Recent Advances in Enzymatic and Chemoenzymatic Cascade Processes
Abstract
1. Introduction
2. Enzymatic Cascade Processes
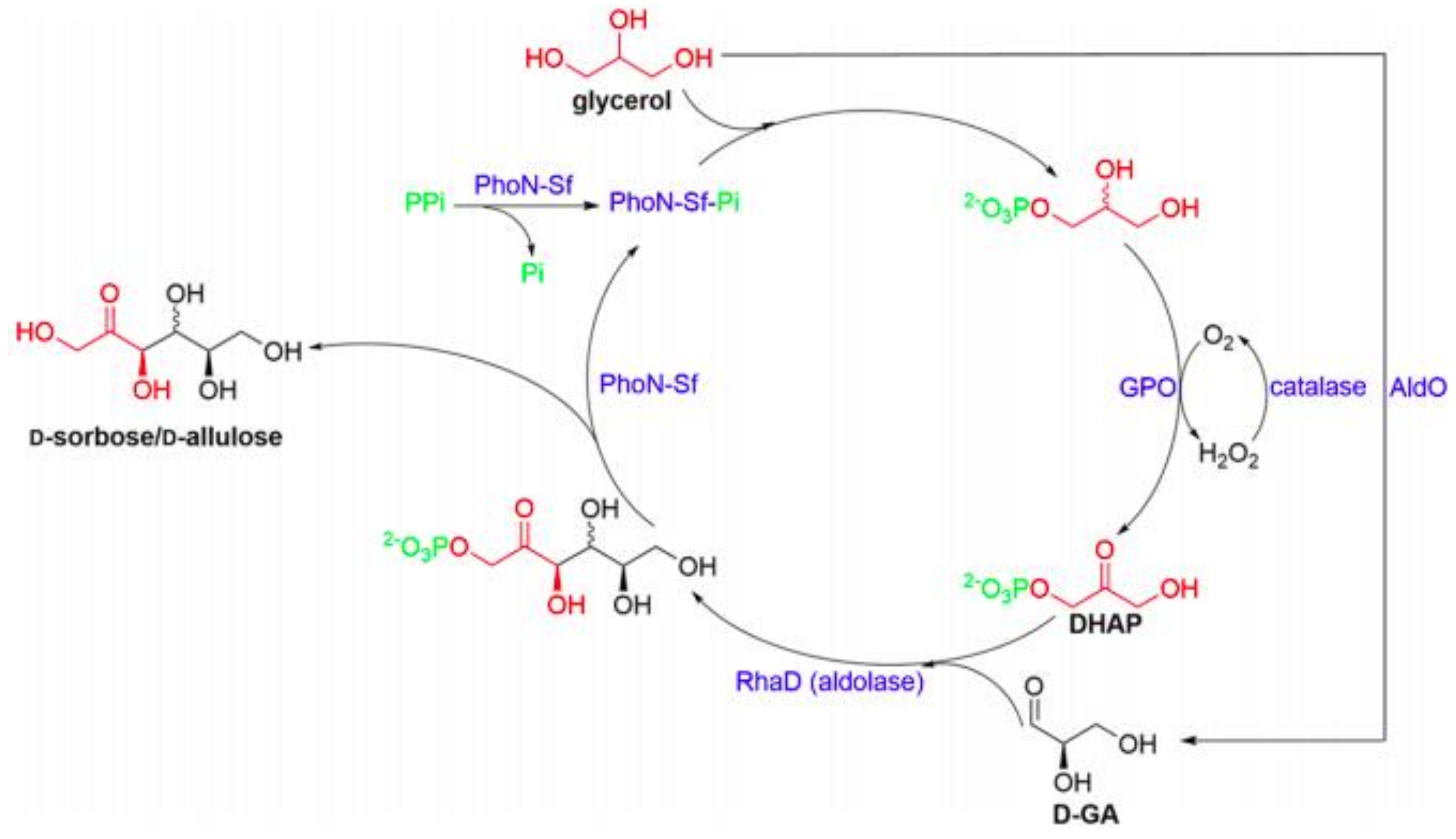
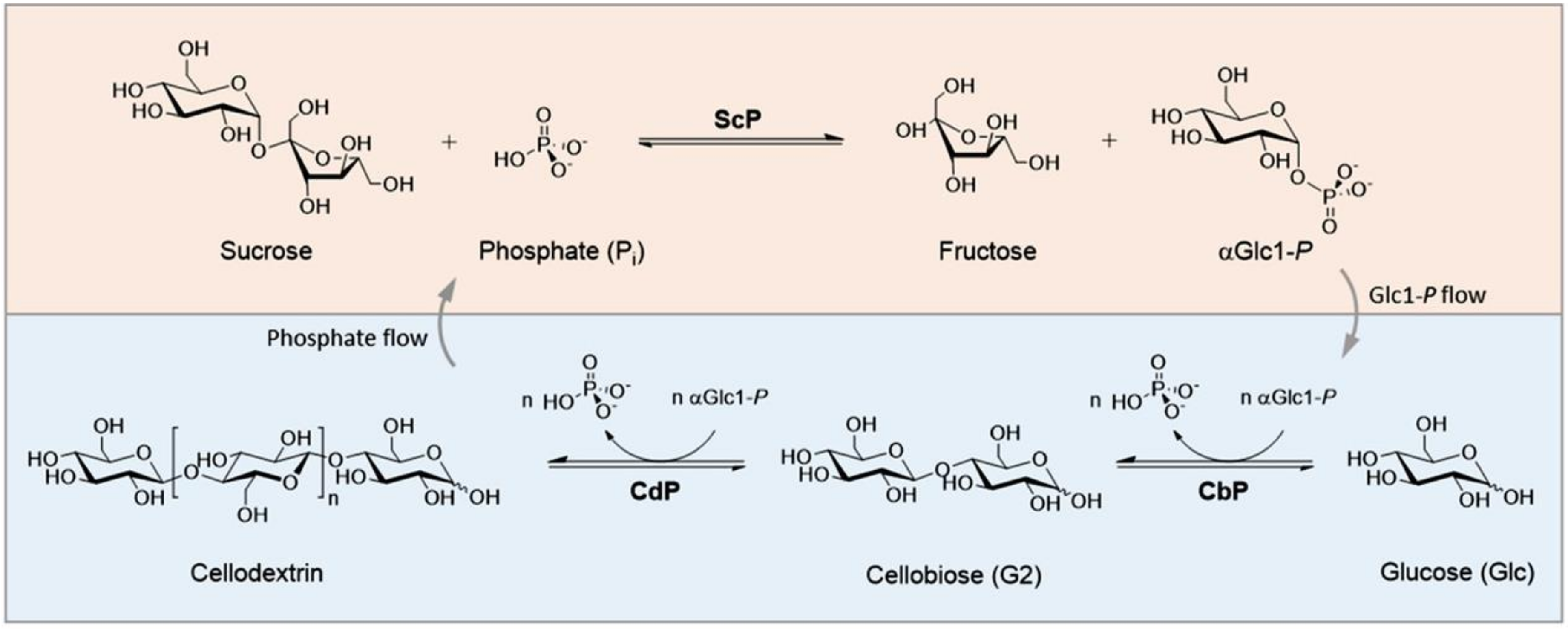
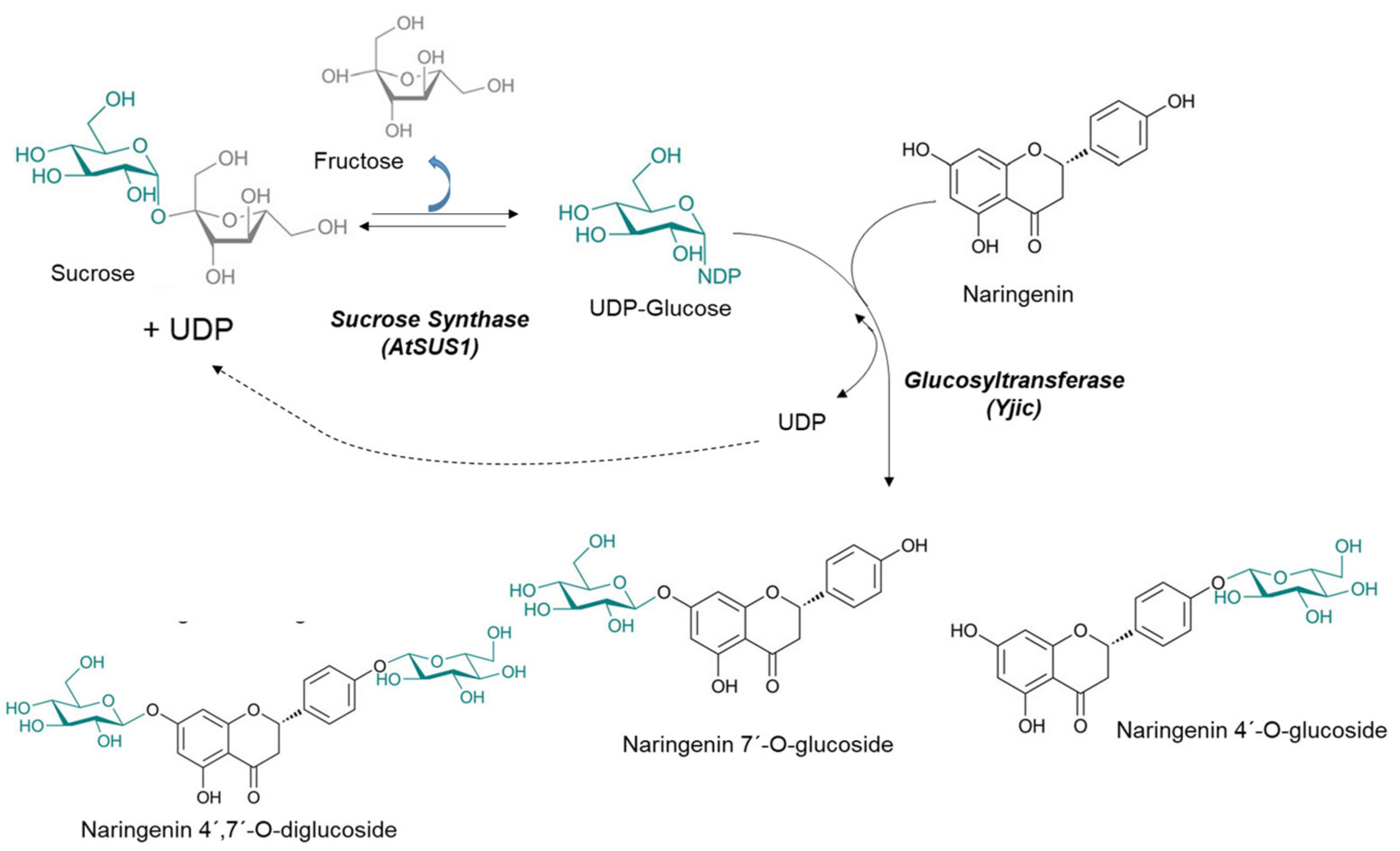
2.1. Glyco-Enzymes
2.2. Cascade Enzymes in Aminoacid Chemistry
3. Chemo-Enzymatic Cascades
4. Solid-Phase Multi-Chemoenzymatic Cascade Reactions
5. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Carlson, J.C.; Li, S.; Gunatilleke, S.S.; Anzai, Y.; Burr, D.A.; Podust, L.M.; Sherman, D.H. Tirandamycin biosynthesis is mediated by co-dependent oxidative enzymes. Nat. Chem. 2011, 3, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Galanie, S.; Entwistle, D.; Lalonde, J. Engineering biosynthetic enzymes for industrial natural product synthesis. Nat. Prod. Rep. 2020, 37, 1122–1143. [Google Scholar] [CrossRef] [PubMed]
- Reetz, M.T. Biocatalysis in organic chemistry and biotechnology: Past, present, and future. J. Am. Chem. Soc. 2013, 135, 12480–12496. [Google Scholar] [CrossRef] [PubMed]
- Shiroodi, R.K.; Gevorgyan, V. Metal-catalyzed double migratory cascade reactions of propargylic esters and phosphates. Chem. Soc. Rev. 2013, 42, 4991–5001. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Lercher, J.A. Upgrading pyrolysis oil over Ni/HZSM-5 by cascade reactions. Angew. Chem. Int. Ed. 2012, 51, 5935–5940. [Google Scholar] [CrossRef] [PubMed]
- Grondal, C.; Jeanty, M.; Enders, D. Organocatalytic cascade reactions as a new tool in total synthesis. Nat. Chem. 2010, 2, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Arnold, F.H. Innovation by evolution: Bringing new chemistry to life (Nobel lecture). Angew. Chem. Int. Ed. 2019, 58, 14420–14426. [Google Scholar] [CrossRef] [PubMed]
- Shylesh, S.; Wagener, A.; Seifert, A.; Ernst, S.; Thiel, W.R. Mesoporous organosilicas with acidic frameworks and basic sites in the pores: An approach to cooperative catalytic reactions. Angew. Chem. Int. Ed. 2010, 49, 184–187. [Google Scholar] [CrossRef]
- Palo-Nieto, C.; Afewerki, S.; Anderson, M.; Tai, C.W.; Berglund, P.; Córdova, A. Integrated heterogeneous metal/enzymatic multiple relay catalysis for eco-friendly and asymmetric synthesis. ACS Catal. 2016, 6, 3932–3940. [Google Scholar] [CrossRef]
- Zhou, Y.B.; Liu, B.W.; Yang, R.H.; Liu, J.W. Filling in the gaps between nanozymes and enzymes: Challenges and opportunities. Bioconjugate Chem. 2017, 28, 2903–2909. [Google Scholar] [CrossRef]
- Singh, S. Nanomaterials Exhibiting Enzyme-Like Properties (Nanozymes): Current Advances and Future Perspectives. Front Chem. 2019, 7, 46. [Google Scholar] [CrossRef]
- Ricca, E.; Brucher, B.; Schrittwieser, J.H. Multi-enzymatic cascade reactions: Overview and perspectives. Adv. Synth. Catal. 2011, 353, 2239–2262. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, H.; Zhao, H. Expanding the boundary of biocatalysis: Design and optimization of in vitro tandem catalytic reactions for biochemical production. Crit. Rev. Biochem. Mol. Biol. 2018, 53, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Muschiol, J.; Peters, C.; Oberleitner, N.; Mihovilovic, M.D.; Bornscheuer, U.T.; Rudroff, F. Cascade catalysis-strategies and challenges en route to preparative synthetic biology. Chem. Commun. 2015, 51, 5798–5811. [Google Scholar] [CrossRef] [PubMed]
- Wilding, K.M.; Schinn, S.M.; Long, E.A.; Bundy, B.C. The emerging impact of cell-free chemical biosynthesis. Curr. Opin. Biotech. 2018, 53, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Wiltschi, B.; Cernava, T.; Dennig, A.; Casas, M.G.; Geier, M.; Gruber, S.; Haberbauer, M.; Heidinger, P.; Herrero Acero, E.; Kratzer, R.; et al. Enzymes revolutionize the bioproduction of value-added compounds: From enzyme discovery to special applications. Biotechnol. Adv. 2020, 40, 107520. [Google Scholar] [CrossRef] [PubMed]
- Arana-Peña, S.; Carballares, D.; Morellon-Sterlling, R.; Berenguer-Murcia, Á.; Alcántara, A.R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Enzyme co-immobilization: Always the biocatalyst designers’ choice…or not? Biotechnol. Adv. 2020, 107584. [Google Scholar] [CrossRef]
- Hwang, E.T.; Lee, S. Multienzymatic Cascade Reactions via Enzyme Complex by Immobilization. ACS Catal. 2019, 9, 4402–4425. [Google Scholar] [CrossRef]
- Cutlan, R.; De Rose, S.; Isupov, M.N.; Littlechild, J.A.; Harmer, N.J. Using enzyme cascades in biocatalysis: Highlight on transaminases and carboxylic acid reductases. Biochim. Biophys. Acta. Proteins. Proteom. 2020, 1868, 140322. [Google Scholar] [CrossRef]
- Perl, J.M.; Sieber, V. Multienzyme Cascade Reactions—Status and Recent Advances. ACS Catal. 2018, 8, 2385–2396. [Google Scholar]
- Cheng, C.; Zuo, X.; Tu, D.; Wan, B.; Zhang, Y. Synthesis of 3,4-Fused Tricyclic Indoles through Cascade Carbopalladation and C-H Amination: Development and Total Synthesis of Rucaparib. Org. Lett. 2020, 22, 4985–4989. [Google Scholar] [CrossRef]
- Mekheimer, R.A.; Al-Sheikh, M.A.; Medrasi, H.Y.; Sadek, K.U. Advancements in the synthesis of fused tetracyclic quinoline derivatives. RSC Adv. 2020, 10, 19867–19935. [Google Scholar] [CrossRef]
- Schmaltz, R.M.; Hanson, S.R.; Wong, C.-H. Enzymes in the Synthesis of Glycoconjugates. Chem. Rev. 2011, 111, 4259–4307. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Edmunds, G.; Gibbons, C.; Zhang, J.; Gadi, M.R.; Zhu, H.; Fang, J.; Liu, X.; Kong, Y.; Wang, P.G. Toward Automated Enzymatic Synthesis of Oligosaccharides. Chem. Rev. 2018, 118, 8151–8187. [Google Scholar] [CrossRef]
- Krasnova, L.; Wong, C.-H. Oligosaccharide Synthesis and Translational Innovation. J. Am. Chem. Soc. 2019, 141, 3735–3754. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, T.; Tian, C.; Zhu, Y.; Zeng, Y.; Men, Y.; Chen, P.; Sun, Y.; Ma, Y. Multi-enzyme systems and recombinant cells for synthesis of valuable saccharides: Advances and perspectives. Biotechnol. Adv. 2019, 37, 107406. [Google Scholar] [CrossRef]
- Li, Z.; Cai, L.; Qi, Q.; Wang, P.G. Enzymatic synthesis of D-sorbose and D-psicose with aldolase RhaD: Effect of acceptor configuration on enzyme stereoselectivity. Bioorg. Med. Chem. Lett. 2011, 21, 7081–7084. [Google Scholar] [CrossRef]
- Fessner, W.-D.; Sinerius, G. Synthesis of Dihydroxyacetone Phosphate (and Isosteric Analogues) by Enzymatic Oxidation; Sugars from Glycerol. Angew. Chem. Int. Ed. 1994, 33, 209–212. [Google Scholar] [CrossRef]
- Burek, B.O.; Bormann, S.; Hollmann, F.; Bloh, J.Z.; Holtmann, D. Hydrogen peroxide driven biocatalysis. Green Chem. 2019, 21, 3232–3249. [Google Scholar] [CrossRef]
- Li, Z.; Li, F.; Cai, L.; Chen, Z.; Qin, L.; Gao, X.-D. One-Pot Multienzyme Synthesis of Rare Ketoses from Glycerol. J. Agric. Food Chem. 2020, 68, 1347–1353. [Google Scholar] [CrossRef]
- Zhu, P.; Zeng, Y.; Chen, P.; Men, Y.; Yang, J.; Yue, X.; Zhang, J.; Zhu, Y.; Sun, Y. A one-pot two-enzyme system on the production of high value-added D-allulose from Jerusalem artichoke tubers. Process Biochem. 2020, 88, 90–96. [Google Scholar] [CrossRef]
- Lorillière, M.; Dumoulin, R.; L’Enfant, M.; Rambourdin, A.; Thery, V.; Nauton, L.; Fessner, W.-D.; Charmantray, F.; Hecquet, L. Evolved Thermostable Transketolase for Stereoselective Two-Carbon Elongation of Non-Phosphorylated Aldoses to Naturally Rare Ketoses. ACS Catal. 2019, 9, 4754–4763. [Google Scholar] [CrossRef]
- Lorillière, M.; Guérard-Hélaine, C.; Gefflaut, T.; Fessner, W.D.; Clapés, P.; Charmantray, F.; Hecquet, L. Convergent in situ Generation of Both Transketolase Substrates via Transaminase and Aldolase Reactions for Sequential One-Pot, Three-Step Cascade Synthesis of Ketoses. ChemCatChem 2020, 12, 812–817. [Google Scholar] [CrossRef]
- Sybesma, W.; Kort, R.; Lee, Y.K. Locally sourced probiotics, the next opportunity for developing countries? Trends Biotechnol. 2015, 33, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Shrotri, A.; Fukuoka, A. Soluble Cello-Oligosaccharides Produced by Carbon-Catalyzed Hydrolysis of Cellulose. ChemSusChem 2019, 12, 2576–2580. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Nidetzky, B. Three-Enzyme Phosphorylase Cascade for Integrated Production of Short-Chain Cellodextrins. Biotechnol. J. 2020, 15, 1900349–1900358. [Google Scholar] [CrossRef]
- Tian, C.; Yang, J.; Zeng, Y.; Zhang, T.; Zhou, Y.; Men, Y.; You, C.; Zhu, Y.; Sun, Y. Biosynthesis of Raffinose and Stachyose from Sucrose via an In Vitro Multienzyme System. Appl. Environ. Microbiol. 2019, 85, 02306-18. [Google Scholar] [CrossRef]
- Hofer, B. Recent developments in the enzymatic O-glycosylation of flavonoids. Appl. Microbiol. Biotechnol. 2016, 100, 4269–4281. [Google Scholar] [CrossRef]
- Tegl, G.; Nidetzky, B. Leloir glycosyltransferases of natural product C-glycosylation: Structure, mechanism and specificity. Biochem. Soc. Trans. 2020, 48, 1583–1598. [Google Scholar] [CrossRef]
- Hu, Y.; Min, J.; Qu, Y.; Zhang, X.; Zhang, J.; Yu, X.; Dai, L. Biocatalytic Synthesis of Calycosin-7-O-β-D-Glucoside with Uridine Diphosphate–Glucose Regeneration System. Catalysts 2020, 10, 258–269. [Google Scholar] [CrossRef]
- Huang, F.-C.; Hinkelmann, J.; Hermenau, A.; Schwab, W. Enhanced production of β-glucosides by in-situ UDP-glucose regeneration. J. Biotechnol. 2016, 224, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Thapa, S.B.; Pandey, R.P.; Bashyal, P.; Yamaguchi, T.; Sohng, J.K. Cascade biocatalysis systems for bioactive naringenin glucosides and quercetin rhamnoside production from sucrose. Appl. Microbiol. Biotechnol. 2019, 103, 7953–7969. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Fokou, P.V.T.; Sharifi-Rad, M.; Zucca, P.; Pezzani, R.; Martins, N.; Sharifi-Rad, J. The therapeutic potential of naringenin: A review of clinical trials. Pharmaceuticals 2019, 12, 11. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-J.; Zhang, D.; Yang, D.-C. Biosynthesis and biotechnological production of ginsenosides. Biotechnol. Adv. 2015, 33, 717–735. [Google Scholar] [CrossRef]
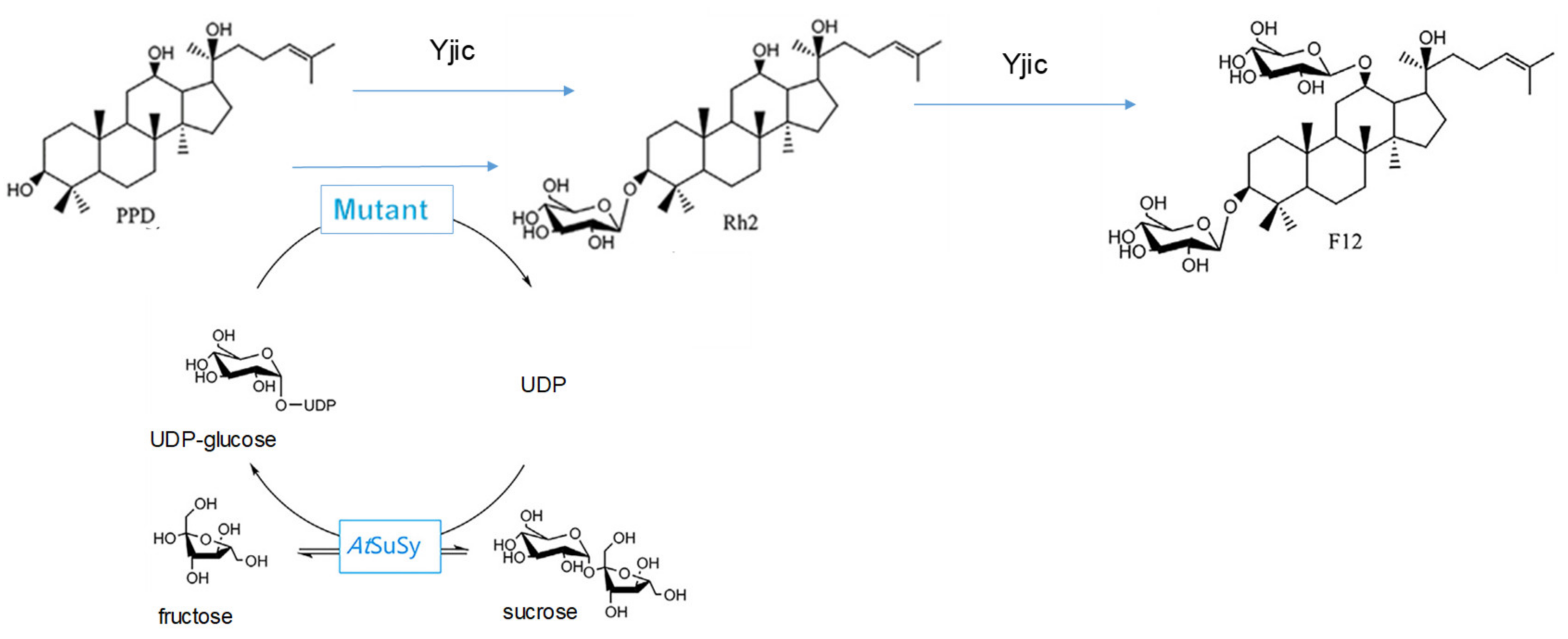
- Ma, W.; Zhao, L.; Ma, Y.; Li, Y.; Qin, S.; He, B. Oriented efficient biosynthesis of rare ginsenoside Rh2 from PPD by compiling UGT-Yjic mutant with sucrose synthase. Int. J. Biol. Macromol. 2020, 146, 853–859. [Google Scholar] [CrossRef]
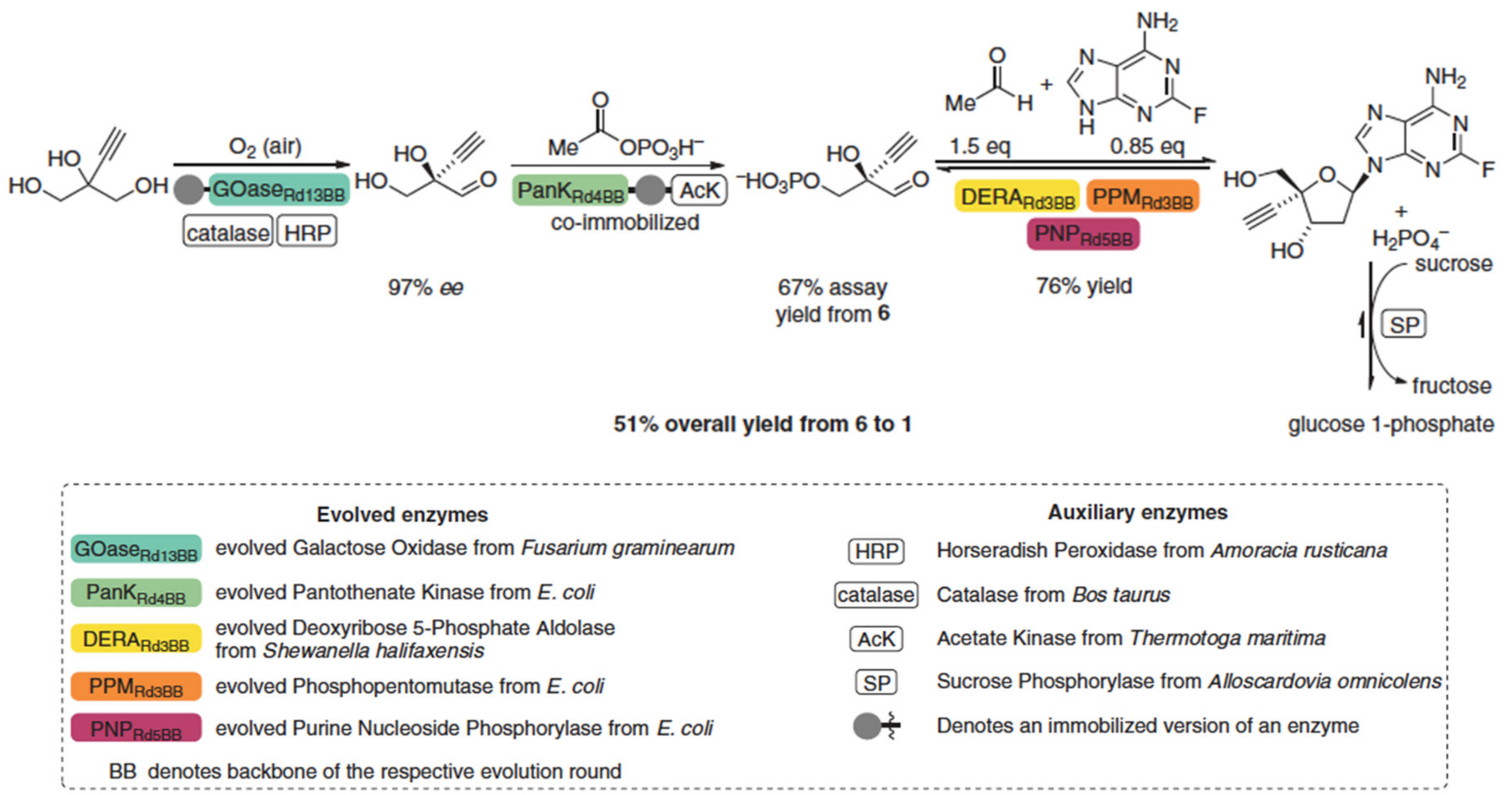
- Huffman, M.A.; Fryszkowska, A.; Alvizo, O.; Borra-Garske, M.; Campos, K.R.; Canada, K.A.; Devine, P.N.; Duan, D.; Forstater, J.H.; Grosser, S.T.; et al. Design of an in vitro biocatalytic cascade for the manufacture of islatravir. Science 2020, 368, 1255–1259. [Google Scholar] [CrossRef]
- Song, W.; Chen, X.; Wu, J.; Xu, J.; Zhang, W.; Liu, J.; Chen, J.; Liu, L. Biocatalytic derivatization of proteinogenic amino acids for fine chemicals. Biotechnol. Adv. 2020, 40, 107496. [Google Scholar] [CrossRef]
- Laurent, V.; Gourbeyre, L.; Uzel, A.; Hélaine, V.; Nauton, L.; Traïkia, M.; Salanoubat, M.; Gefflaut, T.; Lemaire, M.; Guérard-Hélaine, C. Pyruvate Aldolases Catalyze Cross-Aldol Reactions between Ketones: Highly Selective Access to Multi-Functionalized Tertiary Alcohols. ACS Catal. 2020, 10, 2538–2543. [Google Scholar] [CrossRef]
- Gmelch, T.J.; Sperl, J.M.; Sieber, V. Optimization of a reduced enzymatic reaction cascade for the production of L-alanine. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Takamatsu, S.; Tosa, T.; Chibata, I. Production of L-alanine from ammonium fumarate using two microbial cells immobilized with κ-carrageenan. J. Chem. Eng. Jpn. 1985, 18, 66–70. [Google Scholar] [CrossRef][Green Version]
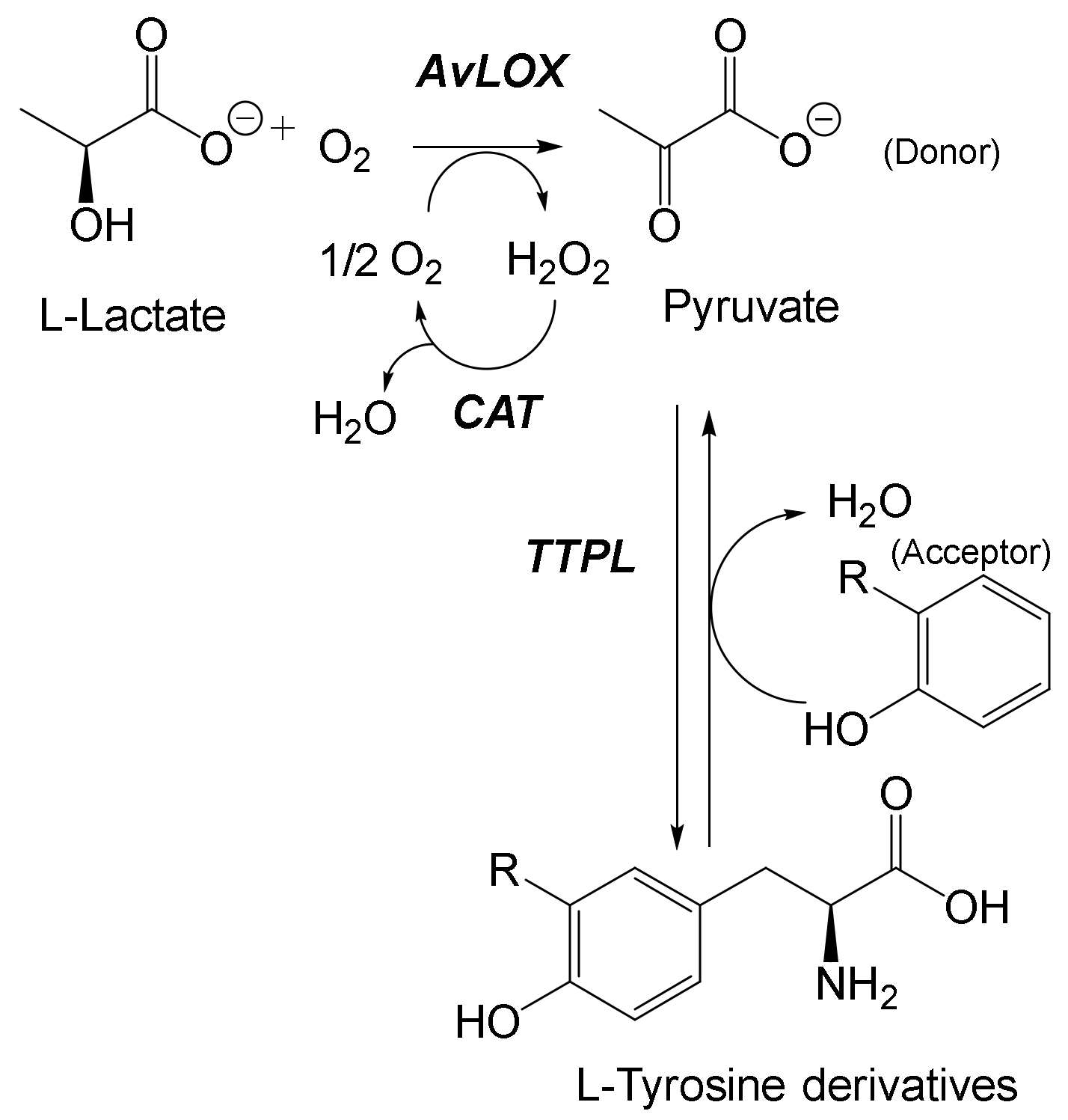
- Li, G.; Lian, J.; Xue, H.; Jiang, Y.; Ju, S.; Wu, M.; Lin, J.; Yang, L. Biocascade Synthesis of L-Tyrosine Derivatives by Coupling a Thermophilic Tyrosine Phenol-Lyase and L-Lactate Oxidase. Eur. J. Org. Chem. 2020, 2020, 1050–1054. [Google Scholar] [CrossRef]
- Fernández-Tejada, A.; Corzana, F.; Busto, J.H.; Avenoza, A.; Peregrina, J.M. Conformational effects of the non-natural α-methylserine on small peptides and glycopeptides. J. Org. Chem. 2009, 74, 9305–9313. [Google Scholar] [CrossRef] [PubMed]
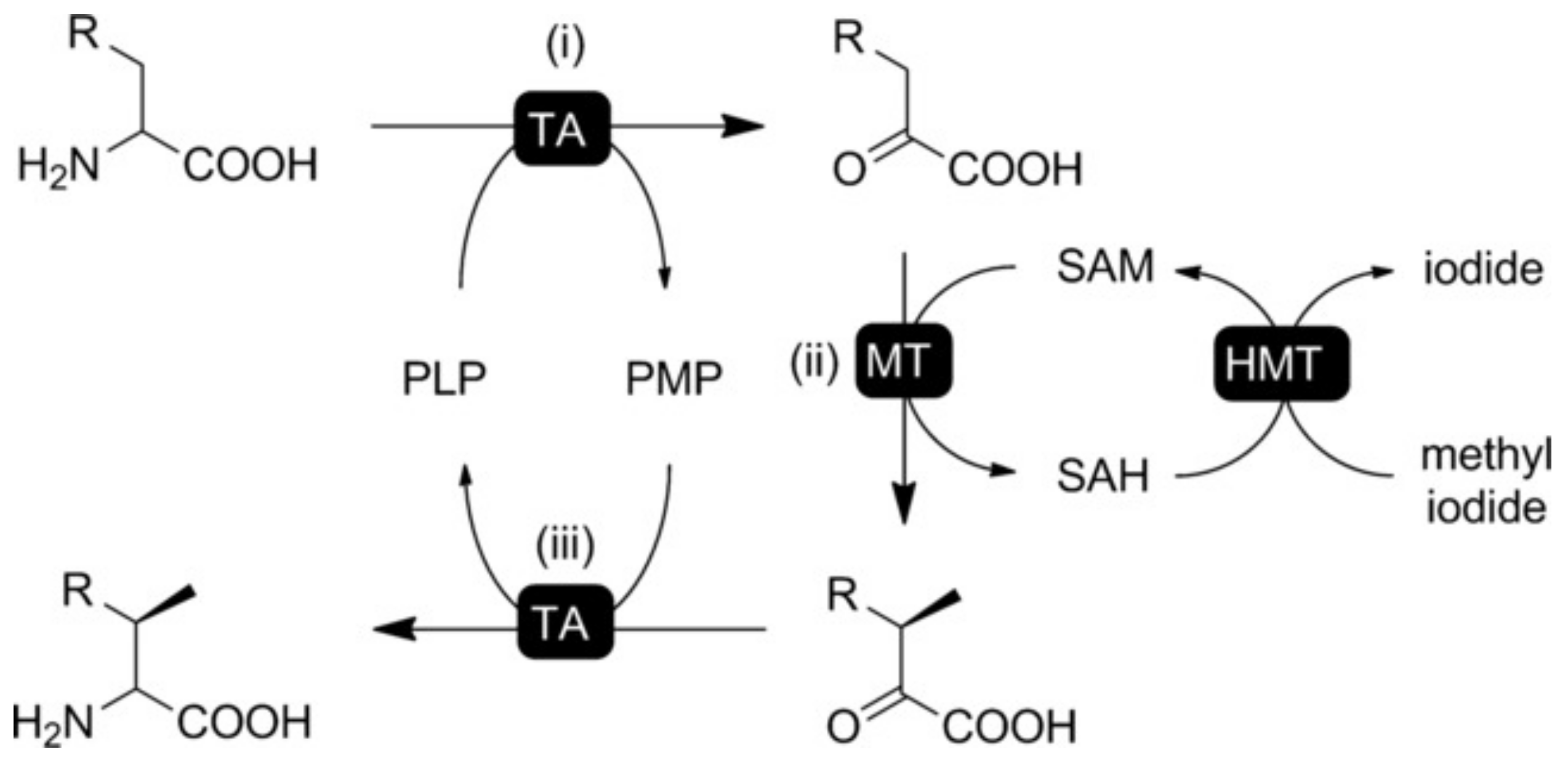
- Liao, C.; Seebeck, F.P. Asymmetric β-Methylation of l- and d-α-Amino Acids by a Self-Contained Enzyme Cascade. Angew. Chem. Int. Ed. 2020, 59, 7184–7187. [Google Scholar] [CrossRef] [PubMed]
- Contente, M.L.; Paradisi, F. Self-sustaining closed-loop multienzyme-mediated conversion of amines into alcohols in continuous reactions. Nat. Catal. 2018, 1, 452–459. [Google Scholar] [CrossRef]
- Burgener, S.; Cortina, N.S.; Erb, T.J. Oxalyl-CoA Decarboxylase Enables Nucleophilic One-Carbon Extension of Aldehydes to Chiral α-Hydroxy Acids. Angew. Chem. Int. Ed. 2020, 59, 5526–5530. [Google Scholar] [CrossRef]
- Groeger, H.; Hummel, W. Combining the ‘two worlds’ of chemocatalysis and biocatalysis towards multi-step one-pot processes in aqueous media. Curr. Opin. Chem. Biol. 2014, 19, 171–179. [Google Scholar] [CrossRef]
- Ríos-Lombardía, N.; García-Álvarez, J.; González-Sabín, J. One-pot combination of metal-and bio-catalysis in water for the synthesis of chiral molecules. Catalysts 2018, 8, 75. [Google Scholar] [CrossRef]
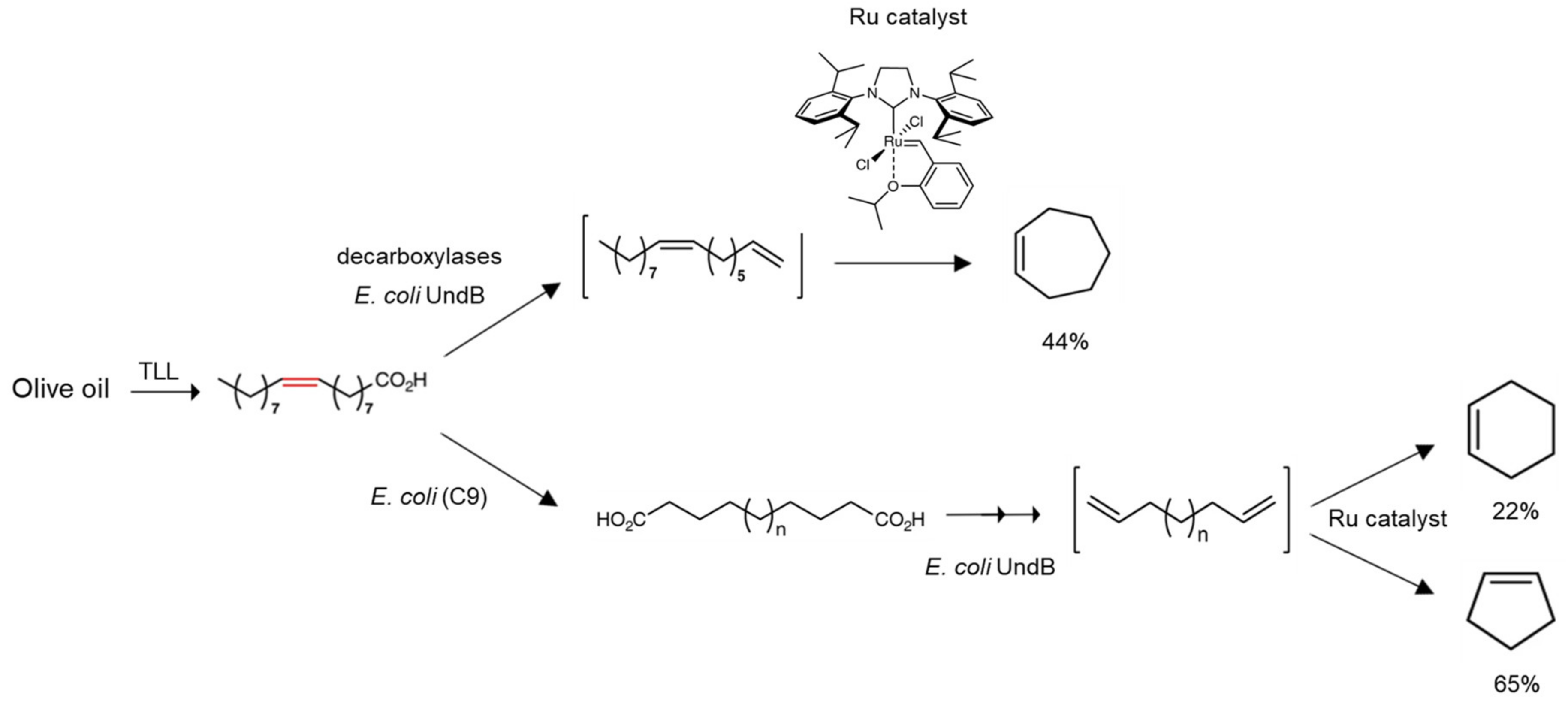
- Wu, S.; Zhou, Y.; Gerngross, D.; Jeschek, M.; Ward, T.R. Chemo-enzymatic cascades to produce cycloalkenes from bio-based resources. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Schuchardt, U.; Cardoso, D.; Seercheli, R.; Pereira, R.; da Cruz, R.S.; Guerreiro, M.C.; Mandelli, D.; Spinacé, E.V.; Pires, E.L. Cyclohexane oxidation continues to be a challenge. Appl. Catal. A Gen. 2001, 97, 1–17. [Google Scholar] [CrossRef]
- Soussan, L.; Pen, N.; Belleville, M.P.; Marcano, J.S.; Paolucci-Jeanjean, D. Alkane biohydroxylation: Interests, constraints and future developments. J. Biotechnol. 2016, 2022, 117–142. [Google Scholar] [CrossRef]
- Hrycay, E.G.; Bandiera, S.M. The monooxygenase, peroxidase, and peroxygenase properties of cytochrome P450. Arch. Biochem. Biophys. 2012, 522, 71–89. [Google Scholar] [CrossRef]
- Wang, Y.; Lan, D.; Durrani, R.; Hollmann, F. Peroxygenases en route to becoming dream catalysts. What are the opportunities and challenges? Curr. Opin. Chem. Biol. 2017, 37, 1–9. [Google Scholar] [CrossRef]
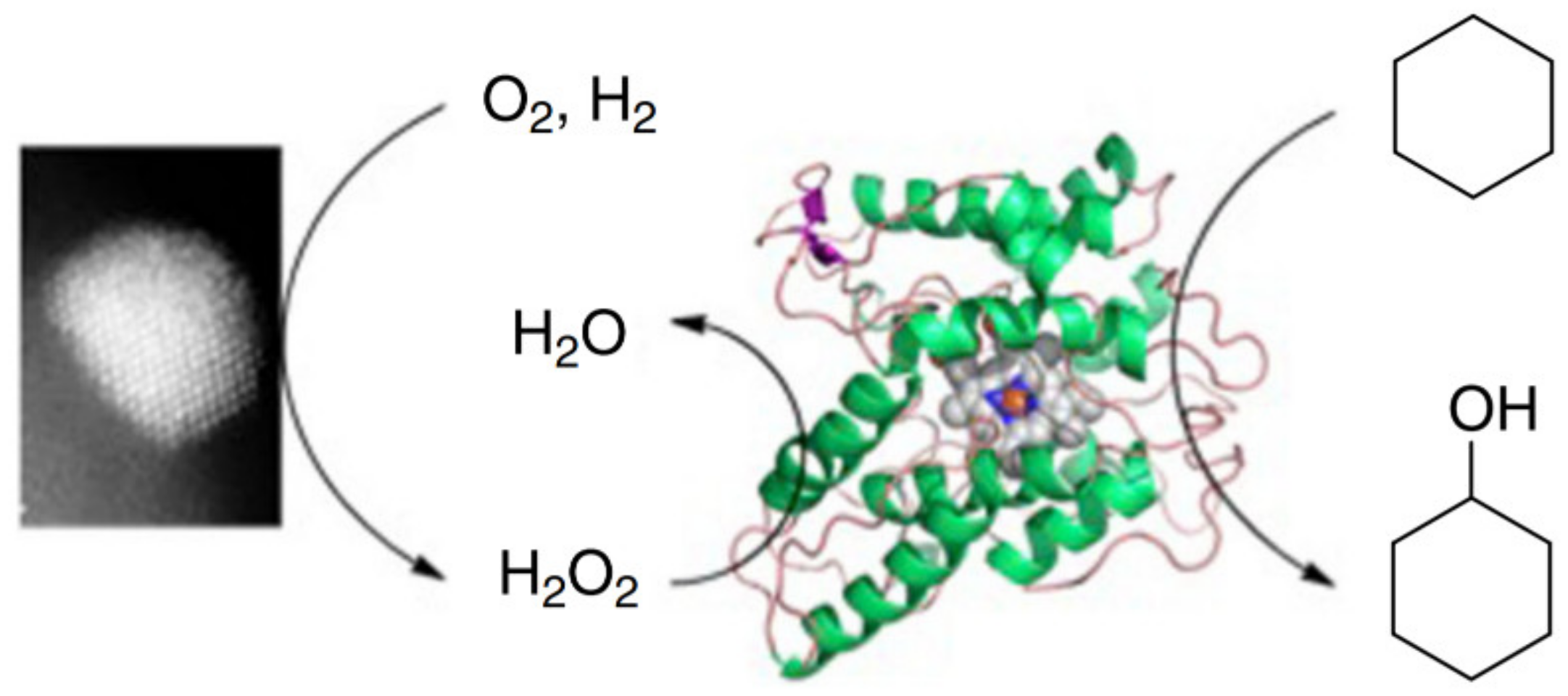
- Freakley, S.J.; Kochius, S.; Van Marwijk, J.; Fenner, C.; Lewis, R.J.; Baldenius, K.; Marais, S.S.; Opperman, D.J.; Harrison, S.T.L.; Alcalde, M.; et al. A chemo-enzymatic oxidation cascade to activate C–H bonds with in situ generated H2O2. Nat. Commun. 2019, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Khanam, H. Bioactive Benzofuran derivatives: A review. Eur. J. Med. Chem. 2015, 97, 483–504. [Google Scholar] [CrossRef] [PubMed]
- Mertens, M.S.; Thomas, F.; Nöth, M.; Moegling, J.; El-Awaad, I.; Sauer, D.F.; Dhoke, G.V.; Xu, W.; Pich, A.; Schwaneberg, U. One-Pot Two-Step Chemoenzymatic Cascade for the Synthesis of a Bis-benzofuran Derivative. Eur. J. Org. Chem. 2019, 2019, 6341–6346. [Google Scholar] [CrossRef]
- Schmidt, S.; Castiglione, K.; Kourist, R. Frontispiece: Overcoming the Incompatibility Challenge in Chemoenzymatic and Multi-Catalytic Cascade Reactions. Chem. Eur. J. 2018, 24. [Google Scholar] [CrossRef]
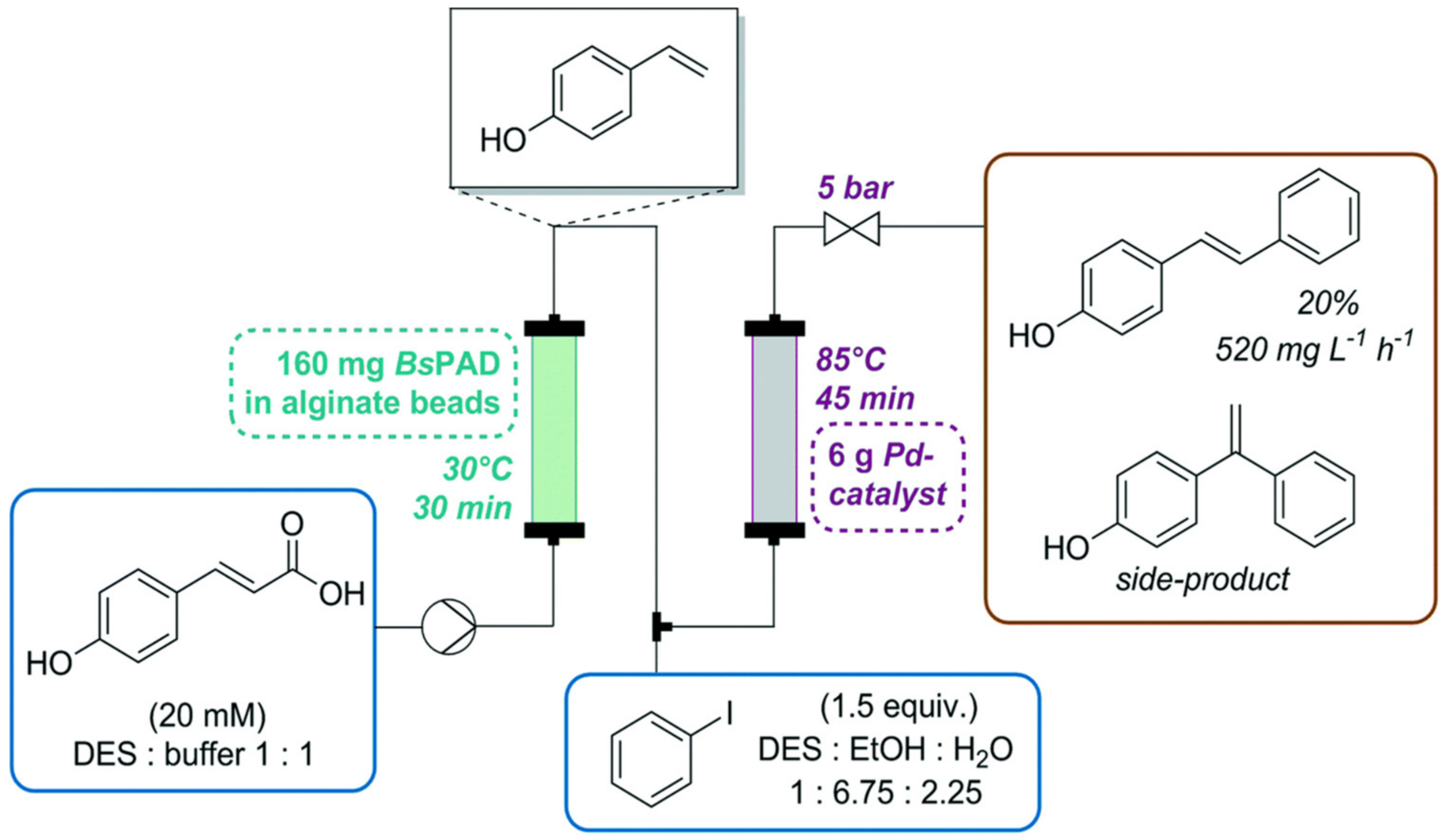
- Cicco, L.; Ríos-Lombardía, N.; Rodríguez-Álvarez, M.J.; Morís, F.; Perna, F.M.; Capriati, V.; García-Álvarez, J.; González-Sabín, J. Programming cascade reactions interfacing biocatalysis with transition-metal catalysis in Deep Eutectic Solvents as biorenewable reaction media. Green Chem. 2018, 20, 3468–3475. [Google Scholar] [CrossRef]
- Grabner, B.; Schweiger, A.K.; Gavric, K.; Kourist, R.; Gruber-Woelfler, H. A chemo-enzymatic tandem reaction in a mixture of deep eutectic solvent and water in continuous flow. React. Chem. Eng. 2020, 5, 263–269. [Google Scholar] [CrossRef]
- Geng, P.B.; Zheng, S.S.; Tang, H.; Zhu, R.M.; Zhang, L.; Cao, S.; Xue, H.G.; Pang, H. Transition metal sulfides based on graphene for electrochemical energy storage. Adv. Energy Mater. 2018, 8, 1703259. [Google Scholar] [CrossRef]
- Losada-García, N.; Rodríguez-Otero, A.; Palomo, J.M. Tailorable synthesis of heterogeneous enzyme–copper nanobiohybrids and their application in the selective oxidation of benzene to phenol. Catal. Sci. Technol. 2020, 10, 196–206. [Google Scholar] [CrossRef]
- Palomo, J.M. Nanobiohybrids: A new concept for metal nanoparticles synthesis. Chem. Commun. 2019, 55, 9583–9589. [Google Scholar] [CrossRef]
- Wei, H.; Wang, E.K. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes. Chem. Soc. Rev. 2013, 42, 6060–6093. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Ge, J.; Liu, Z. Enhanced activity of immobilized or chemically modified enzymes. ACS Catal. 2015, 5, 4503–4513. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, X.; Huang, L.; Zhang, Z.; Dong, S. One-pot synthesis of Fe3O4 nanoparticle loaded 3D porous graphene nanocomposites with enhanced nanozyme activity for glucose detection. ACS Appl. Mater. Interfaces 2017, 9, 7465–7471. [Google Scholar] [CrossRef]
- Ruan, X.; Liu, D.; Niu, X.; Wang, Y.; Simpson, C.D.; Cheng, N.; Du, D.; Lin, Y. 2D Graphene oxide/Fe-MOF nanozyme nest with superior peroxidase-like activity and its application for detection of woodsmoke exposure biomarker. Anal. Chem. 2019, 91, 13847–13854. [Google Scholar] [CrossRef] [PubMed]
- Losada-Garcia, N.; Berenguer-Murcia, A.; Cazorla-Amorós, D.; Palomo, J.M. Efficient production of multi-layer graphene from graphite flakes in water by lipase-graphene sheets conjugation. Nanomaterials 2020, 10, 7. [Google Scholar] [CrossRef]
- Losada-Garcia, N.; Rodriguez-Oliva, I.; Simovic, M.; Bezbradica, D.I.; Palomo, J.M. New Advances in Fabrication of Graphene Glyconanomaterials for Application in Therapy and Diagnosis. ACS Omega 2020, 5, 4362–4369. [Google Scholar] [CrossRef]
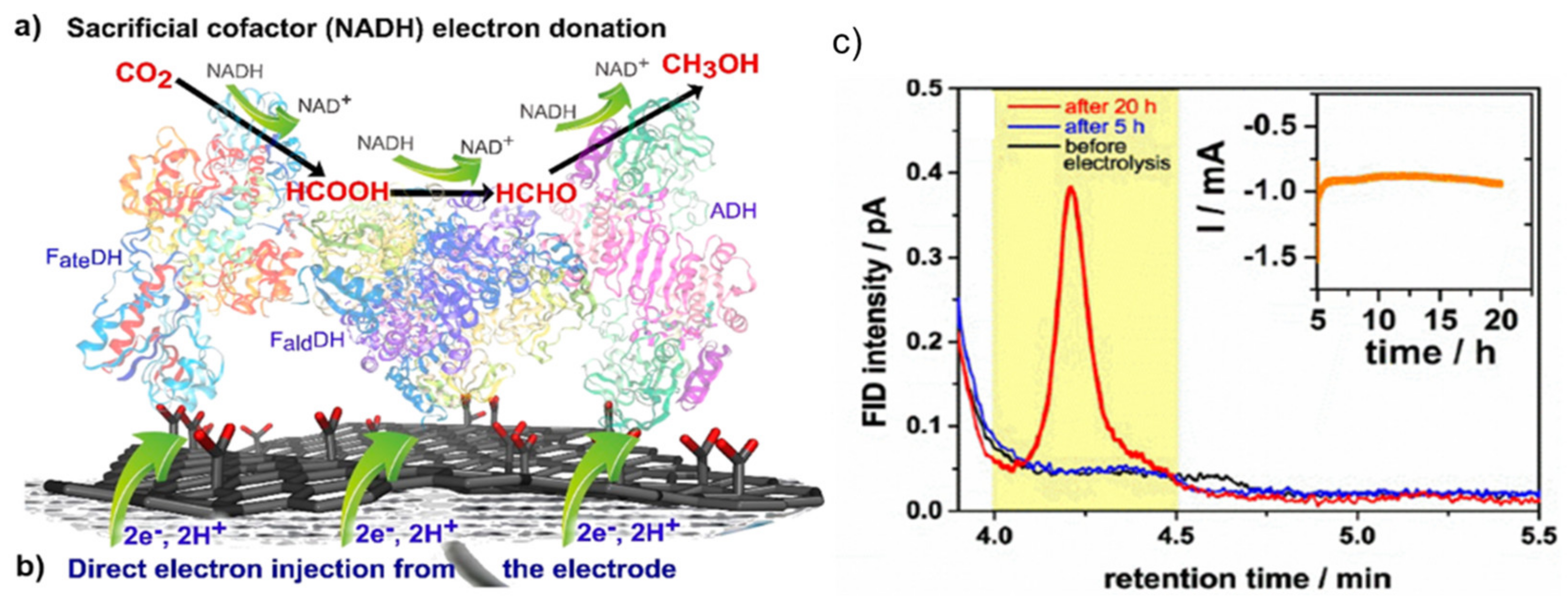
- Malkhandi, S.; Yeo, B.S. Electrochemical conversion of carbon dioxide to high value chemicals using gas-diffusion electrodes. Curr. Opin. Chem. Eng. 2019, 26, 112–121. [Google Scholar] [CrossRef]
- Seelbach, K.; Riebel, B.; Hummel, W.; Kula, M.-R.; Tishkov, V.I.; Egorov, A.M.; Wandrey, C.; Kragl, U. A Novel, Efficient Regenerating Method of NADPH Using a New Formate Dehydrogenase. Tetrahedron Lett. 1996, 37, 1377–1380. [Google Scholar] [CrossRef]
- Alissandratos, A.; Easton, C.J. Biocatalysis for the application of CO2 as a chemical feedstock. Beilstein J. Org. Chem. 2015, 11, 2370–2387. [Google Scholar] [CrossRef]
- Schlager, S.; Neugebauer, H.; Haberbauer, M.; Hinterberger, G.; Sariciftci, N.S. Direct Electrochemical Addressing of Immobilized Alcohol Dehydrogenase for the Heterogeneous Bioelectrocatalytic Reduction of Butyraldehyde to Butanol. ChemCatChem 2015, 7, 967–971. [Google Scholar] [CrossRef]
- Seelajaroen, H.; Bakandritsos, A.; Otyepka, M.; Zbořil, R.; Sariciftci, N.S. Immobilized Enzymes on Graphene as Nanobiocatalyst. ACS Appl. Mater. Interfaces 2019, 12, 250–259. [Google Scholar] [CrossRef]
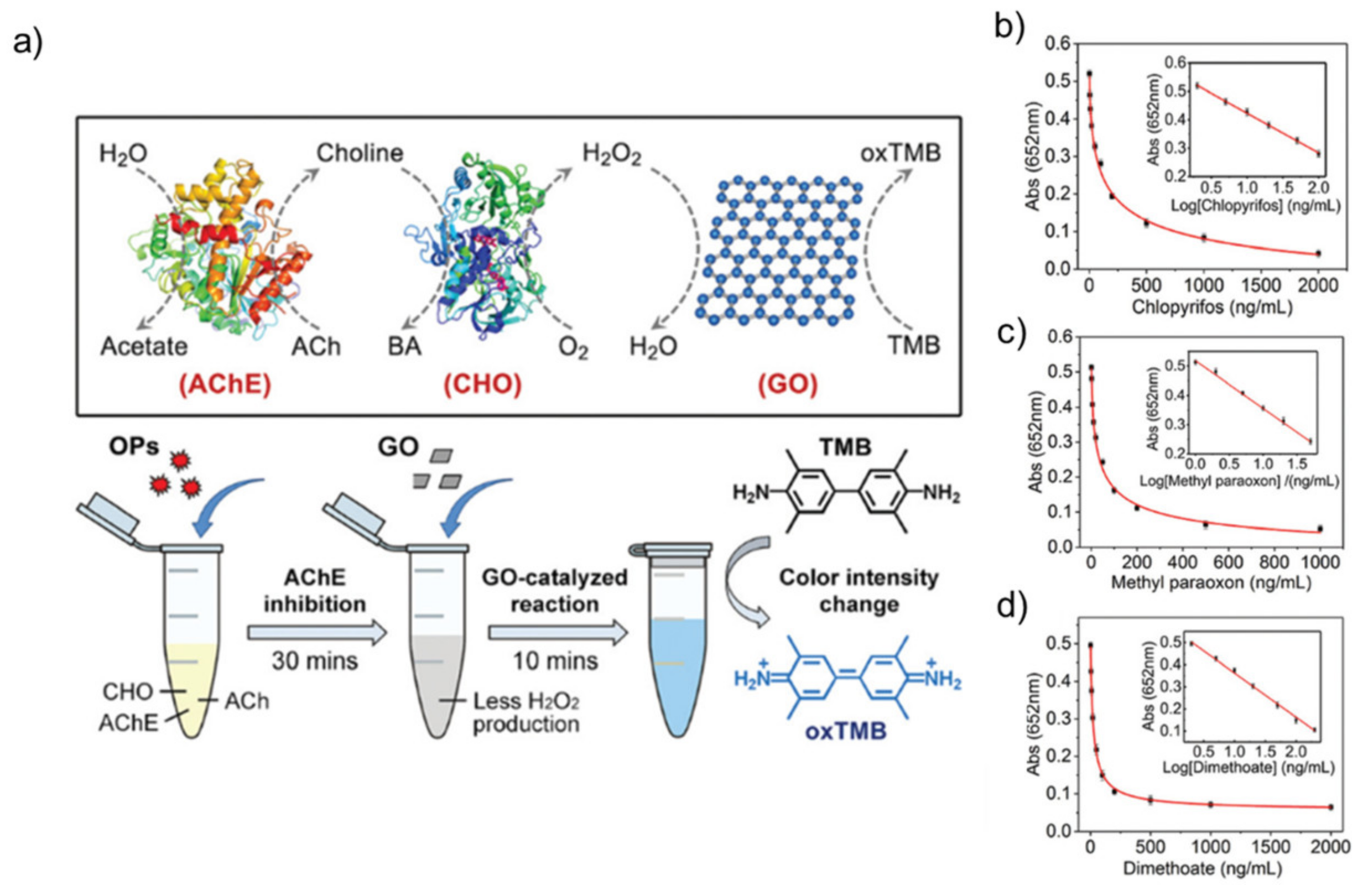
- Chu, S.; Huang, W.; Shen, F.; Li, T.; Li, S.; Xu, W.; Lv, C.; Luo, Q.; Liu, J. Graphene oxide-based colorimetric detection of organophosphorus pesticides via a multi-enzyme cascade reaction. Nanoscale 2020, 12, 5829–5833. [Google Scholar] [CrossRef]
- Periasamy, A.P.; Umasankar, Y.; Chen, S.M. Nanomaterials-acetylcholinesterase enzyme matrices for organophosphorus pesticides electrochemical sensors: A review. Sensors 2009, 9, 4034. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.F.; Wu, W.W.; Li, M.M.; Song, X.; Lv, Y.; Zhang, T.T. A highly stable acetylcholinesterase biosensor based on chitosan-TiO2-graphene nanocomposites for detection of organophosphate pesticides. Biosens. Bioelectron. 2018, 99, 223–229. [Google Scholar] [CrossRef] [PubMed]
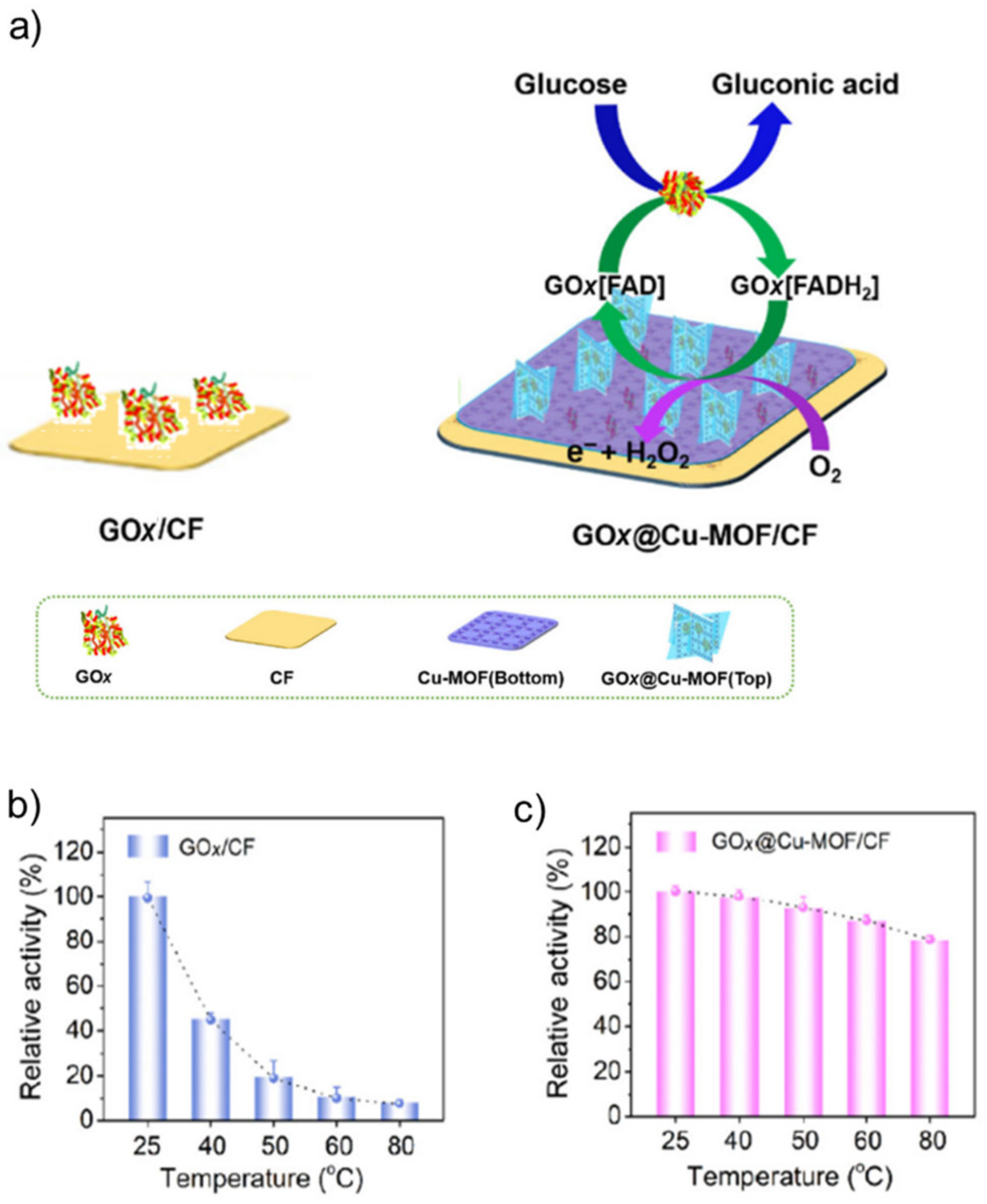
- Wang, Q.Q.; Zhang, X.P.; Huang, L.; Zhang, Z.Q.; Dong, S.J. GOx@ZIF-8 (NiPd) nanoflower: An artificial enzyme system for tandem catalysis. Angew. Chem. Int. Ed. 2017, 56, 16082–16085. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.S.; Huang, S.M.; Kou, X.X.; Wei, S.B.; Huang, S.Y.; Jiang, S.Q.; Shen, J.; Zhu, F.; Ouyang, G.F. A convenient and versatile aminoacid- boosted biomimetic strategy for the nondestructive encapsulation of biomacromolecules within metal−organic frameworks. Angew. Chem. Int. Ed. 2019, 58, 1463–1467. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Zhou, J.; Chen, J.; Xie, Z.; Kuang, Q.; Zheng, L. One-step synthesis of thermally stable artificial multienzyme cascade system for efficient enzymatic electrochemical detection. Nano Res. 2019, 12, 3031–3036. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Losada-Garcia, N.; Cabrera, Z.; Urrutia, P.; Garcia-Sanz, C.; Andreu, A.; Palomo, J.M. Recent Advances in Enzymatic and Chemoenzymatic Cascade Processes. Catalysts 2020, 10, 1258. https://doi.org/10.3390/catal10111258
Losada-Garcia N, Cabrera Z, Urrutia P, Garcia-Sanz C, Andreu A, Palomo JM. Recent Advances in Enzymatic and Chemoenzymatic Cascade Processes. Catalysts. 2020; 10(11):1258. https://doi.org/10.3390/catal10111258
Chicago/Turabian StyleLosada-Garcia, Noelia, Zaida Cabrera, Paulina Urrutia, Carla Garcia-Sanz, Alicia Andreu, and Jose M. Palomo. 2020. "Recent Advances in Enzymatic and Chemoenzymatic Cascade Processes" Catalysts 10, no. 11: 1258. https://doi.org/10.3390/catal10111258
APA StyleLosada-Garcia, N., Cabrera, Z., Urrutia, P., Garcia-Sanz, C., Andreu, A., & Palomo, J. M. (2020). Recent Advances in Enzymatic and Chemoenzymatic Cascade Processes. Catalysts, 10(11), 1258. https://doi.org/10.3390/catal10111258