High Degradation of Trichloroethylene in Water by Nanostructured MeNPs@CALB Biohybrid Catalysts
Abstract
1. Introduction
2. Results and Discussion
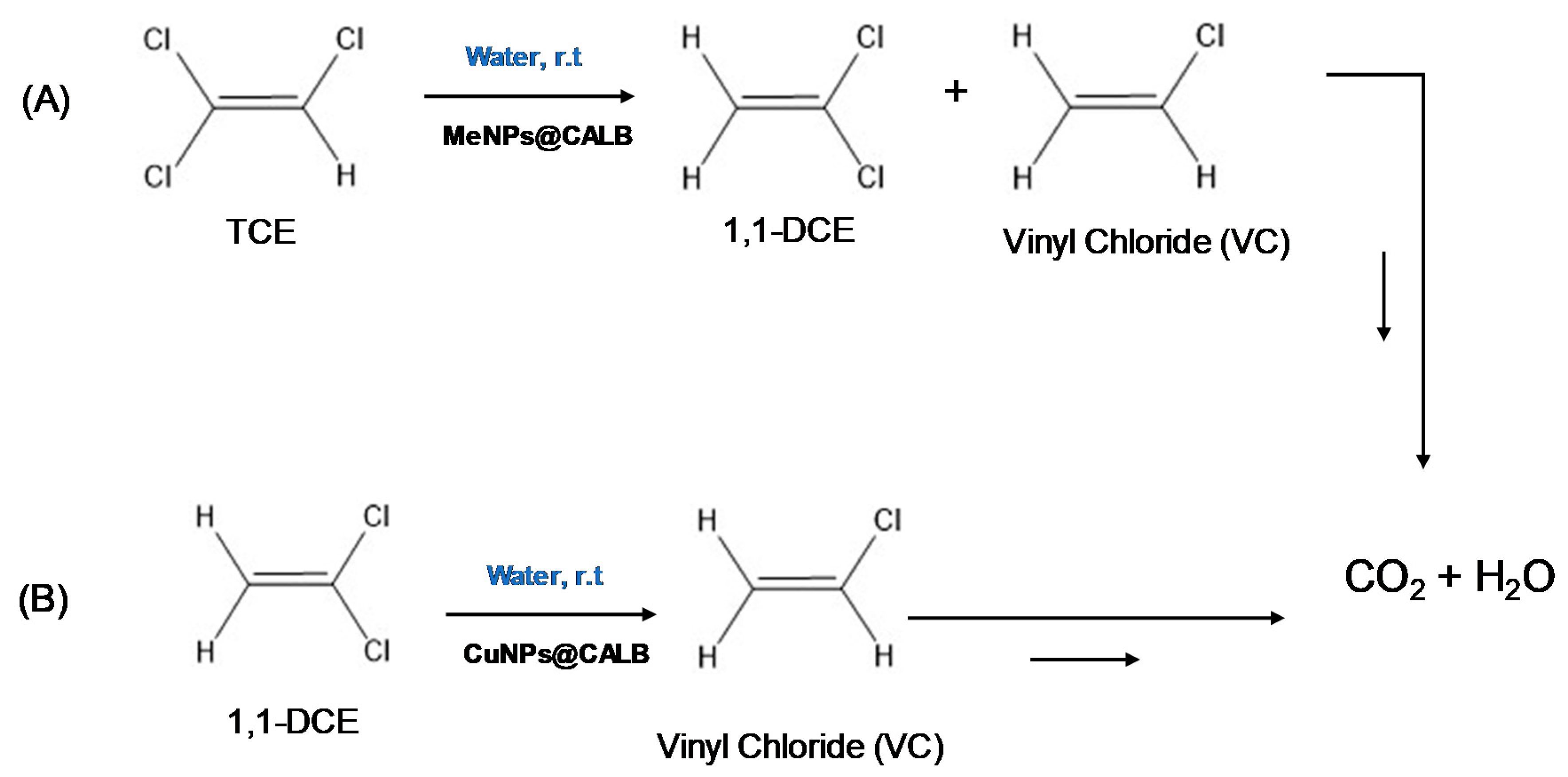
2.1. Trichloroethylene (TCE) Degradation Catalyzed by MeNPs@CALB Biohybrids
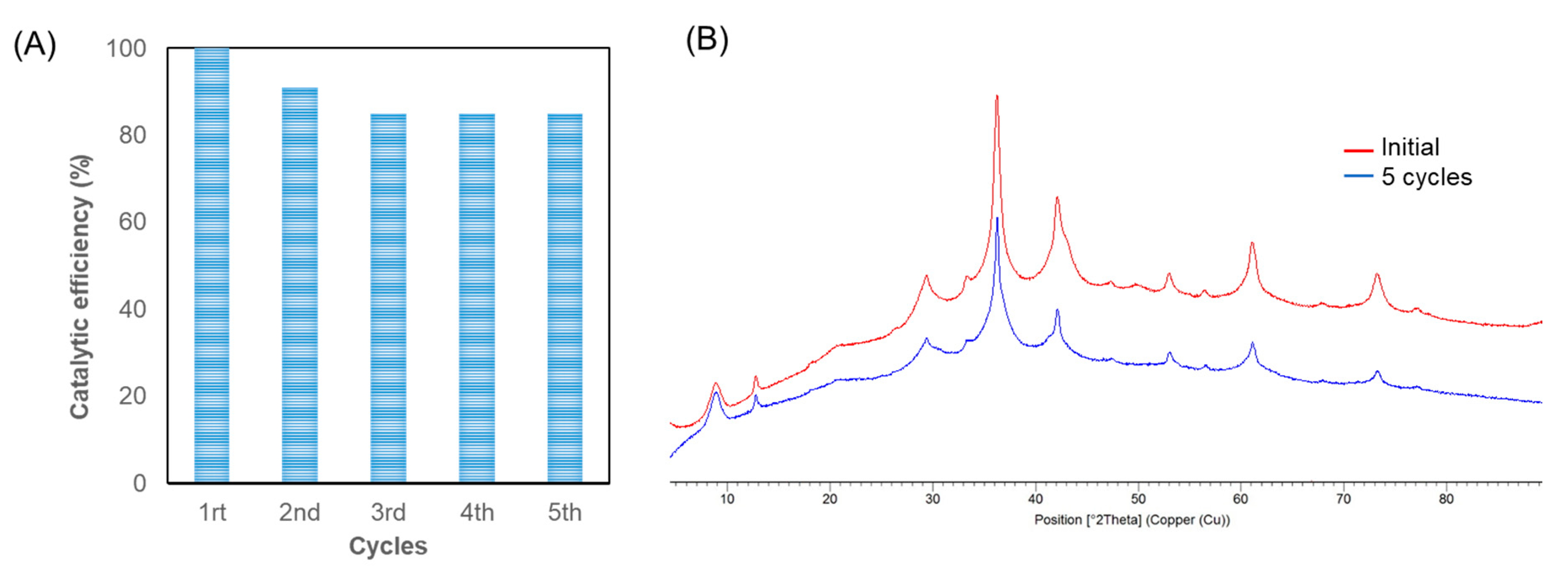
2.2. Reuse of Cu2O@CALB Biohybrid in the Degradation of TCE
2.3. 1,1-Dichloroethylene (1,1-DCE) Degradation Catalyzed by CuNPs@CALB Biohybrids
3. Materials and Methods
3.1. Materials
3.2. General Synthesis of MeNPs@CAL-B Biohybrids
3.2.1. Synthesis and Characterization of FeCO3@CAL-B Biohybrids
3.2.2. Synthesis and Characterization of CuNPs@CAL-B Biohybrids
3.2.3. Synthesis and Characterization of Pd(0)@CAL-B Biohybrids
3.2.4. Synthesis and Characterization of ZnO@CAL-B Biohybrids
3.3. Characterization Techniques
3.4. MeNPs@CALB Biohybrids Catalyzing the Degradation of Trichloroethylene (TCE)
3.5. Reuse of Cu2O@CALB Biohybrid in the Degradation of TCE
3.6. MeNPs@CALB Biohybrids Catalyzing the Degradation of 1,1-Dichloroethylene (1,1-DCE)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chiu, W.A.; Jinot, J.; Scott, C.S.; Makris, S.L.; Cooper, G.S.; Dzubow, R.C.; Bale, A.S.; Evans, M.V.; Guyton, K.Z.; Keshava, N.; et al. Human health effects of trichloroethylene: Key findings and scientific issues. Environ. Health Perspect. 2013, 121, 303–311. [Google Scholar] [CrossRef]
- Kueper, B.H.; Stroo, H.F.; Vogel, C.M.; Ward, C.H. Source zone remediation: The state of the practice. In Chlorinated Solvent Source Zone Remediation; Springer: New York, NY, USA, 2014; pp. 1–27. [Google Scholar]
- Palau, J.; Marchesi, M.; Chambon, J.C.C.; Aravena, R.; Canals, A.; Binning, P.J.; Bjerg, P.L.; Otero, N.; Soler, A. Multi-isotope (carbon and chlorine) analysis for fingerprinting and site characterization at a fractured bedrock aquifer contaminated by chlorinated ethenes. Sci. Total Environ. 2014, 475, 61–70. [Google Scholar] [CrossRef]
- Cucciniello, R.; Intiso, A.; Siciliano, T.; Palomares, A.E.; Martínez-Triguero, J.; Cerrillo, J.L.; Proto, A.; Rossi, F. Oxidative Degradation of Trichloroethylene over Fe2O3-doped Mayenite: Chlorine Poisoning Mitigation and Improved Catalytic Performance. Catalysts 2019, 9, 747. [Google Scholar] [CrossRef]
- Zhang, M.; Dong, J. Phase-transfer catalysis enhanced remediation of trichloroethylene polluted groundwater by potassium permanganate. Environ. Technol. 2019, 1–12. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, H.; Lu, Q.; Li, G.; Zhang, F. A CuNi bimetallic cathode with nanostructured copper array for enhanced hydrodechlorination of trichloroethylene (TCE). Sci. Total Environ. 2018, 635, 1417–1425. [Google Scholar] [CrossRef]
- Tsai, T.T.; Kao, C.M.; Surampalli, R.Y.; Weng, C.H.; Liang, S.H. Treatment of TCE contaminated groundwater using fenton-like oxidation activated with basic oxygen furnace slag. J. Environ. Eng. 2010, 136, 288–294. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. IARC Monographs on the Identification of Carcinogenic Hazards to Humans; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- US Environmental Protection Agency. Edition of the Drinking Water Standards and Health Advisories; EPA 822-R-18-001; EPA Office of Water: Washington, DC, USA, 2018.
- Danish, M.; Gu, X.; Lu, S.; Brusseau, M.L.; Ahmad, A.; Naqvi, M.; Farooq, U.; Zaman, W.Q.; Fu, X.; Miao, Z. An efficient catalytic degradation of trichloroethene in a percarbonate system catalyzed by ultra-fine heterogeneous zeolite supported zero valent iron-nickel bimetallic composite. Appl. Catal. A Gen. 2017, 531, 177–186. [Google Scholar] [CrossRef]
- Yan, J.; Qian, L.; Gao, W.; Chen, Y.; Ouyang, D.; Chen, M. Enhanced Fenton-like degradation of trichloroethylene by hydrogen peroxide activated with nanoscale zero valent iron loaded on biochar. Sci. Rep. 2017, 7, 43051. [Google Scholar] [CrossRef]
- Keijer, T.; Bakker, V.; Slootweg, J.C. Circular chemistry to enable a circular economy. Nat. Chem. 2019, 11, 190–195. [Google Scholar] [CrossRef]
- Serrá, A.; Artal, R.; García-Amorós, J.; Sepúlveda, B.; Gómez, E.; Nogués, J.; Philippe, L. Hybrid Ni@ZnO@ZnS-Microalgae for Circular Economy: A Smart Route to the Efficient Integration of Solar Photocatalytic Water Decontamination and Bioethanol Production. Adv. Sci. 2020, 7, 1902447. [Google Scholar] [CrossRef]
- Gonzalez-Olmos, R.; Anfruns, A.; Aguirre, N.V.; Masaguer, V.; Concheso, A.; Montes-Morán, M.A. Use of by-products from integrated steel plants as catalysts for the removal of trichloroethylene from groundwater. Chemosphere 2018, 213, 164–171. [Google Scholar] [CrossRef]
- Mao, X.; Ciblak, A.; Baek, K.; Amiri, M.; Loch-Caruso, R.; Alshawabkeh, A.N. Optimization of electrochemical dechlorination of trichloroethylene in reducing electrolytes. Water Res. 2012, 46, 1847–1857. [Google Scholar] [CrossRef]
- Huang, B.; Lei, C.; Wei, C.; Zeng, G. Chlorinated volatile organic compounds (Cl-VOCs) in environment sources, potential human health impacts, and current remediation technologies. Environ. Int. 2014, 71, 118–138. [Google Scholar] [CrossRef]
- Li, J.; de Toledo, R.A.; Shim, H. Multivariate optimization for the simultaneous bioremoval of BTEX and chlorinated aliphatic hydrocarbons by Pseudomonas plecoglossicida. J. Hazard. Mater. 2017, 321, 238–246. [Google Scholar] [CrossRef]
- Wang, C.; Ma, X.; Walsh, M.P. Competitive uptake and phytomonitoring of chlorinated contaminant mixtures by Redosier dogwood (Cornus sericea). Int. J. Phytoremediat. 2011, 13, 333–344. [Google Scholar] [CrossRef]
- Xin, J.; Zheng, X.; Han, J.; Shao, H.; Kolditz, O. Remediation of trichloroethylene by xanthan gum-coated microscale zero valent iron (XG-mZVI) in groundwater: Effects of geochemical constituents. Chem. Eng. J. 2015, 271, 164–172. [Google Scholar] [CrossRef]
- Biyoghe Bi Ndong, L.; Ibondou, M.P.; Gu, X.; Lu, S.; Qiu, Z.; Sui, Q.; Mbadinga, S.M. Enhanced photocatalytic activity of TiO2 nanosheets by doping with Cu for chlorinated solvent pollutants degradation. Ind. Eng. Chem. Res. 2014, 53, 1368–1376. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, A.; Gan, Y.; Li, X. Effects of inorganic anions on carbon isotope fractionation during Fenton-like degradation of trichloroethene. J. Hazard. Mater. 2016, 308, 187–191. [Google Scholar] [CrossRef]
- Ahn, J.Y.; Kim, C.; Kim, H.S.; Hwang, K.Y.; Hwang, I. Effects of oxidants on in situ treatment of a DNAPL source by nanoscale zero-valent iron: A field study. Water Res. 2016, 107, 57–65. [Google Scholar] [CrossRef]
- Han, Y.; Yan, W. Reductive dechlorination of trichloroethene by zero-valent iron nanoparticles: Reactivity enhancement through sulfidation treatment. Environ. Sci. Technol. 2016, 50, 12992–13001. [Google Scholar] [CrossRef] [PubMed]
- Srebowata, A.; Tarach, K.; Girman, V.; Góra-Marek, K. Catalytic removal of trichloroethylene from water over palladium loaded microporous and hierarchical zeolites. Appl. Catal. B Environ. 2016, 181, 550–560. [Google Scholar] [CrossRef]
- Yu, X.; Wu, T.; Yang, X.J.; Xu, J.; Auzam, J.; Semiat, R.; Han, Y.F. Degradation of trichloroethylene by hydrodechlorination using formic acid as hydrogen source over supported Pd catalysts. J. Hazard. Mater. 2016, 305, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Rajic, L.; Fallahpour, N.; Podlaha, E.; Alshawabkeh, A. The influence of cathode material on electrochemical degradation of trichloroethylene in aqueous solution. Chemosphere 2016, 147, 98–104. [Google Scholar] [CrossRef]
- Lu, Q.; Jeen, S.W.; Gui, L.; Gillham, R.W. Nitrate reduction and its effects on trichloroethylene degradation by granular iron. Water Res. 2017, 112, 48–57. [Google Scholar] [CrossRef]
- Han, Y.; Liu, C.; Horita, J.; Yan, W. Trichloroethene hydrodechlorination by Pd-Fe bimetallic nanoparticles: Solute-induced catalyst deactivation analyzed by carbon isotope fractionation. Appl. Catal. B Environ. 2016, 188, 77–86. [Google Scholar] [CrossRef]
- Kumar, M.A.; Bae, S.; Han, S.; Chang, Y.; Lee, W. Reductive dechlorination of trichloroethylene by polyvinylpyrrolidone stabilized nanoscale zerovalent iron particles with Ni. J. Hazard. Mater. 2017, 340, 399–406. [Google Scholar] [CrossRef]
- Shan, A.; Farooq, U.; Lyu, S.; Zaman, W.Q.; Abbas, Z.; Ali, M.; Idress, A.; Tang, P.; Li, M.; Sun, Y.; et al. Efficient removal of trichloroethylene in surfactant amended solution by nano Fe0-Nickel bimetallic composite activated sodium persulfate process. Chem. Eng. J. 2020, 386, 123995. [Google Scholar] [CrossRef]
- Benavente, R.; Lopez-Tejedor, D.; Palomo, J.M. Synthesis of a superparamagnetic ultrathin FeCO3 nanorods–enzyme bionanohybrid as a novel heterogeneous catalyst. Chem. Commun. 2018, 54, 6256–6259. [Google Scholar] [CrossRef]
- Losada-García, N.; Rodríguez-Otero, A.; Palomo, J.M. Tailorable synthesis of heterogeneous enzyme–copper nanobiohybrids and their application in the selective oxidation of benzene to phenol. Catal. Sci. Technol. 2020, 10, 196–206. [Google Scholar] [CrossRef]
- Cuenca, T.; Filice, M.; Palomo, J.M. Palladium nanoparticles enzyme aggregate (PANEA) as efficientcatalyst for Suzuki–Miyaura reaction in aqueous media. Enzym. Microb. Technol. 2017, 95, 242–247. [Google Scholar] [CrossRef]
- Wang, X.; Wang, W.; Lowry, G.; Li, X.; Guo, Y.; Li, T. Preparation of palladized carbon nanotubes encapsulated iron composites: Highly efficient dechlorination for trichloroethylene and low corrosion of nanoiron. R. Soc. Open Sci. 2018, 5, 172242. [Google Scholar] [CrossRef] [PubMed]
- Vargas, D.X.; De la Rosa, J.R.; Lucio-Ortiz, C.J.; Hernández-Ramirez, A.; Gerardo, A.; Flores-Escamilla, G.A.; Garcia, C.D. Photocatalytic degradation of trichloroethylene in a continuous annular reactor using Cu-doped TiO2 catalysts by sol–gel synthesis. Appl. Catal. B Environ. 2015, 179, 249–261. [Google Scholar] [CrossRef]
- Li, F.; Vipulanandan, C.; Mohanty, K.K. Microemulsion and solution approaches to nanoparticle iron production for degradation of trichloroethylene. Colloids Surf. A Physicochem. Eng. Asp. 2003, 223, 103–112. [Google Scholar] [CrossRef]
- Jang, D.G.; Ahn, C.H.; Choi, J.S.; Kim, J.H.; Kim, J.K.; Joo, J.C. Enhanced Removal of Trichloroethylene in Water Using Nano-ZnO/Polybutadiene Rubber Composites. Catalysts 2016, 6, 152. [Google Scholar] [CrossRef]
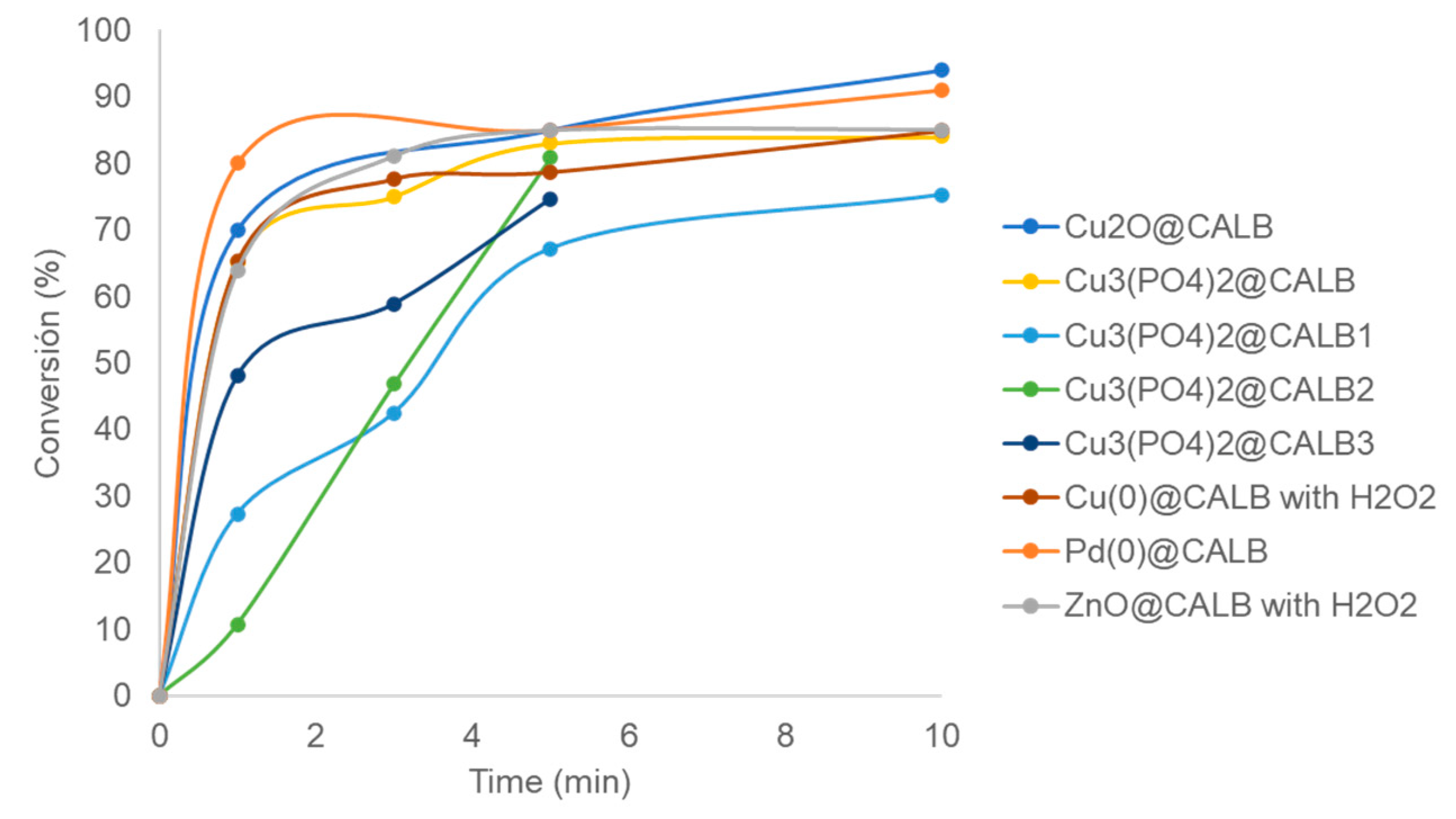
| Entry | Catalyst | H2O2 (mM) b | Time (min) | Degradation Yield (%) c | Presence of Vinyl Chloride d | Degraded (mg L−1) e |
|---|---|---|---|---|---|---|
| 1 | No catalyst | - | - | - | - | - |
| 2 | CALB | - | - | - | - | - |
| 3 | Pd(0)@CALB | 0 | 5 | 85 | no | 112 |
| 4 | FeCO3@CALB | 0 | 300 | 75 | yes | 99 |
| 5 | Cu2O@CALB | 0 | 5 | 85 | no | 112 |
| 6 | Cu3(PO4)2@CALB | 0 | 5 | 83 | no | 109 |
| 7 | Cu3(PO4)2@CALB1 | 0 | 5 | 67 | no | 9 |
| 8 | Cu3(PO4)2@CALB2 | 0 | 5 | 81 | yes | 106 |
| 9 | Cu3(PO4)2@CALB3 | 0 | 5 | 74 | yes | 97 |
| 10 | Cu(0)@CALB | 0 | 60 | 16 | yes | 21 |
| 11 | ZnO@CALB | 0 | 5 | 41 | no | 54 |
| 12 | FeCO3@CALB | 10 | 420 | >90 | no | >119 |
| 13 | Cu2O@CALB | 10 | 5 | 85 | no | 112 |
| 14 | Cu(0)@CALB | 10 | 5 | 78 | yes | 102 |
| 15 | ZnO@CALB | 10 | 5 | 85 | yes | 112 |
| Bionanohybrid | Time (min) b | Yield (%) c | TOF (min−1) d | DCE Degraded (mg L−1) e |
|---|---|---|---|---|
| Cu2O@CALB | 1 | 93.5 | 233.8 | 92.5 |
| Cu3(PO4)2@CALB | 1 | 91.5 | 228.6 | 90.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Losada-Garcia, N.; Rodriguez-Otero, A.; Palomo, J.M. High Degradation of Trichloroethylene in Water by Nanostructured MeNPs@CALB Biohybrid Catalysts. Catalysts 2020, 10, 753. https://doi.org/10.3390/catal10070753
Losada-Garcia N, Rodriguez-Otero A, Palomo JM. High Degradation of Trichloroethylene in Water by Nanostructured MeNPs@CALB Biohybrid Catalysts. Catalysts. 2020; 10(7):753. https://doi.org/10.3390/catal10070753
Chicago/Turabian StyleLosada-Garcia, Noelia, Alba Rodriguez-Otero, and Jose M. Palomo. 2020. "High Degradation of Trichloroethylene in Water by Nanostructured MeNPs@CALB Biohybrid Catalysts" Catalysts 10, no. 7: 753. https://doi.org/10.3390/catal10070753
APA StyleLosada-Garcia, N., Rodriguez-Otero, A., & Palomo, J. M. (2020). High Degradation of Trichloroethylene in Water by Nanostructured MeNPs@CALB Biohybrid Catalysts. Catalysts, 10(7), 753. https://doi.org/10.3390/catal10070753






