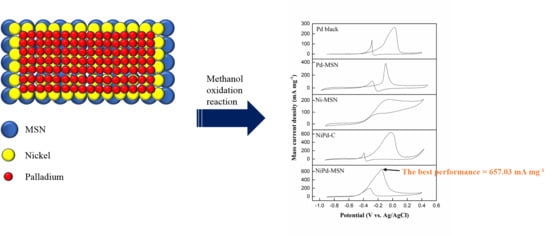
NiPd Supported on Mesostructured Silica Nanoparticle as Efficient Anode Electrocatalyst for Methanol Electrooxidation in Alkaline Media
Abstract
1. Introduction
2. Results and Discussion
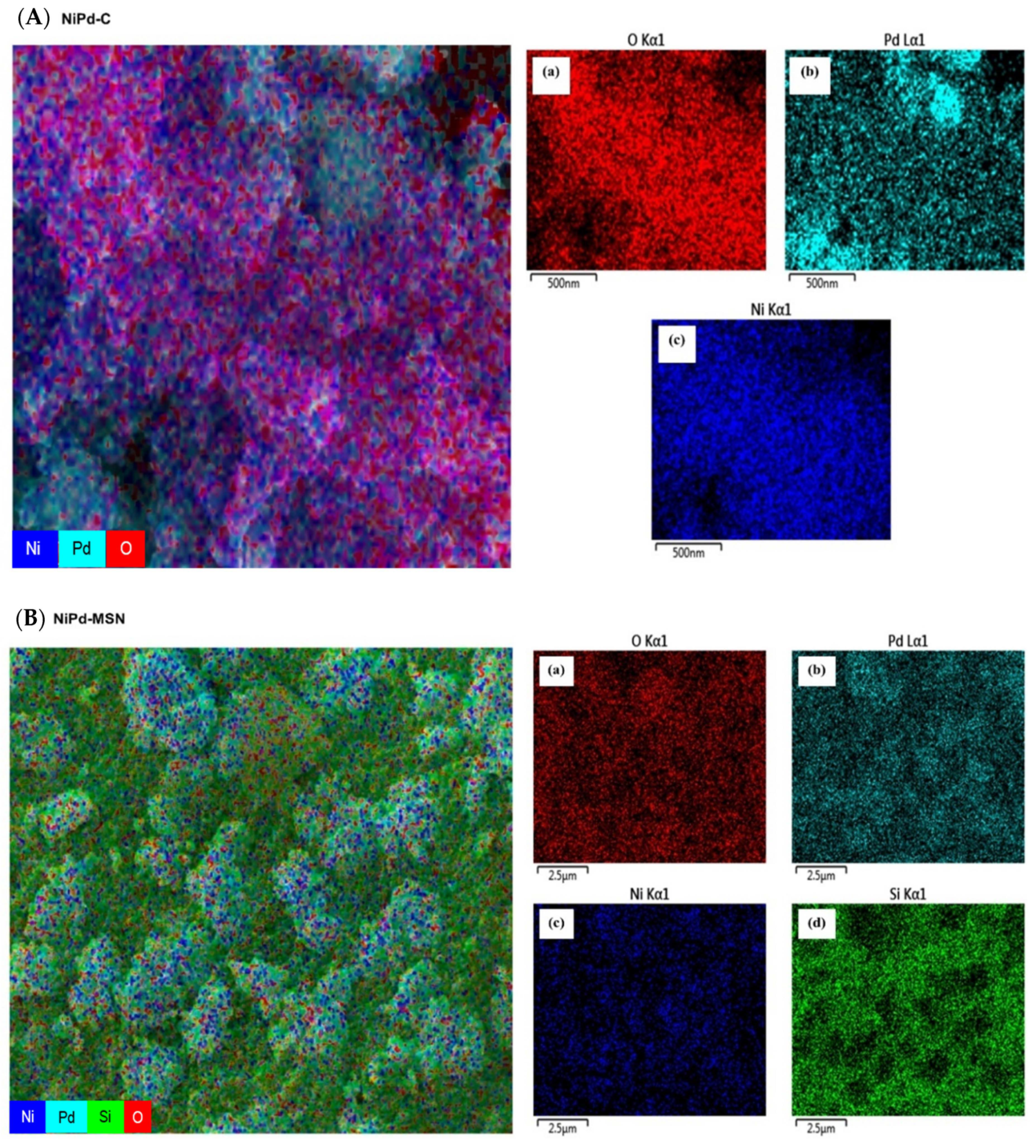
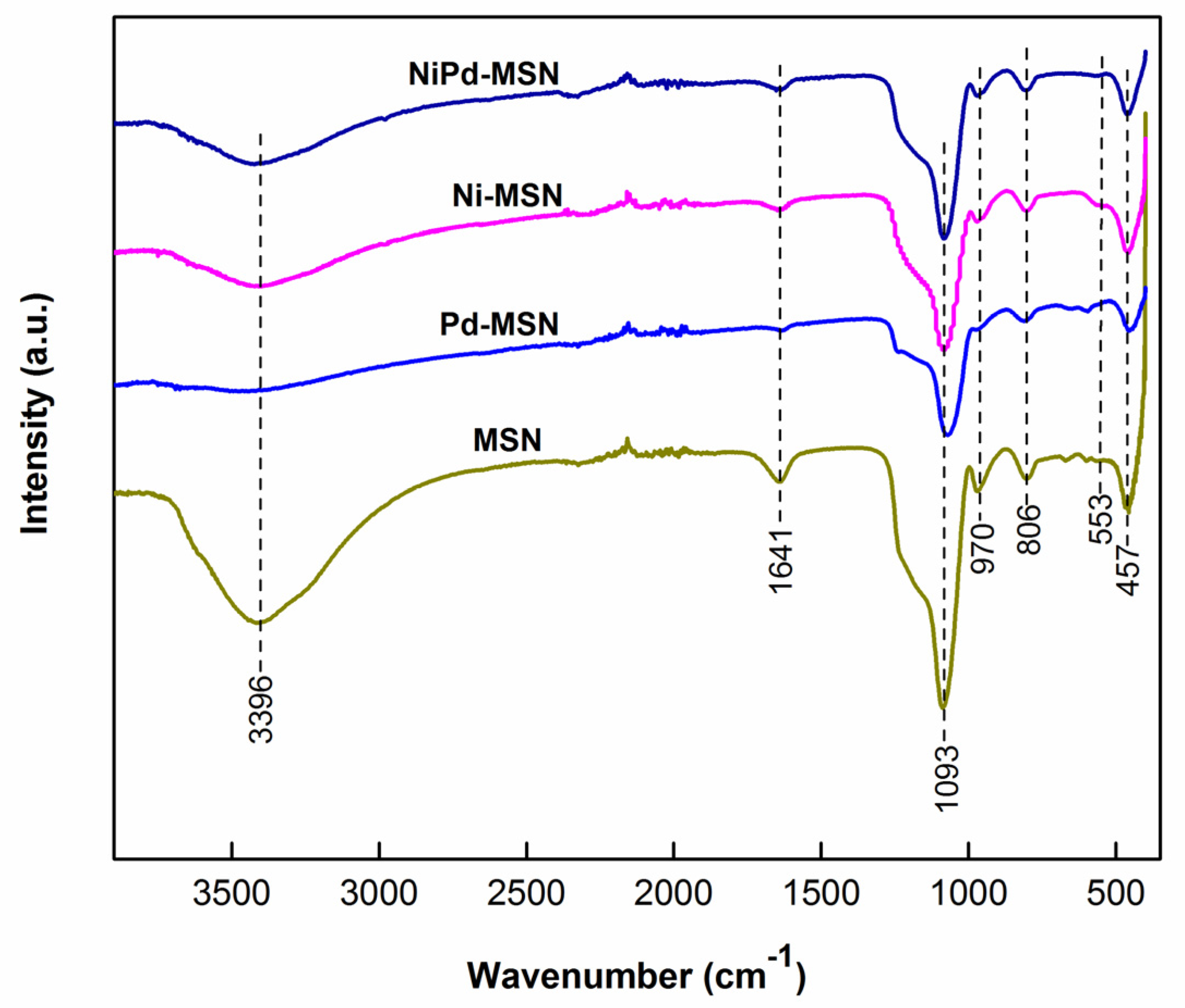
2.1. Structural Characterization
2.2. Electrochemical Characterization
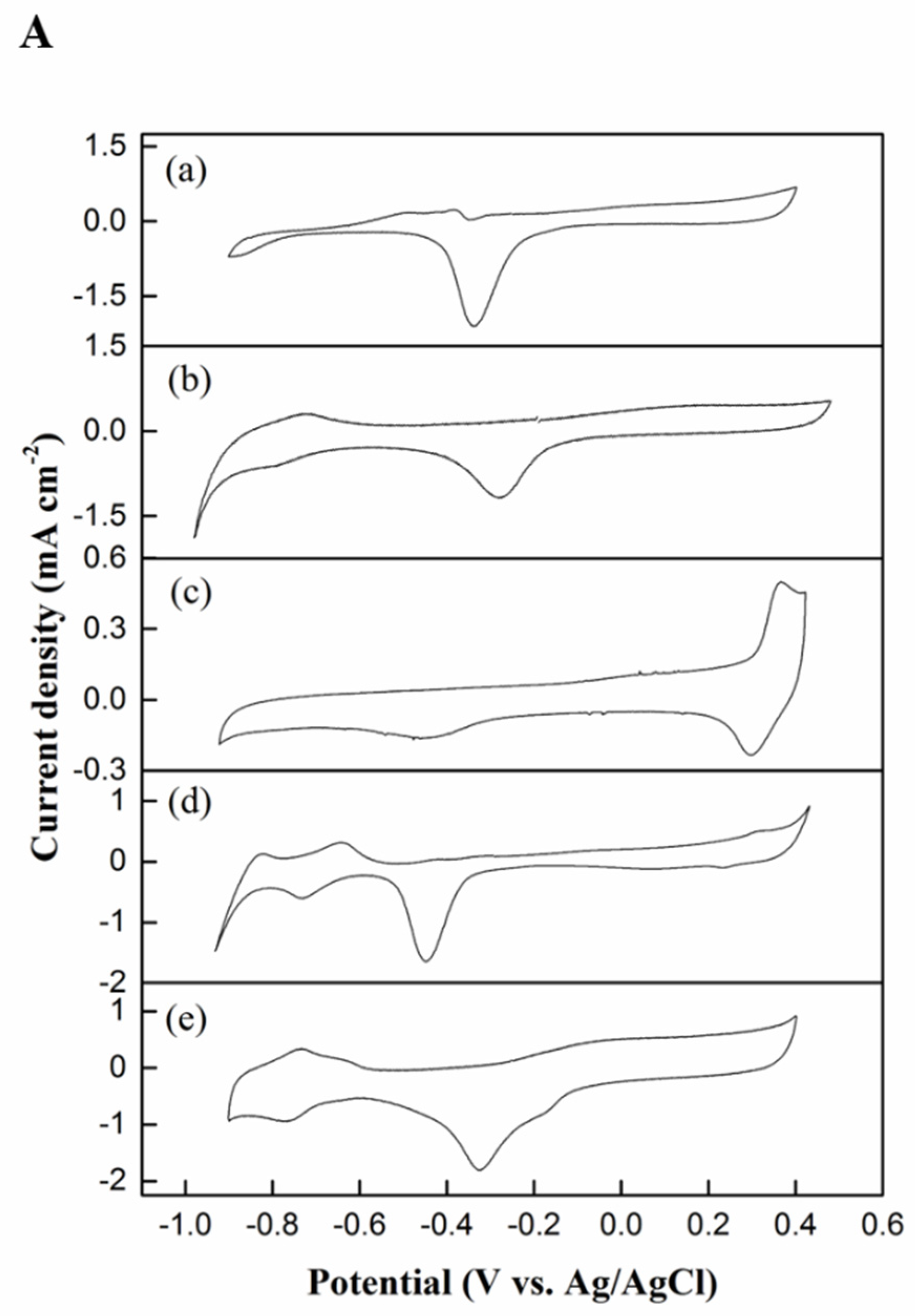
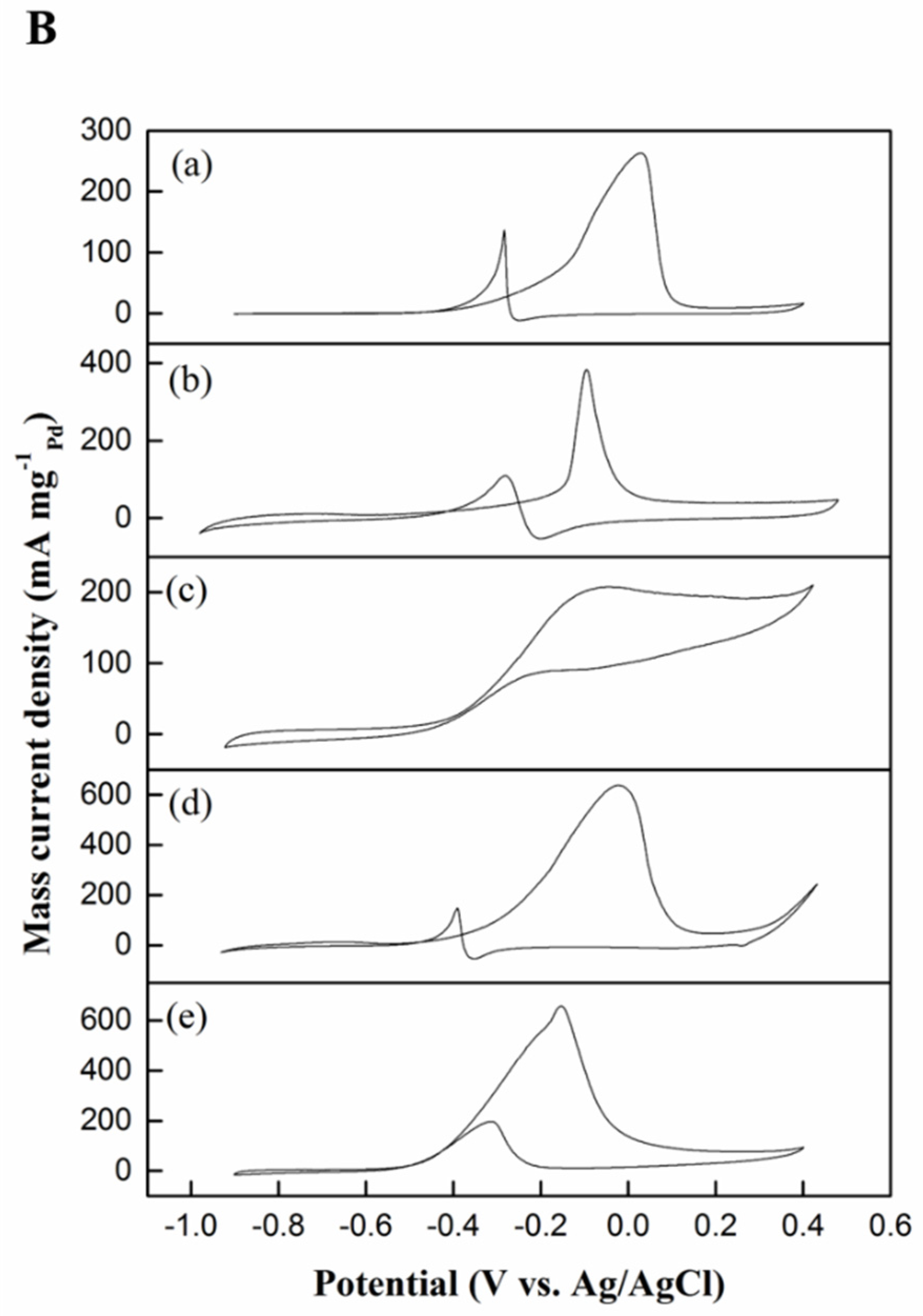
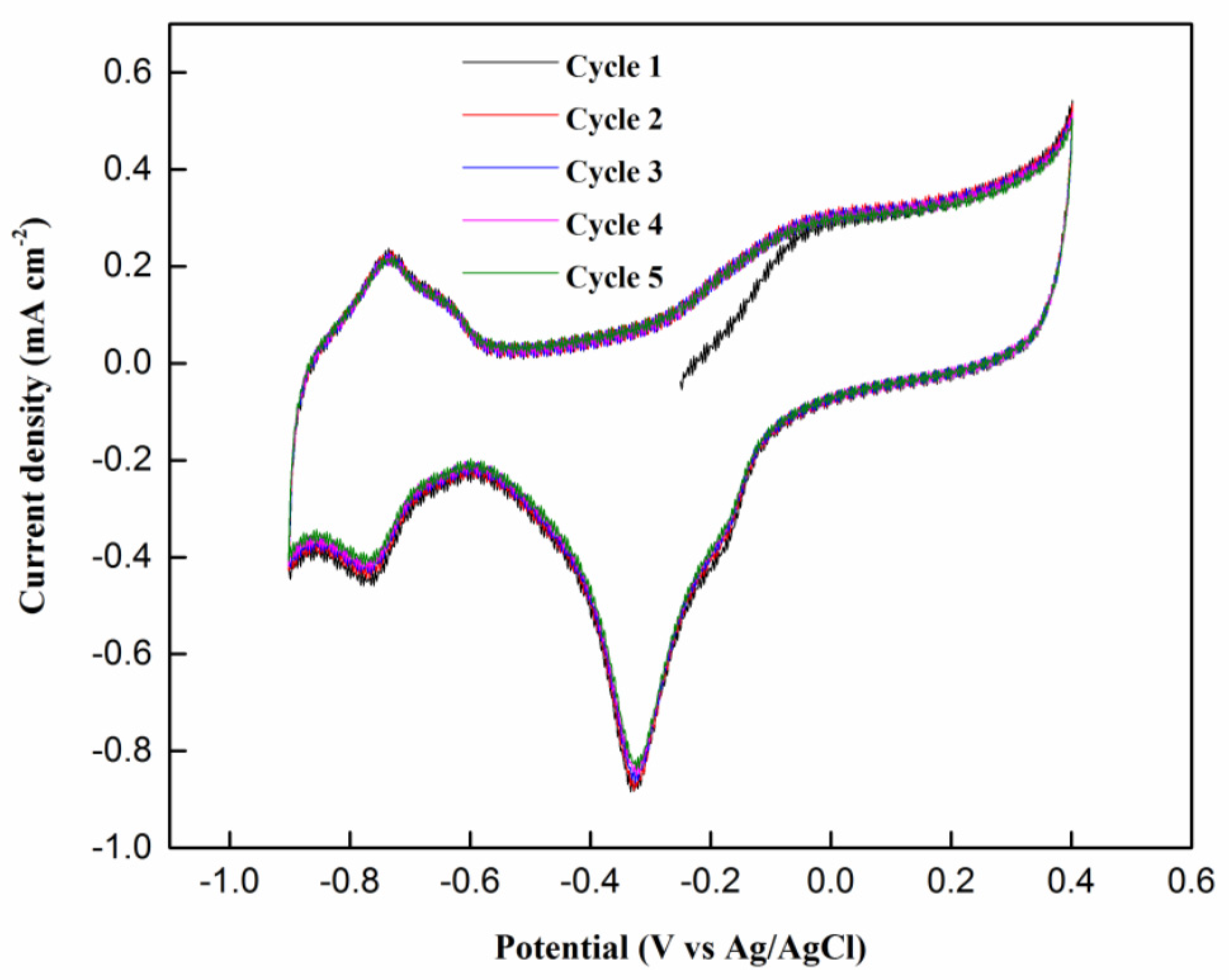
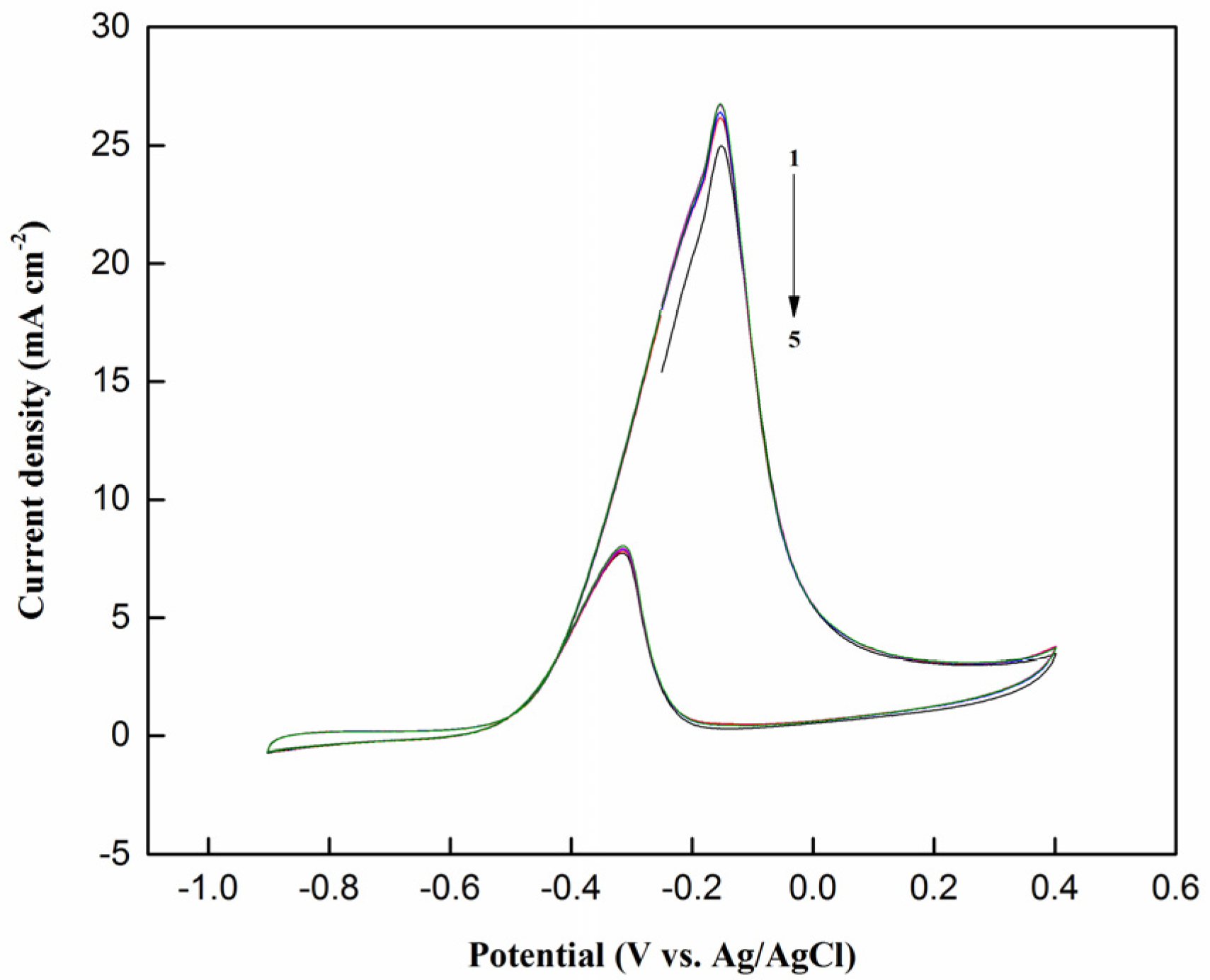
2.2.1. Cyclic Voltammetry Testing
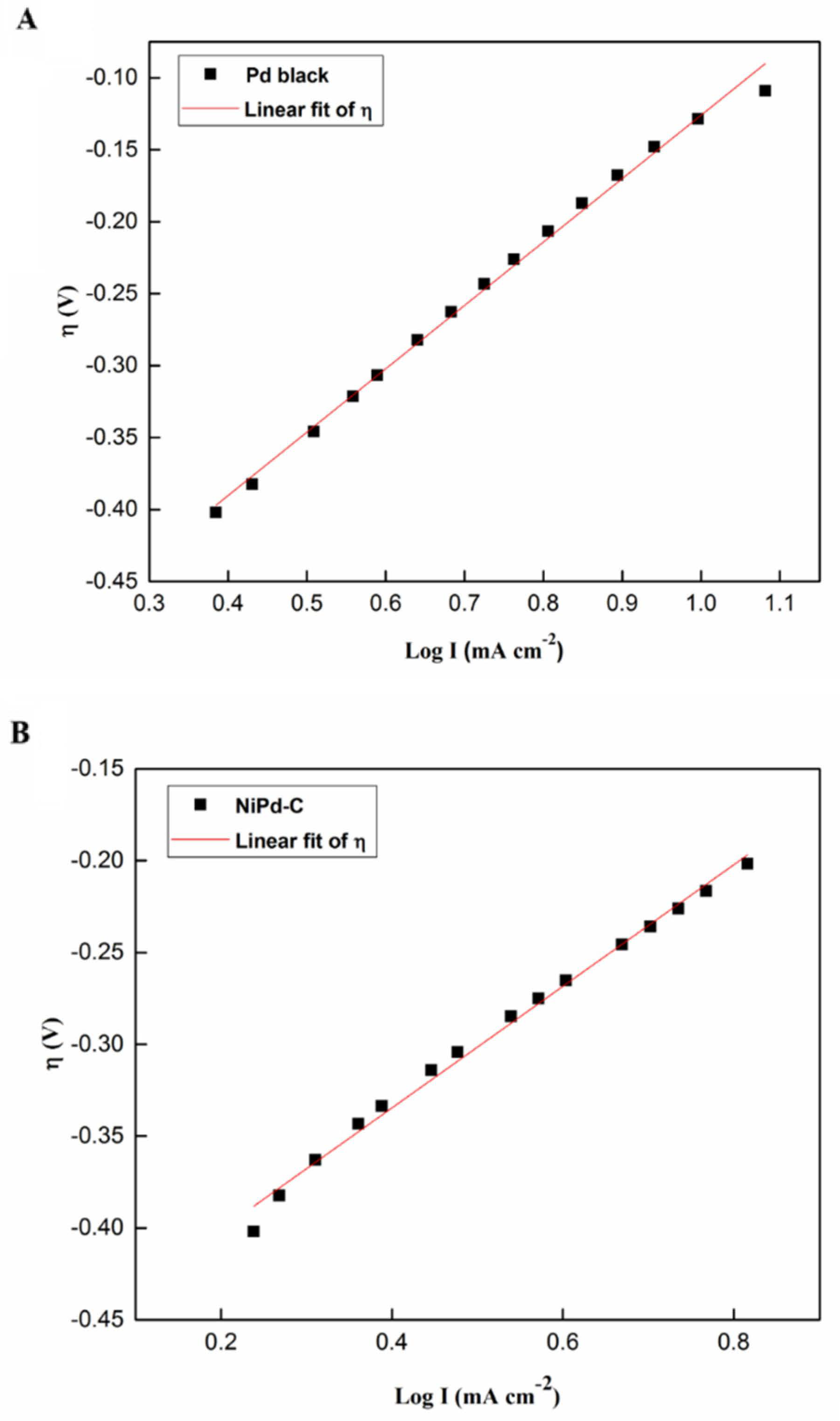
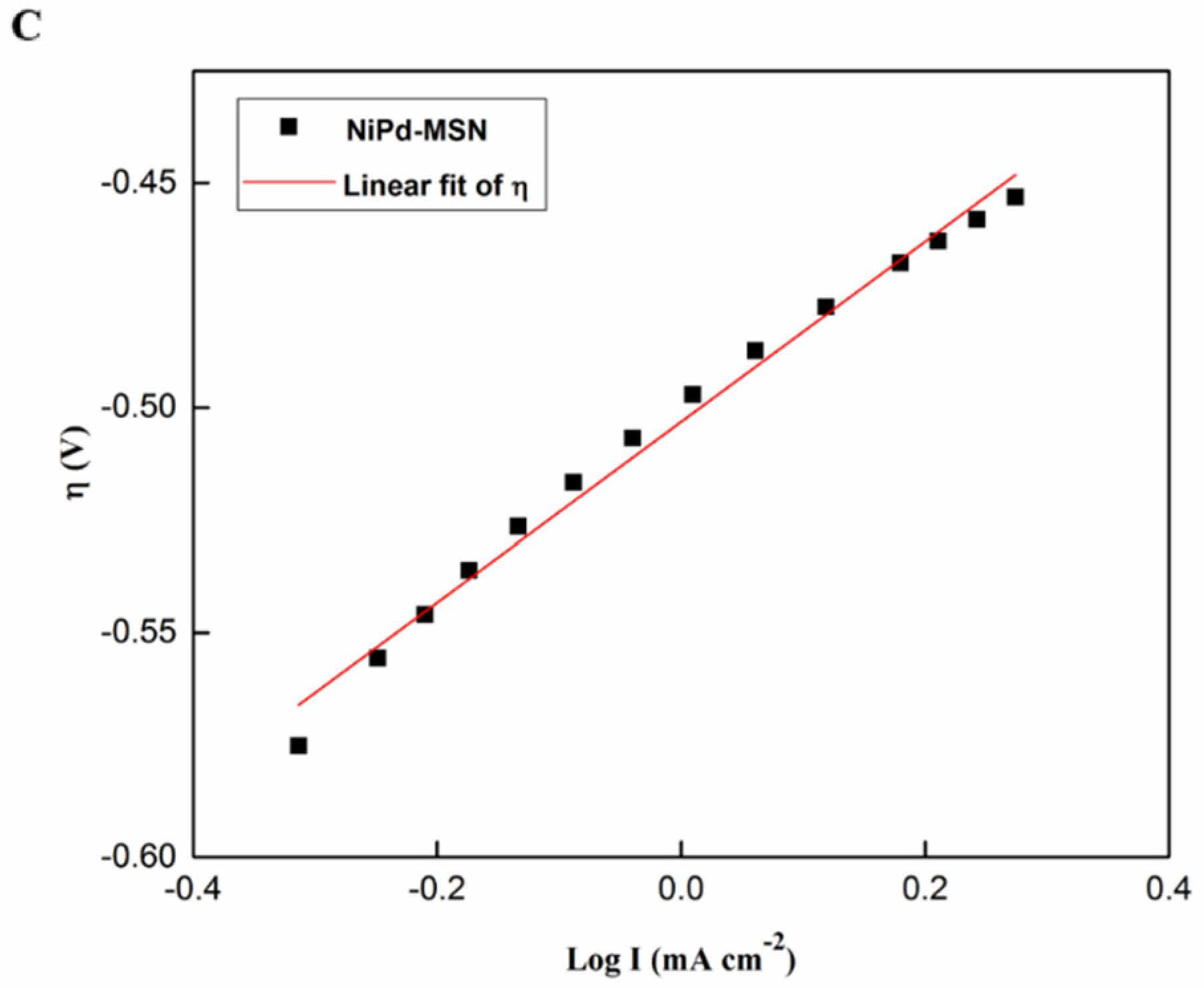
2.2.2. Tafel Slope Analysis
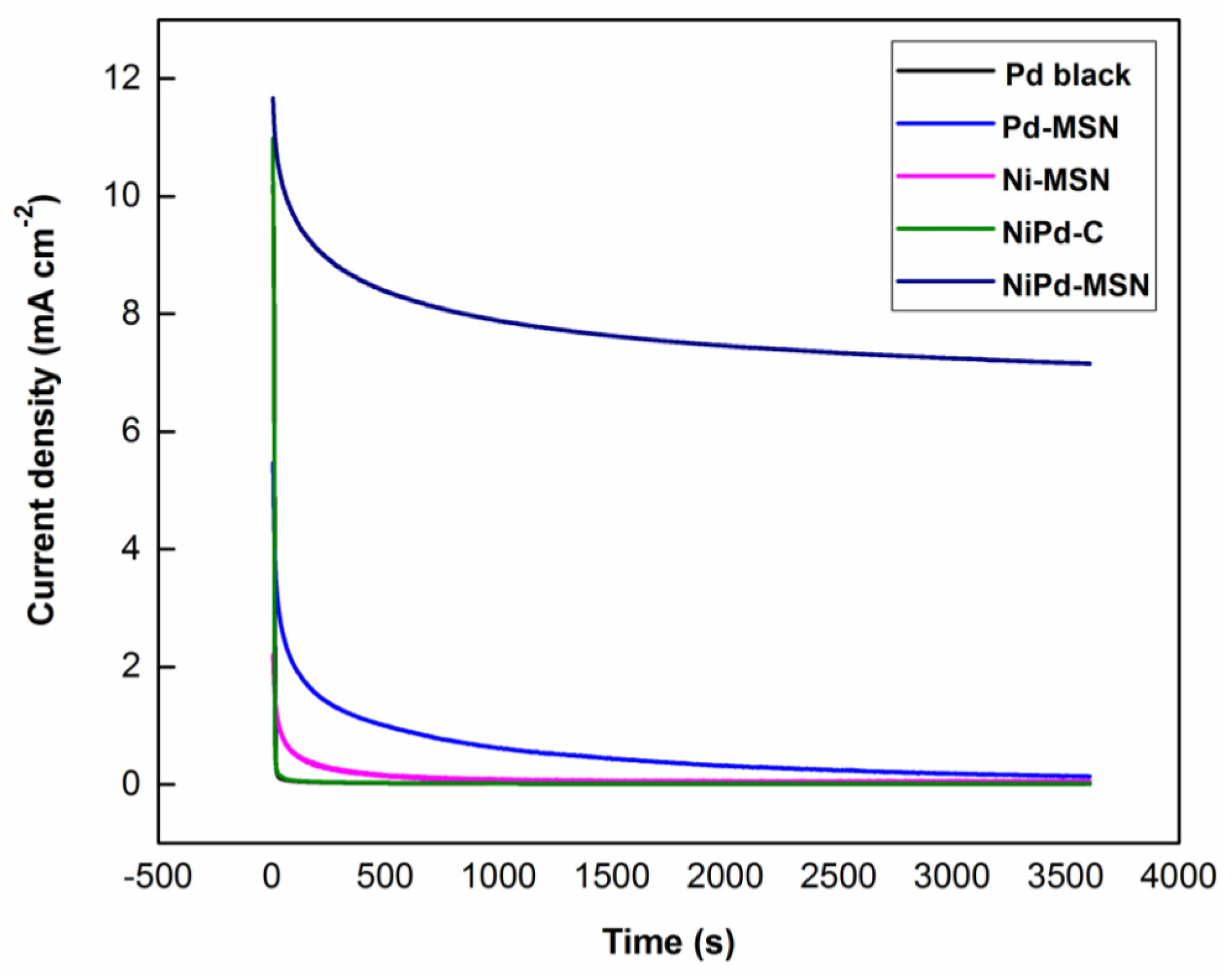
2.2.3. Chronoamperometry Testing
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Synthesis of Mesostructured Silica Nanoparticle (MSN)
3.3. Synthesis of NiPd–MSN
3.4. Physical Characterization
3.5. Electrode Preparation
3.6. Electrochemical Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, Z.B.; Yin, G.P.; Shi, P.F. Effects of ozone treatment of carbon support on Pt-Ru/C catalysts performance for direct methanol fuel cell. Carbon N. Y. 2006, 44, 133–140. [Google Scholar] [CrossRef]
- Yousaf, A.; Zaidi, S.J. Precious metal free Ni/Cu/Mo trimetallic nanocomposite supported on multi-walled carbon nanotubes as highly efficient and durable anode-catalyst for alkaline direct methanol fuel cells. J. Electroanal. Chem. 2018, 823, 98–105. [Google Scholar] [CrossRef]
- Antolini, E.; Gonzalez, E.R. Alkaline direct alcohol fuel cells. J. Power Sources 2010, 195, 3431–3450. [Google Scholar] [CrossRef]
- Geraldes, A.N.; Furtunato Da Silva, D.; Martins Da Silva, J.C.; Antonio De Sá, O.; Spinacé, E.V.; Neto, A.O.; Coelho Dos Santos, M. Palladium and palladium-tin supported on multi wall carbon nanotubes or carbon for alkaline direct ethanol fuel cell. J. Power Sources 2015, 275, 189–199. [Google Scholar] [CrossRef]
- Debe, M.K. Electrocatalyst approaches and challenges for automotive fuel cells. Nature 2012, 486, 43–51. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, X.; Tian, J.; Wang, F.; Zhan, L. Methanol electro-oxidation on Ni@Pd core-shell nanoparticles supported on multi-walled carbon nanotubes in alkaline media. Int. J. Hydrogen Energy 2010, 35, 3249–3257. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, W.; Pang, D.; Xu, Q. Methanol Electrooxidation Reaction in Alkaline Medium on Glassy Carbon Electrode Modified with Ordered Mesoporous Ni/Al2O3. Int. J. Electrochem. Sci. 2017, 12, 2194–2206. [Google Scholar] [CrossRef]
- Lu, S.; Li, H.; Sun, J.; Zhuang, Z. Promoting the methanol oxidation catalytic activity by introducing surface nickel on platinum nanoparticles. Nano Res. 2017, 11, 2058–2068. [Google Scholar] [CrossRef]
- Nieto-Monge, M.J.; Moliner, R. Palladium-nickel catalysts supported on different chemically-treated carbon blacks for methanol oxidation in alkaline media. Int. J. Hydrogen Energy 2016, 41, 19556–19569. [Google Scholar] [CrossRef]
- Jin, C.; Sun, X.; Chen, Z.; Dong, R. Electrocatalytic activity of PdNi/C catalysts for allyl alcohol oxidation in alkaline solution. Mater. Chem. Phys. 2012, 135, 433–437. [Google Scholar] [CrossRef]
- Amin, R.S.; Hameed, R.M.A.; El-khatib, K.M.; Youssef, M.E.; Elzatahry, A.A. Pt–NiO/C anode electrocatalysts for direct methanol fuel cells. Electrochim. Acta 2012, 59, 499–508. [Google Scholar] [CrossRef]
- Zhou, Y.; Gao, Y.; Liu, Y.; Liu, J. High efficiency Pt-CeO2/carbon nanotubes hybrid composite as an anode electrocatalyst for direct methanol fuel cells. J. Power Sources 2010, 195, 1605–1609. [Google Scholar] [CrossRef]
- Hu, F.; Chen, C.; Wang, Z.; Wei, G.; Kang, P. Mechanistic study of ethanol oxidation on Pd–NiO/C electrocatalyst. Electrochim. Acta 2006, 52, 1087–1091. [Google Scholar] [CrossRef]
- Wang, M.; Liu, W.; Huang, C. Investigation of PdNiO/C catalyst for methanol electrooxidation. Int. J. Hydrogen Energy 2009, 34, 2758–2764. [Google Scholar] [CrossRef]
- Ye, K.; Zhou, S.; Zhu, X.; Xu, C.; Kang, P. Stability analysis of oxide (CeO2, NiO, Co3O4 and Mn3O4) effect on Pd/C for methanol oxidation in alkaline medium. Electrochim. Acta 2013, 90, 108–111. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, X.; Hong, L. Physical and electrochemical characterizations of nanostructured Pd/C and PdNi/C catalysts for methanol oxidation. Electrochem. Commun. 2009, 11, 925–928. [Google Scholar] [CrossRef]
- Li, N.; Sun, S.; Chen, S. Studies on the role of oxidation states of the platinum surface in electrocatalytic oxidation of small primary alcohols. J. Electroanal. Chem. 1997, 430, 57–67. [Google Scholar] [CrossRef]
- Tan, J.L.; De Jesus, A.M.; Chua, S.L.; Sanetuntikul, J.; Shanmugam, S.; Tongol, B.J.V.; Kim, H. Preparation and characterization of palladium-nickel on graphene oxide support as anode catalyst for alkaline direct ethanol fuel cell. Appl. Catal. A Gen. 2017, 531, 29–35. [Google Scholar] [CrossRef]
- Mao, Y.-H.; Chen, C.-Y.; Fu, J.-X.; Lai, T.-Y.; Lu, F.-H.; Tsai, Y.-C. Electrodeposition of nickel-copper on titanium nitride for methanol electrooxidation. Surf. Coat. Technol. 2018, 350, 949–953. [Google Scholar] [CrossRef]
- Tang, D.; Zhang, L.; Zhang, Y.; Qiao, Z.A.; Liu, Y.; Huo, Q. Mesoporous silica nanoparticles immobilized salicylaldimine cobalt complexes as high efficient catalysts for polymerization of 1,3-butadiene. J. Colloid Interface Sci. 2012, 369, 338–343. [Google Scholar] [CrossRef]
- Jusoh, N.W.C.; Jalil, A.A.; Triwahyono, S.; Karim, A.H.; Salleh, N.F.; Annuar, N.H.R.; Jaafar, N.F.; Firmansyah, M.L.; Mukti, R.R.; Ali, M.W. Structural rearrangement of mesostructured silica nanoparticles incorporated with ZnO catalyst and its photoactivity: Effect of alkaline aqueous electrolyte concentration. Appl. Surf. Sci. 2015, 330, 10–19. [Google Scholar] [CrossRef]
- Sazegar, M.R.; Jalil, A.A.; Triwahyono, S.; Mukti, R.R.; Aziz, M.; Aziz, M.A.A.; Setiabudi, H.D.; Kamarudin, N.H.N. Protonation of Al-grafted mesostructured silica nanoparticles (MSN): Acidity and catalytic activity for cumene conversion. Chem. Eng. J. 2014, 240, 352–361. [Google Scholar] [CrossRef]
- Aziz, M.A.A.; Jalil, A.A.; Triwahyono, S.; Saad, M.W.A. CO2 methanation over Ni-promoted mesostructured silica nanoparticles: Influence of Ni loading and water vapor on activity and response surface methodology studies. Chem. Eng. J. 2014, 260, 757–764. [Google Scholar] [CrossRef]
- Zainoodin, A.M.; Kamarudin, S.K.; Daud, W.R.W. Electrode in direct methanol fuel cells. Int. J. Hydrogen Energy 2010, 35, 4606–4621. [Google Scholar] [CrossRef]
- Damyanova, S.; Pawelec, B.; Arishtirova, K.; Fierro, J.L.G.; Sener, C.; Dogu, T. MCM-41 supported PdNi catalysts for dry reforming of methane. Appl. Catal. B Environ. 2009, 92, 250–261. [Google Scholar] [CrossRef]
- Karim, A.H.; Jalil, A.A.; Triwahyono, S.; Sidik, S.M.; Kamarudin, N.H.N.; Jusoh, R.; Jusoh, N.W.C.; Hameed, B.H. Amino modified mesostructured silica nanoparticles for efficient adsorption of methylene blue. J. Colloid Interface Sci. 2012, 386, 307–314. [Google Scholar] [CrossRef]
- Karim, A.H.; Jalil, A.A.; Triwahyono, S.; Kamarudin, N.H.N.; Ripin, A. Influence of multi-walled carbon nanotubes on textural and adsorption characteristics of in situ synthesized mesostructured silica. J. Colloid Interface Sci. 2014, 421, 93–102. [Google Scholar] [CrossRef]
- Aziz, M.A.A.; Jalil, A.A.; Triwahyono, S.; Mukti, R.R.; Taufiq-Yap, Y.H.; Sazegar, M.R. Highly active Ni-promoted mesostructured silica nanoparticles for CO2 methanation. Appl. Catal. B Environ. 2014, 147, 359–368. [Google Scholar] [CrossRef]
- Pal, N.; Bhaumik, A. Mesoporous materials: Versatile supports in heterogeneous catalysis for liquid phase catalytic transformations. RSC Adv. 2015, 5, 24363–24391. [Google Scholar] [CrossRef]
- Yan, Z.; Meng, H.; Kang, P.; Meng, Y.; Ji, H. Effect of the templates on the synthesis of hollow carbon materials as electrocatalyst supports for direct alcohol fuel cells. Int. J. Hydrogen Energy 2011, 37, 4728–4736. [Google Scholar] [CrossRef]
- Wang, C.; Yang, F.; Yang, W.; Ren, L.; Zhang, Y.; Jia, X.; Zhang, L.; Li, Y. PdO nanoparticles enhancing the catalytic activity of Pd/carbon nanotubes for 4-nitrophenol reduction. RSC Adv. 2015, 5, 27526–27532. [Google Scholar] [CrossRef]
- Jusoh, N.W.C.; Jalil, A.A.; Triwahyono, S.; Mamat, C.R. Tailoring the metal introduction sequence onto mesostructured silica nanoparticles framework: Effect on physicochemical properties and photoactivity. Appl. Catal. A Gen. 2015, 492, 169–176. [Google Scholar] [CrossRef]
- Qi, T.; Sun, J.; Yang, X.; Yan, F.; Zuo, J. Effects of Chemical State of the Pd Species on H2 Sensing Characteristics of PdOx/SnO2 Based Chemiresistive Sensors. Sensors 2019, 19, 3131. [Google Scholar] [CrossRef]
- Rodríguez, J.L.; Valenzuela, M.A.; Poznyak, T.; Lartundo, L.; Chairez, I. Reactivity of NiO for 2,4-D degradation with ozone: XPS studies. J. Hazard. Mater. 2013, 262, 472–481. [Google Scholar] [CrossRef]
- Gomez-Bolivar, J.; Mikheenko, I.P.; Orozco, R.L.; Sharma, S.; Banerjee, D.; Walker, M.; Hand, R.A.; Merroun, M.L.; Macaskie, L.E. Synthesis of Pd/Ru bimetallic nanoparticles by Escherichia coli and potential as a catalyst for upgrading 5-hydroxymethyl furfural into liquid fuel precursors. Front. Microbiol. 2019, 10, 1276. [Google Scholar] [CrossRef]
- Chao, L.; Qin, Y.; He, J.; Ding, D.; Chu, F. Robust three dimensional N-doped graphene supported Pd nanocomposite as efficient electrocatalyst for methanol oxidation in alkaline medium. Int. J. Hydrogen Energy 2017, 42, 15107–15114. [Google Scholar] [CrossRef]
- Ting, L.; Shyuan, K.; Bakar, A.; Ramli, W.; Daud, W. Synthesis of silver/nitrogen-doped reduced graphene oxide through a one-step thermal solid-state reaction for oxygen reduction in an alkaline medium. J. Power Sources 2016, 324, 412–420. [Google Scholar] [CrossRef]
- Shen, S.Y.; Zhao, T.S.; Xu, J.B.; Li, Y.S. Synthesis of PdNi catalysts for the oxidation of ethanol in alkaline direct ethanol fuel cells. J. Power Sources 2010, 195, 1001–1006. [Google Scholar] [CrossRef]
- Chen, Z.; He, Y.C.; Chen, J.H.; Fu, X.Z.; Sun, R.; Chen, Y.X.; Wong, C.P. PdCu Alloy Flower-like Nanocages with High Electrocatalytic Performance for Methanol Oxidation. J. Phys. Chem. C 2018, 122, 8976–8983. [Google Scholar] [CrossRef]
- Yahya, N.; Kamarudin, S.K.; Karim, N.A.; Basri, S.; Zanoodin, A.M. Nanostructured Pd-Based Electrocatalyst and Membrane Electrode Assembly Behavior in a Passive Direct Glycerol Fuel. Nanoscale Res. Lett. 2019, 14, 52. [Google Scholar] [CrossRef]
- Zhang, Z.; Xin, L.; Sun, K.; Li, W. Pd-Ni electrocatalysts for efficient ethanol oxidation reaction in alkaline electrolyte. Int. J. Hydrogen Energy 2011, 36, 12686–12697. [Google Scholar] [CrossRef]
- Jurzinsky, T.; Bär, R.; Cremers, C.; Tübke, J.; Elsner, P. Highly active carbon supported palladium-rhodium Pd X Rh/C catalysts for methanol electrooxidation in alkaline media and their performance in anion exchange direct methanol fuel cells (AEM-DMFCs). Electrochim. Acta 2015, 176, 1191–1201. [Google Scholar] [CrossRef]
- Yahya, N.; Kamarudin, S.K.; Karim, N.A.; Masdar, M.S.; Loh, K.S.; Lim, K.L. Durability and performance of direct glycerol fuel cell with palladium- aurum/vapor grown carbon nanofiber support. Energy Convers. Manag. 2019, 188, 120–130. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhan, L.; Tian, J.; Nie, S.; Ning, Z. Enhanced electrocatalytic oxidation of methanol on Pd/polypyrrole-graphene in alkaline medium. Electrochim. Acta 2011, 56, 1967–1972. [Google Scholar] [CrossRef]
- Kang, Y.; Wang, W.; Pu, Y.; Li, J.; Chai, D.; Lei, Z. An effective Pd-NiOx-P composite catalyst for glycerol electrooxidation: Co-existed phosphorus and nickel oxide to enhance performance of Pd. Chem. Eng. J. 2017, 308, 419–427. [Google Scholar] [CrossRef]
- Niu, X.; Zhao, H.; Lan, M. Palladium deposits spontaneously grown on nickel foam for electro-catalyzing methanol oxidation: Effect of precursors. J. Power Sources 2016, 306, 361–368. [Google Scholar] [CrossRef]
- Zheng, J.N.; He, L.L.; Chen, F.Y.; Wang, A.J.; Xue, M.W.; Feng, J.J. A facile general strategy for synthesis of palladium-based bimetallic alloyed nanodendrites with enhanced electrocatalytic performance for methanol and ethylene glycol oxidation. J. Mater. Chem. A 2014, 2, 12899–12906. [Google Scholar] [CrossRef]
- Habibi, B.; Mohammadyari, S. Facile synthesis of Pd nanoparticles on nano carbon supports and their application as an electrocatalyst for oxidation of ethanol in alkaline media: The effect of support. Int. J. Hydrogen Energy 2015, 40, 10833–10846. [Google Scholar] [CrossRef]
- Geraldes, A.N.; Da Silva, D.F.; Pino, E.S.; Da Silva, J.C.M.; De Souza, R.F.B.; Hammer, P.; Spinacé, E.V.; Neto, A.O.; Linardi, M.; Dos Santos, M.C. Ethanol electro-oxidation in an alkaline medium using Pd/C, Au/C and PdAu/C electrocatalysts prepared by electron beam irradiation. Electrochim. Acta 2013, 111, 455–465. [Google Scholar]
- Xu, J.B.; Zhao, T.S.; Li, Y.S.; Yang, W.W. Synthesis and characterization of the Au-modified Pd cathode catalyst for alkaline direct ethanol fuel cells. Int. J. Hydrogen Energy 2010, 5, 3–10. [Google Scholar] [CrossRef]
- Mukherjee, P.; Bagchi, J.; Dutta, S.; Bhattacharya, S.K. The nickel supported platinum catalyst for anodic oxidation of ethanol in alkaline medium. Appl. Catal. A Gen. 2015, 506, 220–227. [Google Scholar] [CrossRef]
- Baronia, R.; Goel, J.; Tiwari, S.; Singh, P.; Singh, D.; Singh, S.P.; Singhal, S.K. Efficient electro-oxidation of methanol using PtCo nanocatalysts supported reduced graphene oxide matrix as anode for DMFC. Int. J. Hydrogen Energy 2017, 42, 3–12. [Google Scholar] [CrossRef]
- Semasko, M.; Tamasauskaite-Tamasiunaite Kepeniene, V.; Balciunaite, A.; Vaiciuniene, J.; Drabavicius, A.; Norkus, E. Evaluation of Activity of Platinum-Ruthenium-Cobalt/Graphene Catalysts towards Methanol Oxidation. ECS Trans. 2015, 68, 55–62. [Google Scholar] [CrossRef]
- Liang, Z.X.; Zhao, T.S.; Xu, J.B.; Zhu, L.D. Mechanism study of the ethanol oxidation reaction on palladium in alkaline media. Electrochim. Acta 2009, 54, 2203–2208. [Google Scholar] [CrossRef]
- Ren, Y.; Zhang, S.; Li, H. Electro-oxidation of methanol on SnO2-promoted Pd/MWCNTs catalysts in alkaline solution. Int. J. Hydrogen Energy 2014, 39, 288–296. [Google Scholar] [CrossRef]
- Abdullah, N.; Kamarudin, S.K. Novel Anodic Catalyst Support for Direct Methanol Fuel Cell: Characterizations and Single-Cell Performances. Nanoscale Res. Lett. 2018, 13, 90. [Google Scholar] [CrossRef]
- Dinesh, B.; Saraswathi, R. Enhanced performance of Pt and Pt–Ru supported PEDOT–RGO nanocomposite towards methanol oxidation. Int. J. Hydrogen Energy 2016, 41, 13448–13458. [Google Scholar] [CrossRef]
- Jurzinsky, T.; Kammerer, P.; Cremers, C.; Pinkwart, K.; Tübke, J. Investigation of ruthenium promoted palladium catalysts for methanol electrooxidation in alkaline media. J. Power Sources 2016, 303, 182–193. [Google Scholar] [CrossRef]
- Qi, Z.; Geng, H.; Wang, X.; Zhao, C.; Ji, H.; Zhang, C.; Xu, J.; Zhang, Z. Novel nanocrystalline PdNi alloy catalyst for methanol and ethanol electro-oxidation in alkaline media. J. Power Sources 2011, 196, 5823–5828. [Google Scholar] [CrossRef]
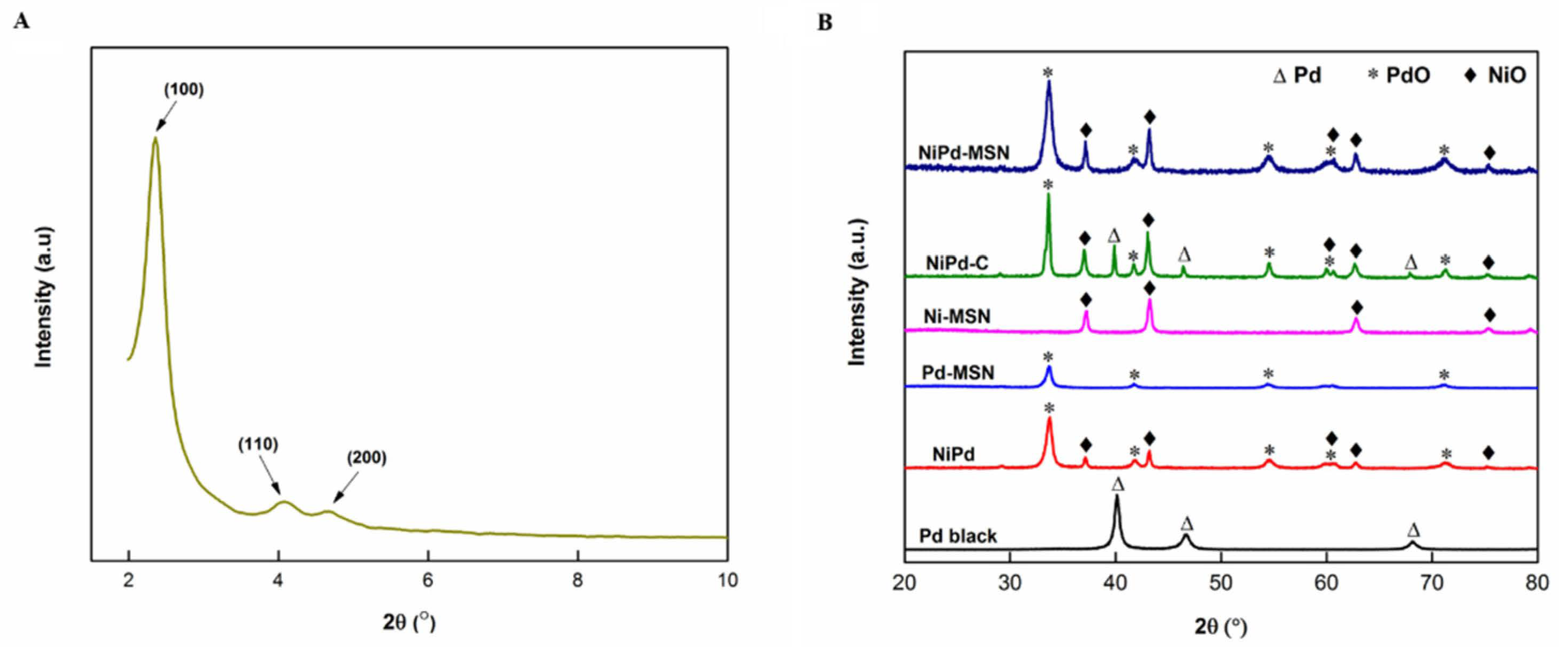


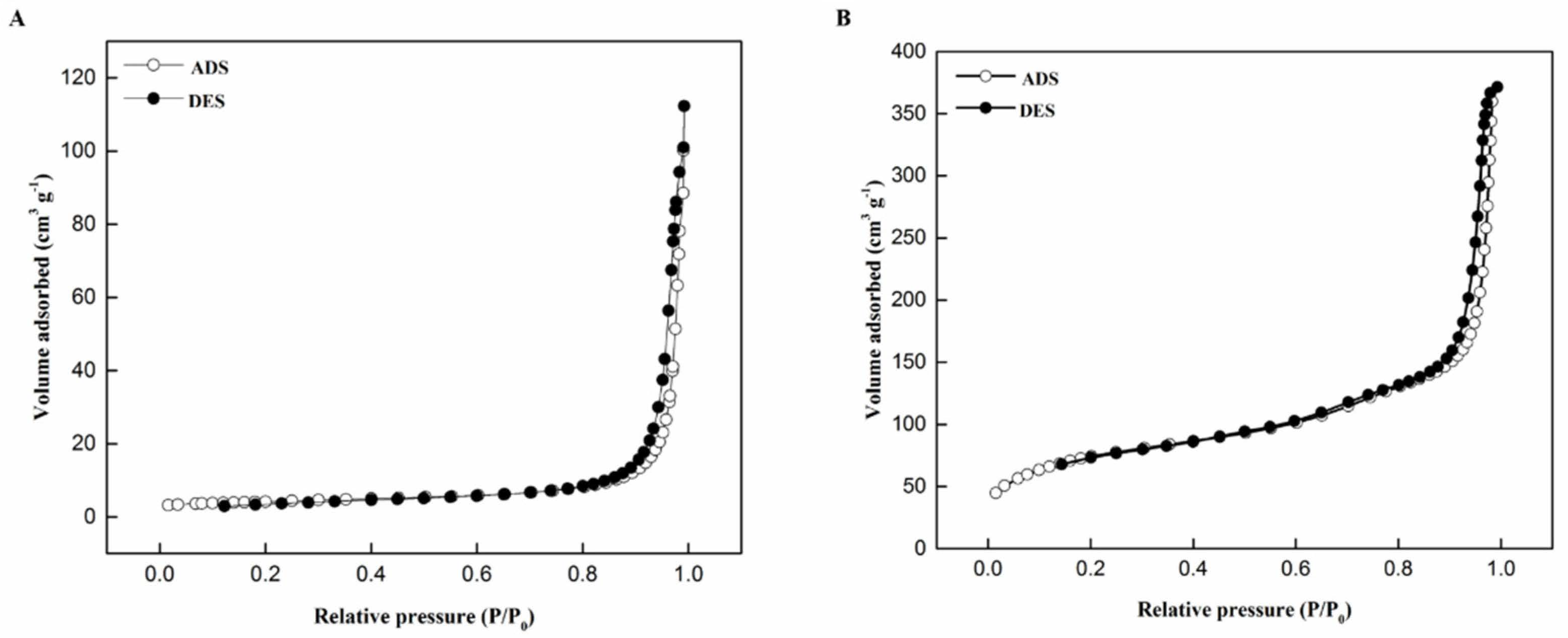
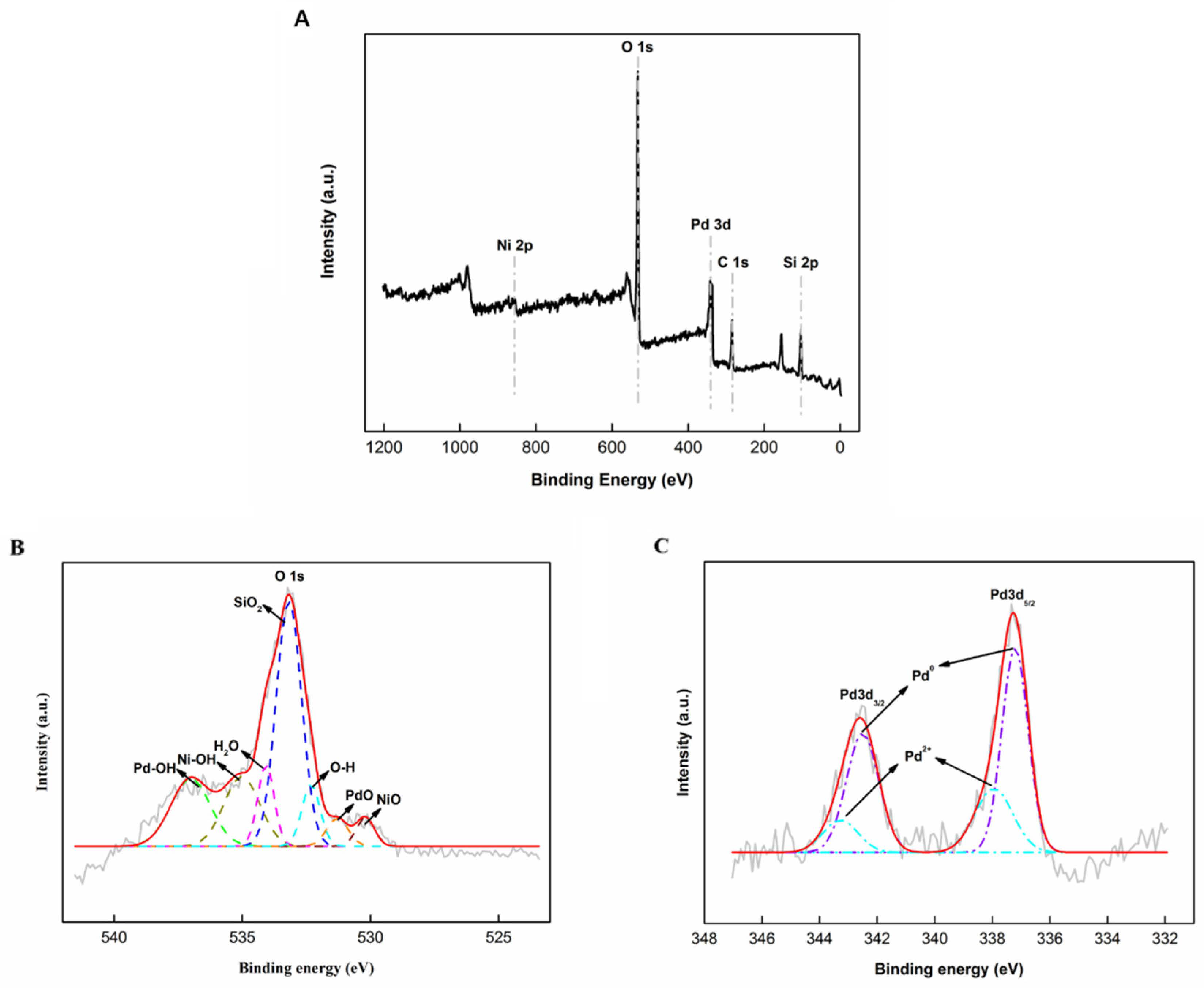

| Catalyst | SBET (m2 g−1) | VTotal (cm3 g−1) | DPore (nm) |
|---|---|---|---|
| MSN | 1001.8 | 0.813 | 3.25 |
| NiPd–C | 14.4 | 0.137 | 3.82 |
| NiPd–MSN | 270.8 | 0.532 | 7.85 |
| Electrocatalyst | ECSA (m2 g−1) | Onset Potential (V) | Oxidation Peak Current, IF (mA mg−1Pd) | Reduction Peak Current, IB (mA mg−1Pd) | IF/IB Ratio |
|---|---|---|---|---|---|
| Pd black | 14.95 | −0.40 | 261.60 | 90.58 | 2.88 |
| Pd–MSN | 8.37 | −0.25 | 371.90 | 106.95 | 3.47 |
| Ni–MSN | NA | −0.45 | 208.55 | 88.32 | 2.36 |
| NiPd–C | 14.50 | −0.30 | 637.59 | 150.88 | 4.22 |
| NiPd–MSN | 19.53 | −0.52 | 657.03 | 175.39 | 3.74 |
| Electrocatalyst | ECSA (m2 g−1) | Onset Potential (V) | Forward Peak Current, IF (mA mg−1) | IF/IB Ratio | Reference |
|---|---|---|---|---|---|
| Pd–NiOx–P/C | 5.76 | −0.48 | 364.00 | 1.52 | [45] |
| Pd–4–Ni foam | NA | −0.562 | 180.8 | NA | [46] |
| PdNi ND | 22.02 | −0.095 | 467.36 | NA | [47] |
| Pd–NiO/C | NA | −0.46 | 248.00 | 0.57 | [15] |
| Pd2Ni3/C | 32.90 | −0.75 | 544.22 | 1.72 | [41] |
| NiPd–C | 14.50 | −0.30 | 637.59 | 4.22 | This study |
| NiPd–MSN | 19.53 | −0.52 | 657.03 | 3.74 | This study |
| Electrocatalyst | Tafel Slope, ba (mV dec−1) | Ionic Exchange Current Density, j0 (mA cm−2) | Standard Deviation, R2 |
|---|---|---|---|
| Pd black | 440 | 0.051 | 0.995 |
| NiPd–C | 331 | 0.388 | 0.993 |
| NiPd–MSN | 201 | 0.398 | 0.989 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mansor, M.; Timmiati, S.N.; Wong, W.Y.; Mohd Zainoodin, A.; Lim, K.L.; Kamarudin, S.K. NiPd Supported on Mesostructured Silica Nanoparticle as Efficient Anode Electrocatalyst for Methanol Electrooxidation in Alkaline Media. Catalysts 2020, 10, 1235. https://doi.org/10.3390/catal10111235
Mansor M, Timmiati SN, Wong WY, Mohd Zainoodin A, Lim KL, Kamarudin SK. NiPd Supported on Mesostructured Silica Nanoparticle as Efficient Anode Electrocatalyst for Methanol Electrooxidation in Alkaline Media. Catalysts. 2020; 10(11):1235. https://doi.org/10.3390/catal10111235
Chicago/Turabian StyleMansor, Muliani, Sharifah Najiha Timmiati, Wai Yin Wong, Azran Mohd Zainoodin, Kean Long Lim, and Siti Kartom Kamarudin. 2020. "NiPd Supported on Mesostructured Silica Nanoparticle as Efficient Anode Electrocatalyst for Methanol Electrooxidation in Alkaline Media" Catalysts 10, no. 11: 1235. https://doi.org/10.3390/catal10111235
APA StyleMansor, M., Timmiati, S. N., Wong, W. Y., Mohd Zainoodin, A., Lim, K. L., & Kamarudin, S. K. (2020). NiPd Supported on Mesostructured Silica Nanoparticle as Efficient Anode Electrocatalyst for Methanol Electrooxidation in Alkaline Media. Catalysts, 10(11), 1235. https://doi.org/10.3390/catal10111235