The Impacts of Fluorine-Doped Tin Oxide Photonic Crystals on a Cadmium Sulfide-Based Photoelectrode for Improved Solar Energy Conversion under Lower Incidence
Abstract
1. Introduction
2. Results and Discussion
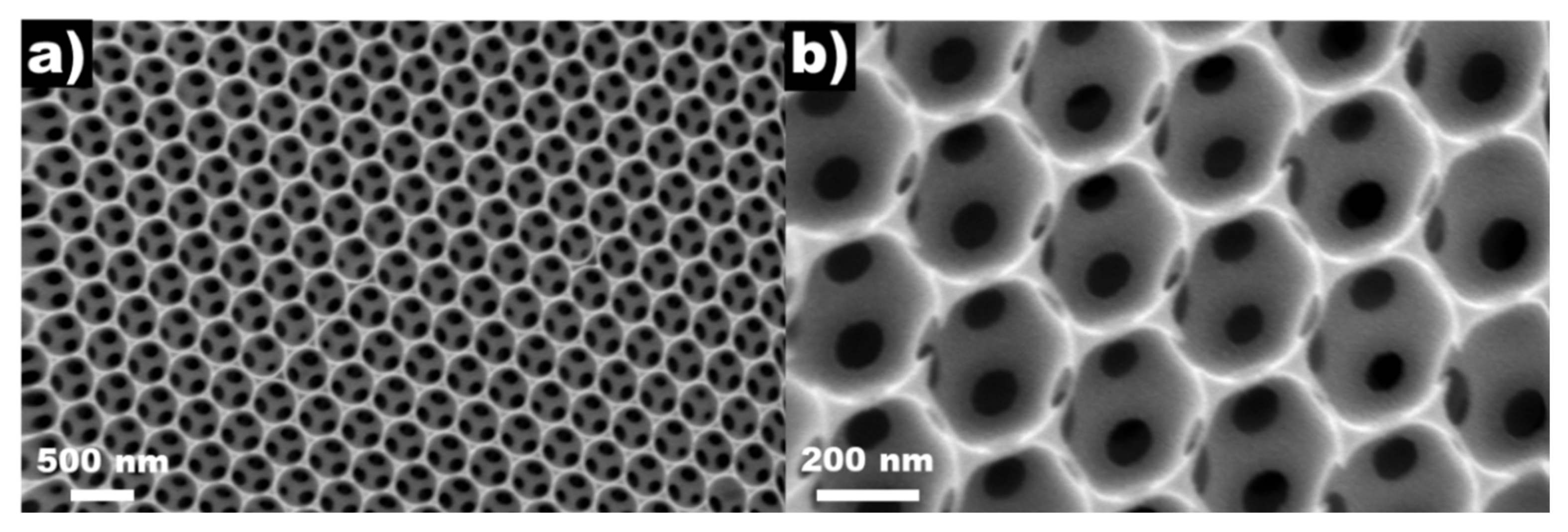
2.1. Geometrical Features
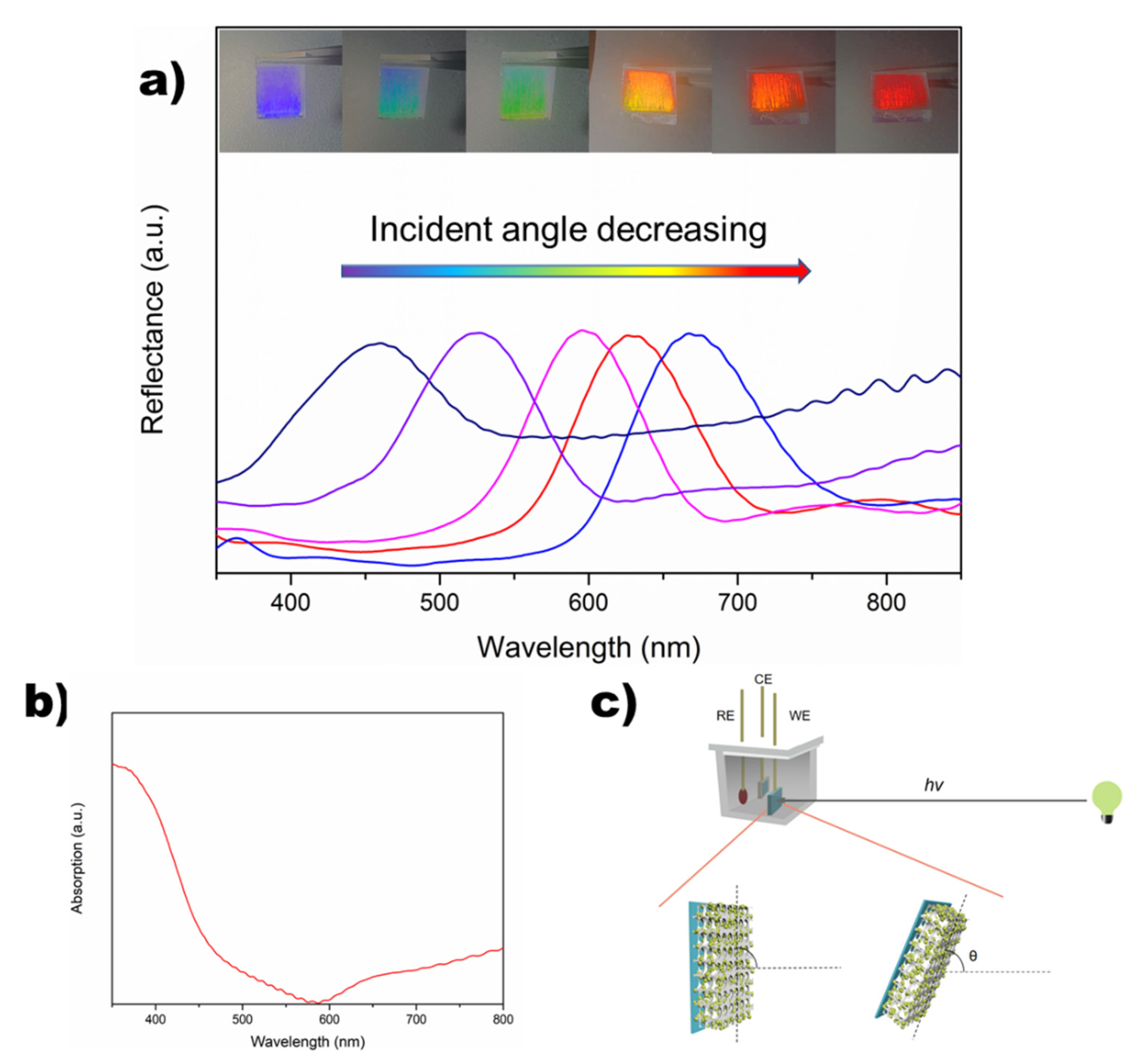
2.2. Optical Features
2.3. Loading the PC FTO Photoelectrode with CdS Nanoparticles
2.4. Integrated PEC Photocurrent Generation from the PC FTO Photoelectrode
3. Experimental
3.1. General
3.2. Polystyrene (PS) Template Film Formation
3.3. Photonic Crystal FTO (PC FTO) Electrode
3.4. Coating of the PC FTO with a Continuous CdS Film
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Archer, M.D.; Nozik, A.J. Nanostructured and Photoelectrochemical Systems for Solar Photon Conversion; Imperial College Press: London, UK, 2008; Volume 3. [Google Scholar]
- Jiang, C.; Moniz, S.J.A.; Wang, A.; Zhang, T.; Tang, J. Photoelectrochemical devices for solar water splitting–materials and challenges. Chem. Soc. Rev. 2017, 46, 4645–4660. [Google Scholar] [CrossRef]
- Walter, M.G.; Warren, E.L.; McKone, J.R.; Boettcher, S.W.; Mi, Q.; Santori, E.A.; Lewis, N.S. Solar Water Splitting Cells. Chem. Rev. 2010, 110, 6446–6473. [Google Scholar] [CrossRef] [PubMed]
- Khan, Q.; Subramanian, A.; Yu, G.; Maaz, K.; Li, D.; Sagar, R.U.R.; Chen, K.; Lei, W.; Shabbir, B.; Zhang, Y. Structure optimization of perovskite quantum dot light-emitting diodes. Nanoscale 2019, 11, 5021–5029. [Google Scholar] [CrossRef]
- Yin, X.L.; Li, L.L.; Jiang, W.J.; Zhang, Y.; Zhang, X.; Wan, L.J.; Hu, J.S. MoS2/CdS Nanosheets-on-Nanorod Heterostructure for Highly Efficient Photocatalytic H2 Generation under Visible Light Irradiation. ACS Appl. Mater. Interfaces 2016, 8, 15258–15266. [Google Scholar] [CrossRef]
- Wang, Y.; Zhen, W.; Zeng, Y.; Wan, S.; Guo, H.; Zhang, S.; Zhong, Q. In situ self-assembly of zirconium metal–organic frameworks onto ultrathin carbon nitride for enhanced visible light-driven conversion of CO2 to CO. J. Mater. Chem. A 2020, 8, 6034–6040. [Google Scholar] [CrossRef]
- Li, Y.; Fu, Z.-Y.; Su, B.-L. Hierarchically Structured Porous Materials for Energy Conversion and Storage. Adv. Funct. Mater. 2012, 22, 4634–4667. [Google Scholar] [CrossRef]
- Collins, G.; Armstrong, E.; McNulty, D.; O’Hanlon, S.; Geaney, H.; O’Dwyer, C. 2D and 3D photonic crystal materials for photocatalysis and electrochemical energy storage and conversion. Sci. Technol. Adv. Mater. 2016, 17, 563–582. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, L. Photonic nanostructures for solar energy conversion. Energy Environ. Sci. 2016, 9, 2511–2532. [Google Scholar] [CrossRef]
- Ming, J.; Liu, A.; Zhao, J.; Zhang, P.; Huang, H.; Lin, H.; Xu, Z.; Zhang, X.; Wang, X.; Hofkens, J.; et al. Hot pi-Electron Tunneling of Metal-Insulator-COF Nanostructures for Efficient Hydrogen Production. Angew. Chem. 2019, 58, 18290–18294. [Google Scholar] [CrossRef]
- Pi, M.; Wang, X.; Zhang, D.; Wang, S.; Chen, S. A 3D porous WP2 nanosheets@carbon cloth flexible electrode for efficient electrocatalytic hydrogen evolution. Front. Chem. Sci. Eng. 2018, 12, 425–432. [Google Scholar] [CrossRef]
- Xiang, L.; Zhao, X. Wet-Chemical Preparation of TiO2-Based Composites with Different Morphologies and Photocatalytic Properties. Nanomaterials 2017, 7, 310. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Chen, M.; Zhang, M. Improved Utilization of Sunlight Through the Incidence Dependence of the Photonic Stop Band: A g-C3N4-Embedded Fluorine-Doped Tin Oxide (FTO) Photonic Crystal Film. ChemPhotoChem 2019, 3, 101–106. [Google Scholar] [CrossRef]
- Ellmer, K. Past achievements and future challenges in the development of optically transparent electrodes. Nat. Photon. 2012, 6, 809–817. [Google Scholar] [CrossRef]
- Nishimoto, N.; Yamada, Y.; Ohnishi, Y.; Imawaka, N.; Yoshino, K. Effect of temperature on the electrical properties of ITO in a TiO2 /ITO film. Phys. Status Solidi (a) 2013, 210, 589–593. [Google Scholar] [CrossRef]
- Yang, Z.; Gao, S.; Li, T.; Liu, F.-Q.; Ren, Y.; Xu, T. Enhanced Electron Extraction from Template-Free 3D Nanoparticulate Transparent Conducting Oxide (TCO) Electrodes for Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2012, 4, 4419–4427. [Google Scholar] [CrossRef]
- Yuan, S.; Huang, H.; Wang, Z.; Zhao, Y.; Shi, L.; Cai, C.; Li, D.D. Improved electron-collection performance of dye sensitized solar cell based on three-dimensional conductive grid. J. Photochem. Photobiol. A Chem. 2013, 259, 10–16. [Google Scholar] [CrossRef]
- Ahmad, M.S.; Pandey, A.; Rahim, N.A. Advancements in the development of TiO2 photoanodes and its fabrication methods for dye sensitized solar cell (DSSC) applications. A review. Renew. Sustain. Energy Rev. 2017, 77, 89–108. [Google Scholar] [CrossRef]
- Tang, M.H.; Chakthranont, P.; Jaramillo, T.F. Top-down fabrication of fluorine-doped tin oxide nanopillar substrates for solar water splitting. RSC Adv. 2017, 7, 28350–28357. [Google Scholar] [CrossRef]
- Xie, Z.; Zhang, Q.; Liu, Q.; Zhai, J.; Diao, X. Enhanced electrochromic performance of 2D grid-structured WO3 thin films. Thin Solid Films 2018, 653, 188–193. [Google Scholar] [CrossRef]
- Li, J.; Qiu, Y.; Wei, Z.; Lin, Q.; Zhang, Q.; Yan, K.; Chen, H.; Xiao, S.; Fan, Z.; Yang, S. A three-dimensional hexagonal fluorine-doped tin oxide nanocone array: A superior light harvesting electrode for high performance photoelectrochemical water splitting. Energy Environ. Sci. 2014, 7, 3651–3658. [Google Scholar] [CrossRef]
- Xie, J.; Yang, P.; Liang, X.; Xiong, J. Self-Improvement of Ti:Fe2O3 Photoanodes: Photoelectrocatalysis Improvement after Long-Term Stability Testing in Alkaline Electrolyte. ACS Appl. Energy Mater. 2018, 1, 2769–2775. [Google Scholar] [CrossRef]
- Yang, Z.; Gao, S.; Li, W.; Vlasko-Vlasov, V.; Welp, U.; Kwok, W.-K.; Xu, T. Three-Dimensional Photonic Crystal Fluorinated Tin Oxide (FTO) Electrodes: Synthesis and Opstical and Electrical Properties. ACS Appl. Mater. Interfaces 2011, 3, 1101–1108. [Google Scholar] [CrossRef]
- Gun, Y.; Song, G.Y.; Quy, V.H.V.; Heo, J.; Lee, H.; Ahn, K.-S.; Kang, S.-H. Joint Effects of Photoactive TiO2 and Fluoride-Doping on SnO2 Inverse Opal Nanoarchitecture for Solar Water Splitting. ACS Appl. Mater. Interfaces 2015, 7, 20292–20303. [Google Scholar] [CrossRef]
- Cho, S.Y.; Kang, S.H.; Yun, G.; Balamurugan, M.; Ahn, K.S. Annealing Effect of Fluorine-Doped SnO2/WO3 Core-Shell Inverse Opal Nanoarchitecture for Photoelectrochemical Water Splitting. J. Korean Phys. Soc. 2017, 70, 162–168. [Google Scholar] [CrossRef]
- Luo, D.; Chen, Q.; Qiu, Y.; Liu, B.; Zhang, M. Carbon Dots-Decorated Bi2WO6 in an Inverse Opal Film as a Photoanode for Photoelectrochemical Solar Energy Conversion under Visible-Light Irradiation. Materials 2019, 12, 1713. [Google Scholar] [CrossRef] [PubMed]
- Stein, A.; Wilson, B.E.; Rudisill, S.G. Design and functionality of colloidal-crystal-templated materials—chemical applications of inverse opals. Chem. Soc. Rev. 2013, 42, 2763–2803. [Google Scholar] [CrossRef]
- Kanai, T.; Sano, K.; Yano, H.; Sawada, T. Independent control of optical stop-band wavelength and width of colloidal photonic crystals. Colloids Surfaces A Physicochem. Eng. Asp. 2016, 506, 586–590. [Google Scholar] [CrossRef]
- Hu, J.; Yang, M.; Ke, X.; Yang, S.; Wang, K.; Huang, H.; Wang, W.; Luo, D.; Zheng, Z.; Huang, L.; et al. Cubic-cubic perovskite quantum dots/PbS mixed dimensional materials for highly efficient CO2 reduction. J. Power Sources 2021, 481, 228838. [Google Scholar] [CrossRef]
- Huang, H.; Yuan, H.; Zhao, J.; Solís-Fernández, G.; Zhou, C.; Seo, J.W.; Hendrix, J.; Debroye, E.; Steele, J.A.; Hofkens, J.; et al. C(sp3)–H Bond Activation by Perovskite Solar Photocatalyst Cell. ACS Energy Lett. 2018, 4, 203–208. [Google Scholar] [CrossRef]
- Huang, H.; Yuan, H.; Janssen, K.P.F.; Solís-Fernández, G.; Wang, Y.; Tan, C.Y.X.; Jonckheere, D.; Debroye, E.; Long, J.; Hendrix, J.; et al. Efficient and Selective Photocatalytic Oxidation of Benzylic Alcohols with Hybrid Organic–Inorganic Perovskite Materials. ACS Energy Lett. 2018, 3, 755–759. [Google Scholar] [CrossRef]
- Liu, H.; Sun, H.; Liu, L.; Hou, X.; Jia, X. Optical properties of porous anodic alumina embedded Cu nanocomposite films. Opt. Mater. 2015, 44, 9–12. [Google Scholar] [CrossRef]
- Keller, D.A.; Barad, H.-N.; Rosh-Hodesh, E.; Zaban, A.; Cahen, D. Can fluorine-doped tin Oxide, FTO, be more like indium-doped tin oxide, ITO? Reducing FTO surface roughness by introducing additional SnO2 coating. MRS Commun. 2018, 8, 1358–1362. [Google Scholar] [CrossRef]
- Banyamin, Z.Y.; Kelly, P.J.; West, G.; Boardman, J. Electrical and Optical Properties of Fluorine Doped Tin Oxide Thin Films Prepared by Magnetron Sputtering. Coatings 2014, 4, 732–746. [Google Scholar] [CrossRef]
- Cui, X.; Wang, Y.; Jiang, G.; Zhao, Z.; Xu, C.; Wei, Y.; Duan, A.; Liu, J.; Gao, J. A photonic crystal-based CdS–Au–WO3 heterostructure for efficient visible-light photocatalytic hydrogen and oxygen evolution. RSC Adv. 2014, 4, 15689–15694. [Google Scholar] [CrossRef]
- Wang, Z.; Li, X.; Ling, H.; Tan, C.K.; Yeo, L.P.; Grimsdale, A.C.; Tok, A. 3D FTO/FTO-Nanocrystal/TiO2 Composite Inverse Opal Photoanode for Efficient Photoelectrochemical Water Splitting. Small 2018, 14, 1800395. [Google Scholar] [CrossRef]
- Wu, X.; Zhou, J.; Xing, W.; Zhang, Y.; Bai, P.; Xu, B.; Zhuo, S.; Xue, Q.; Yan, Z. Insight into high areal capacitances of low apparent surface area carbons derived from nitrogen-rich polymers. Carbon 2015, 94, 560–567. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Ke, X.; Wang, W.; Tu, C.; Luo, D.; Zhang, M. The Impacts of Fluorine-Doped Tin Oxide Photonic Crystals on a Cadmium Sulfide-Based Photoelectrode for Improved Solar Energy Conversion under Lower Incidence. Catalysts 2020, 10, 1252. https://doi.org/10.3390/catal10111252
Wang K, Ke X, Wang W, Tu C, Luo D, Zhang M. The Impacts of Fluorine-Doped Tin Oxide Photonic Crystals on a Cadmium Sulfide-Based Photoelectrode for Improved Solar Energy Conversion under Lower Incidence. Catalysts. 2020; 10(11):1252. https://doi.org/10.3390/catal10111252
Chicago/Turabian StyleWang, Kunqiang, Xi Ke, Weizhe Wang, Chen Tu, Dongxiang Luo, and Menglong Zhang. 2020. "The Impacts of Fluorine-Doped Tin Oxide Photonic Crystals on a Cadmium Sulfide-Based Photoelectrode for Improved Solar Energy Conversion under Lower Incidence" Catalysts 10, no. 11: 1252. https://doi.org/10.3390/catal10111252
APA StyleWang, K., Ke, X., Wang, W., Tu, C., Luo, D., & Zhang, M. (2020). The Impacts of Fluorine-Doped Tin Oxide Photonic Crystals on a Cadmium Sulfide-Based Photoelectrode for Improved Solar Energy Conversion under Lower Incidence. Catalysts, 10(11), 1252. https://doi.org/10.3390/catal10111252



