Hybrid Molybdenum Carbide/Heteroatom-Doped Carbon Electrocatalyst for Advanced Oxygen Evolution Reaction in Hydrogen Production
Abstract
1. Introduction
2. Results and Discussion
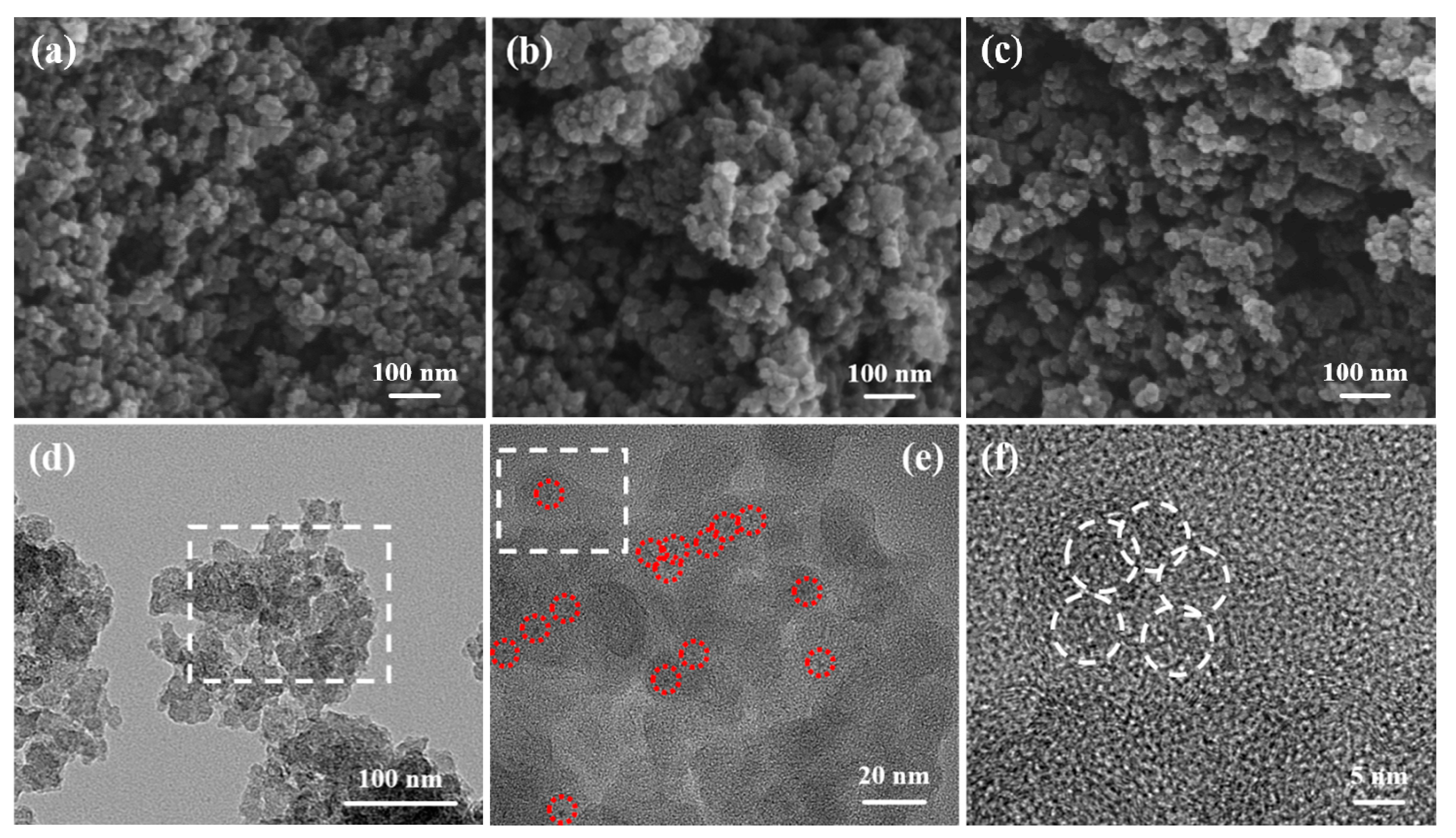
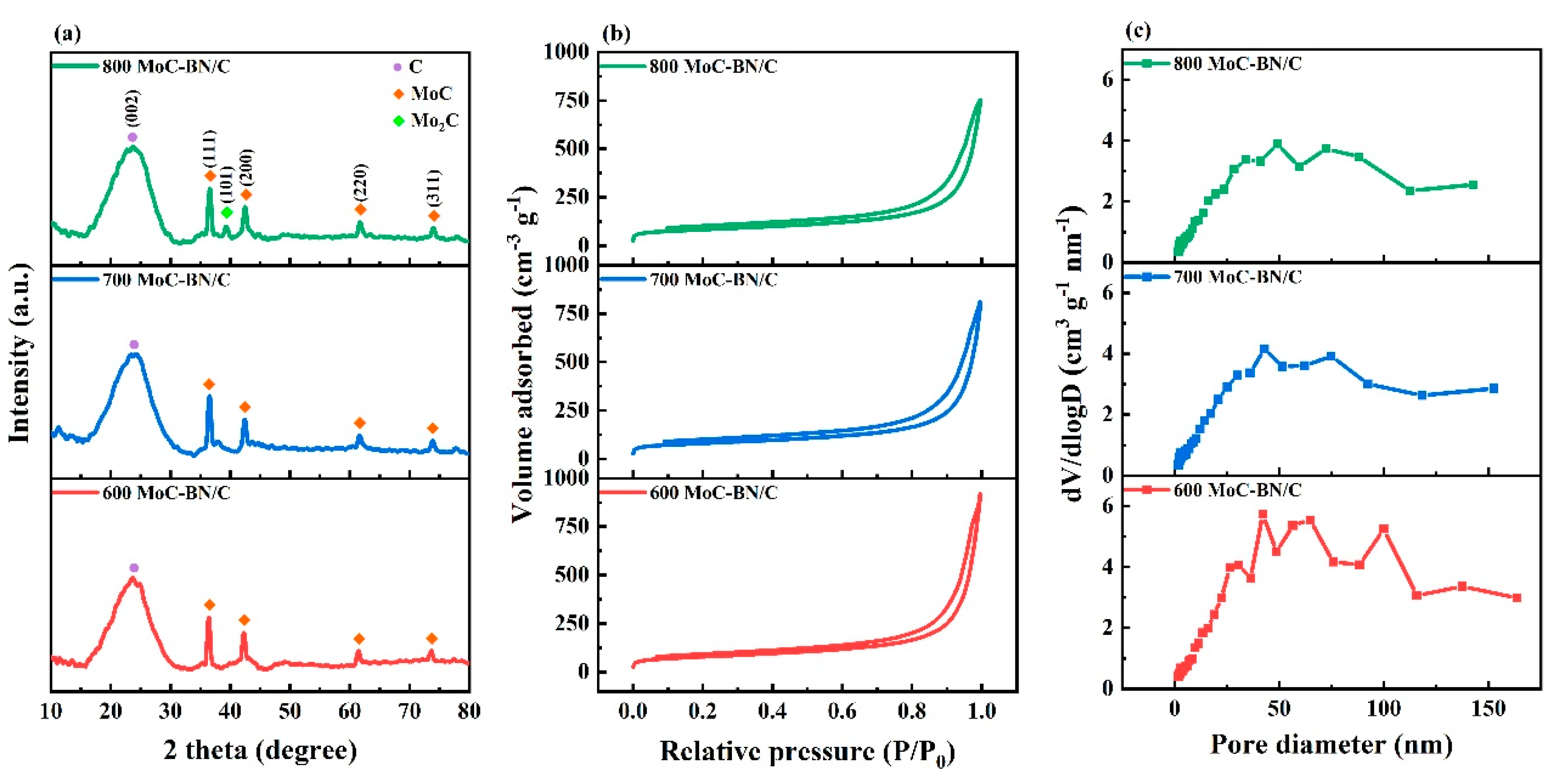
2.1. Morphology and Physical Properties
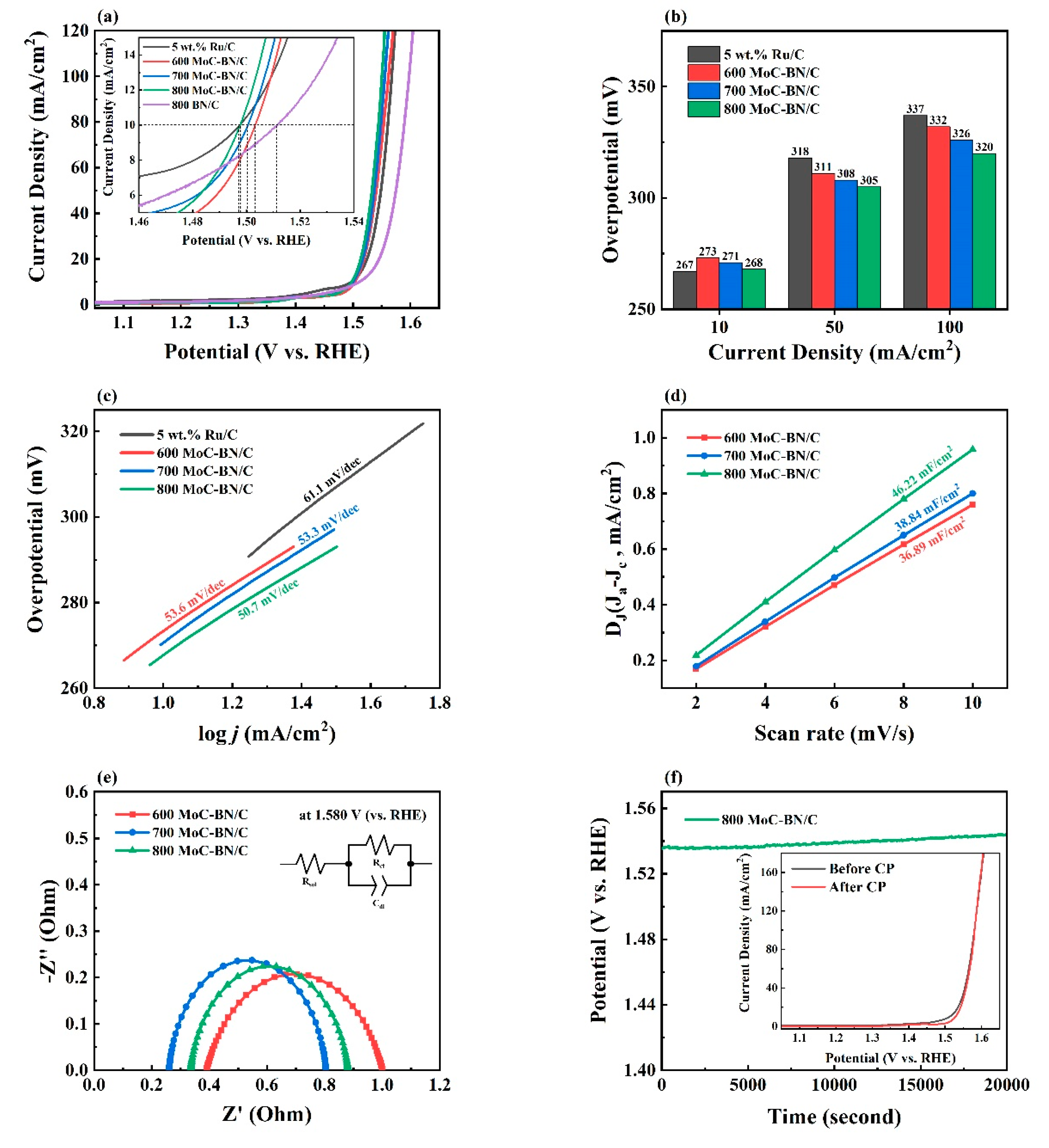
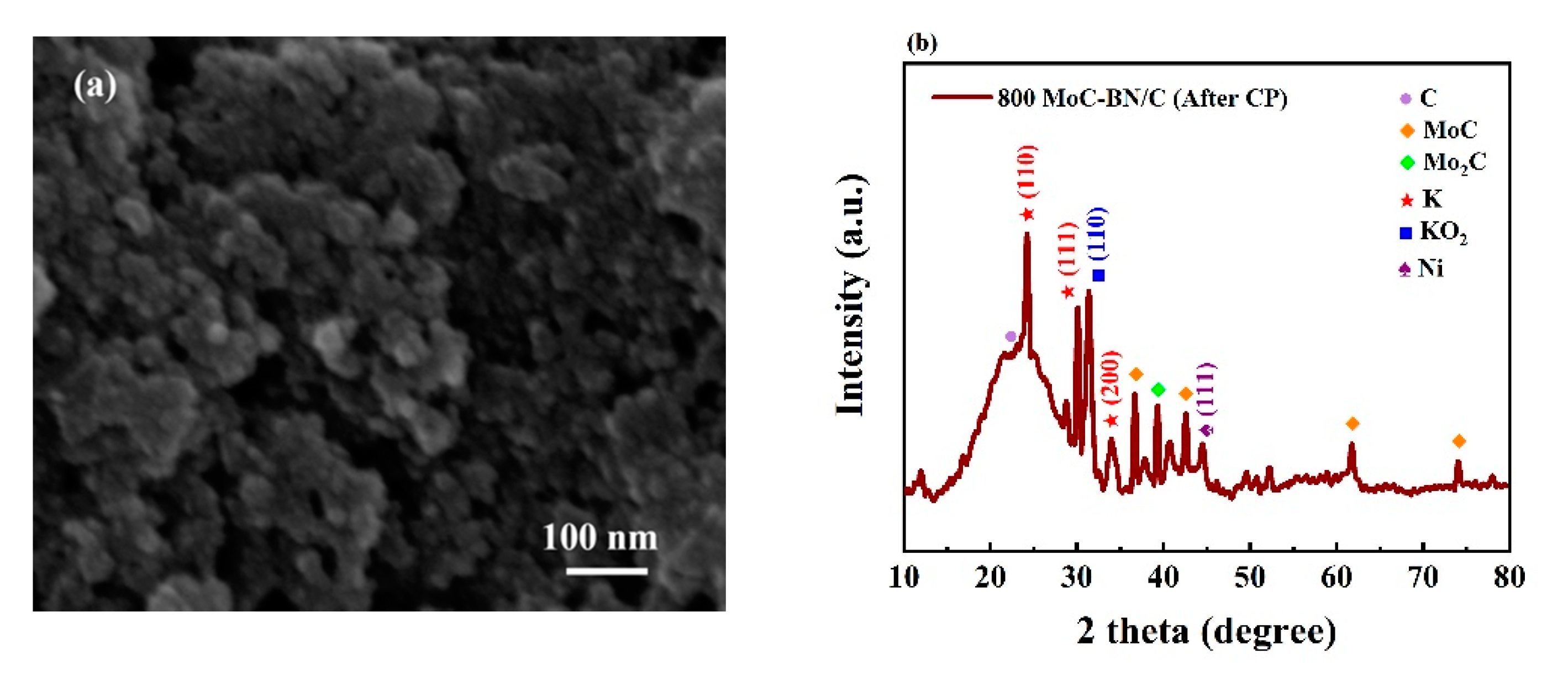
2.2. Electrochemical Performances for the OER
3. Materials and Methods
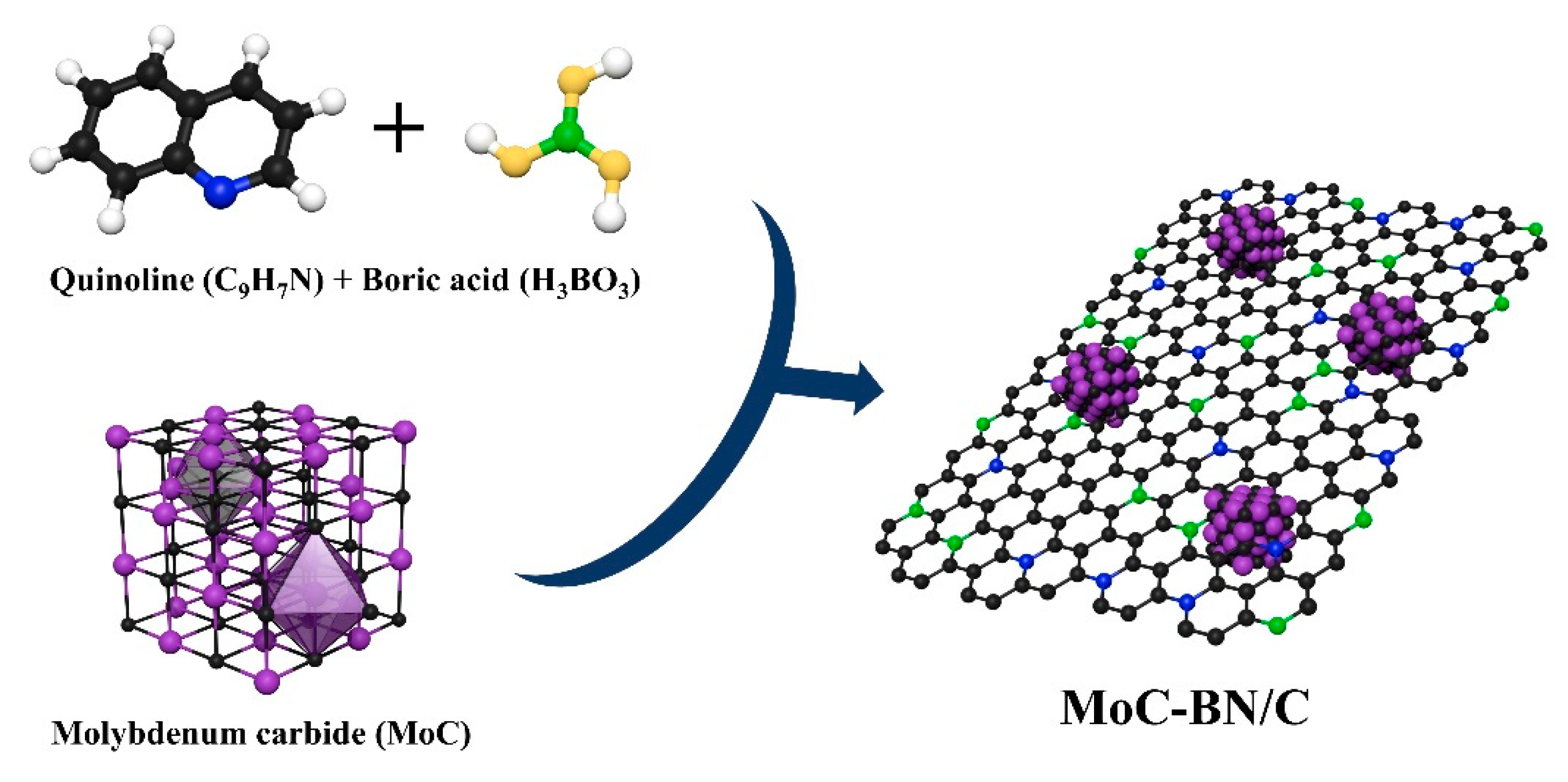
3.1. Synthesis of MoC-BN/C by Plasma Engineering
3.2. Material Characterization
3.3. Electrochemical Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, L.-N.; Hou, K.-P.; Liu, Y.-S.; Qi, Z.-Y.; Zheng, Q.; Lu, Y.-H.; Chen, J.-Y.; Chen, J.-L.; Pao, C.-W.; Wang, S.-B. Efficient hydrogen production from methanol using a single-site Pt1/CeO2 catalyst. J. Am. Chem. Soc. 2019, 141, 17995–17999. [Google Scholar] [CrossRef] [PubMed]
- Chandran, P.; Ghosh, A.; Ramaprabhu, S. High-performance Platinum-free oxygen reduction reaction and hydrogen oxidation reaction catalyst in polymer electrolyte membrane fuel cell. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Pedersen, C.M.; Escudero-Escribano, M.; Velázquez-Palenzuela, A.; Christensen, L.H.; Chorkendorff, I.; Stephens, I.E. Benchmarking Pt-based electrocatalysts for low temperature fuel cell reactions with the rotating disk electrode: Oxygen reduction and hydrogen oxidation in the presence of CO. Electrochim. Acta 2015, 179, 647–657. [Google Scholar] [CrossRef]
- Nwanebu, E.O.; Yao, Y.; Omanovic, S. The influence of Ir content in (Ni0. 4Co0. 6) 1-xIrx-oxide anodes on their electrocatalytic activity in oxygen evolution by acidic and alkaline water electrolysis. J. Electroanal. Chem. 2020, 114122. [Google Scholar] [CrossRef]
- Yan, Y.; Xia, B.Y.; Zhao, B.; Wang, X. A review on noble-metal-free bifunctional heterogeneous catalysts for overall electrochemical water splitting. J. Mater. Chem. A 2016, 4, 17587–17603. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, W.; Chen, Z.G.; Chen, Y.; Su, C.; Tadé, M.O.; Shao, Z. SrNb0. 1Co0. 7Fe0. 2O3− δ perovskite as a next-generation electrocatalyst for oxygen evolution in alkaline solution. Angew. Chem. 2015, 127, 3969–3973. [Google Scholar] [CrossRef]
- Shinde, S.S.; Lee, C.-H.; Sami, A.; Kim, D.-H.; Lee, S.-U.; Lee, J.-H. Scalable 3-D carbon nitride sponge as an efficient metal-free bifunctional oxygen electrocatalyst for rechargeable Zn–air batteries. ACS Nano 2017, 11, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Suntivich, J.; May, K.J.; Perry, E.E.; Shao-Horn, Y. Synthesis and activities of rutile IrO2 and RuO2 nanoparticles for oxygen evolution in acid and alkaline solutions. J. Phys. Chem. Lett. 2012, 3, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-F.; King, G.; Dickerson, R.M.; Papin, P.A.; Gupta, S.; Kellogg, W.R.; Wu, G. Oxygen-deficient BaTiO3− x perovskite as an efficient bifunctional oxygen electrocatalyst. Nano Energy 2015, 13, 423–432. [Google Scholar] [CrossRef]
- Anjum, M.A.R.; Lee, M.H.; Lee, J.S. Boron-and nitrogen-codoped molybdenum carbide nanoparticles imbedded in a BCN network as a bifunctional electrocatalyst for hydrogen and oxygen evolution reactions. ACS Catal. 2018, 8, 8296–8305. [Google Scholar] [CrossRef]
- Filimonenkov, I.S.; Bouillet, C.; Kéranguéven, G.; Simonov, P.A.; Tsirlina, G.A.; Savinova, E.R. Carbon materials as additives to the OER catalysts: RRDE study of carbon corrosion at high anodic potentials. Electrochim. Acta 2019, 321, 134657. [Google Scholar] [CrossRef]
- Murdachaew, G.; Laasonen, K. Oxygen evolution reaction on nitrogen-doped defective carbon nanotubes and graphene. J. Phys. Chem. C 2018, 122, 25882–25892. [Google Scholar] [CrossRef]
- Jiang, H.; Gu, J.; Zheng, X.; Liu, M.; Qiu, X.; Wang, L.; Li, W.; Chen, Z.; Ji, X.; Li, J. Defect-rich and ultrathin N doped carbon nanosheets as advanced trifunctional metal-free electrocatalysts for the ORR, OER and HER. Energy Environ. Sci. 2019, 12, 322–333. [Google Scholar] [CrossRef]
- Gu, J.; Magagula, S.; Zhao, J.; Chen, Z. Boosting ORR/OER activity of graphdiyne by simple heteroatom doping. Small Methods 2019, 3, 1800550. [Google Scholar] [CrossRef]
- Vineesh, T.V.; Kumar, M.P.; Takahashi, C.; Kalita, G.; Alwarappan, S.; Pattanayak, D.K.; Narayanan, T.N. Bifunctional electrocatalytic activity of boron-doped graphene derived from boron carbide. Adv. Energy Mater. 2015, 5, 1500658. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Z.; Xia, Z.; Dai, L. A metal-free bifunctional electrocatalyst for oxygen reduction and oxygen evolution reactions. Nat. Nanotechnol. 2015, 10, 444–452. [Google Scholar] [CrossRef]
- El-Sawy, A.M.; Mosa, I.M.; Su, D.; Guild, C.J.; Khalid, S.; Joesten, R.; Rusling, J.F.; Suib, S.L. Controlling the active sites of sulfur-doped carbon nanotube–graphene nanolobes for highly efficient oxygen evolution and reduction catalysis. Adv. Energy Mater. 2016, 6, 1501966. [Google Scholar] [CrossRef]
- Sun, T.; Wang, J.; Qiu, C.; Ling, X.; Tian, B.; Chen, W.; Su, C. B, N Codoped and Defect-Rich Nanocarbon Material as a Metal-Free Bifunctional Electrocatalyst for Oxygen Reduction and Evolution Reactions. Adv. Sci. 2018, 5, 1800036. [Google Scholar] [CrossRef]
- Huang, X.; Wang, Q.; Jiang, D.; Huang, Y. Facile synthesis of B, N co-doped three-dimensional porous graphitic carbon toward oxygen reduction reaction and oxygen evolution reaction. Catal. Commun. 2017, 100, 89–92. [Google Scholar] [CrossRef]
- Jiang, H.; Zhao, T.; Shi, L.; Tan, P.; An, L. First-principles study of nitrogen-, boron-doped graphene and co-doped graphene as the potential catalysts in nonaqueous Li–O2 batteries. J. Phys. Chem. C 2016, 120, 6612–6618. [Google Scholar] [CrossRef]
- Li, O.L.; Pham, N.N.; Kim, J.; Choi, H.; Lee, D.H.; Yang, Y.; Yao, W.; Cho, Y.-R.; Lee, S.G. Insights on boosting oxygen evolution reaction performance via boron incorporation into nitrogen-doped carbon electrocatalysts. Appl. Surf. Sci. 2020, 528, 146979. [Google Scholar] [CrossRef]
- Cao, X.; Johnson, E.; Nath, M. Identifying high-efficiency oxygen evolution electrocatalysts from Co–Ni–Cu based selenides through combinatorial electrodeposition. J. Mater. Chem. A 2019, 7, 9877–9889. [Google Scholar] [CrossRef]
- Liu, P.F.; Yang, S.; Zheng, L.R.; Zhang, B.; Yang, H.G. Mo 6+ activated multimetal oxygen-evolving catalysts. Chem. Sci. 2017, 8, 3484–3488. [Google Scholar] [CrossRef]
- Regmi, Y.N.; Wan, C.; Duffee, K.D.; Leonard, B.M. Nanocrystalline Mo2C as a bifunctional water splitting electrocatalyst. ChemCatChem 2015, 7, 3911–3915. [Google Scholar] [CrossRef]
- Kim, M.; Kim, S.; Song, D.; Oh, S.; Chang, K.J.; Cho, E. Promotion of electrochemical oxygen evolution reaction by chemical coupling of cobalt to molybdenum carbide. Appl. Catal. B 2018, 227, 340–348. [Google Scholar] [CrossRef]
- Jiang, J.; Liu, Q.; Zeng, C.; Ai, L. Cobalt/molybdenum carbide@ N-doped carbon as a bifunctional electrocatalyst for hydrogen and oxygen evolution reactions. J. Mater. Chem. A 2017, 5, 16929–16935. [Google Scholar] [CrossRef]
- Morishita, T.; Ueno, T.; Panomsuwan, G.; Hieda, J.; Yoshida, A.; Bratescu, M.A.; Saito, N. Fastest formation routes of nanocarbons in solution plasma processes. Sci. Rep. 2016, 6, 36880. [Google Scholar] [CrossRef]
- Panomsuwan, G.; Saito, N.; Ishizaki, T. Electrocatalytic oxygen reduction activity of boron-doped carbon nanoparticles synthesized via solution plasma process. Electrochem. Commun. 2015, 59, 81–85. [Google Scholar] [CrossRef]
- Lee, S.-H. Solution Plasma Synthesis of BNC Nanocarbon for Oxygen Reduction Reaction. J. Korean Inst. Surf. Eng. 2018, 51, 332–336. [Google Scholar] [CrossRef]
- Li, O.L.; Chiba, S.; Wada, Y.; Lee, H.; Ishizaki, T. Selective nitrogen bonding states in nitrogen-doped carbon via a solution plasma process for advanced oxygen reduction reaction. RSC Adv. 2016, 6, 109354–109360. [Google Scholar] [CrossRef]
- Panomsuwan, G.; Chiba, S.; Kaneko, Y.; Saito, N.; Ishizaki, T. In situ solution plasma synthesis of nitrogen-doped carbon nanoparticles as metal-free electrocatalysts for the oxygen reduction reaction. J. Mater. Chem. A 2014, 2, 18677–18686. [Google Scholar] [CrossRef]
- Hyun, K.; Saito, N. The solution plasma process for heteroatom-carbon nanosheets: The role of precursors. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Ishizaki, T.; Wada, Y.; Chiba, S.; Kumagai, S.; Lee, H.; Serizawa, A.; Li, O.L.; Panomsuwan, G. Effects of halogen doping on nanocarbon catalysts synthesized by a solution plasma process for the oxygen reduction reaction. Phys. Chem. Chem. Phys. 2016, 18, 21843–21851. [Google Scholar] [CrossRef]
- Kang, J.; Li, O.L.; Saito, N. A simple synthesis method for nano-metal catalyst supported on mesoporous carbon: The solution plasma process. Nanoscale 2013, 5, 6874–6882. [Google Scholar] [CrossRef]
- Panomsuwan, G.; Chantaramethakul, J.; Chokradjaroen, C.; Ishizaki, T. In situ solution plasma synthesis of silver nanoparticles supported on nitrogen-doped carbons with enhanced oxygen reduction activity. Mater. Lett. 2019, 251, 135–139. [Google Scholar] [CrossRef]
- Kim, S.; Park, H.; Li, O.L. Cobalt Nanoparticles on Plasma-Controlled Nitrogen-Doped Carbon as High-Performance ORR Electrocatalyst for Primary Zn-Air Battery. Nanomaterials 2020, 10, 223. [Google Scholar] [CrossRef]
- Sing, K.S. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Xu, Y.H.; Liu, Q.; Zhu, Y.J.; Liu, Y.H.; Langrock, A.; Zachariah, M.R.; Wang, C.S. Uniform nano-Sn/C composite anodes for lithium ion batteries. Nano Lett. 2013, 13, 470–474. [Google Scholar] [CrossRef]
- Xia, Y.; Zheng, J.M.; Wang, C.M.; Gu, M. Designing principle for Ni-rich cathode materials with high energy density for practical applications. Nano Energy 2018, 49, 434–452. [Google Scholar] [CrossRef]
- Chen, Y.; Niu, Y.; Tian, T.; Zhang, J.; Wang, Y.; Li, Y.; Qin, L.-C. Microbial reduction of graphene oxide by Azotobacter chroococcum. Chem. Phys. Lett. 2017, 677, 143–147. [Google Scholar] [CrossRef]
- Li, O.L.; Chiba, S.; Wada, Y.; Panomsuwan, G.; Ishizaki, T. Synthesis of graphitic-N and amino-N in nitrogen-doped carbon via a solution plasma process and exploration of their synergic effect for advanced oxygen reduction reaction. J. Mater. Chem. A 2017, 5, 2073–2082. [Google Scholar] [CrossRef]
- Peng, H.; Mo, Z.; Liao, S.; Liang, H.; Yang, L.; Luo, F.; Song, H.; Zhong, Y.; Zhang, B. High performance Fe-and N-doped carbon catalyst with graphene structure for oxygen reduction. Sci. Rep. 2013, 3, 1765. [Google Scholar] [CrossRef]
- Mutuma, B.K.; Matsoso, B.J.; Momodu, D.; Oyedotun, K.O.; Coville, N.J.; Manyala, N. Deciphering the structural, textural, and electrochemical properties of activated BN-doped spherical carbons. Nanomaterials 2019, 9, 446. [Google Scholar] [CrossRef]
- Lin, T.W.; Su, C.Y.; Zhang, X.Q.; Zhang, W.; Lee, Y.H.; Chu, C.W.; Lin, H.Y.; Chang, M.T.; Chen, F.R.; Li, L.J. Converting graphene oxide monolayers into boron carbonitride nanosheets by substitutional doping. Small 2012, 8, 1384–1391. [Google Scholar] [CrossRef]
- Sunu, S.; Prabhu, E.; Jayaraman, V.; Gnanasekar, K.; Seshagiri, T.; Gnanasekaran, T. Electrical conductivity and gas sensing properties of MoO3. Sens. Actuators B Chem. 2004, 101, 161–174. [Google Scholar] [CrossRef]
- Wei, H.; Xi, Q.; Chen, X. a.; Guo, D.; Ding, F.; Yang, Z.; Wang, S.; Li, J.; Huang, S. Molybdenum carbide nanoparticles coated into the graphene wrapping n-doped porous carbon microspheres for highly efficient electrocatalytic hydrogen evolution both in acidic and alkaline media. Adv. Sci. 2018, 5, 1700733. [Google Scholar] [CrossRef]
- Li, O.L.; Hayashi, H.; Ishizaki, T.; Saito, N. Enhancement of conductivity in nano carbon balls by the addition of carbon tetrachloride via room temperature solution plasma process. RSC Adv. 2016, 6, 51864–51870. [Google Scholar] [CrossRef]
- Song, F.; Hu, X. Exfoliation of layered double hydroxides for enhanced oxygen evolution catalysis. Nat. Commun. 2014, 5, 1–9. [Google Scholar] [CrossRef]
- Jung, S.; McCrory, C.C.; Ferrer, I.M.; Peters, J.C.; Jaramillo, T.F. Benchmarking nanoparticulate metal oxide electrocatalysts for the alkaline water oxidation reaction. J. Mater. Chem. A 2016, 4, 3068–3076. [Google Scholar] [CrossRef]
- Lin, Y.; Tian, Z.; Zhang, L.; Ma, J.; Jiang, Z.; Deibert, B.J.; Ge, R.; Chen, L. Chromium-ruthenium oxide solid solution electrocatalyst for highly efficient oxygen evolution reaction in acidic media. Nat. Commun. 2019, 10, 1–13. [Google Scholar] [CrossRef]
- Merki, D.; Vrubel, H.; Rovelli, L.; Fierro, S.; Hu, X. Fe, Co, and Ni ions promote the catalytic activity of amorphous molybdenum sulfide films for hydrogen evolution. Chem. Sci. 2012, 3, 2515–2525. [Google Scholar] [CrossRef]
- Zhang, H.; Zheng, J.; Chao, Y.; Zhang, K.; Zhu, Z. Surface engineering of FeCo-based electrocatalysts supported on carbon paper by incorporating non-noble metals for water oxidation. New J. Chem. 2018, 42, 7254–7261. [Google Scholar] [CrossRef]

| 600 MoC-BN/C | 700 MoC-BN/C | 800 MoC-BN/C | |
|---|---|---|---|
| BET surface area | 281.15 m2/g | 284.44 m2/g | 295.16 m2/g |
| Total pore volume | 1.34 cm3/g | 1.16 cm3/g | 1.09 cm3/g |
| Average pore diameter | 11.751 nm | 9.318 nm | 9.231 nm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Lee, D.H.; Yang, Y.; Chen, K.; Liu, C.; Kang, J.; Li, O.L. Hybrid Molybdenum Carbide/Heteroatom-Doped Carbon Electrocatalyst for Advanced Oxygen Evolution Reaction in Hydrogen Production. Catalysts 2020, 10, 1290. https://doi.org/10.3390/catal10111290
Kim J, Lee DH, Yang Y, Chen K, Liu C, Kang J, Li OL. Hybrid Molybdenum Carbide/Heteroatom-Doped Carbon Electrocatalyst for Advanced Oxygen Evolution Reaction in Hydrogen Production. Catalysts. 2020; 10(11):1290. https://doi.org/10.3390/catal10111290
Chicago/Turabian StyleKim, Jihun, Dae Hoon Lee, Yang Yang, Kai Chen, Chunli Liu, Jun Kang, and Oi Lun Li. 2020. "Hybrid Molybdenum Carbide/Heteroatom-Doped Carbon Electrocatalyst for Advanced Oxygen Evolution Reaction in Hydrogen Production" Catalysts 10, no. 11: 1290. https://doi.org/10.3390/catal10111290
APA StyleKim, J., Lee, D. H., Yang, Y., Chen, K., Liu, C., Kang, J., & Li, O. L. (2020). Hybrid Molybdenum Carbide/Heteroatom-Doped Carbon Electrocatalyst for Advanced Oxygen Evolution Reaction in Hydrogen Production. Catalysts, 10(11), 1290. https://doi.org/10.3390/catal10111290





