. 2014, 81, 139–149. [Google Scholar] [CrossRef]
- Pairas, G.N.; Perperopoulou, F.; Tsoungas, P.G.; Varvounis, G. The Isoxazole Ring and Its N-Oxide: A Privileged Core Structure in Neuropsychiatric Therapeutics. ChemMedChem 2017, 12, 408–419. [Google Scholar] [CrossRef]
- Lamberth, C. Oxazole and Isoxazole Chemistry in Crop Protection. J. Heterocycl. Chem. 2018, 55, 2035–2045. [Google Scholar] [CrossRef]
- Sato, K.; Kawasaki, A.; Karuo, Y.; Tarui, A.; Kawai, K.; Omote, M. Synthesis of new fluorescent molecules having an aggregation-induced emission property derived from 4-fluoroisoxazoles. Beilstein J. Org. Chem. 2020, 16, 1411–1417. [Google Scholar] [CrossRef]
- de Brito, A.C.F.; Correa, R.S.; Pinto, A.A.; Matos, M.J.S.; Tenorio, J.C.; Taylor, J.G.; Cazati, T. Synthesis, crystal structure, photophysical properties and theoretical studies of a novel bis(phenylisoxazolyl) benzene derivative. J. Mol. Struct. 2018, 1163, 197–204. [Google Scholar] [CrossRef]
- Irfan, A.; Al-Sehemi, A.G.; Chaudhry, A.R.; Muhammad, S. A first-principles study of the linear and nonlinear optical properties of isoxazole derivatives. J. Theor. Comput. Chem. 2016, 15, 1650060. [Google Scholar] [CrossRef]
- Morita, T.; Yugandar, S.; Fuse, S.; Nakamura, H. Recent progresses in the synthesis of functionalized isoxazoles. Tetrahedron Lett. 2018, 59, 1159–1171. [Google Scholar] [CrossRef]
- Hu, F.; Szostak, M. Recent Developments in the Synthesis and Reactivity of Isoxazoles: Metal Catalysis and Beyond. Adv. Synth. Catal. 2015, 357, 2583–2614. [Google Scholar] [CrossRef]
- Chen, Y.; Li, L.; He, X.; Li, Z. Four-Component Reactions for the Synthesis of Perfluoroalkyl Isoxazoles. ACS Catal. 2019, 9, 9098–9102. [Google Scholar] [CrossRef]
- Thirukovela, N.S.; Balaboina, R.; Botla, V.; Vadde, R. One-pot regioselective synthesis of substituted pyrazoles and isoxazoles in PEG-400/water medium by Cu-free nano-Pd catalyzed sequential acyl Sonogashira coupling–intramolecular cyclization. Catal. Sci. Technol. 2019, 9, 6471–6481. [Google Scholar] [CrossRef]
- Wang, X.-D.; Zhu, L.-H.; Liu, P.; Wang, X.-Y.; Yuan, H.-Y.; Zhao, Y.-L. Copper-Catalyzed Cascade Cyclization Reactions of Diazo Compounds with tert-Butyl Nitrite and Alkynes: One-Pot Synthesis of Isoxazoles. J. Org. Chem. 2019, 84, 16214–16221. [Google Scholar] [CrossRef]
- Liu, H.; Geng, Z.; Zhang, S.; Han, J. One-pot three-component synthesis of 3,5-disubstituted isoxazoles by a coupling-cyclocondensation sequence. Heterocycles 2014, 89, 1221–1227. [Google Scholar] [CrossRef]
- Willy, B.; Rominger, F.; Müller, T.J.J. Novel Microwave-Assisted One-Pot Synthesis of Isoxazoles by a Three-Component Coupling-Cycloaddition Sequence. Synthesis 2008, 293–303. [Google Scholar] [CrossRef]
- Hu, X.-M.; Dong, H.; Li, Y.-D.; Huang, P.; Tiana, Z.; Wang, P.-A. Tandem grinding reactions involving aldol condensation and Michael addition in sequence for synthesis of 3,4,5-trisubstituted isoxazoles. RSC Adv. 2019, 9, 27883–27887. [Google Scholar] [CrossRef]
- Gayon, E.; Quinonero, O.; Lemouzy, S.; Vrancken, E.; Campagne, J.M. Transition-Metal-Catalyzed Uninterrupted Four-Step Sequence to Access Trisubstituted Isoxazoles. Org. Lett. 2011, 13, 6418–6421. [Google Scholar] [CrossRef]
- Berlham, I.B. On an Empirical Correlation between Nuclear Conformation and Certain Fluorescence and Absorption Characteristics of Aromatic Compounds. J. Phys. Chem. 1970, 74, 3085–3093. [Google Scholar] [CrossRef]
- Im, H.S.; Bernstein, E.R. Geometry and torsional motion of biphenyl in the ground and first excited singlet state. J. Chem. Phys. 1988, 88, 7337–7347. [Google Scholar] [CrossRef][Green Version]
- McFarland, S.A.; Finney, N.S. Fluorescent chemosensors based on conformational restriction of a biaryl fluorophore. J. Am. Chem. Soc. 2001, 123, 1260–1261. [Google Scholar] [CrossRef]
- May, L.; Daniel, S.; Müller, T.J.J. Diversity-oriented approach to functional thiophene dyes by Suzuki coupling-lithiation one-pot sequences. Org. Chem. Front. 2020, 7, 329–339. [Google Scholar] [CrossRef]
- Dohe, J.; Koßmann, J.; Müller, T.J.J. Diversity-oriented four-component synthesis of solid state luminescent difluoro oxazaborinines. Dyes Pigments 2018, 157, 198–217. [Google Scholar] [CrossRef]
- Burke, M.D.; Schreiber, S.L. A planning strategy for diversity-oriented synthesis. Angew. Chem. Int. Ed. 2004, 43, 46–58. [Google Scholar] [CrossRef]
- Denißen, M.; Nordmann, J.; Dziambor, J.; Mayer, B.; Frank, W.; Müller, T.J.J. Sequential palladium catalyzed coupling-cyclocondensation-coupling (C3) four-component synthesis of intensively blue luminescent biaryl substituted pyrazoles. RSC Adv. 2015, 5, 33838–33854. [Google Scholar] [CrossRef]
- Lessing, T.; Müller, T.J.J. Sequentially Palladium-Catalyzed Processes in One-Pot Syntheses of Heterocycles. Appl. Sci. 2015, 5, 1803–1836. [Google Scholar] [CrossRef]
- Müller, T.J.J. Sequential Catalysis Involving Metal Catalyzed Cycloisomerizations and Cyclizations. In Molecular Catalysts: Structure and Functional Design; Gade, L.H., Hofmann, P., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2014; pp. 255–279. [Google Scholar]
- Müller, T.J.J. Sequentially Palladium-Catalyzed Processes. Top. Organomet. Chem. 2006, 19, 149–205. [Google Scholar] [CrossRef]
- Gers-Panther, C.F.; Müller, T.J.J. Multicomponent syntheses of heterocycles initiated by catalytic generation of ynones and ynediones. Adv. Heterocycl. Chem. 2016, 120, 67–98. [Google Scholar] [CrossRef]
- Crystallographic data (excluding structure factors) for the structure reported in this paper have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication no. CCDC 2032844 (4k). Copies of the data can be obtained free of charge on application to CCDC, 12 Union Road, Cambridge CB2 1EZ, UK.
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer17, Version 17.5; University of Western Australia: Perth, Australia, 2017; Available online: http://hirshfeldsurface.net (accessed on 4 November 2020).
- McKinnon, J.J.; Spackman, M.A.; Mitchell, A.S. Novel tools for visualizing and exploring intermolecular interactions in molecular crystals. Acta Cryst. 2004, 60, 627–668. [Google Scholar] [CrossRef] [PubMed]
- Spackman, M.A.; McKinnon, J.J. Fingerprinting intermolecular interactions in molecular crystals. Cryst. Eng. Commun. 2002, 4, 378–392. [Google Scholar] [CrossRef]
- McKinnon, J.J.; Jayatilaka, D.; Spackman, M.A. Towards quantitative analysis of intermolecular interactions with Hirshfeld surfaces. Chem. Commun. 2007, 37, 3814–3816. [Google Scholar] [CrossRef] [PubMed]
- Qin, N.W.; Basarić, N.; Hofkens, J.; Ameloot, M.; Pouget, J.; Lefèvre, J.-P.; Valeur, B.; Gratton, E.; van de Ven, M.; Silva, N.D., Jr.; et al. Fluorescence Lifetime Standards for Time and Frequency Domain Fluorescence Spectroscopy. Anal. Chem. 2007, 79, 2137–2149. [Google Scholar] [CrossRef]
- Reynolds, G.A.; Drexhage, K.H. New coumarin dyes with rigidized structure for flashlamp-pumped dye lasers. Opt. Commun. 1975, 13, 222–225. [Google Scholar] [CrossRef]
- Jortner, J. Radiationless transitions. Pure Appl. Chem. 1971, 27, 389–420. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A. Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Becke, A.D. A new mixing of Hartree–Fock and local density-functional theories. J. Chem. Phys. 1993, 98, 1372–1377. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Kim, K.; Jordan, K.D. Comparison of Density Functional and MP2 Calculations on the Water Monomer and Dimer. J. Phys. Chem. 1994, 98, 10089–10094. [Google Scholar] [CrossRef]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Scalmani, G.; Frisch, M.J. Continuous surface charge polarizable continuum models of solvation. I. General formalism. J. Chem. Phys. 2010, 132, 114110. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, M.; Watanabe, T.; Kakihana, M. Internal Rotation of Biphenyl in Solution Studied by IR an NMR Spectra. J. Phys. Chem. 1986, 90, 1752–1755. [Google Scholar] [CrossRef]
- Bauernschmitt, R.; Ahlrichs, R. Treatment of electronic excitations within the adiabatic approximation of time dependent density functional theory. Chem. Phys. Lett. 1996, 256, 454–464. [Google Scholar] [CrossRef]
- Casida, M.E.; Jamorski, C.; Casida, K.C.; Salahub, D.R. Molecular excitation energies to high-lying bound states from time-dependent density-functional response theory: Characterization and correction of the time-dependent local density approximation ionization threshold. J. Chem. Phys. 1998, 108, 4439–4449. [Google Scholar] [CrossRef]
- Stratmann, R.E.; Scuseria, G.E.; Frisch, M.J. An efficient implementation of time-dependent density-functional theory for the calculation of excitation energies of large molecules. J. Chem. Phys. 1998, 109, 8218–8224. [Google Scholar] [CrossRef]
- Yanai, T.; Tew, D.P.; Handy, N.C. A new hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]










| Entry | Acid Chloride 1 | Boronic Acid 3 | 3-Biaryl-substituted Isoxazoles 4 |
|---|---|---|---|
| 1 | R1 = Ph (1a) | R3 = p-MeC6H4 (3a) |  4a (80%) 4a (80%) |
| 2 | R1 = p-MeOC6H4 (1b) | 3a |  4b (66%) 4b (66%) |
| 3 | R1 = o-FC6H4 (1c) | 3a |  4c (36%) 4c (36%) |
| 4 | R1 = p-F3CC6H4 (1d) | 3a |  4d (25%) 4d (25%) |
| 5 | R1 = m-ClC6H4 (1e) | 3a |  4e (26%) 4e (26%) |
| 6 | 1a | R3 = p-MeOC6H4 (3b) |  4f (74%) 4f (74%) |
| 7 | 1a | R3 = p-NCC6H4 (3c) |  4g (71%) 4g (71%) |
| 8 | 1a | R3 = p-Me2NC6H4 (3d) |  4h (27%) 4h (27%) |
| 9 | 1a | R3 = p-OHCC6H4 (3e) |  4i (53%) 4i (53%) |
| 10 | 1a | R3 = p-O2NC6H4 (3f) |  4j (18%) 4j (18%) |
| 11 | 1b | 3c |  4k (51%) 4k (51%) |
| Entry | Alkyne 2 | Boronic Acid 3 | 5-Biaryl-substituted Isoxazoles 5 |
|---|---|---|---|
| 1 | R2 = Ph (2b) | R3 = p-MeC6H4 (3a) |  5a (49%) 5a (49%) |
| 2 | 2b | R3 = p-MeOC6H4 (3b) |  5b (29%) 5b (29%) |
| 3 | R2 = p-F3CC6H4 (2c) | 3b |  5c (13%) 5c (13%) |
| 4 | R2 = p-NCC6H4 (2d) | 3b |  5d (6%) 5d (6%) |
| 5 | 2d | R3 = p-Me2NC6H4 (3d) |  5e (34%) 5e (34%) |
| Compound | Absorption Maxima λmax,abs (nm) (ε (M−1 cm−1)) | Emission Maxima λmax,em (nm) (ΦF) | Stokes Shift a Δ (cm−1) |
|---|---|---|---|
| 4k | 294 (66,000) | 423 (0.17) b | 10,400 |
| 5c | 311 (40,000) | 376 (0.86) b | 5600 |
| 5d | 311 (45,100) | 411 (0.62) b | 7800 |
| 5e | 356 (34,100) | 554 (0.69) c | 10,000 |
| Compound | Emission Maxima λmax,em (nm) (ΦF) |
|---|---|
| 4k | 448 (0.07) a |
| 5c | 391 (<0.01) a |
| 5d | 405 (<0.01) a |
| 5e | 508 (<0.01) b |
| Compound | Torsional Angle α | Torsional Angle β | Torsional Angle γ |
|---|---|---|---|
 4k 4k | 0.5° | 12.8° | 36.3° |
 5d 5d | 0.1° | 9.0° | 34.8° |
| Compound | Experimental λmax,abs (nm) (ε (m−1 cm−1)) | Calculated λmax,abs (nm) (Oscillatory Strength) | Most Dominant Contributions |
|---|---|---|---|
| 4k | 294 (66,000) | 285 (2.187) | HOMO-1→LUMO (65%) HOMO→LUMO+1 (27%) |
| 5c | 311 (40,000) | 302 (1.436) | HOMO→LUMO (67%) HOMO→LUMO+1 (20%) |
| 5d | 311 (45,100) | 303 (1.496) | HOMO→LUMO (37%) HOMO→LUMO+1 (50%) |
| 5e | 356 (34,100) | 333 (2.187) | HOMO→LUMO (30%) HOMO→LUMO+1 (54%) |
| Compound | dFMO (Å) | <HOMO|LUMO> | HOMO→LUMO (%) |
|---|---|---|---|
| 5c | 6.143 | 0.541 | 67 |
| 5d | 9.005 | 0.338 | 37 |
| 5e | 11.136 | 0.221 | 30 |
Sample Availability: Samples of the compounds 4f, 4g, and 5e are available from the authors. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deden, T.; May, L.; Reiss, G.J.; Müller, T.J.J. Rapid Sequentially Palladium Catalyzed Four-Component Synthesis of Novel Fluorescent Biaryl-Substituted Isoxazoles. Catalysts 2020, 10, 1412. https://doi.org/10.3390/catal10121412
Deden T, May L, Reiss GJ, Müller TJJ. Rapid Sequentially Palladium Catalyzed Four-Component Synthesis of Novel Fluorescent Biaryl-Substituted Isoxazoles. Catalysts. 2020; 10(12):1412. https://doi.org/10.3390/catal10121412
Chicago/Turabian StyleDeden, Tobias, Lars May, Guido J. Reiss, and Thomas J. J. Müller. 2020. "Rapid Sequentially Palladium Catalyzed Four-Component Synthesis of Novel Fluorescent Biaryl-Substituted Isoxazoles" Catalysts 10, no. 12: 1412. https://doi.org/10.3390/catal10121412
APA StyleDeden, T., May, L., Reiss, G. J., & Müller, T. J. J. (2020). Rapid Sequentially Palladium Catalyzed Four-Component Synthesis of Novel Fluorescent Biaryl-Substituted Isoxazoles. Catalysts, 10(12), 1412. https://doi.org/10.3390/catal10121412