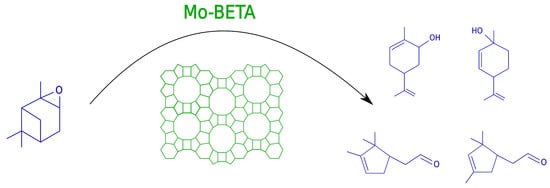
Solvent Influence on Selectivity in α-Pinene Oxide Isomerization Using MoO3-Modified Zeolite BETA
Abstract
1. Introduction
2. Results and Discussion
2.1. Material Preparation and Characterization
2.2. Catalytic Testing
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Edl, W.; Sienel, G.R. Process for the production of epoxides. U.S. Patent No. 4882442A, 30 December 1989. [Google Scholar]
- Jaafari, A.; Tilaoui, M.; Mouse, H.A.; M’bark, L.A.; Aboufatima, R.; Chait, A.; Lepoivre, M.; Zyad, A. Comparative study of the antitumor effect of natural monoterpenes: Relationship to cell cycle analysis. Revista Brasileira Farmacognosia 2012, 22, 534–540. [Google Scholar] [CrossRef]
- Hussain, A.I.; Anwar, F.; Shahid, M.; Ashraf, M.; Przybylski, R. Chemical Composition, and Antioxidant and Antimicrobial Activities of Essential Oil of Spearmint (Mentha spicata L.). J. Essent. Oil Res. 2010, 22, 78–84. [Google Scholar] [CrossRef]
- Castro, J.M.; Linares-Palomino, P.J.; Salido, S.; Altarejos, J.; Nogueras, M.; Sánchez, A. Enantiospecific synthesis, separation and olfactory evaluation of all diastereomers of a homologue of the sandalwood odorant Polysantol. Tetrahedron 2005, 61, 11192–11203. [Google Scholar] [CrossRef]
- Arbushow, B. Studium der Isomerisation von Terpen-oxyden, I. Mitteil.: Isomerisation des α-Pinen-oxydes bei der Reaktion von Reformatsky. Chem. Ber. 1935, 68, 1430–1435. [Google Scholar] [CrossRef]
- Duetz, W.; Fjällman, A.; Ren, S.; Jourdat, C.; Witholt, B. Biotransformation of D-Limonene to (+) trans-Carveol by Toluene-Grown Rhodococcus opacus PWD4 Cells. Appl. Environ. Microbiol. 2001, 67, 2829–2832. [Google Scholar] [CrossRef] [PubMed]
- Coelho, J.V.; de Meireles, A.L.P.; da Silva Rocha, K.A.; Pereira, M.C.; Oliveira, L.C.A.; Gusevskaya, E.V. Isomerization of α-pinene oxide catalyzed by iron-modified mesoporous silicates. Appl. Catal. A Gen. 2012, 443–444, 125–132. [Google Scholar] [CrossRef]
- Kumar, N.; Mäki-Arvela, P.; Diaz, S.F.; Aho, A.; Demidova, Y.; Linden, J.; Shepidchenko, A.; Tenhu, M.; Salonen, J.; Laukkanen, P.; et al. Isomerization of α-Pinene Oxide Over Iron-Modified Zeolites. Top. Catal. 2013, 56, 696–713. [Google Scholar] [CrossRef]
- Ravasio, N.; Zaccheria, F.; Gervasini, A.; Messi, C. A new, Fe based, heterogeneous Lewis acid: Selective isomerization of α-pinene oxide. Catal. Commun. 2008, 9, 1125–1127. [Google Scholar] [CrossRef]
- Štekrová, M.; Kumar, N.; Aho, A.; Sinev, I.; Grunert, W.; Dahl, J.; Roine, J.; Arzumanov, S.S.; Mäki-Arvela, P.; Murzin, D.Y. Isomerization of alpha-pinene oxide using Fe-supported catalysts: Selective synthesis of campholenic aldehyde. Appl. Catal. A Gen. 2014, 470, 162–176. [Google Scholar] [CrossRef]
- Sánchez-Velandia, J.E.; Villa, A.L. Isomerization of α- and β-pinene epoxides over Fe or Cu supported MCM-41 and SBA-15 materials. Appl. Catal. A Gen. 2019, 580, 17–27. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Alvaro, M.; Chevreau, H.; Horcajada, P.; Devic, T.; Serre, C.; Garcia, H. Iron(iii) metal–organic frameworks as solid Lewis acids for the isomerization of α-pinene oxide. Catal. Sci. Technol. 2012, 2, 324–330. [Google Scholar] [CrossRef]
- Fellenz, N.A.; Bengoa, J.F.; Marchetti, S.G.; Gervasini, A. Influence of the Brönsted and Lewis acid sites on the catalytic activity and selectivity of Fe/MCM-41 system. Appl. Catal. A Gen. 2012, 435-436, 187–196. [Google Scholar] [CrossRef]
- Vyskočilová, E.; Hašková, L.; Červený, L. Solvent-induced selectivity in α-pinene oxide isomerization catalyzed by Fe-modified zeolite beta. Chem. Pap. 2019, 73, 1621–1627. [Google Scholar] [CrossRef]
- Štekrová, M.; Kumar, N.; Diaz, S.F.; Mäki-Arvela, P.; Murzin, D.Y. H- and Fe-modified zeolite beta catalysts for preparation of trans-carveol from α-pinene oxide. Catal. Today 2015, 241, 237–245. [Google Scholar] [CrossRef]
- Kunkeler, P.J.; van der Waal, J.C.; Bremmer, J.; Zuurdeeg, B.J.; Downing, R.S.; van Bekkum, H. Application of zeolite titanium Beta in the rearrangement of α-pinene oxide to campholenic aldehyde. Catal. Lett. 1998, 53, 135–138. [Google Scholar] [CrossRef]
- Panadero, M.P.; Velty, A. Readily available Ti-beta as an efficient catalyst for greener and sustainable production of campholenic aldehyde. Catal. Sci. Technol. 2019, 9, 4293–4303. [Google Scholar] [CrossRef]
- Pitínová-Štekrová, M.; Eliášová, P.; Weissenberger, T.; Shamzy, M.; Musilová, Z.; Čejka, J. Highly selective synthesis of campholenic aldehyde over Ti-MWW catalysts by α-pinene oxide isomerization. Catal. Sci. Technol. 2018, 8, 4690–4701. [Google Scholar] [CrossRef]
- Costa, V.V.; da Silva Rocha, K.A.; de Sousa, L.F.; Robles-Dutenhefner, P.A.; Gusevskaya, E.V. Isomerization of α-pinene oxide over cerium and tin catalysts: Selective synthesis of trans-carveol and transsobrerol. J. Mol. Catal. A Chem. 2011, 345, 69–74. [Google Scholar] [CrossRef]
- Štekrová, M.; Matoušková, M.; Vyskočilová, E.; Červený, L. Selective preparation of campholenic aldehyde over heterogenized methyltrioxorhenium. Res. Chem. Intermed. 2015, 41, 9003–9013. [Google Scholar] [CrossRef]
- Štekrová, M.; Kumar, N.; Mäki-Arvela, P.; Ardashov, O.V.; Volcho, K.P.; Salakhutdinov, N.F.; Murzin, D.Y. Selective Preparation of trans-Carveol over Ceria Supported Mesoporous Materials MCM-41 and SBA-15. Materials 2013, 6, 2103–2118. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Rocha, K.A.; Kozhevnikov, I.V.; Gusevskaya, E.V. Isomerisation of α-pinene oxide over silica supported heteropoly acid H3PW12O40. Appl. Catal. A 2005, 294, 106–110. [Google Scholar] [CrossRef]
- Da Silva Rocha, K.A.; Hoehne, J.L.; Gusevskaya, E.V. Phosphotungstic Acid as a Versatile Catalyst for the Synthesis of Fragrance Compounds by α-Pinene Oxide Isomerization: Solvent-Induced Chemoselectivity. Chem. Eur. J. 2008, 14, 6166–6172. [Google Scholar] [CrossRef] [PubMed]
- Sidorenko, A.Y.; Ignatovich, Z.V.; Ermolinskaya, A.L.; Kravtsova, A.V.; Baranovskii, A.V.; Koroleva, E.V.; Agabekov, V.E. Synthesis of Fencholenic Aldehyde from α-pinene Epoxide on Modified Clays. Chem. Nat. Compd. 2018, 54, 893–897. [Google Scholar] [CrossRef]
- Vrbková, E.; Šteflová, B.; Sekerová, L.; Krupka, J.; Vyskočilová, E.; Červený, L. Contribution to MoO 3–SiO 2 and WO 3–SiO 2 utilization—Active catalysts in jasmine aldehyde, 2-hexyl-1, 3-dioxolane and methyllaurate synthesis. React. Kinet. Mech. Catal. 2020, 129, 645–658. [Google Scholar] [CrossRef]
- Vrbková, E.; Šteflová, B.; Vyskočilová, E.; Červený, L. Heterogeneous Mo/W/Zn–SiO2 based catalysts in nopol (2-(6,6-dimethyl-2-bicyclo[3.1.1]hept-2-enyl)ethanol) synthesis. React. Kinet. Mech. Catal. 2020, 131, 213–232. [Google Scholar] [CrossRef]
- Wehrer, W.; Bigey, L.; Hilaire, L. Catalytic reactions of n-hexane and 1-hexene on molybdenum dioxide. Appl. Catal. A 2003, 243, 109–119. [Google Scholar] [CrossRef]
- Bouchy, C.; Pham-Huu, C.; Heinrich, B.; Chaumont, C.; Ledoux, M.J. Microstructure and Characterization of a Highly Selective Catalyst for the Isomerization of Alkanes: A Molybdenum Oxycarbide. J. Catal. 2000, 190, 92–103. [Google Scholar] [CrossRef]
- Lamic, A.F.; Shin, C.H.; Djega-Mariadassou, G.; Potvin, C. Characterization of Mo2C–WO2 composite catalysts for bifunctional isomerization: A new pulse method to quantify acid sites. Appl. Catal. A 2006, 302, 5–13. [Google Scholar] [CrossRef]
- York, A.P.E.; Pham-Huu, C.; Del Gallo, P.; Ledoux, M.J. Molybdenum oxycarbide hydrocarbon isomerization catalysts: Cleaner fuels for the future. Catal. Today 1997, 35, 51–57. [Google Scholar] [CrossRef]
- Ohno, T.; Li, Z.; Sakai, N.; Sakagami, H.; Takahashi, N.; Matsuda, T. Heptane isomerization over molybdenum oxides obtained by H2 reduction of HxMoO3 with different hydrogen contents. Appl. Catal. A 2010, 389, 52–59. [Google Scholar] [CrossRef]
- Bruno, S.M.; Valente, A.A.; Pillinger, M.; Amelse, J.; Romão, C.C.; Gonçalves, I.S. Efficient Isomerization of α-Pinene Oxide to Campholenic Aldehyde Promoted by a Mixed-Ring Analogue of Molybdenocene. ACS Sustain. Chem. Eng. 2019, 7, 13639–13645. [Google Scholar] [CrossRef]
- Caullet, P.; Hazm, J.; Guth, J.; Joly, J.; Lynch, J.; Raatz, F. Synthesis of zeolite Beta from nonalkaline fluoride aqueous aluminosilicate gels. Zeolites 1992, 12, 240–250. [Google Scholar] [CrossRef]
- Hansen, S.; Andersson, A. Electron microscopy of some molybdenum oxide phases after use as catalysts in oxidative ammonolysis and ammoxidation of toluene. J. Solid State Chem. 1988, 75, 225–243. [Google Scholar] [CrossRef]
- Sekerová, L.; Vyskočilová, E.; Červený, L. Prins cyclization of isoprenol with various aldehydes using MoO3/SiO2 as a catalyst. React. Kinet. Mech. Catal. 2017, 121, 83–95. [Google Scholar] [CrossRef]
- Gutmann Acceptor and Donor number. Available online: http://www.stenutz.eu/chem/solv21.php (accessed on 12 August 2020).
- Solvent Properties Chart. Available online: https://depts.washington.edu/eooptic/linkfiles/dielectric_chart%5B1%5D.pdf (accessed on 12 August 2020).
- Munack, A.; Schmidt, L.; Schröder, O.; Schaper, K.; Pabst, C.; Krahl, J. Alcohols as a means to inhibit the formation of precipitates in blends of biodiesel and fossil diesel fuel. Agric. Eng. Int. 2015, 2015, 226–233. [Google Scholar]
- Dielectric Constants of Liquids. Available online: https://www.engineeringtoolbox.com/liquid-dielectric-constants-d_1263.html (accessed on 31 August 2020).
- Dielectric Constants of Common Materials. Available online: https://www.kabusa.com/Dilectric-Constants.pdf (accessed on 31 August 2020).
- Mäki-Arvela, P.; Shcherban, N.; Lozachmeur, C.; Russo, V.; Wärnå, J.; Murzin, D.Y. Isomerization of α-Pinene Oxide: Solvent Effects, Kinetics and Thermodynamics. Catal. Lett. 2019, 149, 203–214. [Google Scholar] [CrossRef]
- Vrbková, E.; Šteflová, B.; Zapletal, M.; Vyskočilová, E.; Červený, L. Tungsten oxide-based materials as effective catalysts in isopulegol formation by intramolecular Prins reaction of citronellal. Res. Chem. Intermed. 2020, 46, 4047–4059. [Google Scholar] [CrossRef]














| Material | MoO3 (%) | Mo (%) calculated | SiO2 (%) | Al2O3 (%) | Others (%) |
|---|---|---|---|---|---|
| 20Mo450 | 32.8 | 21.6 | 63.7 | 3.4 | 0.1 |
| 20Mo450RT | 36.0 | 23.7 | 60.8 | 3.1 | 0.1 |
| 20Mo500 | 32.0 | 21.1 | 64.7 | 3.2 | 0.1 |
| 20Mo550 | 33.2 | 21.9 | 63.5 | 3.2 | 0.1 |
| 20Mo600 | 33.9 | 22.3 | 62.9 | 3.1 | 0.1 |
| Material | SBET m2/g | St-plot m2/g | Total Pore Volume cm3/g | t-Plot Micropore Volume cm3/g | Ratio of Micropores (%) |
|---|---|---|---|---|---|
| BETA38 | 539.62 | 136.15 | 0.341 | 0.210 | 61.6 |
| 20Mo450 | 272.81 | 58.70 | 0.181 | 0.110 | 60.8 |
| 20Mo500 | 239.46 | 53.98 | 0.171 | 0.096 | 56.1 |
| 20Mo550 | 154.33 | 43.47 | 0.139 | 0.057 | 41.0 |
| 20Mo600 | 16.50 | 12.43 | 0.057 | 0.002 | 3.5 |
| 20Mo450RT | 269.30 | 61.61 | 0.184 | 0.107 | 58.1 |
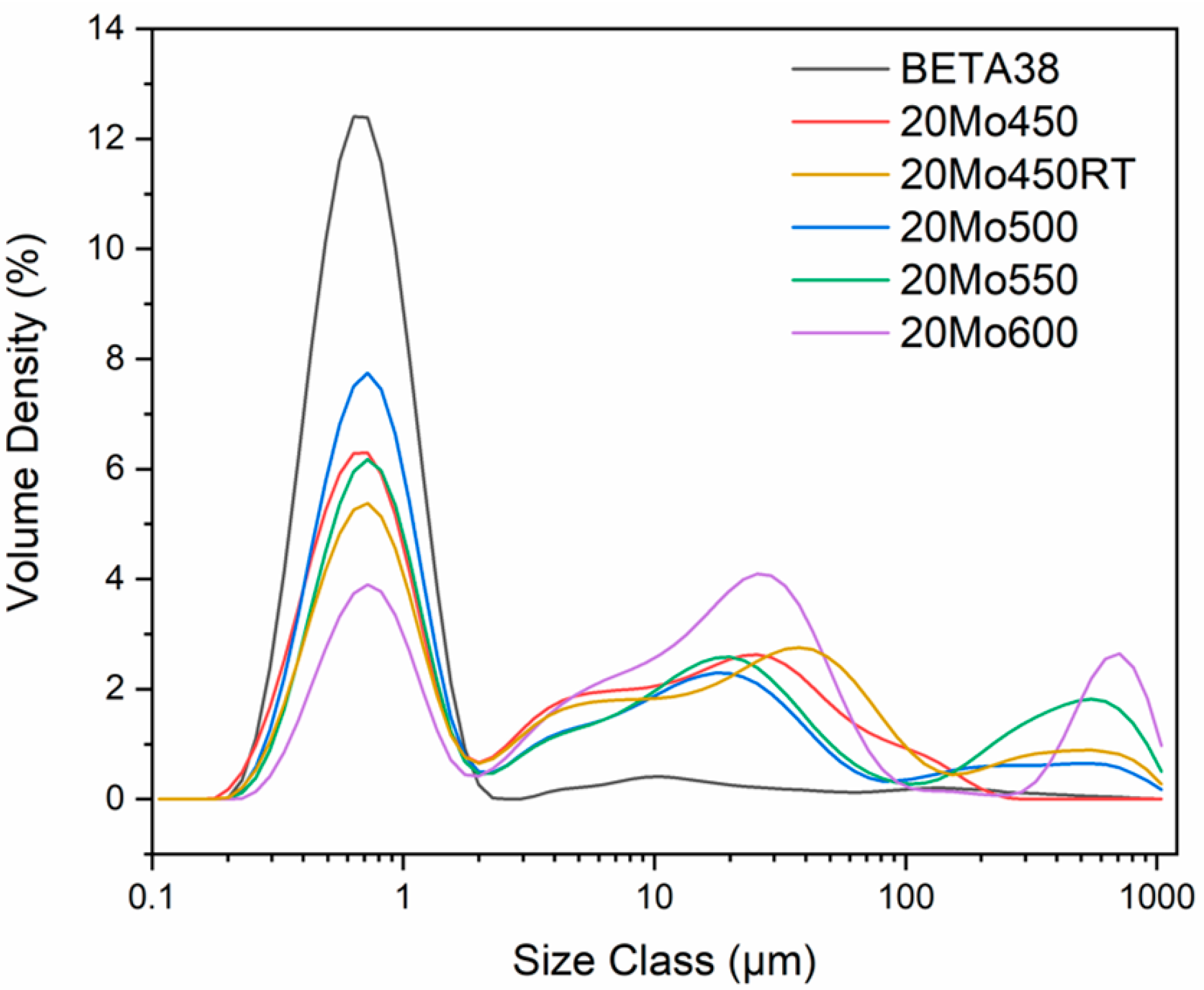
| Material | Span Value | Particle Size (µm) | ||
|---|---|---|---|---|
| Dv(10) | Dv(50) | Dv(90) | ||
| BETA38 | 1.6 | 0.39 | 0.71 | 1.6 |
| 20Mo450 | 22.2 | 0.43 | 1.64 | 35.2 |
| 20Mo450RT | 22.5 | 0.44 | 1.94 | 44.2 |
| 20Mo500 | 47.9 | 0.46 | 1.74 | 55.0 |
| 20Mo550 | 81.9 | 0.50 | 4.74 | 389.0 |
| 20Mo600 | 43.8 | 0.58 | 16.20 | 710.0 |
| Solvent | Solvent Type | Donor Number (kJ/mol) | Dielectric Constant (Relative Permitivity) | Initial Reaction Rate (mmol/gcat.min) | APO Conversion at 3 h (%) | CA Selectivity at 3 h (%) | TCA Selectivity at 3 h (%) | PMD Selectivity at 3 h (%) |
|---|---|---|---|---|---|---|---|---|
| cyclohexane | nonpolar | 0 | 2.02 | 2.40 | 99 | 34.6 | 17.4 | 13.9 |
| toluene | nonpolar | 0.1 | 2.38 | 5.43 | 100 | 34.2 | 14.8 | 14.0 |
| 1,4 dioxane | nonpolar | 14.3 | 2.25 | 6.63 | 100 | 28.7 | 18.0 | 11.0 |
| nitromethane | polar aprotic | 2.7 | 35.87 | 4.68 | 86 | 44.9 | 10.7 | 12.3 |
| butan-1-ol | polar protic | 19.5 | 17.8 | 13.11 | 100 | 3.2 | 7.0 | 1.9 |
| propan-1-ol | polar protic | 19.8 | 21.8 | 13.11 | 100 | 3.3 | 6.4 | 2.7 |
| propan-2-ol | polar protic | 21.1 | 17.9 | 13.11 | 100 | 18.2 | 14.2 | 5.4 |
| dichlorbenzene | polar aprotic | 3 | 9.93 | 2.67 | 100 | 42.1 | 13.3 | 13.1 |
| benzonitrile | polar aprotic | 11.9 | 26 | 0.71 | 16 | 66.5 | 9.8 | 0.0 |
| acetonitrile | polar aprotic | 14 | 37.5 | 0.68 | 10 | 62.7 | 4.9 | 2.9 |
| ethylacetate | polar aprotic | 17.1 | 6.02 | 2.67 | 97 | 37.1 | 15.8 | 13.9 |
| pentan-2-one | polar aprotic | 17.1 | 18.2 | 11.32 | 100 | 35.1 | 15.5 | 12.0 |
| butan-2-one | polar aprotic | 17.4 | 18.5 | 11.95 | 100 | 30.8 | 10.4 | 9.1 |
| cyclohexanone | polar aprotic | 18 | 18.2 | 8.34 | 100 | 37.4 | 15.0 | 16.6 |
| tetrahydrofuran | polar aprotic | 20 | 7.58 | 6.74 | 100 | 31.7 | 28.9 | 6.5 |
| cyclohexanol | polar protic | 25 | 15 | 5.58 | 100 | 24.1 | 21.1 | 5.1 |
| N,N’-dimethylformamide | polar aprotic | 26.6 | 36.7 | 1.01 | 27 | 26.5 | 43.8 | 14.0 |
| N-methylpyrrolidone | polar aprotic | 27.3 | 32.17 | 0.85 | 41 | 26.1 | 42.9 | 12.2 |
| N,N’-dimethylacetamide | polar aprotic | 27.8 | 37.8 | 4.65 | 44 | 25.3 | 45.5 | 11.5 |
| dimethylsulfoxide | polar aprotic | 29.8 | 46.7 | 0.97 | 45 | 25.3 | 53.6 | 12.7 |
| pyridine | polar aprotic | 33.1 | 12.4 | 0.14 | 3 | 55.6 | 18.5 | 0.0 |
| Material Denotation | Temperature of Wet Impregnation | Calcination Temperature (°C) |
|---|---|---|
| 20Mo450RT | room temperature | 450 |
| 20Mo450 | 60 °C | 450 |
| 20Mo500 | 500 | |
| 520Mo550 | 550 | |
| 20Mo600 | 600 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vrbková, E.; Vyskočilová, E.; Lhotka, M.; Červený, L. Solvent Influence on Selectivity in α-Pinene Oxide Isomerization Using MoO3-Modified Zeolite BETA. Catalysts 2020, 10, 1244. https://doi.org/10.3390/catal10111244
Vrbková E, Vyskočilová E, Lhotka M, Červený L. Solvent Influence on Selectivity in α-Pinene Oxide Isomerization Using MoO3-Modified Zeolite BETA. Catalysts. 2020; 10(11):1244. https://doi.org/10.3390/catal10111244
Chicago/Turabian StyleVrbková, Eva, Eliška Vyskočilová, Miloslav Lhotka, and Libor Červený. 2020. "Solvent Influence on Selectivity in α-Pinene Oxide Isomerization Using MoO3-Modified Zeolite BETA" Catalysts 10, no. 11: 1244. https://doi.org/10.3390/catal10111244
APA StyleVrbková, E., Vyskočilová, E., Lhotka, M., & Červený, L. (2020). Solvent Influence on Selectivity in α-Pinene Oxide Isomerization Using MoO3-Modified Zeolite BETA. Catalysts, 10(11), 1244. https://doi.org/10.3390/catal10111244